Syringomyelia (SM) is defined as a condition that results in the development of fluid-containing cavities within the parenchyma of the spinal cord as a consequence of abnormal cerebrospinal fluid movement through the foramen magnum (1). Although the exact etiology and pathogenesis are unknown, SM is thought to develop secondary to an obstruction of cerebrospinal fluid (CSF) flow at the level of the foramen magnum (2). A cause of SM in veterinary medicine is a reduced volume of the caudal fossa secondary to an inappropriately small occipital bone (2,3). This malformation of the caudal fossa is known as a Chiari-like malformation (CM), a condition that appears similar to Chiari type I malformation in humans. Other documented etiologies causing SM in the dog include spinal trauma (4) and neoplasia in the region of the brainstem or foramen magnum (5,6). Recent data suggest that CM in the Cavalier King Charles spaniel (CKCS) is inherited (3,7). The incidence of CM in the CKCS breed is an estimated 95% and current studies suggest that SM is present in more than 50% of dogs with CM (7) with approximately 35% of affected dogs exhibiting clinical signs (2). Along with the CKCS breed, this condition has been reported in Pekingese dogs, Maltese terriers, miniature dachshunds, fox terriers, lhasa apsos, pomeranians, Yorkshire terriers, and a Samoyed dog (4,8–10). Occasionally, the presence of CM/SM is found as incidental findings while investigating another neurologic condition (11).
The 2 following cases, recently presented to the Ontario Veterinary College Teaching Hospital, illustrate the variability in clinical signs that can be observed in dogs afflicted with CM/SM.
Case 1
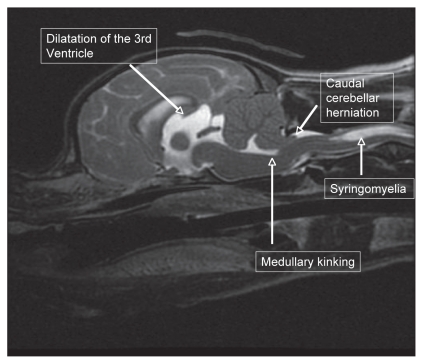
A 3-year-old, spayed female CKCS was presented with a progressive 6-month history of mild paraparesis, cervical hyperesthesia, and frequent episodes of aggressively scratching at her neck and face. Magnetic resonance imaging (MRI) of the brain revealed CM with severe cerebellar crowding secondary to a caudal occipital malformation, kinking of the brainstem, caudal cerebellar herniation, occlusion of CSF passage through the foramen magnum, and SM affecting the cervical spinal cord (Figure 1). Treatment was initiated with prednisone 0.5 mg/kg orally twice daily and gabapentin (Neurontin, Pfizer Canada, Kirkland, Quebec) 10 mg/kg orally 3 times daily. Specific to the central nervous system, corticosteroids are thought to decrease the production of phospholipase A2 (11), a powerful regulator of inflammatory cascades, inhibit the expression of cytokines, and decrease the release of substance P (13,14). Gabapentin and Pregabalin (Lyrica, Pfizer), second generation antiepileptics, have shown promise for alleviating pain within the central nervous system (15,16). While their mechanisms of analgesia remain unknown, they are believed to decrease the release of glutamate, an excitatory neurotransmitter, via binding to the alpha2-delta subunit on voltage dependant calcium channels in the dorsal horn (17,18). Convincing evidence has shown that these calcium channels are involved in the persistence of pain (19). At 1-month and 3-month recheck examinations her cervical pain had significantly improved and her scratching episodes had ceased. She has since been tapered off prednisone but remains on gabapentin.
Figure 1.
Sagittal T2-weighted magnetic resonance imaging of the brain and cranial cervical spine revealing crowding of the caudal fossa, kinking of the medulla, caudal cerebellar herniation, and syringomyelia affecting the cervical spinal cord.
Case 2
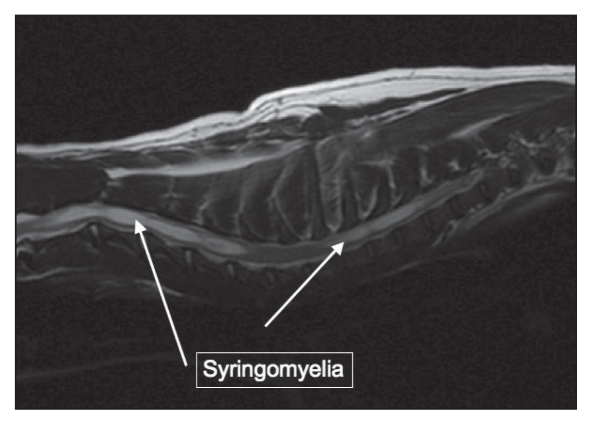
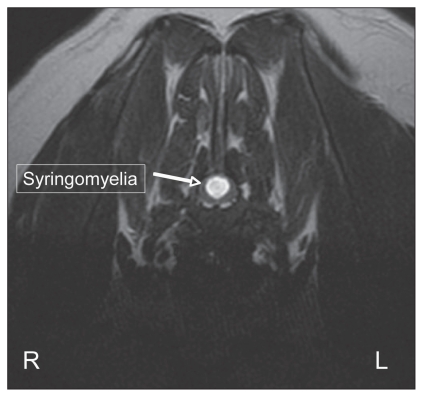
A 2-year-old, castrated male CKCS was presented with a 2-month history of progressive pelvic limb ataxia, right thoracic limb monoparesis, cervical hyperesthesia, and scratching at his right shoulder. Magnetic resonance imaging of the brain revealed CM with severe cerebellar crowding, kinking of the caudal brainstem and moderate ventricular dilatation (not shown). He was affected with severe SM, predominantly in the caudal cervical spine affecting spinal cord white and grey matter, presumably accounting for the right thoracic limb monoparesis (Figures 2 and 3). Treatment was initiated with prednisone 0.5 mg/kg orally twice daily and gabapentin 10 mg/kg orally twice daily. At a 3-month recheck examination, his pelvic limb ataxia had improved; however, he remained monoparetic in the right thoracic limb and mildly painful in his cervical spine.
Figure 2.
Sagittal T2-weighted magnetic resonance image of the cervical and cranial thoracic spinal cord revealing syringomyelia extending from C1–C5 and T1–T3.
Figure 3.
Transverse T2-weighted magnetic resonance image at the region of T1. Syringomyelia is noted causing neural destruction of both the grey and white matters of the cervical spinal cord at this region.
Discussion
Syringomyelia (SM) that accompanies a Chiari-like malformation (CM) is thought to be a consequence of abnormal CSF flow at the foramen magnum secondary to a decrease in caudal fossa volume and compression of the subarachnoid space (20–22). The causative mechanism of SM is a relative increase in pulse pressure in the spinal cord compared to that in the nearby subarachnoid space due to an induced pressure difference secondary to an obstruction in the subarachnoid space (23). Normally, the pulse pressure in the CSF is transferred to the spinal cord and equals the inherent pressure within the cord and the 2 cancel out to maintain a homeostatic environment. However, when an obstruction in the subarachnoid space is present such as a caudally displaced cerebellum in CM type I in humans, there is a mismatch of the pulse pressure between the CSF and spinal cord (24,25). This leads to a medullary-subarachnoid pressure dissociation with transiently higher pressure in the spinal cord than the CSF allowing a distention of parenchmyal tissue and injury to the tissue causing an extravasation of plasma infiltrate into the syrinx (24–26).
The most consistent clinical sign seen in SM-affected dogs is pain localized to the cervical spine (11,27,28). Many owners report that their dog exhibits postural pain characterized by sudden vocalizing after jumping (28). Syringomyelia-affected dogs behave as if they experience allodynia (pain arising in response to an otherwise nonpainful stimulus) and dysesthesia (intense burning sensation) (28). For example, affected dogs appear to dislike being touched in certain areas around their ears, neck, top of head, and they may be unable to tolerate grooming or wearing a neck collar (28). Allodynia and dysesthesia are 2 characteristics consistent with neuropathic pain syndrome described by humans affected with CM/SM (29).
Neuropathic pain syndrome, a clinical syndrome due to abnormal somatosensory processing in the peripheral and central nervous systems, is complex and relies on anatomical, physiological, and neurochemical causes. Once initially thought to be primarily due to damage to the spinothalamic tract (21,30) or the spinoreticular tract (31), researchers studying pain-related somatosensory evoked potentials following CO2 laser stimulation in humans. It was concluded that the spinothalamic tract is intact in most patients, while the cellular function of the dorsal grey horn of the spinal cord is impaired (32).
While the exact pathophysiology is unknown, there are 3 important phenomena critical to the development of neuropathic pain, central sensitization, central disinhibition, and phenotypic change (33–35). Central sensitization is the state of heightened sensitivity of dorsal grey matter neurons during repetitive C-fiber nociceptive stimulation such that their threshold of activation is reduced, and their responsiveness to synaptic inputs is augmented (36). Long-term effects of central sensitization are due to an augmented release of glutamate, an excitatory amino acid, and substance P, a peptide thought to be responsible for pain modulation and perception. These neurotransmitters activate voltage-gated calcium channels resulting in a calcium influx from voltage sensitive ion channels as well as from intracellular stores. The intracellular calcium increase results in activation of calcium-dependent kinases such as protein kinase C and A, and tyrosine kinase (37). These changes activate phosphorylation of membrane receptors and ion channels that can alter neuron excitability for minutes to hours after the initiating stimulus (38). Central disinhibition results from the loss of spinal cord inhibitory interneuron transmitters such as GABA and glycine and consequently shifts the balance towards an increased excitatory state, which can manifest clinically as spontaneous or evoked pain to an otherwise innocuous stimulus (allodynia) (36,39,40).
Currently, a diagnosis of CM and SM in dogs is made most often with MRI of the brain and spinal cord (3,4,11). Obtaining both sagittal and transverse images is necessary for identifying the characteristic changes associated with CM and for measuring the width and length of SM. Magnetic resonance imaging changes consistent with CM in dogs include a small caudal fossa secondary to a hypoplastic or dysplastic occipital bone, cerebellar crowding, compression and/or herniation of the cerebellar vermis and medulla through the foramen magnum, and minimal to absent CSF signal between the caudal cerebellum and brainstem (2,11,27,28). Additionally, kinking of the brainstem at the level of the cerebellum and ventricular dilation may be present (11,27,28). Magnetic resonance imaging findings consistent with SM in dogs include detecting a distinct cavity of fluid within the parenchyma of the spinal cord. On T1-weighted imaging this fluid will appear hypointense to isointense to surrounding spinal cord tissue and hyperintense to surrounding spinal cord tissue on T2-weighted imaging (41). Transverse and parasaggital images allow for the assessment of width, dorsal horn involvement, and longitudinal extent of the cavity (28). Maximum syrinx width is the strongest predictor of pain, scratching behavior, and scoliosis; 95% of CKCS with a maximum syrinx width of 0.64 cm or more will have associated clinical signs (28).
Treatment of dogs with CM/SM is aimed at medically or surgically relieving pain and other neurologic signs thought to be associated with the condition. Depending on the severity of clinical signs, 3 categories of drugs that have shown some benefit include analgesics (NSAIDs, Gabapentin, Tramadol), drugs targeted at decreasing CSF production (Omeprazole, Acetazolamide), and corticosteroids (1,2). For dogs that exhibit signs consistent with neurogenic pain (neck and back pain on palpation, abnormal scratching, episodes of sudden vocalizing, allodynia) the authors recommend Gabapentin at 10 mg/kg orally every 8 to 12 h as a primary treatment. Side effects include sedation and occasional vomiting and anorexia. For episodes of severe pain nonresponsive to Gabapentin, Prednisone is added at anti-inflammatory doses of 0.5 mg/kg orally every 12 to 24 h as it effectively targets pain mediators such as substance P in the central nervous system (13,14). The length of treatment depends on clinical response. While the majority of affected dogs are maintained for the long-term on Gabapentin, Prednisone is usually discontinued after approximately 7 to 10 d, as long as the clinical symptoms are controlled. However, for recurrent episodes of acute and severe neck and back pain, Prednisone is continued for a longer period of time at the lowest acceptable dose. Owners with dogs that are severely affected and/or are unresponsive to medical management may consider surgery.
Surgical management is frequently performed in humans with CM/SM to alleviate clinical symptoms (42). The most common procedure is a foramen magnum (FM) decompression (suboccipital decompression). This procedure involves the removal of a portion of the supraoccipital bone overlying the cerebellum and the rostral extent of C1. The largest case series of dogs undergoing this procedure reported 81.25% of dogs had improvement or resolution of clinical signs (43). However, the study also found that 25% of dogs undergoing suboccipital decompression had a recurrence of clinical signs within the follow-up period, and this recurrence was presumed to be due to scar tissue formation at the surgical site. This recurrence rate is consistent with that reported in humans (42). A modified surgical technique, FM decompression with cranioplasty, aims to alleviate scar tissue formation by securing a titanium mesh to the perimeter of the supraoccipital craniectomy (43). Early reports of this procedure are favorable; however, long-term data are unknown.
Overall, the prognosis for CM/SM-affected dogs depends on the severity of clinical signs and on the response to medication. Chiari-like malformation and syringomyelia is a progressive condition in those dogs that are affected clinically. Some dogs will need constant dose adjustments to adequately treat their symptoms. Unfortunately, some dogs afflicted with severe and disabling pain do not respond to medical management and are not surgical candidates, in which cases a thorough evaluation of their quality of life is necessary.
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office ( hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Rusbridge C, Jeffery ND. Pathophysiology and treatment of neuropathic pain associated with syringomyelia. Vet J. 2008;175:164–172. doi: 10.1016/j.tvjl.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Rusbridge C, Greitz D, Iskandar BJ. Syringomyelia: Current concepts in pathogensis, diagnosis and treatment. JVIM. 2006;20:469–479. doi: 10.1892/0891-6640(2006)20[469:sccipd]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 3.Rusbridge C, Knowler SP. Inheritance of occipital bone hypoplasia (Chiari type I malformation) in Cavalier King Charles spaniels. J Vet Intern Med. 2004;18:673–678. doi: 10.1892/0891-6640(2004)18<673:ioobhc>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 4.Taga A, Taura T, Nakaichi N, Wada N, Hasegawa T. Magnetic resonance imaging of syringomyelia in five dogs. J Small Anim Prac. 2000;41:362–365. doi: 10.1111/j.1748-5827.2000.tb03221.x. [DOI] [PubMed] [Google Scholar]
- 5.Jung DI, Park C, Kang BT, et al. Acquired cervical syringomyelia secondary to a brainstem meningioma in a maltese dog. Vet Med Sci. 2006;68:1235–1238. doi: 10.1292/jvms.68.1235. [DOI] [PubMed] [Google Scholar]
- 6.Costa RC, Parent JM, Poma R, Duque MC. Cervical syringohydromyelia secondary to a brainstem tumor in a dog. J Am Vet Med Assoc. 2004;225:1061–1064. doi: 10.2460/javma.2004.225.1061. [DOI] [PubMed] [Google Scholar]
- 7.Rusbridge C, Knowler SP. Hereditary aspects of occipital bone hypoplasia and syringomyelia (chiari-like malformation) in Cavalier King Charles spaniels. Vet Rec. 2003;153:107–112. doi: 10.1136/vr.153.4.107. [DOI] [PubMed] [Google Scholar]
- 8.Cauzinille L, Kornegay JN. Acquired syringomyelia in a dog. J Am Vet Med Assoc. 1992;201:1225–1228. [PubMed] [Google Scholar]
- 9.Schmahl W, Kaiser E. Hydrocephalus, syringomyelia, and spinal cord angiodysgenesis in a lhasa apso dog. Vet Pathol. 1984;21:252–254. doi: 10.1177/030098588402100220. [DOI] [PubMed] [Google Scholar]
- 10.Furneaux RW, Doige CE, Kaye MM. Syringomyelia and spina bifida occulta in a Samoyed dog. Can Vet J. 1973;14:317–321. [PMC free article] [PubMed] [Google Scholar]
- 11.Lu D, Lamb CR, Pfeiffer M, Targett P. Neurologic signs and results of magnetic resonance imaging in 40 Cavalier King Charles spaniels with chiari type I-like malformations. Vet Rec. 2003:260–263. doi: 10.1136/vr.153.9.260. [DOI] [PubMed] [Google Scholar]
- 12.Nolon AM. Pharmacology of analgesic drugs. In: Flecknell PA, Waterman-Pearson A, editors. Pain Management in Animals. WB Saunders; London: 2000. pp. 21–52. [Google Scholar]
- 13.Barnes PJ. Anti-inflammatory actions of glucocorticoids: Molecular mechanisms. Clin Sci. 1998;94:557–572. doi: 10.1042/cs0940557. [DOI] [PubMed] [Google Scholar]
- 14.Wong HK, Tan KJ. Effects of corticosteroids on nerve root recovery after spinal nerve root compression. Clin Orthop. 2002;403:248–252. doi: 10.1097/00003086-200210000-00036. [DOI] [PubMed] [Google Scholar]
- 15.Backonja MM. Use of anticonvulsants for the treatement of neuropathic pain. Neurology. 2002;59:14–17. doi: 10.1212/wnl.59.5_suppl_2.s14. [DOI] [PubMed] [Google Scholar]
- 16.Gray P, William B, Cramond T. Successful use of gabapentin in acute pain management following burn injury: A case series. Pain Med. 2008;9:371–376. doi: 10.1111/j.1526-4637.2006.00149.x. [DOI] [PubMed] [Google Scholar]
- 17.Coderre TJ, Kumar N, Lefebvre CD, Yu JS. Evidence that gabapentin reduces neuropathic pain by inhibiting the spinal release of glutamate. J Neurochem. 2005;94:1131–1139. doi: 10.1111/j.1471-4159.2005.03263.x. [DOI] [PubMed] [Google Scholar]
- 18.Gee NS, Brown JP, Dissanayake VU, Offord J, Thurlow R, Woodruff GN. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the alpha2delta subunit of a calcium channel. J Biol Chem. 1996;271:5768–5776. doi: 10.1074/jbc.271.10.5768. [DOI] [PubMed] [Google Scholar]
- 19.Shimoyama M, Shimoyama N, Hori Y. Gabapentin affects glutamatergic excitatory neurotransmission in the rat dorsal horn. Pain. 2000;85:405–414. doi: 10.1016/S0304-3959(99)00283-3. [DOI] [PubMed] [Google Scholar]
- 20.Klekamp J. The pathophysiology of syringomyelia — historical overview and current concept. Acta Neurochir (Wien) 2002;144:649–664. doi: 10.1007/s00701-002-0944-3. [DOI] [PubMed] [Google Scholar]
- 21.Milhorat TS, Capocelli AL, Anzil AP, Kotzen RM, Milhorat RH. Pathological basis of spinal cord cavitation in syringomyelia: Analysis of 105 autopsy cases. J Neurosurg. 1995;82:802–812. doi: 10.3171/jns.1995.82.5.0802. [DOI] [PubMed] [Google Scholar]
- 22.Williams H. A unifying hypothesis for hydrocephalus, chiari malformation, syringomyelia, anencephaly and spina bifida. Cerebrospinal Fluid Res. 2008;5:7. doi: 10.1186/1743-8454-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greitz D, Hellwig D, Krauss J, et al. Unraveling the riddle of syringomyelia. Neurosurg Rev. 2006;294:251–264. doi: 10.1007/s10143-006-0029-5. [DOI] [PubMed] [Google Scholar]
- 24.Josephson A, Greitz D, Klason T, Olson L, Spenger C. A spinal the-cal sac constriction model supports the theory that induced pressure gradients in the cord cause edema and cyst formation. Neurosurgery. 2001;48:636–646. doi: 10.1097/00006123-200103000-00039. [DOI] [PubMed] [Google Scholar]
- 25.Greitz D. CSF flow at the craniocervical junction: Increased systolic and diastolic pressure gradients as the cause of cystic cord lesions. In: Kenez J, editor. Imaging of the Craniocervical Junction. Udine Milano: Edizione del Centauro; 1995. pp. 19–23. [Google Scholar]
- 26.Levine D. The pathogenesis of syringomyelia associated with lesions at the foramen magnum: A critical review of existing theories and proposal of a new hypothesis. J Neurol Sci. 2004;220:3–21. doi: 10.1016/j.jns.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 27.Rusbridge C, MacSweeny J, Davies J, et al. Syringomyelia in the Cavalier King Charles spaniels. J Am Anim Hosp Assoc. 2000;36:34–41. doi: 10.5326/15473317-36-1-34. [DOI] [PubMed] [Google Scholar]
- 28.Rusbridge C, Carruthers H, Dubé MP, Holmes M, Jeffery ND. Syringomyelia in Cavalier King Charles spaniels: The relationship between syrinx dimensions and pain. J Small Anim Pract. 2007;48:432–436. doi: 10.1111/j.1748-5827.2007.00344.x. [DOI] [PubMed] [Google Scholar]
- 29.Milhorat TH, Kotzen RM, Mu HTM, Capocelli AL, Milhorat H. Dysesthetic pain in patients with syringomyelia. Neurosurgery. 1996;35:940–947. doi: 10.1097/00006123-199605000-00017. [DOI] [PubMed] [Google Scholar]
- 30.Ducreux D, Attal N, Parker F, Bouhassira D. Mechanisms of central neuropathic pain: A combined psychophysical and fMRI study in syringomyelia. Brain. 2006;129:963–976. doi: 10.1093/brain/awl016. [DOI] [PubMed] [Google Scholar]
- 31.De Broucker T, Cesaro P, Willer JC, Le Bars D. Diffuse noxious inhibitory controls in man. Involvement of the spinoreticular tract. Brain. 1990;113:1223–34. doi: 10.1093/brain/113.4.1223. [DOI] [PubMed] [Google Scholar]
- 32.Kakigi R, Shibasaki H, Kuroda Y, et al. Pain-related somatosensory evoked potentials in syringomyelia. Brain. 1991;114:1871–1889. doi: 10.1093/brain/114.4.1871. [DOI] [PubMed] [Google Scholar]
- 33.Costigan M, Woolf CJ. Pain: molecular mechanisms. J Pain. 2000;1:35–44. doi: 10.1054/jpai.2000.9818. [DOI] [PubMed] [Google Scholar]
- 34.Yaksh TL. Behavioral and autonomic correlates of the tactile evoked allodynia produced by spinal glycine inhibition: Effects of modulatory receptor systems and excitatory amino acid antagonists. Pain. 1989;37:111–123. doi: 10.1016/0304-3959(89)90160-7. [DOI] [PubMed] [Google Scholar]
- 35.Neumann S, Doubell TP, Leslie T, Woolf CJ. Inflammatory pain hypersensitivity mediated by phenotypic switch in myelinated primary sensory neurons. Nature. 1996;384:360–364. doi: 10.1038/384360a0. [DOI] [PubMed] [Google Scholar]
- 36.Woolf CJ, Salter MW. Neuronal plasticity: Increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 37.Ji RR, Woolf CJ. Neuronal plasticity and signal transduction in noci-ceptive neurons: Implications for the initiation and maintenance of pathological pain. Neurobiol Dis. 2001;8:1–10. doi: 10.1006/nbdi.2000.0360. [DOI] [PubMed] [Google Scholar]
- 38.Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306:686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- 39.Marvizón JC, Grady EF, Stefani E, Bunnett NW, Mayer EA. Substance P release in the dorsal horn assessed by receptor internalization: NMDA receptors counteract a tonic inhibition by GABA(B) receptors. Eur J Neurosci. 1999;2:417–426. doi: 10.1046/j.1460-9568.1999.00445.x. [DOI] [PubMed] [Google Scholar]
- 40.Woolf CJ. Dissecting out mechanisms responsible for peripheral neuropathic pain: Implications for diagnosis and therapy. Life Sci. 2004;74:2605–2610. doi: 10.1016/j.lfs.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 41.Grossman R, Youssem D. Neuroradiology — The Requisites. 2nd ed. Philadelphia: Mosby; 2003. Techniques in neuroimaging; pp. 1–35. [Google Scholar]
- 42.Tubbs RS, McGirt MJ, Oakes WJ. Surgical experience in 130 pediatric patients with chiari malformations. J Neurosurg. 2003;99:291–296. doi: 10.3171/jns.2003.99.2.0291. [DOI] [PubMed] [Google Scholar]
- 43.Dewey C, Berg J, Barone G, et al. Foramen magnum decompression for treatment of caudal occipital malformation syndrome in dogs. J Am Vet Med Assoc. 2005;227:1270–1275. doi: 10.2460/javma.2005.227.1270. [DOI] [PubMed] [Google Scholar]