Abstract
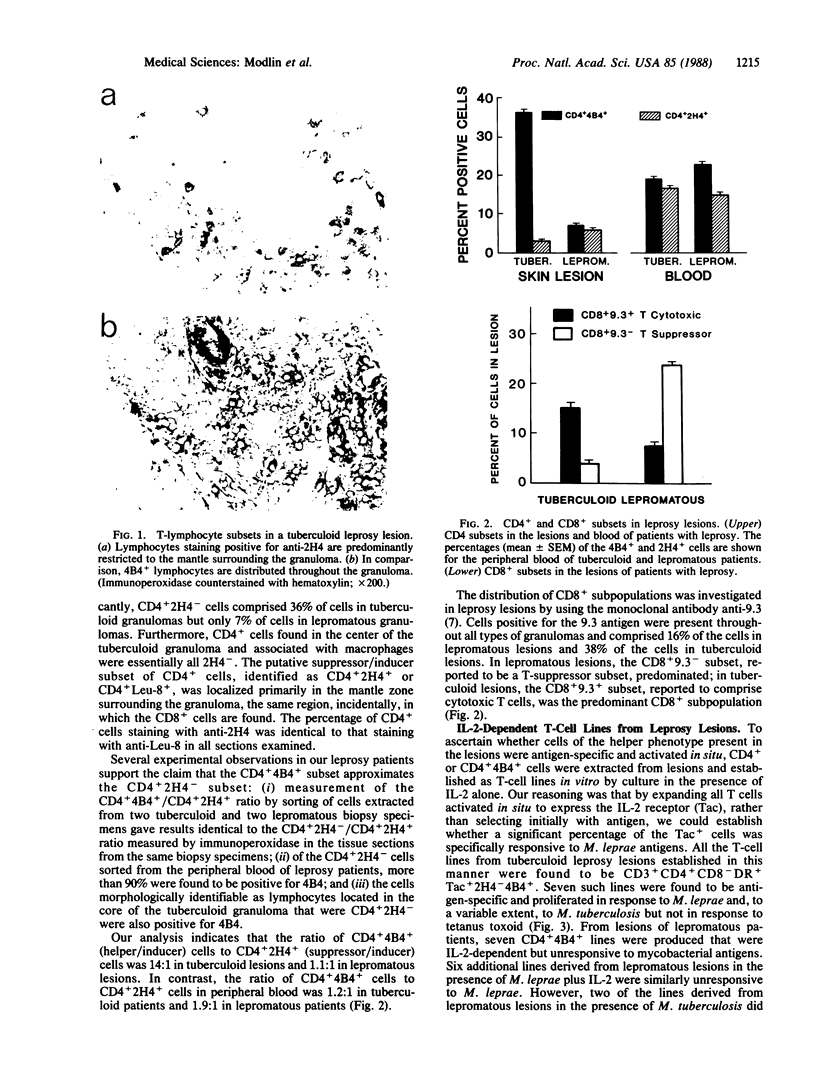
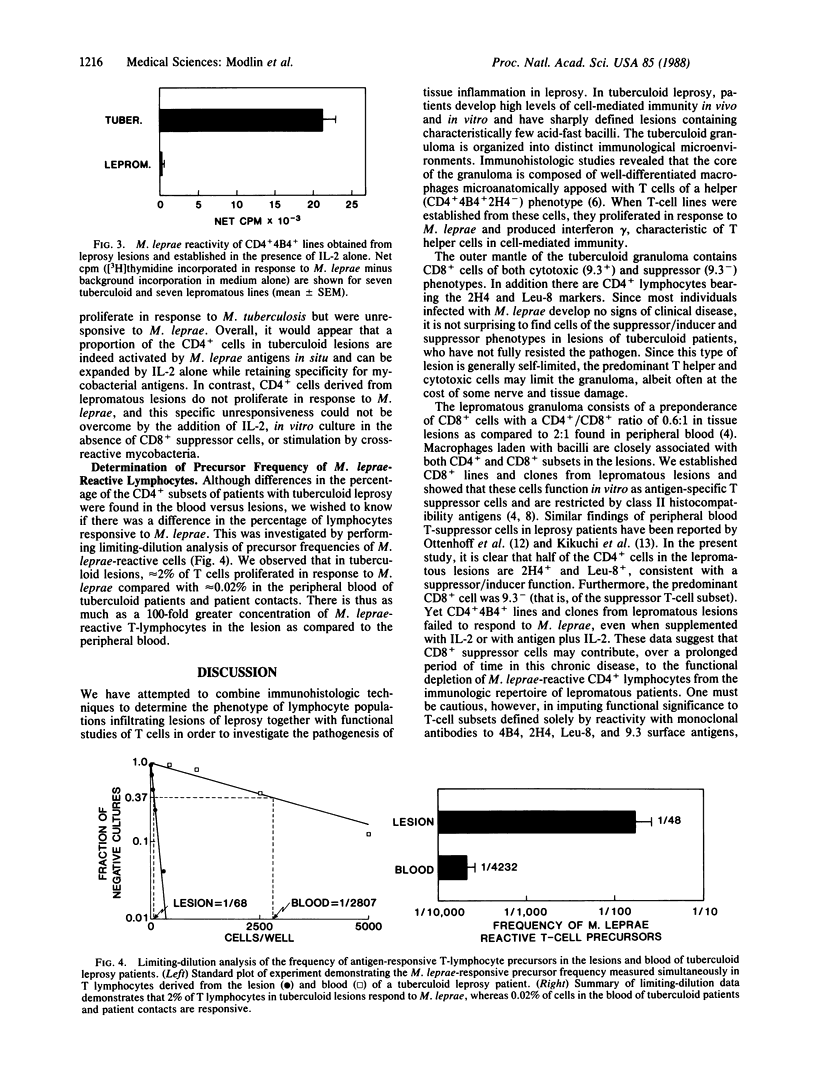
The clinical forms of leprosy constitute a spectrum that correlates closely with the degree of cell-mediated immunity. Patients with tuberculoid leprosy develop strong cell-mediated responses and have only a few, localized lesions, whereas patients with multibacillary lepromatous leprosy are specifically unresponsive to antigens of Myobacterium leprae. T cells of the CD4+ subset predominate in tuberculoid lesions, whereas CD8+ cells predominate in lepromatous lesions. Monoclonal antibodies that distinguish subpopulations of CD4+ and CD8+ cells were used to analyze the distribution of T cells infiltrating lesions across the disease spectrum. In lepromatous lesions, T cells of T-suppressor phenotype (9.3-) were the predominant CD8+ cells and suppressor/inducer cells (2H4+, Leu-8+) represented half of the CD4+ subset. In tuberculoid lesions, helper T cells (CD4+4B4+) outnumbered suppressor/inducer T cells by 14:1, compared with a ratio of 1.2:1 in peripheral blood. Analysis of the precursor frequency of antigen-reactive T cells permitted us to estimate that there was a 100-fold enrichment of T cells able to proliferate in response to M. leprae antigens in tuberculoid lesions (2/100), when compared with blood from the same patients. The methods used here to characterize the T-lymphocyte subsets and frequency of antigen-reactive T cells in leprosy may be useful in analyzing immunological reactions occurring in lesions of other inflammatory and autoimmune diseases.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Convit J., Aranzazu N., Ulrich M., Pinardi M. E., Reyes O., Alvarado J. Immunotherapy with a mixture of Mycobacterium leprae and BCG in different forms of leprosy and in Mitsuda-negative contacts. Int J Lepr Other Mycobact Dis. 1982 Dec;50(4):415–424. [PubMed] [Google Scholar]
- Damle N. K., Childs A. L., Doyle L. V. Immunoregulatory T lymphocytes in man. Soluble antigen-specific suppressor-inducer T lymphocytes are derived from the CD4+CD45R-p80+ subpopulation. J Immunol. 1987 Sep 1;139(5):1501–1508. [PubMed] [Google Scholar]
- Jalkanen S., Reichert R. A., Gallatin W. M., Bargatze R. F., Weissman I. L., Butcher E. C. Homing receptors and the control of lymphocyte migration. Immunol Rev. 1986 Jun;91:39–60. doi: 10.1111/j.1600-065x.1986.tb01483.x. [DOI] [PubMed] [Google Scholar]
- Kansas G. S., Engleman E. G. Phenotypic identification of suppressor-effector, suppressor-amplifier and suppressor-inducer T cells of B cell differentiation in man. Eur J Immunol. 1987 Apr;17(4):453–457. doi: 10.1002/eji.1830170403. [DOI] [PubMed] [Google Scholar]
- Kikuchi I., Ozawa T., Hirayama K., Sasazuki T. An HLA-linked gene controls susceptibility to lepromatous leprosy through T cell regulation. Lepr Rev. 1986 Dec;57 (Suppl 2):139–142. doi: 10.5935/0305-7518.19860064. [DOI] [PubMed] [Google Scholar]
- Modlin R. L., Hofman F. M., Horwitz D. A., Husmann L. A., Gillis S., Taylor C. R., Rea T. H. In situ identification of cells in human leprosy granulomas with monoclonal antibodies to interleukin 2 and its receptor. J Immunol. 1984 Jun;132(6):3085–3090. [PubMed] [Google Scholar]
- Modlin R. L., Hofman F. M., Taylor C. R., Rea T. H. T lymphocyte subsets in the skin lesions of patients with leprosy. J Am Acad Dermatol. 1983 Feb;8(2):182–189. doi: 10.1016/s0190-9622(83)70021-6. [DOI] [PubMed] [Google Scholar]
- Modlin R. L., Kato H., Mehra V., Nelson E. E., Fan X. D., Rea T. H., Pattengale P. K., Bloom B. R. Genetically restricted suppressor T-cell clones derived from lepromatous leprosy lesions. 1986 Jul 31-Aug 6Nature. 322(6078):459–461. doi: 10.1038/322459a0. [DOI] [PubMed] [Google Scholar]
- Modlin R. L., Mehra V., Wong L., Fujimiya Y., Chang W. C., Horwitz D. A., Bloom B. R., Rea T. H., Pattengale P. K. Suppressor T lymphocytes from lepromatous leprosy skin lesions. J Immunol. 1986 Nov 1;137(9):2831–2834. [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Boyd A. W., Hagan M., Brown H. M., Kornacki M. M., Schlossman S. F. The isolation and characterization of the human helper inducer T cell subset. J Immunol. 1985 Jun;134(6):3762–3769. [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Distaso J. A., Aldrich W. R., Schlossman S. F. The isolation and characterization of the human suppressor inducer T cell subset. J Immunol. 1985 Mar;134(3):1508–1515. [PubMed] [Google Scholar]
- Ottenhoff T. H., Elferink D. G., Klatser P. R., de Vries R. R. Cloned suppressor T cells from a lepromatous leprosy patient suppress Mycobacterium leprae reactive helper T cells. 1986 Jul 31-Aug 6Nature. 322(6078):462–464. doi: 10.1038/322462a0. [DOI] [PubMed] [Google Scholar]
- Ridley D. S., Jopling W. H. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966 Jul-Sep;34(3):255–273. [PubMed] [Google Scholar]
- Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J Immunol. 1981 Apr;126(4):1614–1619. [PubMed] [Google Scholar]
- Van Voorhis W. C., Kaplan G., Sarno E. N., Horwitz M. A., Steinman R. M., Levis W. R., Nogueira N., Hair L. S., Gattass C. R., Arrick B. A. The cutaneous infiltrates of leprosy: cellular characteristics and the predominant T-cell phenotypes. N Engl J Med. 1982 Dec 23;307(26):1593–1597. doi: 10.1056/NEJM198212233072601. [DOI] [PubMed] [Google Scholar]
- Yamada H., Martin P. J., Bean M. A., Braun M. P., Beatty P. G., Sadamoto K., Hansen J. A. Monoclonal antibody 9.3 and anti-CD11 antibodies define reciprocal subsets of lymphocytes. Eur J Immunol. 1985 Dec;15(12):1164–1168. doi: 10.1002/eji.1830151204. [DOI] [PubMed] [Google Scholar]
- van Oers M. H., Pinkster J., Zeijlemaker W. P. Quantification of antigen-reactive cells among human T lymphocytes. Eur J Immunol. 1978 Jul;8(7):477–484. doi: 10.1002/eji.1830080706. [DOI] [PubMed] [Google Scholar]