Abstract
The versatility of Ca2+ as an intracellular messenger derives largely from the spatial organization of cytosolic Ca2+ signals, most of which are generated by regulated openings of Ca2+-permeable channels. Most Ca2+ channels are expressed in the plasma membrane (PM). Others, including the almost ubiquitous inositol 1,4,5-trisphosphate receptors (IP3R) and their relatives, the ryanodine receptors (RyR), are predominantly expressed in membranes of the sarcoplasmic or endoplasmic reticulum (ER). Targeting of these channels to appropriate destinations underpins their ability to generate spatially organized Ca2+ signals. All Ca2+ channels begin life in the cytosol, and the vast majority are then functionally assembled in the ER, where they may either remain or be dispatched to other membranes. Here, by means of selective examples, we review two issues related to this trafficking of Ca2+ channels via the ER. How do cells avoid wayward activity of Ca2+ channels in transit as they pass from the ER via other membranes to their final destination? How and why do some cells express small numbers of the archetypal intracellular Ca2+ channels, IP3R and RyR, in the PM?
Local and Global Ca2+ Signals
The plasma membrane (PM)1 and the membranes of many intracellular organelles separate the cytosol, with its low resting free Ca2+ concentration of ∼100 nM, from environments with very different free Ca2+ concentrations and electrical potentials. The resulting steep Ca2+ gradients are poised to allow Ca2+ to flow rapidly down its electrochemical gradient whenever Ca2+-permeable channels within these membranes are open. For the PM and most organelles, notably the endoplasmic reticulum (ER) and organelles derived from it, the gradients are directed toward the cytosol. Regulated opening of Ca2+ channels within these membranes is the means by which most extracellular and intracellular signals evoke the increases in cytosolic Ca2+ concentration that regulate almost every aspect of cellular activity (1,2). For other membranes, the inner membrane of mitochondria and perhaps of chloroplasts (3), the Ca2+ gradients are directed away from the cytosol so that channels within these membranes, MiCa within the inner mitochondrial membrane, for example (4), mediate uptake of Ca2+ from the cytosol. Each of these membranes is also home to proteins that transport Ca2+ in the opposite direction, against its electrochemical gradient, the Ca2+-ATPases of the PM (PMCA), ER (SERCA), and the Golgi and secretory vesicles (SPCA) (5−7), for example. The competing activities of Ca2+ channels and the pumps and exchangers that move Ca2+ up its electrochemical gradient ultimately determine the cytosolic Ca2+ concentration, but it is the Ca2+-permeable channels that mediate the most rapid Ca2+ exchanges and which are most commonly acutely regulated by signaling pathways.
Each of these Ca2+-permeable channels allows Ca2+ to pass through a central pore traversing a biological membrane. They differ, however, in whether under physiological conditions they effectively allow only Ca2+ to pass [e.g., voltage-gated Ca2+ channels (Cav) and the Orai proteins that mediate store-operated Ca2+ entry] or also allow other cations to pass [e.g., IP3 receptors (IP3R), ryanodine receptors (RyR), and nicotinic acetylcholine receptors]. The difference, defined by the structure of the selectivity filter (8), is significant because it determines whether the channels, in addition to mediating Ca2+ fluxes, can also regulate membrane potential (most important at the PM) and conduct the counterions required to allow electrogenic movement of Ca2+(9).
Because the cytosol of all cells contains high concentrations of Ca2+ buffers, Ca2+ diffuses more slowly in cytosol than in free solution (10,11). An important consequence is that as Ca2+ flows rapidly through an open Ca2+ channel, it creates a local cloud with a high cytosolic Ca2+ concentration: each active channel creates its own local Ca2+ signal (12). These spatially organized Ca2+ signals are important because different Ca2+-binding proteins selectively associate with different Ca2+ channels, so that Ca2+ passing through one channel may regulate different events to Ca2+ passing through another (2). Store-operated Ca2+ entry (SOCE) (13), for example, has been reported to regulate selectively the Ca2+-sensitive adenylyl cyclases (14), nitric oxide synthase (15), and gene expression in rat basophilic leukemia cells (16). Cardiac IP3R associate with a Ca2+-regulated protein kinase that also regulates IP3R activity [Ca2+-calmodulin-dependent protein kinase IIδ (CaMKIIδ)], so that release of Ca2+ via IP3R may selectively activate an enzyme that then feeds back to inhibit IP3R activity (17). Ca2+ entry via Cav channels (Cav1 and Cav2) selectively regulates the activity of the channel itself (18). The latter highlights another key feature of many Ca2+ channels, namely their regulation by cytosolic Ca2+. This provides feedback regulation of Ca2+ signaling, and it allows Ca2+ channels to evoke regenerative Ca2+ signals (19). The latter are important because they underpin the versatility of Ca2+ as an intracellular messenger, permitting it to function either locally or globally (2). It follows from this discussion that the versatility of Ca2+ as a ubiquitous intracellular messenger derives in large part from putting Ca2+-permeable channels into the right place; only then can they deliver spatially organized Ca2+ signals.
Putting Ca2+ Channels in the Right Place
A few proteins, mostly those related to transcription and translation, but also components of a H+ channel (ATP synthase), are encoded by DNA within mitochondria and chloroplasts. These proteins are synthesized within these organelles and so reach their final destination without traversing the cytoplasm. All other proteins are encoded by nuclear DNA. These proteins are synthesized in the cytosol from where they are dispatched to other destinations. Targeting of proteins has attracted the most attention (20,21), but many proteins are guided toward their final destination before translation begins by selective targeting of mRNA (22).
After transcription and mRNA processing within the nucleus, mature mRNA is exported via nuclear pores to the cytosol. Even this step may contribute to mRNA targeting. Synthesis of mRNA encoding some subunits of nicotinic acetylcholine receptors, for example, is restricted to the nuclei lying immediately beneath the neuromuscular junction of multinucleate muscle fibers (23). More generally, transport of mRNA within the cytosol, mediated by binding of proteins to sequences within the 3′-untranslated region, probably plays the major role in selective trafficking of mRNA (22,24,25). Such transport can direct mRNA to associate with the membranes of specific organelles. The mRNAs for approximately half of all mitochondrial proteins, for example, including the mRNA for another subunit of the ATP synthase, are directed to the outer mitochondrial membrane (25). Other mRNAs are selectively addressed to nuclear membranes, the ER (26), and even to specific subdomains of the ER (27). Transport of mRNA also occurs over much larger distances, allowing its selective targeting to specific cytosolic destinations such as dendrites (24), immature axons (28), and polarized regions of developing embryos (22). The mRNAs for IP3R1 (29), glutamate receptors, and a major neuronal Ca2+ sensor (CaMKIIα) are transported to dendrites by microtubules within “mRNA granules” that include ribosomes and additional proteins. Within these “ready-to-translate” granules (27), translation is probably repressed until the complex reaches its destination, where protein synthesis can proceed close to the final destination (24,28). The advantages of selectively transporting mRNA, rather than protein, have been much discussed (22,30,31). Clearly, mRNA transport can complement the additional targeting provided by proteins, but other advantages include cost (cheaper to move mRNA than all the proteins to which it will give rise), restricting production of potentially toxic proteins to their target site, speeding the rate at which protein synthesis can adapt to local needs (e.g., within postsynaptic structures) (24), facilitating cotranslational assembly of multisubunit proteins, and establishing protein gradients, notably during development. For Ca2+ channels destined to function in membranes derived from the ER, mRNA targeting may also provide a means of ensuring that functional channels are assembled near their final destination and so spend less time en route via organelles within which they may function inappropriately.
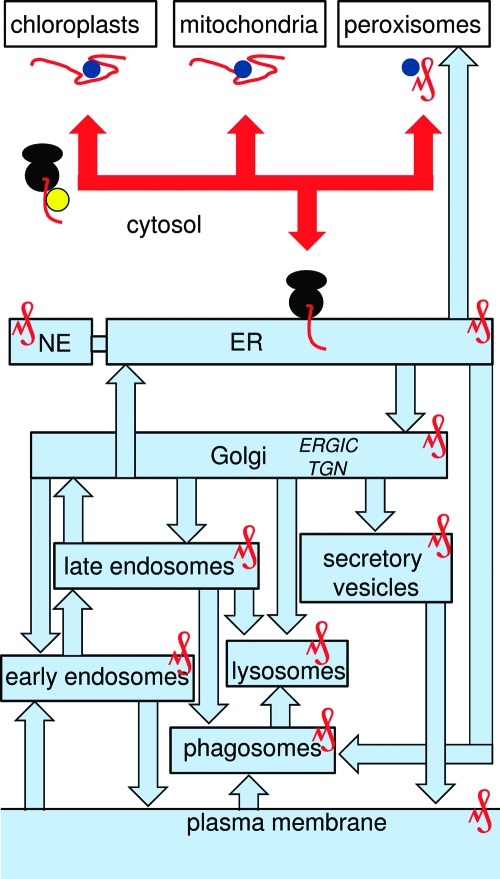
All proteins encoded by nuclear DNA, even those encoded by mRNA that associates with specific membranes, begin life in the cytoplasm where protein synthesis is initiated. Integral membrane proteins, which comprise ∼30% of all proteins, are then directed to specific intracellular membranes. These decisions are dictated by short signal sequences, address labels, within the nascent protein chain (32). The identities of these sequences are generally better understood for proteins destined for the lumen of organelles than for those targeted to membranes (20,21). The sequences, whether N-terminal (which are often cleaved later) or internal, are recognized by cytosolic proteins, which then interact with receptors in the target membrane. There are rather few immediate destinations for integral membrane proteins: the membranes of mitochondria or, in plants, of chloroplasts; peroxisomes; and the ER (Figure 1). With the exception of peroxisomes, where membrane proteins can be fully synthesized and assembled before being dispatched from the cytosol to the peroxisome import machinery (33), all membrane proteins are imported into membranes from the cytosol in a state that is at least partially unfolded. Mitochondrial and chloroplast proteins are fully translated, held in their unfolded state by association with cytosolic chaperones, and then dispatched to the appropriate membrane, where they fold, integrate into the lipid bilayer, and assemble with other proteins. For both organelles, signal sequences guide the protein through the outer and, where appropriate, inner membrane via protein translocases (“translocons”). The transmembrane regions of the folded protein can then either escape laterally from the translocase into the lipid bilayer or reinsert into the outer or inner membrane after release into the intermembrane space or matrix, respectively (34,35). The key point, in the context of this review, is that membrane proteins destined for mitochondria, chloroplasts, or peroxisomes are functionally assembled at their final destination: they need not visit other organelles once they become functional. The situation is very different for the many membranes that acquire their proteins from the ER.
Figure 1.
Targeting channels to different membranes from the cytosol. Protein synthesis begins in the cytosol before targeting to specific organelles (red lines). This can occur post-translationally as unfolded proteins (mitochondria and chloroplasts) or fully folded proteins (peroxisomes), each protein protected by chaperones (blue circles). Most ER targeting occurs cotranslationally, mediated by SRP (yellow) binding to a signal sequence. Once inserted into the ER membrane, proteins assume the topology that they will retain through all subsequent trafficking steps. Some proteins may pass to the nuclear envelope (NE) or be exported directly from the ER to peroxisomes, but all other proteins pass via the ER−Golgi intermediate compartment (ERGIC) to the Golgi before being sorted to various destinations from the trans-Golgi network (TGN). Routes to and from the PM are shown.
Targeting Ca2+ Channels to the ER and Beyond
Most ER proteins are cotranslationally targeted. The signal sequence is recognized as it emerges from the ribosome by the signal recognition particle (SRP), translation is arrested, and it resumes only after SRP has conveyed the ribosome-nascent chain complex to the ER and engaged the Sec61 translocon. The growing protein is then threaded through the translocon. A lateral gate within the translocon appears to allow proteins to become exposed to the lipid bilayer as they pass through the translocon, so that hydrophobic transmembrane helices can diffuse, alone or in pairs, through the gate and into the ER membrane (36,37). There, they can assemble with other helices or protein subunits. Some ER-targeted proteins, those with C-terminal signal sequences, follow the same route but after translation is complete (38). An essential point is that the topology of every membrane protein—dictated by whether it has a cleavable signal sequence, the number of transmembrane helices, and their preference for aligning with the more positively charged end in the cytosol—is determined once and for all by the initial integration events (39). This applies to all membrane proteins, but it deserves particular emphasis for those targeted to the ER, from where many proteins will progress to other organelles. Because this later trafficking involves moving the protein within its membrane, the topology of the protein is invariably preserved: the surface of the protein that was cytosolic when it first integrated into the ER membrane remains so wherever the protein goes (39) (Figure 1).
A safeguard against errors in the long sequence from DNA to assembled protein is provided by the proofreading abilities of the ER. The ER-associated degradation (ERAD) pathway ensures that only properly folded proteins are allowed to remain within the ER or progress onward. Misfolded proteins are recognized by chaperones, exported back to the cytosol, probably through the Sec61 translocon, polyubiquitylated, and then degraded by proteasomes (40). Most proteins that reach the ER probably follow this ERAD pathway, with only a small fraction passing scrutiny. This provides a “fail-safe” mechanism for ensuring that only functional and assembled proteins remain within the ER or pass onward. However, for some proteins, the overzealous ERAD pathway underlies a devastating pathology. In the cystic fibrosis transmembrane regulator (CFTR), a Cl− channel, the point mutation most commonly associated with cystic fibrosis (ΔF508) does not prevent the channel from functioning but does trigger ERAD-mediated degradation (41). The functional protein is thereby prevented from reaching the PM, where it is required to mediate Cl− transport. The key point for this discussion is that most channels, which are invariably multimeric or pseudomultimeric (8), assemble into their functional complexes within the ER membrane.
All membrane proteins, with the exception of those in mitochondria and chloroplasts and most within peroxisomes, reach their final destination via the ER. The major routes are shown in Figure 1. Proteins destined for the inner nuclear membrane are probably recognized as they are synthesized in the ER, bind importin-α-16, and then pass directly from the ER to the outer nuclear membrane, which is continuous with the ER, and from there to the inner nuclear membrane via the nuclear pore complex (42). Some membrane proteins may be trafficked directly from the ER to peroxisomes in preperoxisome vesicles (43) and perhaps also to phagosomes (44), but all other membrane proteins pass through the Golgi en route to their destination. Here too, the ER exerts stringent quality control, retaining protein subunits until they assemble appropriately with partners. This is often achieved by having ER retention signals concealed only when the multimeric protein has properly assembled (45−47).
N-linked glycosylation, a feature of many membrane proteins, begins in the ER, and the modifications continue throughout the Golgi apparatus, assuming their final form before leaving the trans-Golgi network (TGN). From here, proteins are sorted into secretory vesicles or endosomes (48), and perhaps directly into secretory lysosomes (49). Secretory vesicles and early endosomes provide routes to the PM, where membrane fusion inserts proteins into the PM (50). Endocytosis, and in some cells phagocytosis, brings membrane proteins back from the PM into intracellular organelles, early endosomes and phagosomes, respectively. The latter are destined for lysosomes, where most proteins are degraded, but some proteins, like P2X4 receptors, protected by extensive glycosylation (51,52), survive the hostile lysosomal environment and may later return to the PM by exocytosis from lysosomes (51). Early endosomes can deliver proteins back to the PM or, via late endosomes, to the Golgi or lysosomes (Figure 1).
Conserved motifs within membrane proteins play a large part in sorting, but additional mechanisms, like their membrane-spanning α-helices and association with other retained proteins, also contribute (20,21). The sorting motifs are recognized by cytoplasmic coat proteins, which concentrate them into defined membrane regions and tag the region for onward movement to a specific membrane. A cytosolic K(X)KXX sequence, for example, serves both to retain proteins within the ER and, after binding COPI coat protein, to return escapees to the ER from the cis-Golgi. Budding of the membrane from the donor organelle, transport of the resulting vesicle along microtubules or the actin cytoskeleton (50,53), and fusion with the target organelle deliver the membrane protein to its next destination. Details of these processes, the essential role of small GTPases (54), the identities of the many sorting sequences (32), the role of lipids in these sorting events (55), and the behavior of the SNAREs that mediate vesicle fusion (56) are described elsewhere (20,21,32).
Even proteins destined to function in a specific membrane are dynamically shuffled between compartments: escaped ER proteins are trafficked back from the Golgi, a PM protein may be endocytosed and then reappear at the PM, and some proteins take very circuitous routes to their final destination. In polarized epithelia, for example, many proteins are directly dispatched to the apical or basolateral PM after being sorted at the TGN (53), but in hepatocytes, all PM proteins are first sent to the basolateral PM, from where apical proteins are selectively endocytosed and sent to the apical PM (57). These observations highlight the fact that most ion channels must pass through several different membranes to reach their destination and even then are likely to make periodic excursions into other membranes. How does the cell ensure effective control of Ca2+ channels as they pass through these different cellular compartments?
Getting Ca2+ Channels Harmlessly to Their Destinations
As proteins progress through membrane compartments, they are exposed to very different membrane and luminal environments. The lipid composition of the asymmetric bilayer differs in the different membranes (58), and this causes radical changes in both its physical (thickness, fluidity, curvature, etc.) and chemical properties (presence of polyphosphoinositides, etc.) (59) (Table 1). The membrane becomes thicker and more rigid between the ER, TGN, and PM as it comes to include more cholesterol and sphingolipids; phosphoinositides in the cytosolic leaflet also become more phosphorylated (60). Even within continuous membranes, subdomains (rafts, caveolae, etc.) that differ in their lipid and protein composition form (58,61). As different proteins become segregated into the lumen and membranes, different organelles come to provide different luminal environments (proteins, pH, redox state, etc.), and they generate different electrical potentials across the membrane (Table 1). All of these factors can affect the behavior of resident ion channels.
Table 1. Some Key Properties of Cell Membranes.
| membrane thickness (nm) | Ema (mV) | pHb | redox potentiald | cholesterol:phospholipid ratio | |
|---|---|---|---|---|---|
| mitochondria outer | 2.13 (253)e | 0 | 7.2 | 0.06 (254) | |
| mitochondria inner | 140 | 8.5 | − (255) | 0.12 (254) | |
| ER | 3.75 (256) | ∼0 (88−90) | 7.2 (257) | ++ (258) | 0.065−0.237 (254) |
| Golgi | 3.95 (256) | ∼0 (91) | 6.0−6.7 (257) | 0.25 (259) | |
| secretory vesicles | 3.83 (253)e | −76 (92) | 5.2 (257) | ||
| PM | 3.56 (256) (basolateral), 4.25 (256) (apical) | −60 | 7.4c | ++ (260) | 0.42 (259), 0.46−0.76 (254) |
| early endosomes | 6.3−6.5 257,261 | + (262) | 0.80 (259) | ||
| late endosomes | 5−6 257,261 | + (262) | 0.69 (259) | ||
| lysosomes | 3.83 (253)e | 72 (263) | 4.5−5.5 257,261 | + (262) | 0.38 (264) |
| phagosome | 3.83 (253)e | −27 (265) | 8 | ||
| cytosol | − | − | 7.2 | − (266) | − |
As the hydrophobic regions of membrane-spanning proteins snuggle into the hydrophobic bilayer, both adjust their structures: the bilayer locally adjusts its thickness, and the protein may modestly rearrange its structure (59). This “hydrophobic matching” is one of the mechanisms proposed to allow sorting of proteins into different membranes (62), but it also has functional consequences for ion channel activity: the bilayer is an “allosteric regulator of membrane function” (59). Nicotinic acetylcholine receptors, for example, come closest to mimicking their native behavior when reconstituted into vesicles in which the lipid composition resembles that of the PM (63). The single-channel conductance (γ) of Ca2+-activated K+ channels (BK channels) varies with membrane thickness (64), and IP3R in the nuclear envelope and PM differ in their γ (65,66). Many ion channels, including some Cav channels, several P2X receptors, and many TRP proteins (67) require phosphatidylinositol 4,5-bisphosphate (PIP2) for activity. These channels may be silenced by a lack of PIP2 until they reach the PM. This brings us to a key question, and the focus of the next section of this review: how, as proteins pass through membrane compartments, does the cell cope with functional Ca2+ channels passing through many organelles before reaching their final destination? How, for example, do the ER, Golgi, and secretory vesicles cope with a fully assembled Cav channel as it makes its way to its final destination in the PM?
The scale of the problem is evident from Table 2, which provides selected examples of Ca2+ channels and their usual distributions. The PM includes the greatest diversity of Ca2+ channels, and many appear to function exclusively within the PM. However, some channels function in more than one membrane: IP3R, RyR, and TRPP2 (47) in the ER and PM (see later), or P2X4 receptors in the PM and lysosomes (51), for example. It is clear that for at least some of these channels, the same gene encodes the channel in each membrane compartment, but the extent to which post-transcriptional processing contributes to expression in different membranes is largely unresolved. In subsequent sections, we consider two issues: (1) the means whereby so many Ca2+ channels reach post-ER membranes, most commonly the PM, without perturbing the behavior of intervening organelles and (2) evidence that IP3R and RyR, which are almost entirely expressed in the ER or sarcoplasmic reticulum (SR), are expressed in the PM of some cells.
Table 2. Distributions of a Selection of Ca2+-Conducting Channels.
| channel | major location | additional locations | activating (inhibitory) regulation |
|---|---|---|---|
| IP3R | ER | PM (65), Golgi (113), nucleus (115), secretory vesicles (118,119) | IP3, Ca2+, ATP, cAMP, PKA, PKC, luminal chromogranin, Bcl-2, Bcl-XL, RACK1, Gβγ, NCS-1 (Mg2+, Ca2+, PIP2, IRBIT, calmodulin, ankyrin, ERp44) (111,198) |
| RyR | ER | PM (185), secretory vesicles (118,169), endosomes (170) | Ca2+, cyclic ADP ribose, ATP, Ca2+-calmodulin-dependent protein kinase, PKA, FKBP12 (Mg2+, Ca2+, calmodulin) (199) |
| TPC1 | lysosomes (233) | PM (267) | NAADP (233) |
| TRPP2 | ER (47) | PM (47) | Ca2+, casein kinase 2, α-actinin, ATP (cytosolic H+) (268) |
| TRPM8 | PM (269,270) | ER (271) | cold, depolarization, PIP2(272) (spermine) (273) |
| TRPC7 | PM (274) | TGN and Golgi (275) | G-protein-coupled receptors, diacyglycerol (276) (calmodulin, cytosolic Ca2+) (277) |
| TRPV1 | PM (278) | ER (78) | capsaicin, luminal H+, heat, ATP, PKA, protein kinase C (75,279) (PIP2) (279) |
| TRPA1 | PM (280) | cold, PIP2(281) | |
| TRPML1 | lysosomes, endosomes (282) | PM (283) | (Luminal H+) (282) |
| Cav | PM | secretory vesicles (93) | depolarization, PIP2, PKA, spermine (depolarization, arachidonic acid) (284,285) |
| NMDA receptor | PM | glutamate, glycine, PIP2 (extracellular: oxidation, H+, Mg2+, Zn2+) (286,287) | |
| CNG channel | PM | cAMP, cGMP | |
| P2X4 receptor | PM | lysosomes (51) | ATP (extracellular H+) (288) |
| pannexin-1 | PM | ER (289) | depolarization, mechanical stress, Ca2+ (ATP, cytosolic H+) (290) |
| connexins | PM | cytosolic Ca2+, mechanical stress, depolarization (extracellular: Ca2+, Mg2+; cytosolic H+) (290) | |
| Orai1−3 | PM | STIM1, extracellular Ca2+ (cytosolic Ca2+) (147) | |
| nicotinic receptor | PM | acetylcholine |
Getting Ca2+ Channels to the Plasma Membrane without Wayward Activity
For many Ca2+-permeable channels, avoiding wayward activity as they pass through different membranes is probably straightforward. Most ligand-gated ion channels, like nicotinic acetylcholine, ionotropic glutamate, and 5HT3 receptors, open very infrequently in the absence of their agonist (68). They are presumably protected from activation in inappropriate organelles because the closed state is very stable and their ligand-binding sites are concealed within the lumen of the intermediate organelles. The Orai proteins that mediate SOCE are activated by interaction with stromal interaction proteins (STIMs), and this probably requires that the two proteins be in opposing membranes, the PM and ER, respectively (69). STIM1 is also expressed in the PM (70−72), from where it could conceivably activate Orai in transit through intracellular organelles, but STIM1 activates Orai only when it sheds Ca2+ from its luminal EF-hand, which would never occur for the extracellular EF-hand of STIM1 in the PM. Inappropriate activation of intracellular Orai is, therefore, probably prevented because its activation requires that STIM1 in the ER be presented to Orai in the PM at places where the two membranes are closely apposed.
However, for other Ca2+-permeable channels, binding of a cytosolic ligand is sufficient to stimulate opening: cGMP or cAMP binding to cyclic nucleotide-gated (CNG) channels (73), or a decrease in the cytosolic Mg2+ concentration for TRPM6 (74), for example. Several Ca2+-permeable channels are activated by stimuli that freely cross membranes: capsaicin and related endogenous compounds are lipophilic and activate TRPV1 (75), and several TRP proteins (TRPV1−4, TRPM2, TRPM8, and TRPA) are activated by changes in temperature (76). It is hard to envisage how these channels can entirely avoid activation en route to the PM, although polymodal regulation of many channels, notably TRP channels (76), may provide some defense. It is perhaps not surprising, therefore, that some of these channels destined for the PM, including TRPM8 (77), TRPV1 (78), and TRPP2 (47), do mediate release of Ca2+ from intracellular stores. The physiological significance of the activities of these channels “in transit” is generally unresolved, but the differential distribution of TRPM8 between the PM and ER has been implicated in the progression of prostate cancer (77).
For the remainder of this section, we have chosen to examine a specific example, Cav channels, and consider their likely behavior as they pass from the ER through the Golgi and secretory vesicles to the PM, where most are located and where their opening is regulated by membrane potential and numerous modulatory signals (79). These important channels are almost entirely responsible for transducing changes in membrane potential into signals (increases in cytosolic Ca2+ concentration) that can regulate intracellular activities. Trafficking of some Cav channels (Cav1 and Cav2) to the PM is facilitated by association of the pore-forming α1 subunits with auxiliary Cavβ subunits, which may mask an ER retention signal within the α1 subunits (80). Mutations within the α1A subunit of the Cav2.1 channel trap associated subunits within the ER and gives rise to episodic ataxia type 2 (81). Whether the pathology reflects aberrant activity of Cav2.1 within the ER or loss of activity at the PM is unresolved, but in either case, the importance of effective trafficking to the PM is clear.
Members of each of the three major families of Cav channels [Cav1 (L-type), Cav2 (N-, P/Q-, and R-type), and Cav3 (T-type)] are inactivated (at very different rates) by sustained depolarization to potentials of approximately −60 to −10 mV (Cav1), −120 to −20 mV (Cav2), and −100 to −60 mV (Cav3) (8,82). At the resting membrane potential of the PM (typically approximately −60 mV), most of these channels will not be inactivated and can open during transient depolarizations. Many ion channels, including Cav channels (83), CNG channels (84), and glutamate receptors (85,86), are blocked by polyamines, like spermine, and can mediate ion fluxes only when changes in membrane potential dislodge the polyamine. For Ca2+-permeable AMPA receptors, an accessory protein, stargazin, both guides the receptors to the PM and relieves the block by polyamines (87), perhaps thereby ensuring effective block of the channels until they leave the ER. The important point is that activation of Cav channels requires transient depolarization from a hyperpolarized membrane potential for activation. However, the potential across the membranes of the ER (88−90) and Golgi (91) is probably close to 0 mV, sufficient to inactivate most Cav channels. However, secretory vesicles appear to maintain a membrane potential more like that of the PM (−76 mV) (92), and with individual vesicles showing considerable variability in membrane potential (−11 to −160 mV), it seems likely that Cav channels within secretory vesicles may be exposed to potential changes that would allow their activation. Because secretory vesicles, in common with every other intracellular Ca2+ store, contain only a limited pool of Ca2+, Cav within them could contribute to Ca2+ signaling only if bouts of activation were interspersed with periods of closure to allow the vesicles to refill with Ca2+. The possibility that Cav channels might be activated within secretory vesicles assumes further significance in light of evidence that a large reservoir of functional Cav channels may be retained within the secretory vesicles of neuroblastoma cells, from where trafficking to the PM is dynamically regulated (93). Whether other factors, like low pH or the absence of PIP2 (see below), silence Cav channels in secretory vesicles or whether they are active and responsive to changes in vesicle membrane potential deserves further study.
The pH to which the luminal/extracellular surface of membrane proteins is exposed also changes dramatically as they are transported from the ER to the PM (Table 1). Many Ca2+ channels, including Cav1 (94), CNG channels (95), TRPV5, and TRPV6 (96), are inhibited by the low pH encountered within the secretory pathway. Other Ca2+ channels, such as TRPV1 (97), TRPM6 (98), and TRPM7 (99), are stimulated by this low pH, although this may be accompanied by a reduced Ca2+ flux as H+ within the permeation pathway attenuates Ca2+ binding and increases the relative permeability to monovalent cations (99).
The different lipid compositions of the PM and earlier membranes (Table 1) may also restrain the activity of Ca2+ channels in transit. PIP2 is enriched in the inner leaflet of the PM and is essential for the activity of Cav2 channels (100). The activity of Cav1 channels is enhanced by cholesterol (101), the concentration of which increases as membranes progress toward the PM (Table 1). Similar interactions may inhibit the activities of other Ca2+ channels until they reach the PM: many TRP channels are stimulated by PIP2(102) or the diacylglycerol produced by its hydrolysis (103), and the activities of CNG channels (104) and TRPC3 (105) are enhanced by cholesterol.
Finally, Cav and many other Ca2+ channels have their responses tuned by association with additional proteins, such that successful targeting of these proteins to the PM can unmask latent channel activity. We have already discussed the role of Cavβ subunits in coordinating PM targeting and the activity of Cav channels (80). Palmitoylation of Cav channels likewise both regulates their trafficking to the PM and enhances their activity (106−108). Anchoring of cyclic AMP-dependent protein kinase (PKA) at the PM by A-kinase-anchoring protein (AKAP15) positions PKA close to Cav1 channels, allowing phosphorylation to increase their activity (109).
Clearly, Cav channels can reach the PM without overly perturbing cytosolic or luminal Ca2+ regulation as they pass through intervening organelles. A few general themes emerge. Cav (and other) channels in transit may be inactive because the intervening environment is hostile (e.g., inactivation by sustained depolarization, lipids, pH, etc.). A single modification (e.g., association with a β-subunit or palmitoylation) may both enhance activity and simultaneously provide an express ticket to the PM, thereby ensuring that the most active channels reach the PM without lingering in the ER. Additional PM proteins (e.g., AKAP-anchored PKA) may enhance the activity of juxtaposed channels. Whether these, or additional mechanisms, are wholly effective in silencing Cav channels in transit seems to be unresolved. Do Cav channels, for example, mediate voltage-gated Ca2+ fluxes in secretory vesicles? Do they, or other Ca2+ channels in transit, contribute to the unresolved leak of basal Ca2+ from the ER? What are the physiological roles of those Ca2+ channels in transit that are not completely silenced as they make their way to the PM?
Counting IP3 Receptors into the Plasma Membrane
Most IP3R in most cells are expressed within ER membranes, or the nuclear envelope, which is continuous with them (110,111). This pattern of expression is maintained by several ER retention signals within the six C-terminal transmembrane domains of IP3R (112). Nevertheless, there is persuasive evidence that functional IP3R can also be expressed within the Golgi apparatus (113,114), the nucleoplasmic reticulum (115), and, more contentiously, secretory vesicles (116−119). In this section, we consider evidence that IP3R, the archetypal intracellular Ca2+ channels, are also functionally expressed in the PM of some cells, notably DT40 cells, a prelymphocyte cell line (120), and mammalian B-lymphocytes (65).
Several studies had suggested that IP3R might be expressed within the PM (reviewed in refs (65) and (121)), but the results were inconclusive and in many cases likely to reflect the activities of intracellular IP3R. Subcellular fractionation showed IP3R to copurify with PM markers (122−124), probably because IP3R within the ER are closely associated with the PM (123,125). The same explanation might account for the presence of IP3R immunostaining close to the PM of oviductal ciliated cells (126). Cell surface labeling suggested the presence of IP3R within the PM (127−130). Patch-clamp recording identified IP3-activated, Ca2+-permeable channels in the PM of some cells (131−136), and a protein purified from a liver PM fraction behaved as an IP3-gated channel when reconstituted into lipid bilayers (137). However, the electrophysiological properties of these channels were very different from those of IP3R either in nuclear membranes (66,110,111) or after reconstitution of intracellular IP3R into bilayers (138,139). With the exception of IP3R in olfactory cilia (131), which differ from intracellular IP3R in their sensitivity to ruthenium red, insensitivity to ATP, and lower conductance, the other reports of IP3-activated PM channels failed to establish whether the channels were actually IP3R within the PM or more likely other channels indirectly activated by stimulation of IP3R within the ER. Our work with DT40 cells (65,140) and subsequent work by others (141) have provided conclusive evidence that functional IP3R can be expressed in the PM of at least some cells.
Stimulation of DT40 cells with either anti-IgM to activate the B-cell receptor (BCR) or trypsin to activate the protease-activated receptor (PAR) leads to activation of phospholipase C, formation of IP3, and thereby release of Ca2+ from intracellular stores mediated by IP3R (120,142). DT40 cells are uniquely suited to analyses of these signaling pathways because Kurosaki and his colleagues generated a DT40 cell line (DT40-KO cells) in which the genes for all three IP3R subtypes are disrupted (142). DT40-KO cells are the only vertebrate cells unequivocally devoid of functional IP3R and therefore the only null background in which recombinant IP3R can be functionally expressed free of the complexity arising from association with residual native IP3R (120,143). The inability of either anti-IgM or trypsin to evoke Ca2+ release in DT40-KO cells and restoration of that response by expression of recombinant IP3R firmly establishes the role of the IP3R in mediating Ca2+ release (65).
The same stimuli that evoke release of Ca2+ also evoke entry of Ca2+ across the PM of DT40 cells. In most cells, such Ca2+ entry is commonly mediated by SOCE (144,145). The defining feature of SOCE is that depletion of intracellular Ca2+ stores provides a sufficient stimulus for its activation (144). Thapsigargin, which inhibits SERCA and thereby causes loss of Ca2+ from the ER without engaging the signaling pathways used by receptors, is often used to activate SOCE (Figure 2A). In many cells, the electrophysiological manifestation of SOCE is the Ca2+ release-activated current (ICRAC), which is characterized by its inward rectification, Ca2+ selectivity, and low unitary conductance, block by low concentrations of Gd3+, and activation by depleted Ca2+ stores (146,147). The molecular architecture of the SOCE pathway has recently been established (13), with STIM1 emerging as the luminal Ca2+ sensor in the ER membrane (148,149), and Orai proteins as the pore-forming subunits of the channel in the PM (150). After Ca2+ is lost from the ER, the luminal EF-hand of STIM1 loses Ca2+, causing it to cluster. The clustered polybasic C-termini of STIM1 can then interact with PIP2 in the PM, allowing a stretch of conserved residues within the cytosolic tail of STIM1, the CRAC activation domain (CAD), to stimulate opening of Orai (69,151). Further details of the mechanisms of activation of SOCE (13), including the possible involvement of TRP proteins (152), the possibility that biochemical signals might link STIM1 to activation of Orai (153), the relative roles of STIM1 and STIM2 (154,155), and the possibility that STIM might activate Orai by causing its assembly into a tetrameric channel (156), are discussed elsewhere.
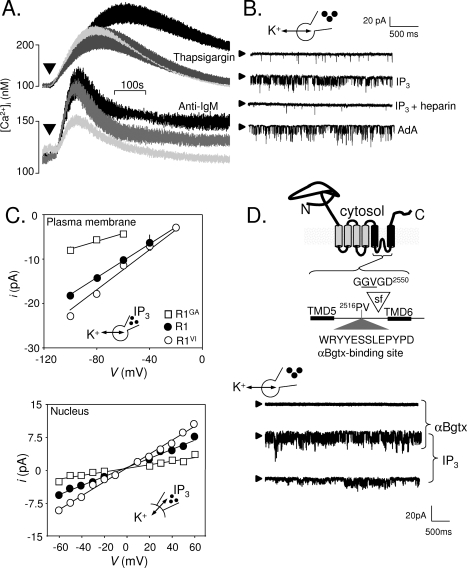
Figure 2.
Expression of IP3 receptors in the plasma membrane of DT40 cells. (A) Thapsigargin (top) stimulates Ca2+ release (palest line, in Ca2+-free medium) and Ca2+ entry (black line, in Ca2+-containing medium). The latter, SOCE, is entirely blocked by 300 nM GdCl3 (gray line). The bottom panel shows that activation of the BCR with anti-IgM stimulates both Ca2+ release and Ca2+ entry, but the latter is only partially inhibited by GdCl3. (B) Whole-cell patch-clamp recoding from DT40 cells with IP3, IP3 with heparin, or adenophostin A in the patch pipette. The holding potential was −100 mV; arrowheads denote the closed state. (C) Current−voltage (i−V) relationships for the IP3-stimulated currents recorded from the PM or nuclear envelope of DT40-KO cells stably transfected with wild-type IP3R1 (R1) or IP3R1 with mutants in the putative pore (G2547A, R1GA; V2548I, R1VI). The point mutations similarly affected γK of the IP3-activated currents in both settings. (D) The six TMDs of a single IP3R subunit are shown to highlight the putative selectivity filter (sf) and the engineered αBgtx-binding site. In whole-cell patch-clamp recordings from DT40 cells expressing IP3R1 with this αBgtx-binding site, intracellular IP3 stimulated channel openings, and both Po and γK were increased by extracellular αBgtx. Reproduced from ref (65) with permission. Copyright 2006. American Academy for the Advancement of Science.
SOCE, with the properties of ICRAC(157,158) and mediated by STIM1 (158) and Orai1/2 (159), is expressed in DT40 cells, and it can be activated by thapsigargin (65,120,159) (Figure 2A). However, whereas this thapsigargin-activated SOCE is entirely blocked by low concentrations of Gd3+(65) or La3+(159), the Ca2+ entry evoked by activation of the BCR is only partially blocked: typically, ∼50% of the Ca2+ entry evoked by the BCR appears not to be mediated by SOCE (Figure 2A) (65,159). Both components of Ca2+ entry require functional IP3R: activation of the BCR evokes neither Ca2+ release nor Ca2+ entry in DT40-KO cells (65,121,142,159). Others have recently suggested that a non-SOCE pathway in DT40 cells is evoked only by activation of the BCR, that the need for IP3R is limited to its ability to empty intracellular Ca2+ stores, and that activation of the pathway requires a BCR-evoked signal and STIM1, but not Orai1 (159). These observations together with earlier work suggesting an essential role for IP3R in Ca2+ entry that was independent of its ability to conduct Ca2+(160) and evidence implicating TRPC7 in the non-SOCE pathway (161) have added complexity and considerable confusion to our understanding of Ca2+ entry in DT40 cells (120). Here we focus on evidence that DT40 cells express functional IP3R in the PM and suggest that these may mediate Ca2+ entry via the non-SOCE pathway.
In whole-cell patch-clamp recording from DT40 cells, IP3 stimulates opening of very small numbers of large-conductance cation channels (1.9 ± 0.2 channels/cell). These channels are absent from DT40-KO cells. They are inactive in cells stimulated with IP3 in the presence of its competitive antagonist, heparin. They are activated by adenophostin A, another agonist of IP3R. Like intracellular IP3R, they are also regulated by ATP and Ca2+(65) (Figure 2B). Furthermore, in common with the non-SOCE Ca2+ entry pathway, the IP3-activated PM channels are insensitive to concentrations of Gd3+ that block SOCE. Expression in DT40-KO cells of either IP3R1 or IP3R3 restores both the non-SOCE Ca2+ entry pathway and the IP3-activated PM channels. However, expression of IP3R1 with a point mutation in the putative pore (D2550A) that prevents IP3-evoked Ca2+ release fails also to restore either Ca2+ entry mediated by the non-SOCE pathway (but see ref (160)) or IP3-activated PM cation channels (65). Similar channels are detected in perforated-patch recordings from DT40 cells after stimulation of the BCR, and these channels are activated with a time course similar to that of the Gd3+-insensitive Ca2+ entry evoked by the BCR in intact cells. Collectively, these results demonstrate that the PM channels activated by IP3 do not reflect further activation of SOCE, which might have resulted from more complete emptying of intracellular Ca2+ stores by IP3: the channels are Gd3+-insensitive, their electrophysiological properties are entirely different from ICRAC, and IP3 activates the channels similarly before or after store depletion (65). The coincident behaviors of the IP3-activated PM channels and the Ca2+ signals mediated by the non-SOCE pathway further suggest that the latter might be mediated by the PM channels. A key question, however, is the identity of the PM channels: are they IP3R working within the PM, or are they other channels activated via IP3 binding to IP3R in their conventional location, the ER?
Several lines of evidence provide conclusive evidence that IP3R are themselves functional within the PM. Mutation of residues within the putative pore of IP3R1 causes changes in the unitary conductance (γ) that can be easily resolved by patch-clamp recording from intracellular IP3R within the outer nuclear envelope of DT40 cells (66,110). The latter is continuous with the ER and allows single-channel recording from intracellular channels that would otherwise be inaccessible. The important point is that the same mutations cause similar changes in γ for intracellular IP3R and for IP3-activated cation channels in the PM (65,141) (Figure 2C). Clearly, if the effects of IP3 on the PM were mediated solely by its interaction with intracellular IP3R, pore mutations within the IP3R would not be expected to affect the γ of the PM channels. We also introduced a binding site for α-bungarotoxin (αBgtx) into the luminal loop linking the last pair of transmembrane domains (TMD5−6) of IP3R1 and expressed it in DT40-KO cells (Figure 2D). From the known topology of the IP3R, we expected the αBgtx-binding site to be luminal for IP3R within the ER, but extracellular for IP3R expressed in the PM. In whole-cell patch-clamp recordings from DT40 cells expressing these IP3R, intracellular IP3 again activated cation channels in the PM, but their γ and open probability (Po) were both increased by addition of extracellular, but not intracellular, αBgtx (65). These results establish that the recombinant IP3R must straddle the PM. Finally, others have expressed IP3R with mutations in sites that are phosphorylated by PKA and shown that the Po of the PM cation channels activated by adenophostin A is increased by forskolin (to activate PKA) for wild-type IP3R; IP3R with phospho-mimetic mutations are hyperactive, and those with mutations that prevent phosphorylation have much reduced activity (141). Collectively, these results demonstrate unequivocally that functional IP3R are expressed in the PM of DT40 cells. We stress, however, that IP3R are not expressed in the PM of all cells; we have not, for example, detected IP3R in the PM of either Sf9 or human embryonic kidney (HEK) cells.
Functional IP3R in the PM of DT40 cells are expressed at an exceptionally low density, just 1.9 ± 0.2 channels/cell (65), and we have never, even in cells massively overexpressing intracellular IP3R, detected more than five functional IP3R in the PM. There are some additional examples of cells expressing such tiny numbers of channels in the PM, ∼10 intermediate conductance Ca2+-activated K+ channels (IKCa1) in a resting T-cell (162), for example, or two or three RyR-like channels in rat portal vein myocytes (163) (see later), but most channels expressed in the PM occur at much higher densities (typically >100 channels/μm2 or several thousand channels per cell) (8). An intriguing feature of IP3R expression in the PM is that in several hundred recordings, we never failed to detect at least one IP3R in the PM (65,140). If these IP3R had been randomly inserted into the PM with very low probability, perhaps reflecting “leakage” from the ER, for example, we would have expected (on the basis of the Poisson distribution) ∼28% of cells to lack PM IP3R. These results suggest that functional IP3R are reliably counted into the PM. This may reflect either counting of proteins into the PM or a regulatory process that only ever allows very few of a larger number or resident channels to be active within the PM.
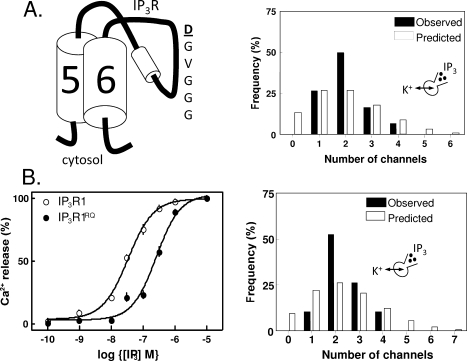
In seeking to address mechanisms that might allow such reliable counting of PM IP3R, we considered that feedback regulation of IP3R trafficking to the PM from active IP3R within the PM was the most likely possibility. An IP3R in which a single residue (D2550A) was mutated to create a pore that is blocked whenever its luminal surface is bathed in Ca2+ provides a channel that is effectively “pore-dead” throughout its normal life cycle as it passes from ER to PM, yet these channels were effectively counted in appropriate numbers (∼2 IP3R/cell) into the PM (Figure 3A). Another possibility was that a feedback signal arose from an earlier step in the activation of IP3R by IP3, a conformational change in the IP3-binding site, for example. We therefore expressed a mutant IP3R (R568Q) in which the affinity for IP3 was reduced by ∼10-fold, arguing that at resting levels of IP3 such IP3R would now be only 10% as likely as normal IP3R to bind IP3, but these IP3R, like those that were pore-dead, were also reliably counted into the PM with ∼2 IP3R/cell (Figure 3B) (140). These rather puzzling results suggest that very small numbers of functional IP3R are reliably counted into the PM in the apparent absence of any obvious feedback regulatory mechanism.
Figure 3.
Counting IP3 receptors into the plasma membrane. (A) A point mutation within the putative pore region of IP3R1 (D2550A, highlighted) causes luminal/extracellular Ca2+ to block the channel, but it does not prevent cells from reliably counting IP3R into the PM. The histogram shows the observed and predicted (from the Poisson distribution) numbers of functional IP3R detected in each cell and establishes that IP3R are not randomly inserted into the PM. (B) A point mutation within the IP3-binding core (R568Q, IP3R1RQ) reduces the binding affinity of the IP3R for IP3 by 10-fold, evidenced by radioligand binding analyses (not shown) and the 10-fold decrease in the sensitivity of Ca2+ release to IP3 (left). The reduced sensitivity to IP3 does not impair the reliability with which IP3R are functionally expressed in the PM (right). Reproduced with permission from ref (140). Copyright 2008. American Society for Biochemistry and Molecular Biology.
Functional Ryanodine Receptors in the Plasma Membrane
RyR are the closest relatives of IP3R, with which they share both structural (164,165) and functional properties (166), and like IP3R, they are expressed predominantly within the membranes of the ER (or SR in muscle), where they are retained by ER retention signals within the TMDs (167). However, as with IP3R, evidence that RyR may also be expressed in post-ER membranes, and in the PM of some cells, is accumulating.
Within pancreatic β-cells, for example, or insulinoma cells derived from them, functional RyR appear to be expressed within the membranes of insulin-containing secretory vesicles (168,169) and/or endosomes (170), whereas neither organelle expresses IP3R (171). In chromaffin cells, both RyR and IP3R appear to escape the ER and function as Ca2+ release channels within secretory vesicles (118). Perhaps more contentious is the possibility that RyR may be expressed in the PM (172). In this section, we briefly review the evidence that RyR are functionally expressed in the PM of some cells, and in the concluding section, we consider the possible physiological significance of RyR and IP3R in the PM.
An early suggestion that RyR might be expressed in the PM came from studies of osteoclasts (173). Osteoclasts probably express functional RyR. These may, as they do elsewhere, amplify, by Ca2+-induced Ca2+ release, the Ca2+ signals generated by activation of other Ca2+ channels within intracellular stores or the PM (174,175). However, the evidence that RyR are also expressed in the PM of osteoclasts deserves close examination because the conclusion is important. Extracellular ruthenium red, an antagonist of RyR, blocked Ni2+-evoked Ca2+ signals (173). Ni2+ is known to activate the extracellular Ca2+-sensing receptor (CaR), a G-protein-coupled receptor (176), although the role of CaR in osteoclasts has been contentious. The inhibition by ruthenium red of Ni2+-evoked Ca2+ signals need not reflect an action at PM RyR, because ruthenium red is membrane-impermeant and its most substantial effects on RyR appear to be mediated by binding to its cytosolic surface (177). It is perhaps more likely that ruthenium red, a polycation, interacts with the Ca2+-sensing receptor (CaR), which is known to be regulated by polyvalent metal ions and polyamines (176). This interpretation would also be consistent with evidence that ruthenium red itself evoked Ca2+ signals in osteoclasts (178). Ni2+ was suggested, although without quantitative analysis, to inhibit binding of [3H]ryanodine to osteoblasts (173), but these binding assays do not distinguish intracellular RyR from those in the PM. Because ryanodine binding is use-dependent, any effect of agents that increase the cytosolic Ca2+ concentration on [3H]ryanodine binding might simply reflect regulation of intracellular RyR by Ca2+. Confocal microscopy identified peripheral immunostaining for RyR (173), but the resolution is insufficient to resolve whether RyR were in the PM or within ER lying close to it. An anti-peptide antiserum (Ab129) raised to 22 residues within a region toward the C-terminus of RyR2 immunostained intact osteoclasts (173). Because the N-terminus of the RyR is cytosolic (179), the limited information provided in the original publication (173), namely that the epitope lies between TMD6 and TMD7 of the originally proposed 10-TMD model of RyR (180), would place the epitope within the cytosol. Subsequent work revealed that Ab129 was raised to residues 4676−4699 of rabbit RyR2 (181), and the revised six-TMD models of RyR (167,179) would place the sequence between TMD2 and TMD3 (181), which again places the epitope within the cytosol. Immunostaining of intact cells with this antiserum is therefore unlikely to reflect its reaction with a PM RyR. Subsequent studies, using valinomycin to manipulate membrane potential, indicated that hyperpolarization increased the susceptibility to blockade by ryanodine of the Ca2+ release evoked by Ni2+(182). This was interpreted as evidence that the RyR might sense membrane potential (182) either directly (because it was resident within the PM) (175,178) or indirectly via conformational coupling to another PM voltage sensor. Evidence that other G-protein-coupled receptors are regulated by membrane potential (183) raises the possibility that the voltage sensor might even be the CaR itself. The significance of the latter findings (182), which provide no decisive evidence of a functional RyR in the PM, is diminished by the observation that in osteoclasts not treated with valinomycin, responses to Ni2+ were insensitive to ryanodine (182). Finally, patch-clamp recordings from excised patches of the PM of osteoclasts resolved small numbers of Ba2+-permeable channels with unexpectedly high Po values (0.5−0.95) in the absence of any known agonists of RyR, but that activity was massively attenuated by application of cytosolic ruthenium red or an antiserum to a cytosolic epitope of the RyR (175). Neither agent is likely to be specific, although the observation that both inhibit the active channels is suggestive of a RyR, but the results are perplexing. The authors show that with symmetrical Ba2+-containing solutions, the reversal potential is (as might be expected) 0 mV, but most traces, including those with massive channel activity, show excised patches at a holding potential of 0 mV using symmetrical Ba2+-containing solutions. Under these conditions, there should be no currents. The inescapable conclusion is that the electrophysiological evidence in support of RyR in the PM of osteoclasts lacks credibility (175). We suggest that although Zaidi and his colleagues were among the first to suggest that RyR might be expressed in the PM (173), neither the original nor subsequent reports provide compelling evidence.
High-conductance PM cation channels resembling RyR have been detected in other cell types. In smooth muscle from portal vein, for example, caffeine, ryanodine, Ca2+, or extracellular stimuli that evoke an increase in cytosolic Ca2+ concentration stimulated opening of high-conductance (γK ∼ 190 pS) cation channels that were permeable to Ca2+ and Ba2+, and only modestly selective between cations (PCa/PNa ∼ 21) [although more so than conventional RyR (184)] (163). It is also noteworthy that the γ of these PM channels is substantially lower than that of RyR reconstituted into lipid bilayers (184) but similar to that of RyR in the PM of insulinoma cells (γK ∼ 169 pS) (185) (see later). In whole-cell recordings, no more than three simultaneous openings of these channels were detected, suggesting that very few were expressed in the PM. These authors may be correct in cautiously concluding that the PM channels are not RyR themselves, but other Ca2+-sensitive channels activated in response to Ca2+ released from intracellular RyR (163), but it remains entirely plausible that these channels result from RyR within the PM that are regulated by physiological stimuli (norepinephrine or acetylcholine) (163). Other studies of gastric (186) or arterial (187) smooth muscle detected caffeine-stimulated nonselective cation channels in the PM, but their insensitivity to ryanodine (186), low conductance, or lack of permeability to bivalent cations (187) suggests that these are probably not RyR in the PM. In cardiac myocytes, too, caffeine or an increased cytosolic Ca2+ concentration stimulated opening of cation channels with high conductance (γNa ∼ 400 pS) and permeability to Ba2+188,189. The most persuasive evidence that these might be RyR in the PM is the ability of ryanodine, as it does for RyR in bilayers (172,177), to lock the channels into a subconducting state (189). Collectively, these observations provide persuasive evidence that cardiac myocytes may express just one to four functional RyR within the PM (189). The possibility that ventricular myocytes express small numbers of RyR in the PM clearly deserves further study both to strengthen the conclusion and to address likely physiological roles.
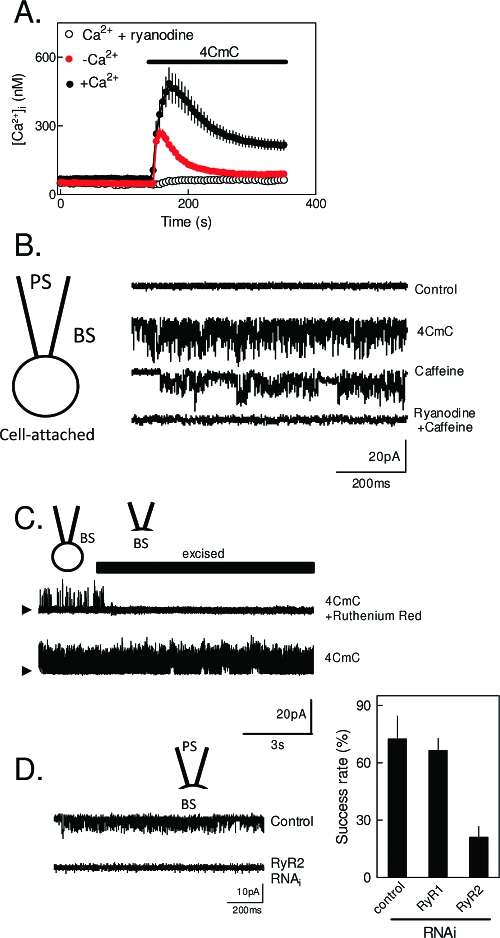
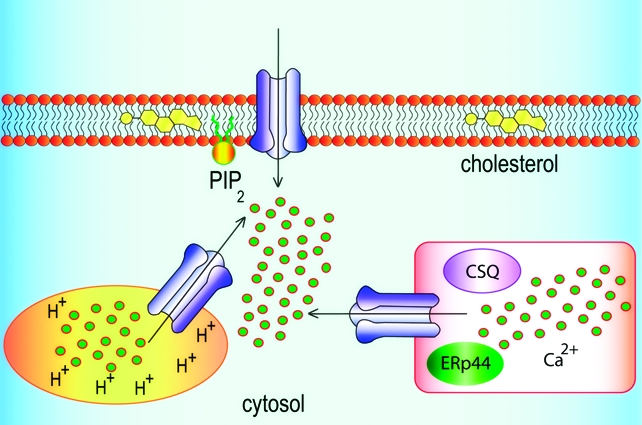
Our analyses of PM RyR2 have concentrated on RINm5F insulinoma cells (185), where 4-chloro-m-cresol (4CmC), at concentrations likely to be selective for activation of RyR1 and RyR2, evokes both Ca2+ release and Ca2+ entry, the latter via a pathway that appears not to involve SOCE. Both responses are blocked by inactivating RyR by prior incubation with ryanodine (Figure 4A). From PCR analyses, IP3R (almost entirely IP3R3) are the major (∼97%) intracellular Ca2+ channels in RINm5F cells, but they also express RyR (almost entirely RyR2). After subcellular fractionation, IP3R3 is concentrated in the ER-enriched fractions, while ∼20% of RyR2 is concentrated in fractions that include the PMCA (i.e., they include PM) but exclude IP3R3. In both cell-attached and excised patch-clamp recordings from RINm5F cells, 4CmC and caffeine activate relatively nonselective, high-conductance (γK = 169 pS) cation channels that are inhibited by prior incubation with ryanodine (Figure 4B). The same channels are inhibited by the membrane-impermeant inhibitor of RyR, ruthenium red, when it is applied to the cytosolic surface of the RyR, but not when applied to the extracellular surface (Figure 4C). There seem, although the estimate is only approximate, to be ∼10 of these channels per cell. We (185) and others (172) have noted that neither γ of these channels nor the effects of high concentrations of ryanodine on γ faithfully replicate the exhaustively studied behavior of RyR in lipid bilayers. RyR in bilayers typically have γ values for K+, Na+, and Cs+ that are 3−4-fold larger than those observed in our recordings from the PM (185) (practical difficulties have prevented us from measuring γ values for bivalent cations of the PM channels), and ryanodine usually locks RyR into a subconducting state (190). These disparities raise the possibility that the PM channels, while sharing key permeability properties and considerable pharmacology with RyR (activation by caffeine, 4CmC; inhibition by ryanodine and cytosolic ruthenium red; insensitivity to nifedipine and clotrimazole), may reflect the activity of an unrelated channel. Alternatively, it may be, as has been reported for IP3R (111), that the behavior of RyR in a native membrane (the PM) is not faithfully replicated in a synthetic lipid bilayer. Our most direct evidence that the 4CmC-activated channels detected in the PM are RyR2 is provided by RNAi, which selectively attenuated expression of IP3R3, RyR1, or RyR2 by ∼60−70% (185). Loss of RyR2 alone attenuated the Ca2+ signals and Mn2+ entry evoked by 4CmC and substantially reduced (though it did not abolish) the detection of 4CmC-activated cation channels in the PM (Figure 4D) (185). Collectively, these results provide persuasive evidence that RyR2 is functionally expressed in the PM of RINm5F insulinoma cells. At present, we have only preliminary data to suggest that primary pancreatic β-cells might also express RyR in the PM (185).
Figure 4.
Functional ryanodine receptors in the plasma membrane of RINm5F insulinoma cells. (A) Ca2+ signals evoked in populations of cells by 4CmC (1 mM) with or without prior treatment with ryanodine (400 μM) and in either normal or Ca2+-free medium. The results are consistent with activation of RyR causing both Ca2+ release and Ca2+ entry. (B) Cell-attached recordings from cells with cesium methanesulfonate in both bathing (BS) and pipette (PS) solutions at a holding potential of −100 mV. Caffeine (1 mM), 4CmC (1 mM), or ryanodine (400 μM) was included in BS as indicated. Arrowheads denote the closed state. (C) 4CmC activates channels in the cell-attached mode, which are then rapidly inhibited when the patch is excised into BS containing the membrane-impermeant inhibitor of RyR, ruthenium red (10 μM). (D) Selective inhibition of RyR2 expression using RNAi attenuates the electrical activity evoked by 4CmC. Typical records for control and RyR2-RNAi-treated cells are shown, and the success rate for detecting 4CmC-activated channels in the PM is shown for mock-transfected cells or cells transfected with RNAi for RyR1 or RyR2. Reproduced with permission from ref (185). Copyright 2009. American Society for Biochemistry and Molecular Biology.
Three issues arise from evidence that at least some cells might express functional RyR in the PM. First, how do RyR, with multiple determinants of ER retention (167), selectively escape the ER to reside, in small numbers, in the PM? Second, all electrophysiological analyses of RyR have hitherto used proteins reconstituted into lipid bilayers because the ER and SR where most native RyR reside are, for all practical purposes, inaccessible to patch-clamp recording. RyR in the PM provide opportunities to explore their behavior with patch-clamp techniques in a native membrane. Third, and the topic of the final section, what are the physiological roles of RyR and IP3R in the PM?
Physiology of “Intracellular” Ca2+ Channels in the Plasma Membrane
Within their usual setting, the membranes of the ER or SR, Ca2+ is probably the only permeant cation of IP3R and RyR with an appreciable electrochemical gradient. Despite the weak cation selectivity of both channels (PCa/PK ∼ 7) (111,184), they are therefore likely to conduct mainly Ca2+ when they open within these membranes. The SERCA, by selectively accumulating Ca2+ into these organelles, ensures that IP3R and RyR behave as intracellular Ca2+ channels; in effect, the role of selecting ions has been delegated to the SERCA. The situation is very different when IP3R or RyR find themselves in the PM, where their opening is likely to provide routes for inward fluxes of Ca2+ and Na+ and outward fluxes of K+. Furthermore, gating of RyR is modulated by membrane potential with steps to hyperpolarized or depolarized potentials of more than ∼40 mV causing an initial activation and then inactivation (191). The effects of membrane potential on IP3R are less clear, with suggestions that IP3R activity is either unaffected (192,193) or weakly enhanced by depolarization (194,195). Depolarization and hyperpolarization have also been reported to increase γ by relieving the Mg2+ block of the channel (196). These effects of membrane potential on RyR and perhaps IP3R have been rather neglected because within the ER/SR such regulation is unlikely to be significant, but it may be very important when IP3R or RyR are expressed in the PM. Within the PM, therefore, IP3R and RyR may regulate both membrane potential and Ca2+ entry, and their activity may also be regulated by membrane potential.
The behavior of IP3R (111,197,198) and RyR (199,200) is modulated by their association with many different accessory proteins. These provide additional levels of regulation of channel gating, directly by the associated proteins (199,201,202), via phosphorylation (203,204), or via the proteins serving as sensors of, for example, redox potential (205) or Ca2+(206). Other associated proteins, for example, IRBIT (207,208) and CaMKII (17), are directly regulated by the active channels. These accessory proteins include those, like chromogranin (209), ERp44 (205), and calsequestrin (200), that are expressed within the ER/SR lumen and others, like calnexin (210), junctin, and triadin (200,211), that are expressed only in ER/SR membranes. Each of these channels therefore provides a scaffold around which a signaling complex is assembled, which then defines the complex integrative behavior of IP3R and RyR. The components of these signaling complexes must be different for channels in the ER/SR and PM, not least because the latter are devoid of luminal proteins. As proteins pass through post-ER compartments, attached carbohydrates are further processed, and these modifications may also affect the behavior of IP3R and RyR that progress to the PM. It has, for example, been suggested that PM IP3R in lymphocytes are enriched in sialic acid and differ from intracellular IP3R in their affinity for IP3(129). The key points are that IP3R and RyR within the PM may, by assembling into different signaling complexes, differ from their intracellular counterparts both in their regulation and in the downstream proteins to which they signal.
Ca2+ channels within the ER/SR access a finite Ca2+ store, whereas those within the PM have access to an unlimited pool of extracellular Ca2+. The difference is likely to affect the duration of the Ca2+ signals evoked by IP3R and RyR in the two settings, and the impact of feedback regulation by luminal Ca2+ concentration on channel gating (206,212). Finally, because Ca2+ signals can be locally decoded, the Ca2+ released by channels within the ER/SR is likely to recruit the activity of different Ca2+ sensors to those arising from PM channels. Ca2+ signals emanating from IP3R (or RyR) within the PM will, therefore, have different spatiotemporal profiles compared to those arising from the same channels with the ER/SR, and each may thereby regulate different cellular responses.
In the remainder of this final section, we consider the likely physiological significance of these effects of RyR and IP3R in the PM.
Bone mass in adults is maintained by the counteracting activities of the osteoblasts that deposit bone and the osteoclasts that resorb it. Numerous feedback loops coordinate the activities of these two cell types (213), one of which is inhibition of the resorptive activity of osteoclasts by high local extracellular Ca2+ concentrations, which trigger an increase in cytosolic Ca2+ concentration (214). CaR, with its huge extracellular region that binds Ca2+(176), mediates the responses of many cells, like osteoblasts (215) and parathyroid chief cells (216), to changes in extracellular Ca2+ concentration. The role of the CaR in osteoclasts has been more contentious, although osteoclasts express CaR (217,218) and loss of CaR severely compromises their responses to extracellular Ca2+ concentration (218). These observations are consistent with CaR, via activation of phospholipase C and an increase in cytosolic Ca2+ concentration, playing a role in feedback inhibition of osteoclast activity by extracellular Ca2+219,220. Zaidi and his colleagues have suggested that RyR2 within the PM of osteoclasts may further contribute to these Ca2+-regulated pathways, minimally by providing a route for entry of Ca2+ across the PM, but perhaps also by providing an additional sensor for extracellular Ca2+221,222. The latter suggestion derives from the observation that RyR within the more typical setting, the SR, are regulated by luminal Ca2+(223). Several arguments suggest that the luminal surface of RyR2 is unlikely to serve as a sensor of extracellular Ca2+ in osteoclasts. First, the affinity of the luminal Ca2+-binding site on RyR2 (KDCa ∼ 40 μM) (212) is far too high, even allowing for some competition with extracellular Mg2+, to respond to the changes in the extracellular Ca2+ concentration (several millimolar) to which osteoclasts respond. Second, it seems likely though perhaps not proven that accessory proteins, such as luminal calsequestrin (224), mediate regulation of RyR2 by luminal Ca2+. Such proteins would be unlikely to associate with the extracellular surface of RyR in the PM. Finally, the contributions from intracellular Ca2+ stores and Ca2+ entry (182,225) and the pharmacology of the intracellular Ca2+ signals evoked in osteoclasts (stimulation by extracellular Ca2+, Cd2+, and Ni2+) (226) seem more likely to reflect initiation of the Ca2+ signals by CaR (227,228) rather than RyR. It seems very unlikely, therefore, that a PM RyR serves as an extracellular Ca2+ sensor in osteoclasts. Indeed, until there is more compelling evidence in support of a functional RyR in the PM of osteoclasts, whether the RyR plays any direct role in mediating Ca2+ entry, rather than simply fulfilling its more conventional role as an intracellular Ca2+ channel, in osteoclasts remains an open question.
Secretion of insulin from pancreatic β-cells is regulated by the synergistic actions of glucose and gut hormones (incretins) that stimulate cAMP formation. Glucose metabolism causes an increase in cytosolic ATP concentration, which closes KATP channels, leading, via an unidentified leak channel, to depolarization of the PM and thereby activation of Cav channels, an increase in cytosolic Ca2+ concentration, and exocytosis of insulin-containing vesicles (229). Treatment of type 2 diabetes mellitus with sulfonylureas, which close KATP channels, affirms the importance of this pathway in insulin secretion, but it is clear that glucose can also stimulate insulin release via pathways, presently ill-defined, that do not require closure of KATP channels (230−232). One of several possibilities (233) is that this second pathway requires RyR. Functional RyR, most likely RyR2, are certainly expressed in insulinoma and pancreatic β-cells (170,234), and stimulation of RyR can evoke insulin release (170,235,236). Whether RyR are required for glucose-evoked insulin release is less clear (168,170,236−242). There is, however, evidence that development of type 2 diabetes is associated with a loss of functional RyR2 (170,243,244).
Minimally, RyR within β-cells seem able, via Ca2+-induced Ca2+ release, to amplify the Ca2+ signals evoked by glucose-stimulated Ca2+ entry (234,245−247). Because the sensitivity of RyR to Ca2+ is modulated by many additional signals, including cyclic ADP ribose, ATP, cAMP, and redox state, other signaling pathways, the incretins, for example, may regulate the gain on this relationship between Ca2+ entry and its amplification by intracellular Ca2+ stores. RyR within the PM might fulfill a similar role by coordinating responses from different signaling pathways and transducing them into opening of a channel that would both depolarize the PM (by allowing Na+ entry) and provide a direct route for Ca2+ entry. Expressing a very high-conductance, nonselective cation channel (the RyR) in the PM of an electrically excitable cell (the β-cell) might seem to be a dangerous undertaking, but it is worth recalling that RyR are also regulated by membrane potential, such that they rapidly inactivate after step changes to either hyperpolarizing or depolarizing potentials (248). It may therefore be that within the PM, RyR transiently open only when provided with coincident stimuli: a step change in membrane potential and delivery of an appropriate cytosolic signal, such as Ca2+ or cyclic ADP ribose. Similar considerations might be important determinants of RyR activity in cardiac myocytes (188,189) or vascular smooth muscle (163). A further level of control might be imposed by dynamic trafficking of RyR to and from the PM of β-cells. Within insulinoma cells, RyR are expressed within insulin-containing vesicles (249) and/or endosomes (170), suggesting that both secretion of insulin and the subsequent retrieval of the membrane by endocytosis (250) may be intimately associated with regulated expression of RyR in the PM. Further work is required to extend results with insulinoma cells to primary β-cells and so establish whether they too express functional RyR2 in the PM, to determine whether PM expression of RyR2 is dynamically regulated, and to establish the consequences for β-cell physiology of gating RyR within the PM.
The evidence that IP3R are expressed in the PM of DT40 cells is compelling (65,120,140,141,143,251). Rather less secure, because it rests more on correlative evidence (see above), is our suggestion that IP3R in the PM are entirely responsible for the BCR-evoked Ca2+ entry that occurs via a non-SOCE pathway (Figure 2A). That conclusion implies that the two or three IP3R found in the PM of each DT40 cell are responsible for approximately half the Ca2+ entry evoked by the BCR (Figure 2A) (120). Our earlier analysis (65), in which we used the measured Ca2+ conductance of the PM IP3R (γCa ∼ 9 pS), its open probability when maximally activated by IP3 (Po ∼ 0.24), and the number of IP3R expressed in the PM (∼2) to estimate the likely flux of Ca2+ (∼4 × 105 Ca2+/s) through the PM IP3R, suggested that two IP3R in the PM are sufficient to mediate the Gd3+-insensitive Ca2+ entry evoked by activation of the BCR (Figure 2A). The remaining Ca2+ entry, via SOCE, occurs via some 10000 or more Orai channels (65,252). We speculate, although without specific evidence yet, that similar amounts of Ca2+ gushing into the cell via just two or three IP3R is likely to generate very different local Ca2+ signals and regulate a different response to that dribbling into the cell via 10000 low-conductance Orai channels (16). The effects of the two pathways on membrane potential are also likely to differ: opening of IP3R within the PM is likely to cause depolarization, whereas Orai channels are exquisitely Ca2+-selective and unlikely to regulate membrane potential directly. Whether IP3R in the PM, where they may be closely associated with the signaling machinery that generates IP3, respond differently to intracellular IP3R when cells are stimulated is another issue that needs to be resolved.
Supported by grants from the Wellcome Trust [085295], and the Biotechnology and Biological Sciences Research Council, U.K.
Footnotes
Abbreviations: AKAP, A-kinase-anchoring protein; BCR, B-cell receptor; CaMKII, Ca2+-calmodulin-dependent protein kinase II; CaR, Ca2+-sensing receptor; Cav, voltage-gated Ca2+ (channel); 4CmC, 4-chloro-m-cresol; CNG, cyclic nucleotide-gated (channel); ER, endoplasmic reticulum; ERAD, ER-associated degradation; γ, single-channel conductance; IP3, inositol 1,4,5-trisphosphate; IP3R, IP3 receptor(s); PIP2, phosphatidylinositol 4,5-bisphosphate; PKA, cyclic AMP-dependent protein kinase; PM, plasma membrane; PMCA, plasma membrane Ca2+-ATPase; RyR, ryanodine receptor(s); SERCA, SR/ER Ca2+-ATPase; SPCA, secretory pathway Ca2+-ATPase; SOCE, store-operated Ca2+ entry; SR, sarcoplasmic reticulum; SRP, signal recognition particle; STIM, stromal interaction protein; TGN, trans-Golgi network; TRP, transient receptor potential.
References
- Berridge M. J.; Bootman M. D.; Roderick H. L. (2003) Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 4, 517–529. [DOI] [PubMed] [Google Scholar]
- Berridge M. J.; Lipp P.; Bootman M. D. (2000) The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1, 11–21. [DOI] [PubMed] [Google Scholar]
- Xiong T. C.; Bourque S.; Lecourieux D.; Amelot N.; Grat S.; Briere C.; Mazars C.; Pugin A.; Ranjeva R. (2006) Calcium signaling in plant cell organelles delimited by a double membrane. Biochim. Biophys. Acta 1763, 1209–1215. [DOI] [PubMed] [Google Scholar]
- Kirichok Y.; Krapavinsky G.; Clapham D. E. (2004) The mitochondrial calcium uniporter is a highly selective ion channel. Nature 427, 360–364. [DOI] [PubMed] [Google Scholar]
- Toyoshima C. (2008) Structural aspects of ion pumping by Ca2+-ATPase of sarcoplasmic reticulum. Arch. Biochem. Biophys. 476, 3–11. [DOI] [PubMed] [Google Scholar]
- Brini M. (2009) Plasma membrane Ca2+-ATPase: From a housekeeping function to a versatile signaling role. Pfluegers Arch. 457, 657–664. [DOI] [PubMed] [Google Scholar]
- Van Baelen K.; Dode L.; Vanoevelen J.; Callewaert G.; De Smedt H.; Missiaen L.; Parys J. B.; Raeymaekers L.; Wuytack F. (2004) The Ca2+/Mn2+ pumps in the Golgi apparatus. Biochim. Biophys. Acta 1742, 103–112. [DOI] [PubMed] [Google Scholar]
- Hille B. (2001) Ionic Channels of Excitable Membranes, 3rd ed., Sinauer Associates, Inc., Sunderland, MA. [Google Scholar]
- Gillespie D.; Fill M. (2008) Intracellular calcium release channels mediate their own countercurrent: The ryanodine receptor case study. Biophys. J. 95, 3706–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allbritton N. L.; Meyer T.; Stryer L. (1992) Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science 258, 1812–1815. [DOI] [PubMed] [Google Scholar]
- Zhou Z.; Neher I. (1993) Mobile and immobile calcium buffers in bovine adrenal chromaffin cells. J. Physiol. 469, 245–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcke M. (2004) Reading patterns in living cells: The physics of Ca2+ signaling. Adv. Phys. 53, 255–440. [Google Scholar]
- Lewis R. S. (2007) The molecular choreography of a store-operated calcium channel. Nature 446, 284–287. [DOI] [PubMed] [Google Scholar]
- Willoughby D.; Cooper D. M. (2007) Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol. Rev. 87, 965–1010. [DOI] [PubMed] [Google Scholar]
- Dudzinski D. M.; Igarashi J.; Greif D.; Michel T. (2006) The regulation and pharmacology of endothelial nitric oxide synthase. Annu. Rev. Pharmacol. Toxicol. 46, 235–276. [DOI] [PubMed] [Google Scholar]
- Di Capite J.; Ng S. W.; Parekh A. B. (2009) Decoding of cytoplasmic Ca2+ oscillations through the spatial signature drives gene expression. Curr. Biol. 19, 853–858. [DOI] [PubMed] [Google Scholar]
- Bare D. J.; Kettlun C. S.; Liang M.; Bers D. M.; Mignery G. A. (2005) Cardiac type-2 inositol 1,4,5-trisphosphate receptor: Interaction and modulation by CaMKII. J. Biol. Chem. 280, 15912–15920. [DOI] [PubMed] [Google Scholar]
- Tadross M. R.; Dick I. E.; Yue D. T. (2008) Mechanism of local and global Ca2+ sensing by calmodulin in complex with a Ca2+ channel. Cell 133, 1228–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant J. S.; Parker I. (2001) Role of elementary Ca2+ puffs in generating repetitive Ca2+ oscillations. EMBO J. 20, 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalbey R. E., and Von Heijne G. (2002) Protein targeting, transport and translocation, Academic Press, New York. [Google Scholar]
- Dalbey R. E., Koehler C. M., and Tamanoi F., Eds. (2007) The enzymes. Molecular machines involved in protein transport across cellular membranes, Vol. 25, Academic Press, London. [Google Scholar]
- St Johnston D. (2005) Moving messages: The intracellular localization of mRNAs. Nat. Rev. Mol. Cell Biol. 6, 363–375. [DOI] [PubMed] [Google Scholar]
- Simon A. M.; Hoppe P.; Burden S. J. (1992) Spatial restriction of AChR gene expression to subsynaptic nuclei. Development 114, 545–553. [DOI] [PubMed] [Google Scholar]
- Dahm R.; Kiebler M.; Macchi P. (2007) RNA localisation in the nervous system. Semin. Cell Dev. Biol. 18, 216–223. [DOI] [PubMed] [Google Scholar]
- Margeot A.; Garcia M.; Wang W.; Tetaud E.; di Rago J. P.; Jacq C. (2005) Why are many mRNAs translated to the vicinity of mitochondria: A role in protein complex assembly?. Gene 354, 64–71. [DOI] [PubMed] [Google Scholar]
- Schmid M.; Jaedicke A.; Du T. G.; Jansen R. P. (2006) Coordination of endoplasmic reticulum and mRNA localization to the yeast bud. Curr. Biol. 16, 1538–1543. [DOI] [PubMed] [Google Scholar]
- Choi S. B.; Wang C.; Muench D. G.; Ozawa K.; Franceschi V. R.; Wu Y.; Okita T. W. (2000) Messenger RNA targeting of rice seed storage proteins to specific ER subdomains. Nature 407, 765–767. [DOI] [PubMed] [Google Scholar]
- Hengst U.; Jaffrey S. R. (2007) Function and translational regulation of mRNA in developing axons. Semin. Cell Dev. Biol. 18, 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannai H.; Fukatsu K.; Mizutani A.; Natsume T.; Iemura S.; Ikegami T.; Inoue T.; Mikoshiba K. (2004) An RNA-interacting protein, SYNCRIP (heterogeneous nuclear ribonuclear protein Q1/NSAP1) is a component of mRNA granule transported with inositol 1,4,5-trisphosphate receptor type 1 mRNA in neuronal dendrites. J. Biol. Chem. 279, 53427–53434. [DOI] [PubMed] [Google Scholar]
- Du T. G.; Schmid M.; Jansen R. P. (2007) Why cells move messages: The biological functions of mRNA localization. Semin. Cell Dev. Biol. 18, 171–177. [DOI] [PubMed] [Google Scholar]
- Jansen R. P. (2001) mRNA localization: Message on the move. Nat. Rev. Mol. Cell Biol. 2, 247–256. [DOI] [PubMed] [Google Scholar]
- van Vliet C.; Thomas E. C.; Merino-Trigo A.; Teasdale R. D.; Gleeson P. A. (2003) Intracellular sorting and transport of proteins. Prog. Biophys. Mol. Biol. 83, 1–45. [DOI] [PubMed] [Google Scholar]
- Platta H. W.; Erdmann R. (2007) The peroxisomal protein import machinery. FEBS Lett. 581, 2811–2819. [DOI] [PubMed] [Google Scholar]
- Benz J. P.; Soll J.; Bolter B. (2009) Protein transport in organelles: The composition, function and regulation of the Tic complex in chloroplast protein import. FEBS Lett. 276, 1166–1176. [DOI] [PubMed] [Google Scholar]
- Neupert W.; Herrmann J. M. (2007) Translocation of proteins into mitochondria. Annu. Rev. Biochem. 76, 723–749. [DOI] [PubMed] [Google Scholar]
- Hessa T.; Meindl-Beinker N. M.; Bernsel A.; Kim H.; Sato Y.; Lerch-Bader M.; Nilsson I.; White S. H.; von Heijne G. (2007) Molecular code for transmembrane-helix recognition by the Sec61 translocon. Nature 450, 1026–1030. [DOI] [PubMed] [Google Scholar]
- White S. H.; von Heijne G. (2008) How translocons select transmembrane helices. Annu. Rev. Biophys. Bioeng. 37, 23–42. [DOI] [PubMed] [Google Scholar]
- Abell B. M.; Pool M. R.; Schelenker O.; Sinning I.; High S. (2004) Signal recognition particle mediates post-translational targeting in eukaryotes. EMBO J. 23, 2755–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. (2006) Membrane-protein topology. Nat. Rev. Mol. Cell Biol. 7, 909–918. [DOI] [PubMed] [Google Scholar]
- Vembar S. S.; Brodsky J. L. (2008) One step at a time: Endoplasmic reticulum-associated degradation. Nat. Rev. Mol. Cell Biol. 9, 944–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove D. E.; Rosser M. F.; Ren H. Y.; Naren A. P.; Cyr D. M. (2009) Mechanisms for rescue of correctable folding defects in CFTRΔF508. Mol. Biol. Cell 20, 4059–4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksena S.; Summers M. D.; Burks J. K.; Johnson A. E.; Braunagel S. C. (2006) Importin-α-16 is a translocon-associated protein involved in sorting membrane proteins to the nuclear envelope. Nat. Struct. Mol. Biol. 13, 500–508. [DOI] [PubMed] [Google Scholar]
- Tabak H. F.; van der Zand A.; Braakman I. (2008) Peroxisomes: Minted by the ER. Curr. Opin. Cell Biol. 20, 393–400. [DOI] [PubMed] [Google Scholar]
- Groves E.; Dart A. E.; Covarelli V.; Caron E. (2008) Molecular mechanisms of phagocytic uptake in mammalian cells. Cell. Mol. Life Sci. 65, 1957–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C.; Filipeanu C. M.; Duvernay M. T.; Wu G. (2007) Regulation of G protein-coupled receptor export trafficking. Biochim. Biophys. Acta 1768, 853–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell-Lagnado R. D.; Qureshi O. S. (2008) Assembly and trafficking of P2X purinergic receptors. Mol. Membr. Biol. 25, 321–331. [DOI] [PubMed] [Google Scholar]
- Giamarchi A.; Padilla F.; Coste B.; Raoux M.; Crest M.; Honore E.; Delmas P. (2006) The versatile nature of the calcium-permeable cation channel TRPP2. EMBO Rep. 7, 787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis M. A.; Luini A. (2008) Exiting the Golgi complex. Nat. Rev. Mol. Cell Biol. 9, 273–284. [DOI] [PubMed] [Google Scholar]
- Blott E. J.; Griffiths G. M. (2002) Secretory lysosomes. Nat. Rev. Mol. Cell Biol. 3, 122–131. [DOI] [PubMed] [Google Scholar]
- Seaman M. N. (2008) Endosome protein sorting: Motifs and machinery. Cell. Mol. Life Sci. 65, 2842–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi O. S.; Paramasivam A.; Yu J. C.; Murrell-Lagnado R. D. (2007) Regulation of P2X4 receptors by lysosomal targeting, glycan protection and exocytosis. J. Cell Sci. 120, 3838–3849. [DOI] [PubMed] [Google Scholar]
- Kundra R.; Kornfeld S. (1999) Asparagine-linked oligosaccharides protect Lamp-1 and Lamp-2 from intracellular proteolysis. J. Biol. Chem. 274, 31039–31046. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E.; Kreitzer G.; Musch A. (2005) Organization of vesicular trafficking in epithelia. Nat. Rev. Mol. Cell Biol. 6, 233–247. [DOI] [PubMed] [Google Scholar]
- Fukuda M. (2008) Regulation of secretory vesicle traffic by Rab small GTPases. Cell. Mol. Life Sci. 65, 2801–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen P. J. (2008) Endosomal sorting and signalling: An emerging role for sorting nexins. Nat. Rev. Mol. Cell Biol. 9, 574–582. [DOI] [PubMed] [Google Scholar]
- Sudhof T. C.; Rothman J. E. (2009) Membrane fusion: Grappling with SNARE and SM proteins. Science 323, 474–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostov K. E.; Verges M.; Altschuler Y. (2000) Membrane traffic in polarized epithelial cells. Curr. Opin. Cell Biol. 12, 483–490. [DOI] [PubMed] [Google Scholar]
- Munro S. (2003) Lipid rafts: Elusive or illusive?. Cell 115, 377–388. [DOI] [PubMed] [Google Scholar]
- Andersen O. S.; Koeppe R. E. II (2007) Bilayer thickness and membrane protein function: An energetic perspective. Annu. Rev. Biophys. Biomol. Struct. 36, 107–130. [DOI] [PubMed] [Google Scholar]
- Vicinanza M.; D'Angelo G.; Di Campli A.; De Matteis M. A. (2008) Phosphoinositides as regulators of membrane trafficking in health and disease. Cell. Mol. Life Sci. 65, 2833–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J. A.; Halverson-Tamboli R. A.; Rasenick M. M. (2007) Lipid raft microdomains and neurotransmitter signalling. Nat. Rev. Neurosci. 8, 128–140. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. B.; Munro S. (1993) Sorting of membrane proteins in the secretory pathway. Cell 75, 603–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado M.; Eibl H.; Barrantes F. J. (1984) Functional properties of the acetylcholine receptor incorporated in model lipid membranes. Differential effects of chain length and head group of phospholipids on receptor affinity states and receptor-mediated ion translocation. J. Biol. Chem. 259, 9188–9198. [PubMed] [Google Scholar]
- Yuan C.; O'Connell R. J.; Feinberg-Zadek P. L.; Johnston L. J.; Treistman S. N. (2004) Bilayer thickness modulates the conductance of the BK channel in model membranes. Biophys. J. 86, 3620–3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellis O.; Dedos S.; Tovey S. C.; Rahman T.-U.; Dubel S. J.; Taylor C. W. (2006) Ca2+ entry through plasma membrane IP3 receptors. Science 313, 229–233. [DOI] [PubMed] [Google Scholar]
- Rahman T.; Taylor C. W. (2009) Dynamic regulation of IP3 receptor clustering and activity by IP3. Channels 3, 336–332. [DOI] [PubMed] [Google Scholar]
- Suh B. C.; Hille B. (2008) PIP2 is a necessary cofactor for ion channel function: How and why?. Annu. Rev. Biophys. 37, 175–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lape R.; Colquhoun D.; Sivilotti L. G. (2008) On the nature of partial agonism in the nicotinic receptor superfamily. Nature 454, 722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C. Y.; Hoover P. J.; Mullins F. M.; Bachhawat P.; Covington E. D.; Raunser S.; Walz T.; Garcia K. C.; Dolmetsch R. E.; Lewis R. S. (2009) STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell 136, 876–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewavitharana T.; Deng X.; Soboloff J.; Gill D. L. (2007) Role of STIM and Orai proteins in the store-operated calcium signaling pathway. Cell Calcium 42, 173–182. [DOI] [PubMed] [Google Scholar]
- Mignen O.; Thompson J. L.; Shuttleworth T. J. (2007) STIM1 regulates Ca2+ entry via arachidonate-regulated Ca2+-selective (ARC) channels without store depletion or translocation to the plasma membrane. J. Physiol. 579, 703–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardin I.; Lopez J. J.; Redondo P. C.; Salido G. M.; Rosado J. A. (2009) Store-operated Ca2+ entry is sensitive to the extracellular Ca2+ concentration through plasma membrane STIM1. Biochim. Biophys. Acta 1793, 1614–1622. [DOI] [PubMed] [Google Scholar]
- Craven K. B.; Zagotta W. N. (2006) CNG and HCN channels: Two peas, one pod. Annu. Rev. Physiol. 68, 375–401. [DOI] [PubMed] [Google Scholar]
- Voets T.; Nilius B.; Hoefs S.; van der Kemp A. W.; Droogmans G.; Bindels R. J.; Hoenderop J. G. (2004) TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J. Biol. Chem. 279, 19–25. [DOI] [PubMed] [Google Scholar]
- Vriens J.; Appendino G.; Nilius B. (2009) Pharmacology of vanilloid transient receptor potential cation channels. Mol. Pharmacol. 75, 1262–1279. [DOI] [PubMed] [Google Scholar]
- Nilius B.; Owsianik G.; Voets T.; Peters J. A. (2007) Transient receptor potential cation channels in disease. Physiol. Rev. 87, 165–217. [DOI] [PubMed] [Google Scholar]
- Bidaux G.; Flourakis M.; Thebault S.; Zholos A.; Beck B.; Gkika D.; Roudbaraki M.; Bonnal J. L.; Mauroy B.; Shuba Y.; Skryma R.; Prevarskaya N. (2007) Prostate cell differentiation status determines transient receptor potential melastatin member 8 channel subcellular localization and function. J. Clin. Invest. 117, 1647–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner H.; Fleig A.; Stokes A.; Kinet J.-P.; Penner R. (2003) Discimination of intracellular calcium store subcompartments using TRPV1 (transient receptor potential channel, vanilloid subfamily member 1) release channel activity. Biochem. J. 371, 341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A. (2000) Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 16, 521–555. [DOI] [PubMed] [Google Scholar]
- Dolphin A. C. (2003) β subunits of voltage-gated calcium channels. J. Bioenerg. Biomembr. 35, 599–620. [DOI] [PubMed] [Google Scholar]
- Jeng C. J.; Sun M. C.; Chen Y. W.; Tang C. Y. (2008) Dominant-negative effects of episodic ataxia type 2 mutations involve disruption of membrane trafficking of human P/Q-type Ca2+ channels. J. Cell. Physiol. 214, 422–433. [DOI] [PubMed] [Google Scholar]
- Talavera K.; Nilius B. (2006) Biophysics and structure-function relationship of T-type Ca2+ channels. Cell Calcium 40, 97–114. [DOI] [PubMed] [Google Scholar]
- Gomez M.; Hellstrand P. (1995) Effects of polyamines on voltage-activated calcium channels in guinea-pig intestinal smooth muscle. Pfluegers Arch. 430, 501–507. [DOI] [PubMed] [Google Scholar]
- Lynch J. W. (1999) Rectification of the olfactory cyclic nucleotide-gated channel by intracellular polyamines. J. Membr. Biol. 170, 213–227. [DOI] [PubMed] [Google Scholar]
- Bowie D.; Mayer M. L. (1995) Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron 15, 453–462. [DOI] [PubMed] [Google Scholar]
- Turecek R.; Vlcek K.; Petrovic M.; Horak M.; Vlachova V.; Vyklicky L. Jr. (2004) Intracellular spermine decreases open probability of N-methyl-d-aspartate receptor channels. Neuroscience 125, 8879–8887. [DOI] [PubMed] [Google Scholar]
- Soto D.; Coombs I. D.; Kelly L.; Farrant M.; Cull-Candy S. G. (2007) Stargazin attenuates intracellular polyamine block of calcium-permeable AMPA receptors. Nat. Neurosci. 10, 1260–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J. M.; Wang J.; Eisenberg R. S. (1989) K+-selective channel from sarcoplasmic reticulum of split lobster muscle fibers. J. Gen. Physiol. 94, 261–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo A. V.; Shuman H.; Somlyo A. P. (1977) Composition of sarcoplasmic reticulum in situ by electron probe X-ray microanalysis. Nature 268, 556–558. [DOI] [PubMed] [Google Scholar]
- Oetliker H. (1982) An appraisal of the evidence for a sarcoplasmic reticulum membrane potential and its relation to calcium release in skeletal muscle. J. Muscle Res. Cell Motil. 3, 247–272. [DOI] [PubMed] [Google Scholar]
- Schapiro F. B.; Grinstein S. (2000) Determinants of the pH of the Golgi complex. J. Biol. Chem. 275, 21025–21032. [DOI] [PubMed] [Google Scholar]
- Breckenridge L. J.; Almers W. (1987) Currents through the fusion pore that forms during exocytosis of a secretory vesicle. Nature 328, 814–817. [DOI] [PubMed] [Google Scholar]
- Passafaro M.; Rosa P.; Sala C.; Clementi F.; Sher E. (1996) N-type Ca2+ channels are present in secretory granules and are transiently translocated to the plasma membrane during regulated exocytosis. J. Biol. Chem. 271, 30096–30104. [DOI] [PubMed] [Google Scholar]
- Prod'hom B.; Pietrobon D.; Hess P. (1987) Direct measurement of proton transfer rates to a group controlling the dihydropyridine-sensitive Ca2+ channel. Nature 329, 243–246. [DOI] [PubMed] [Google Scholar]
- Morrill J. A.; MacKinnon R. (1999) Isolation of a single carboxyl-carboxylate proton binding site in the pore of a cyclic nucleotide-gated channel. J. Gen. Physiol. 114, 71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh B. I.; Sun T. J.; Lee J. Z.; Chen H. H.; Huang C. L. (2003) Mechanism and molecular determinant for regulation of rabbit transient receptor potential type 5 (TRPV5) channel by extracellular pH. J. Biol. Chem. 278, 51044–51052. [DOI] [PubMed] [Google Scholar]
- Caterina M. J.; Schumacher M. A.; Tominaga M.; Rosen T. A.; Levine J. D.; Juliu D. (1997) The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 389, 816–824. [DOI] [PubMed] [Google Scholar]
- Li M.; Jiang J.; Yue L. (2006) Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J. Gen. Physiol. 127, 525–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J.; Li M.; Yue L. (2005) Potentiation of TRPM7 inward currents by protons. J. Gen. Physiol. 126, 137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L.; Bauer C. S.; Zhen X. G.; Xie C.; Yang J. (2002) Dual regulation of voltage-gated calcium channels by PtdIns(4,5)P2. Nature 419, 947–952. [DOI] [PubMed] [Google Scholar]
- Sen L.; Bialecki R. A.; Smith E.; Smith T. W.; Colucci W. S. (1992) Cholesterol increases the L-type voltage-sensitive calcium channel current in arterial smooth muscle cells. Circ. Res. 71, 1008–1014. [DOI] [PubMed] [Google Scholar]
- Rohacs T. (2009) Phosphoinositide regulation of non-canonical transient receptor potential channels. Cell Calcium 45, 554–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann T.; Obukhov A. G.; Schaefer M.; Harteneck C.; Gudermann T.; Schultz G. (1999) Direct activaton of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 397, 259–263. [DOI] [PubMed] [Google Scholar]
- Ding X. Q.; Fitzgerald J. B.; Matveev A. V.; McClellan M. E.; Elliott M. H. (2008) Functional activity of photoreceptor cyclic nucleotide-gated channels is dependent on the integrity of cholesterol- and sphingolipid-enriched membrane domains. Biochemistry 47, 3677–3687. [DOI] [PubMed] [Google Scholar]
- Graziani A.; Rosker C.; Kohlwein S. D.; Zhu M. X.; Romanin C.; Sattler W.; Groschner K.; Poteser M. (2006) Cellular cholesterol controls TRPC3 function: Evidence from a novel dominant-negative knockdown strategy. Biochem. J. 396, 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley J. H.; Cahill A. L.; Currie K. P.; Fox A. P. (2000) The role of dynamic palmitoylation in Ca2+ channel inactivation. Proc. Natl. Acad. Sci. U.S.A. 97, 9293–9298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien A. J.; Gao T.; Perez-Reyes E.; Hosey M. M. (1998) Membrane targeting of L-type calcium channels. Role of palmitoylation in the subcellular localization of the β2a subunit. J. Biol. Chem. 273, 23590–25597. [DOI] [PubMed] [Google Scholar]
- Qin N.; Platano D.; Olcese R.; Costantin J. L.; Stefani E.; Birnbaumer L. (1998) Unique regulatory properties of the type 2a Ca2+ channel β subunit caused by palmitoylation. Proc. Natl. Acad. Sci. U.S.A. 95, 4690–4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P. C.; Scott J. D.; Catterall W. A. (1998) Regulation of ion channels by cAMP-dependent protein kinase and A-kinase anchoring proteins. Curr. Opin. Neurobiol. 8, 330–334. [DOI] [PubMed] [Google Scholar]
- Rahman T.-U.; Skupin A.; Falcke M.; Taylor C. W. (2009) Clustering of IP3 receptors by IP3 retunes their regulation by IP3 and Ca2+. Nature 458, 655–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foskett J. K.; White C.; Cheung K. H.; Mak D. O. (2007) Inositol trisphosphate receptor Ca2+ release channels. Physiol. Rev. 87, 593–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker A. K. T.; Gergely F. V.; Taylor C. W. (2004) Targeting of inositol 1,4,5-trisphosphate receptors to the endoplasmic reticulum by multiple signals within their transmembrane domains. J. Biol. Chem. 279, 23797–23805. [DOI] [PubMed] [Google Scholar]
- Pinton P.; Pozzan T.; Rizzuto R. (1998) The Golgi apparatus is an inositol 1,4,5-trisphosphate-sensitive Ca2+ store, with functional properties distinct from those of the endoplasmic reticulum. EMBO J. 17, 5298–5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missiaen L.; Van Acker K.; Parys J. B.; De Smedt H.; Van Baelen K.; Weidema A. F.; Vanoevelen J.; Raeymaekers L.; Renders J.; Callewaert G.; Rizzuto R.; Wuytack F. (2001) Baseline cytosolic Ca2+ oscillations derived from a non-endoplasmic reticulum Ca2+ store. J. Biol. Chem. 276, 39161–39170. [DOI] [PubMed] [Google Scholar]
- Echevarria W.; Leite M. F.; Guerra M. T.; Zipfel W. R.; Nathanson M. H. (2003) Regulation of calcium signals in the nucleus by a nucleoplasmic reticulum. Nat. Cell Biol. 5, 440–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko O. V.; Gerasimenko J. V.; Belan P. V.; Petersen O. H. (1996) Inositol trisphosphate and cyclic ADP-ribose-mediated release of Ca2+ from single isolated pancreatic zymogen granules. Cell 84, 473–480. [DOI] [PubMed] [Google Scholar]
- Yule D. I.; Ernst S. A.; Ohnishi H.; Wojcikiewicz R. J. H. (1997) Evidence that zymogen granules are not a physiologically relevant calcium pool. Defining the distribution of inositol 1,4,5-trisphosphate receptors in pancreatic acinar cells. J. Biol. Chem. 272, 9093–9098. [DOI] [PubMed] [Google Scholar]
- Santodomingo J.; Vay L.; Camacho M.; Hernandez-Sanmiguel E.; Fonteriz R. I.; Lobaton C. D.; Montero M.; Moreno A.; Alvarez J. (2008) Calcium dynamics in bovine adrenal medulla chromaffin cell secretory granules. Eur. J. Neurosci. 28, 1265–1274. [DOI] [PubMed] [Google Scholar]
- Yoo S. H.; Albanesi J. P. (1990) Inositol 1,4,5-trisphosphate-triggered Ca2+ release from bovine adrenal medullary secretory vesicles. J. Biol. Chem. 265, 13446–13448. [PubMed] [Google Scholar]
- Taylor C. W.; Rahman T.; Tovey S. C.; Dedos S. G.; Taylor E. J. A.; Velamakanni S. (2009) IP3 receptors: Some lessons from DT40 cells. Immunol. Rev. 231, 23–44. [DOI] [PubMed] [Google Scholar]
- Vazquez G.; Wedel B. J.; Bird G. S. J.; Joseph S. K.; Putney J. W. (2002) An inositol 1,4,5-trisphosphate receptor-dependent cation entry pathway in DT40 B lymphocytes. EMBO J. 21, 4531–4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemette G.; Balla T.; Baukal A. J.; Catt K. J. (1988) Characterization of inositol 1,4,5-trisphosphate receptors and calcium mobilization in a hepatic plasma membrane fraction. J. Biol. Chem. 263, 4541–4548. [PubMed] [Google Scholar]
- Rossier M. F.; Bird G. S. J.; Putney J. W. Jr. (1991) Subcellular distribution of the calcium-storing inositol 1,4,5-trisphosphate-sensitive organelle in rat liver. Possible linkage to the plasma membrane through actin microfilaments. Biochem. J. 274, 643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp A. H.; Snyder S. H.; Nigam S. K. (1992) Inositol 1,4,5-trisphosphate receptors: Localization in epithelial tissue. J. Biol. Chem. 267, 7444–7449. [PubMed] [Google Scholar]
- Smith I. F.; Wiltgen S. M.; Parker I. (2009) Localization of puff sites adjacent to the plasma membrane: Functional and spatial characterization of Ca2+ signaling in SH-SY5Y cells utilizing membrane-permeant caged IP3. Cell Calcium 45, 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera N. P.; Morales B.; Villalon M. (2004) Plasma and intracellular membrane inositol 1,4,5-trisphosphate receptors mediate the Ca2+ increase associated with the ATP-induced increase in ciliary beat frequency. Am. J. Physiol. 287, C1114–C1124. [DOI] [PubMed] [Google Scholar]
- El-Daher S. S.; Patel Y.; Siddiqua A.; Hassock S.; Edmunds S.; Maddison B.; Patel G.; Goulding D.; Lupu F.; Wojcikiewicz J. H.; Authi K. S. (2000) Distinct localization and function of 1,4,5IP3 receptor subtypes and the 1,3,4,5IP4 receptor GAP1IP4BP in highly purified human platelet membranes. Blood 95, 3412–3422. [PubMed] [Google Scholar]
- Khan A. A.; Steiner J. P.; Klein M. G.; Schneider M. F.; Snyder S. H. (1992) IP3 receptor: Localization to plasma membrane of T cells and cocapping with the T cell receptor. Science 257, 815–818. [DOI] [PubMed] [Google Scholar]
- Khan A. A.; Steiner J. P.; Snyder S. H. (1992) Plasma membrane inositol 1,4,5-trisphosphate receptor of lymphocytes: Selective enrichment in sialic acid and unique binding specificity. Proc. Natl. Acad. Sci. U.S.A. 89, 2849–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura A.; Tojyo Y.; Turner R. J. (2000) Evidence that type I, II and III inositol-trisphosphate receptors can occur as integral membrane proteins. J. Biol. Chem. 275, 27488–27498. [DOI] [PubMed] [Google Scholar]
- Fadool D. A.; Ache B. W. (1992) Plasma membrane inositol 1,4,5-trisphosphate-activated channels mediate signal transuction in lobster olfactory neurons. Neuron 9, 907–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M.; Gardner P. (1987) Ion channels activated by inositol 1,4,5-trisphosphate in plasma membrane of human T-lymphocytes. Nature 326, 301–304. [DOI] [PubMed] [Google Scholar]
- Kuno M.; Maeda N.; Mikoshiba K. (1994) IP3-activated calcium-permeable channels in the inside-out patches of cultured cerebellar Purkinje cells. Biochem. Biophys. Res. Commun. 199, 1128–1135. [DOI] [PubMed] [Google Scholar]
- Mozhayeva G. N.; Naumov A. P.; Kuryshev Y. A. (1990) Inositol 1,4,5-trisphosphate activates two types of Ca2+-permeable channels in human carcinoma cells. FEBS Lett. 277, 233–234. [DOI] [PubMed] [Google Scholar]
- Ueda H.; Tamura S.; Fukushima N.; Katada T.; Ui M.; Satoh M. (1996) Inositol 1,4,5-trisphosphate-gated calcium transport through plasma membranes in nerve terminals. J. Neurosci. 16, 2891–2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaca L.; Kunze D. L. (1995) IP3-activated Ca2+ channels in the plasma membrane of cultured vascular endothelial cells. Am. J. Physiol. 269, C733–C738. [DOI] [PubMed] [Google Scholar]
- Mayrleitner M.; Schäffer R.; Fleischer S. (1994) Purified IP3 receptor from liver plasma membrane displays IP3 activated and IP4 inhibited calcium channel activity. Biophys. J. 66, A278. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I. (2005) The inositol 1,4,5-trisphosphate receptors. Cell Calcium 38, 261–272. [DOI] [PubMed] [Google Scholar]
- Thrower E. C.; Hagar R. E.; Ehrlich B. E. (2001) Regulation of Ins(1,4,5)P3 receptor isoforms by endogenous modulators. Trends Pharmacol. Sci. 22, 580–586. [DOI] [PubMed] [Google Scholar]
- Dellis O.; Rossi A. M.; Dedos S. G.; Taylor C. W. (2008) Counting functional IP3 receptors into the plasma membrane. J. Biol. Chem. 283, 751–755. [DOI] [PubMed] [Google Scholar]
- Wagner L. E. II; Joseph S. K.; Yule D. I. (2008) Regulation of single inositol 1,4,5-trisphosphate receptor channel activity by protein kinase A phosphorylation. J. Physiol. 586, 3577–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara H.; Kurosaki M.; Takata M.; Kurosaki T. (1997) Genetic evidence for involvement of type 1, type 2 and type 3 inositol 1,4,5-trisphosphate receptors in signal transduction through the B-cell antigen receptor. EMBO J. 16, 3078–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betzenhauser M. J.; Wagner L. E. II; Won J. H.; Yule D. I. (2008) Studying isoform-specific inositol 1,4,5-trisphosphate receptor function and regulation. Methods 46, 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney J. W. Jr. (1986) A model for receptor-regulated calcium entry. Cell Calcium 7, 1–12. [DOI] [PubMed] [Google Scholar]
- Putney J. W. Jr. (2007) Recent breakthroughs in the molecular mechanism of capacitative calcium entry (with thoughts on how we got here). Cell Calcium 42, 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh A. B.; Penner R. (1997) Store depletion and calcium influx. Physiol. Rev. 77, 901–930. [DOI] [PubMed] [Google Scholar]
- Prakriya M. (2009) The molecular physiology of CRAC channels. Immunol. Rev. 231, 88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J.; Kim M. L.; Heo W. D.; Jones J. T.; Myers J. W.; Ferrell J. E. Jr.; Meyer T. (2005) STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 15, 1235–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S. L.; Yu Y.; Roos J.; Kozak J. A.; Deerinck T. J.; Ellisman M. H.; Stauderman K. A.; Cahalan M. D. (2005) STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 437, 902–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feske S.; Gwack Y.; Prakriya M.; Srikanth S.; Puppel S. H.; Tanasa B.; Hogan P. G.; Lewis R. S.; Daly M.; Rao A. (2006) A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441, 179–185. [DOI] [PubMed] [Google Scholar]
- Luik R. M.; Wang B.; Prakriya M.; Wu M. M.; Lewis R. S. (2008) Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature 454, 538–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W.; Yuan J. P.; Kim M. S.; Choi Y. J.; Huang G. N.; Worley P. F.; Muallem S. (2008) STIM1 gates TRPC channels, but not Orai1, by electrostatic interaction. Mol. Cell 32, 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolotina V. M. (2008) Orai, STIM1 and iPLA2β: A view from a different perspective. J. Physiol. 586, 3035–3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandman O.; Liou J.; Park W. S.; Meyer T. (2007) STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell 131, 1327–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird G. S.; Hwang S. Y.; Smyth J. T.; Fukushima M.; Boyles R. R.; Putney J. W. Jr. (2009) STIM1 is a calcium sensor specialized for digital signaling. Curr. Biol. 19, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penna A.; Demuro A.; Yeromin A. V.; Zhang S. L.; Safrina O.; Parker I.; Cahalan M. D. (2008) The CRAC channel consists of a tetramer formed by Stim-induced dimerization of Orai dimers. Nature 456, 116–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakriya M.; Lewis R. S. (2001) Potentiation and inhibition of Ca2+ release-activated Ca2+ channels by 2-aminoethyldiphenyl borate (2-APB) occurs independently of IP3 receptors. J. Physiol. 536, 3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba Y.; Hayashi K.; Fujii Y.; Mizushima A.; Watarai H.; Wakamori M.; Numaga T.; Mori Y.; Iino M.; Hikida M.; Kurosaki T. (2006) Coupling of STIM1 to store-operated Ca2+ entry through its constitutive and inducible movement in the endoplasmic reticulum. Proc. Natl. Acad. Sci. U.S.A. 103, 16704–16709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita T.; Tanimura A.; Baba Y.; Kurosaki T.; Tojyo Y. (2009) A Stim1-dependent, noncapacitative Ca2+-entry pathway is activated by B-cell-receptor stimulation and depletion of Ca2+. J. Cell Sci. 122, 1220–1208. [DOI] [PubMed] [Google Scholar]
- van Rossum D. B.; Patterson R. L.; Kiselyov K.; Boehning D.; Barrow R. K.; Gill D. L.; Snyder S. H. (2004) Agonist-induced Ca2+ entry determined by inositol 1,4,5-trisphosphate recognition. Proc. Natl. Acad. Sci. U.S.A. 101, 2323–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lievremont J. P.; Numaga T.; Vazquez G.; Lemonnier L.; Hara Y.; Mori E.; Trebak M.; Moss S. E.; Bird G. S.; Mori Y.; Putney J. W. Jr. (2005) The role of canonical transient receptor potential 7 in B-cell receptor-activated channels. J. Biol. Chem. 280, 35346–35351. [DOI] [PubMed] [Google Scholar]
- Ghanshani S.; Wulff H.; Miller M. J.; Rohm H.; Neben A.; Gutman G. A.; Cahalan M. D.; Chandy K. G. (2000) Up-regulation of the IKCa1 potassium channel during T-cell activation. Molecular mechanism and functional consequences. J. Biol. Chem. 275, 37137–37149. [DOI] [PubMed] [Google Scholar]
- Loirand G.; Pacaud P.; Baron A.; Mironneau C.; Mironneau J. (1991) Large conductance calcium-activated non-selective cation channel in smooth muscle cells isolated from rat portal vein. J. Physiol. 437, 461–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amador F. J.; Liu S.; Ishiyama N.; Plevin M. J.; Wilson A.; Maclennan D. H.; Ikura M. (2009) Crystal structure of type I ryanodine receptor amino-terminal β-trefoil domain reveals a disease-associated mutation “hot spot” loop. Proc. Natl. Acad. Sci. U.S.A. 106, 11040–11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. W.; da Fonseca P. C. A.; Morris E. P. (2004) IP3 receptors: The search for structure. Trends Biochem. Sci. 29, 210–219. [DOI] [PubMed] [Google Scholar]
- Sitsapesan R., and Williams A. J. (1998) The Structure and Function of Ryanodine Receptors, Imperial College Press, London. [Google Scholar]
- Meur G.; Parker A. K. T.; Gergely F. V.; Taylor C. W. (2007) Targeting and retention of type 1 ryanodine receptors to the endoplasmic reticulum. J. Biol. Chem. 282, 23096–23103. [DOI] [PubMed] [Google Scholar]
- Mitchell K. J.; Lai F. A.; Rutter G. A. (2003) Ryanodine receptor type 1 and nicotinic acid adenine dinucleotide phosphate receptors mediate Ca2+ release from insulin-containing vesicles in living pancreatic β-cells (MIN6). J. Biol. Chem. 278, 11057–11064. [DOI] [PubMed] [Google Scholar]
- Mitchell K. J.; Pinton P.; Varadi A.; Tacchetti C.; Ainscow E. K.; Pozzan T.; Rizzuto R.; Rutter G. A. (2001) Dense core secretory vesicles revealed as a dynamic Ca2+ store in neuroendocrine cells with a vesicle-associated membrane protein aequorin indicator. J. Cell Biol. 155, 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. D.; Kuang S.; Misler S.; Polonsky K. S. (2004) Ryanodine receptors in human pancreatic beta cells: Localization and effects on insulin secretion. FASEB J. 18, 878–880. [DOI] [PubMed] [Google Scholar]
- Prentki M.; Biden T. J.; Janjic D.; Irvine R. F.; Berridge M. J.; Wollheim C. B. (1984) Rapid mobilization of Ca2+ from rat insulinoma microsomes by inositol-1,4,5-trisphosphate. Nature 309, 562–564. [DOI] [PubMed] [Google Scholar]
- Sitsapesan R. (2009) In pursuit of ryanodine receptors gating in the plasma membrane of RINm5F pancreatic β-cells. Islets 1, 82–84. [DOI] [PubMed] [Google Scholar]
- Zaidi M.; Shankar V. S.; Tunwell R.; Adebanjo O. A.; Mackrill J.; Pazianas M.; O'Connell D.; Simon B. J.; Rifkin B. R.; Venkitaraman A. R.; Huang C. L.-H.; Lai F. A. (1995) A ryanodine receptor-like molecule expressed in the osteoclast plasma membrane functions in extracellular Ca2+ sensing. J. Clin. Invest. 96, 1582–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar V. S.; Pazianas M.; Huang C. L.; Simon B.; Adebanjo O. A.; Zaidi M. (1995) Caffeine modulates Ca2+ receptor activation in isolated rat osteoclasts and induces intracellular Ca2+ release. Am. J. Physiol. 268, F447–F454. [DOI] [PubMed] [Google Scholar]
- Moonga B. S.; Li S.; Iqbal J.; Davidson R.; Shankar V. S.; Bevis P. J. R.; Inzerillo A.; Abe E.; Huang C. L.-H.; Zaidi M. (2002) Ca2+ influx through osteoclastic plasma membrane ryanodine receptor. Am. J. Physiol. 282, F921–F932. [DOI] [PubMed] [Google Scholar]
- Bai M. (2004) Structure-function relationship of the extracellular calcium-sensing receptor. Cell Calcium 35, 197–207. [DOI] [PubMed] [Google Scholar]
- Ma J. (1993) Block by ruthenium red of the ryanodine-activated calcium release channel of skeletal muscle. J. Gen. Physiol. 102, 1031–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adebanjo O. A.; Shankar V. S.; Pazianas M.; Simon B. J.; Lai F. A.; Huang C. L.; Zaidi M. (1996) Extracellularly applied ruthenium red and cADP ribose elevate cytosolic Ca2+ in isolated rat osteoclasts. Am. J. Physiol. 270, F469–F475. [DOI] [PubMed] [Google Scholar]
- Du G. G.; Sandu B.; Khanna V. K.; Guo X. H.; MacLennan D. (2002) Topology of the Ca2+ release channel of skeletal muscle sarcoplasmic reticulum (RyR1). Proc. Natl. Acad. Sci. U.S.A. 99, 16725–16730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorzato F.; Fujii J.; Otsu K.; Phillips M.; Green N. M.; Lai F. A.; Meissner G.; MacLennan D. H. (1990) Molecular cloning of cDNA encoding human and rabbit forms of the Ca2+ release channel (ryanodine receptor) of skeletal muscle sarcoplasmic reticulum. J. Biol. Chem. 265, 2244–2256. [PubMed] [Google Scholar]
- Tunwell R. E. A.; Wickenden C.; Bertrand B. M. A.; Shevchenko V. I.; Walsh M. B.; Allen P. D.; Lai F. A. (1996) The human cardiac muscle ryanodine receptor-calcium release channel: Identificaiton, primary structure and topological analysis. Biochem. J. 318, 477–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi M.; Shankar V. S.; Towhidul Alam A. S.; Moonga B. S.; Pazianas M.; Huang C. L. (1992) Evidence that a ryanodine receptor triggers signal transduction in the osteoclast. Biochem. Biophys. Res. Commun. 188, 1332–1326. [DOI] [PubMed] [Google Scholar]
- Mahaut-Smith M. P.; Martinez-Pinna J.; Gurung I. S. (2008) A role for membrane potential in regulating GPCRs?. Trends Pharmacol. Sci. 29, 421–429. [DOI] [PubMed] [Google Scholar]
- Williams A. J. (2002) Ion conduction and selectivity in the ryanodine receptor channel. Front. Biosci. 7, 1–8. [DOI] [PubMed] [Google Scholar]
- Rosker C.; Meur G.; Taylor E. J. A.; Taylor C. W. (2009) Functional ryanodine receptors in the plasma membrane of RINm5F pancreatic β-cells. J. Biol. Chem. 284, 5186–5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero A.; Fay F. S.; Singer J. J. (1994) Caffeine activates a Ca2+-permeable, nonselective cation channel in smooth muscle cells. J. Gen. Physiol. 104, 375–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q.; Hogg R. C.; Large W. A. (1993) A monovalent ion-selective cation current activated by noradrenaline in smooth muscle cells of rabbit ear artery. Pfluegers Arch. 423, 28–33. [DOI] [PubMed] [Google Scholar]
- Zhang Y. A.; Tuft R. A.; Lifshitz L. M.; Fogarty K. E.; Singer J. J.; Zou H. (2007) Caffeine-activated large-conductance plasma membrane cation channels in cardiac myocytes: Characteristics and significance. Am. J. Physiol. 293, H2448–H2461. [DOI] [PubMed] [Google Scholar]
- Kondo R. P.; Weiss J. N.; Goldhaber J. I. (2000) Putative ryanodine receptors in the sarcolemma of ventricular myocytes. Pfluegers Arch. 440, 125–131. [DOI] [PubMed] [Google Scholar]
- Lindsay A. R.; Tinker A.; Williams A. J. (1994) How does ryanodine modify ion handling in the sheep cardiac sarcoplasmic reticulum Ca2+-release channel?. J. Gen. Physiol. 104, 425–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver D. R.; Lamb G. D. (1998) Inactivation of Ca2+ release channels (ryanodine receptors RyR1 and RyR2) with rapid steps in [Ca2+] and voltage. Biophys. J. 74, 2352–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusnier C.; Cardenas C.; Hidalgo J.; Jaimovich E. (2006) Single-channel recording of inositol trisphosphate receptor in the isolated nucleus of a muscle cell line. Biol. Res. 39, 541–553. [DOI] [PubMed] [Google Scholar]
- Ehrlich B. E.; Watras J. (1988) Inositol 1,4,5-trisphosphate activates a channel from smooth muscle sarcoplasmic reticulum. Nature 336, 583–586. [DOI] [PubMed] [Google Scholar]
- Stehno-Bittel L.; Lückhoff A.; Clapham D. E. (1995) Calcium release from the nucleus by InsP3 receptor channels. Neuron 14, 163–167. [DOI] [PubMed] [Google Scholar]
- Watras J.; Bezprozvanny I.; Ehrlich B. E. (1991) Inositol 1,4,5-trisphosphate-gated channels in cerebellum: Presence of multiple conductance states. J. Neurosci. 11, 3239–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak D.-O.; McBride S.; Raghiram V.; Yue Y.; Joseph S. K.; Foskett J. K. (2000) Single-channel properties in endoplasmic reticulum membrane of recombinant type 3 inositol trisphosphate receptor. J. Gen. Physiol. 115, 241–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson R. L.; Boehning D.; Snyder S. H. (2004) Inositol 1,4,5-trisphosphate receptors as signal integrators. Annu. Rev. Biochem. 73, 437–465. [DOI] [PubMed] [Google Scholar]
- Choe C.; Ehrlich B. E. (2006) The inositol 1,4,5-trisphosphate receptor (IP3R) and its regulators: Sometimes good and someimes bad teamwork. Sci. STKE, re15. [DOI] [PubMed] [Google Scholar]
- Zalk R.; Lehnart S. E.; Marks A. R. (2007) Modulation of the ryanodine receptor and intracellular calcium. Annu. Rev. Biochem. 76, 367–385. [DOI] [PubMed] [Google Scholar]
- Bers D. M. (2004) Macromolecular complexes regulating cardiac ryanodine receptor function. J. Mol. Cell. Cardiol. 37, 417–429. [DOI] [PubMed] [Google Scholar]
- van Rossum D. B.; Patterson R. L.; Cheung K. H.; Barrow R. K.; Syrovatkina V.; Gessell G. S.; Burkholder S. G.; Watkins D. N.; Foskett J. K.; Snyder S. H. (2006) DANGER: A novel regulatory protein of IP3-receptor activity. J. Biol. Chem. 281, 37111–37116. [DOI] [PubMed] [Google Scholar]
- Li C.; Wang X.; Vais H.; Thompson C. B.; Foskett J. K.; White C. (2007) Apoptosis regulation by Bcl-xL modulation of mammalian inositol 1,4,5-trisphosphate receptor channel isoform gating. Proc. Natl. Acad. Sci. U.S.A. 104, 12565–12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiselhöringer A.; Werner M.; Sigl K.; Smital P.; Wörner R.; Acheo L.; Stieber J.; Weinmeister J.; Feil R.; Feil S.; Wegener J.; Hofmann F.; Schlossmann J. (2004) IRAG is essential for relaxation of receptor-triggered smooth muscle contraction by cGMP kinase. EMBO J. 23, 4222–4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce J. I. E.; Shuttleworth T. J.; Giovannucci D. R.; Yule D. I. (2002) Phosphorylation of inositol 1,4,5-trisphosphate receptors in parotid acinar cells. A mechanism for the synergistic effects of cAMP on Ca2+ signaling. J. Biol. Chem. 277, 1340–1348. [DOI] [PubMed] [Google Scholar]
- Higo T.; Hattori M.; Nakamura T.; Natsume T.; Michikawa T.; Mikoshiba K. (2005) Subtype-specific and ER lumenal environment-dependent regulation of inositol 1,4,5-trisphosphate receptor type 1 by ERp44. Cell 120, 85–98. [DOI] [PubMed] [Google Scholar]
- Taylor C. W.; Laude A. J. (2002) IP3 receptors and their regulation by calmodulin and cytosolic Ca2+. Cell Calcium 32, 321–334. [DOI] [PubMed] [Google Scholar]
- Shirakabe K.; Priori G.; Yamada H.; Ando H.; Horita S.; Fujita T.; Fujimoto I.; Mizutani A.; Seki G.; Mikoshiba K. (2006) IRBIT, an inositol 1,4,5-trisphosphate receptor-binding protein, specifically binds to and activates pancreas-type Na+/HCO3− cotransporter 1 (pNBC1). Proc. Natl. Acad. Sci. U.S.A. 103, 9542–9547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D.; Shcheynikov N.; Zeng W.; Ohana E.; So I.; Ando H.; Mizutani A.; Mikoshiba K.; Muallem S. (2009) IRBIT coordinates epithelial fluid and HCO3− secretion by stimulating the transporters pNBC1 and CFTR in the murine pancreatic duct. J. Clin. Invest. 119, 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrower E. C.; Choe C. U.; So S. H.; Jeon S. H.; Ehrlich B. E.; Yoo S. H. (2003) A functional interaction between chromogranin B and inositol 1,4,5-trisphosphate receptor/Ca2+ channel. J. Biol. Chem. 278, 49699–49708. [DOI] [PubMed] [Google Scholar]
- Joseph S. K.; Boehning D.; Bokkala S.; Watkins R.; Widjaja J. (1999) Biosynthesis of inositol trisphosphate receptors: Selective association with the molecular chaperone calnexin. Biochem. J. 342, 153–161. [PMC free article] [PubMed] [Google Scholar]
- Treves S.; Vukcevic M.; Maj M.; Thurnheer R.; Mosca B.; Zorzato F. (2009) Minor sarcoplasmic reticulum membrane components that modulate excitation-contraction coupling in striated muscles. J. Physiol. 587, 3071–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver D. R.; Honen B. N. (2008) Luminal Mg2+, a key factor controlling RYR2-mediated Ca2+ release: Cytoplasmic and luminal regulation modeled in a tetrameric channel. J. Gen. Physiol. 132, 429–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada S.; Rodan G. A. (2003) Control of osteoblast function and regulation of bone mass. Nature 423, 349–355. [DOI] [PubMed] [Google Scholar]
- Zaidi M.; Datta H. K.; Patchell A.; Moonga B.; MacIntyre I. (1989) 'Calcium-activated' intracellular calcium elevation: A novel mechanism of osteoclast regulation. Biochem. Biophys. Res. Commun. 163, 1461–1465. [DOI] [PubMed] [Google Scholar]
- Dvorak M. M.; Riccardi D. (2004) Ca2+ as an extracellular signal in bone. Cell Calcium 35, 249–255. [DOI] [PubMed] [Google Scholar]
- Brown E. M. (1991) Extracellular Ca2+ sensing, regulation of parathyroid cell function, and role of Ca2+ and other ions as extracellular (first) messengers. Physiol. Rev. 71, 371–411. [DOI] [PubMed] [Google Scholar]
- Kanatani M.; Sugimoto T.; Kanzawa M.; Yano S.; Chihara K. (1999) High extracellular calcium inhibits osteoclast-like cell formation by directly acting on the calcium-sensing receptor existing in osteoclast precursor cells. Biochem. Biophys. Res. Commun. 261, 144–148. [DOI] [PubMed] [Google Scholar]
- Mentaverri R.; Yano S.; Chattopadhyay N.; Petit L.; Kifor O.; Kamel S.; Terwilliger E. F.; Brazier M.; Brown E. M. (2006) The calcium sensing receptor is directly involved in both osteoclast differentiation and apoptosis. FASEB J. 20, 2562–2564. [DOI] [PubMed] [Google Scholar]
- Bennett B. D.; Alvarez U.; Hruska K. A. (2001) Receptor-operated osteoclast calcium sensing. Endocrinology 142, 1968–1974. [DOI] [PubMed] [Google Scholar]
- Yoshida N.; Sato T.; Kobayashi K.; Okada Y. (1998) High extracellular Ca2+ and Ca2+-sensing receptor agonists activate nonselective cation conductance in freshly isolated rat osteoclasts. Bone 22, 495–501. [DOI] [PubMed] [Google Scholar]
- Zaidi M.; Adebanjo O. A.; Moonga B. S.; Sun L.; Huang C. L. (1999) Emerging insights into the role of calcium ions in osteoclast regulation. J. Bone Miner. Res. 14, 669–674. [DOI] [PubMed] [Google Scholar]
- Huang C. L.; Sun L.; Fraser J. A.; Grace A. A.; Zaidi M. (2007) Similarities and contrasts in ryanodine receptor localization and function in osteoclasts and striated muscle cells. Ann. N.Y. Acad. Sci. 1116, 255–270. [DOI] [PubMed] [Google Scholar]
- Laver D. R. (2009) Luminal Ca2+ activation of cardiac ryanodine receptors by luminal and cytoplasmic domains. Eur. Biophys. J. 39, 19–26. [DOI] [PubMed] [Google Scholar]
- Terentyev D.; Nori A.; Santoro M.; Viatchenko-Karpinski S.; Kubalova Z.; Gyorke I.; Terentyeva R.; Vedamoorthyrao S.; Blom N. A.; Valle G.; Napolitano C.; Williams S. C.; Volpe P.; Priori S. G.; Gyorke S. (2006) Abnormal interactions of calsequestrin with the ryanodine receptor calcium release channel complex linked to exercise-induced sudden cardiac death. Circ. Res. 98, 1151–1158. [DOI] [PubMed] [Google Scholar]
- Shankar V. S.; Bax C. M. R.; Bax B. E.; Alam A. S. M. T.; Moonga B. S.; Simon B.; Pazianos M.; Huang C. L.-H.; Zaidi M. (1993) Activation of the Ca2+ “receptor” on the osteoclast by Ni2+ elicits cytosolic Ca2+ signals: Evidence for receptor activation and inactivation, intracellular Ca2+ redistribution and divalent cation modulation. J. Cell. Physiol. 155, 120–129. [DOI] [PubMed] [Google Scholar]
- Shankar V. S.; Bax C. M.; Alam A. S.; Bax B. E.; Huang C. L.; Zaidi M. (1992) The osteoclast Ca2+ receptor is highly sensitive to activation by transition metal cations. Biochem. Biophys. Res. Commun. 187, 913–918. [DOI] [PubMed] [Google Scholar]
- Chang W.; Shoback D. (2004) Extracellular Ca2+-sensing receptors: An overview. Cell Calcium 35, 183–196. [DOI] [PubMed] [Google Scholar]
- Handlogten M. E.; Shiraishi N.; Awata H.; Huang C.; Miller R. T. (2000) Extracellular Ca2+-sensing receptor is a promiscuous divalent cation sensor that responds to lead. Am. J. Physiol. 279, F1083–F1091. [DOI] [PubMed] [Google Scholar]
- Ashcroft F. M. (2007) The Walter B. Cannon Physiology in Perspective Lecture, 2007. ATP-sensitive K+ channels and disease: From molecule to malady. Am. J. Physiol. 293, E880–E889. [DOI] [PubMed] [Google Scholar]
- Henquin J. C.; Ravier M. A.; Nenquin M.; Jonas J. C.; Gilon P. (2003) Hierarchy of the β-cell signals controlling insulin secretion. Eur. J. Clin. Invest. 33, 742–750. [DOI] [PubMed] [Google Scholar]
- Seghers V.; Nakazaki M.; DeMayo F.; Aguilar-Bryan L.; Bryan J. (2000) Sur1 knockout mice. A model for KATP channel-independent regulation of insulin secretion. J. Biol. Chem. 275, 9270–9277. [DOI] [PubMed] [Google Scholar]
- Straub S. G.; Cosgrove K. E.; Ammala C.; Shepherd R. M.; O'Brien R. E.; Barnes P. D.; Kuchinski N.; Chapman J. C.; Schaeppi M.; Glaser B.; Lindley K. J.; Sharp G. W.; Aynsley-Green A.; Dunne M. J. (2001) Hyperinsulinism of infancy: The regulated release of insulin by KATP channel-independent pathways. Diabetes 50, 329–339. [DOI] [PubMed] [Google Scholar]
- Calcraft P. J.; Ruas M.; Pan Z.; Cheng X.; Arredouani A.; Hao X.; Tang J.; Rietdorf K.; Teboul L.; Chuang K. T.; Lin P.; Xiao R.; Wang C.; Zhu Y.; Lin Y.; Wyatt C. N.; Parrington J.; Ma J.; Evans A. M.; Galione A.; Zhu M. X. (2009) NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature 459, 596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M. S. (2002) The ryanodine receptor calcium channel of β-cells: Molecular regulation and physiological significance. Diabetes 51, 1299–1309. [DOI] [PubMed] [Google Scholar]
- Xie L.; Zhang M.; Zhou W.; Wu Z.; Ding J.; Chen L.; Xu T. (2006) Extracellular ATP stimulates exocytosis via localized Ca2+ release from acidic stores in rat pancreatic β cells. Traffic 7, 429–439. [DOI] [PubMed] [Google Scholar]
- Shigeto M.; Katsura M.; Matsuda M.; Ohkuma S.; Kaku K. (2007) Nateglinide and mitiglinide, but not sulfonylureas, induce insulin secretion through a mechanism mediated by calcium release from endoplasmic reticulum. J. Pharmacol. Exp. Ther. 322, 1–7. [DOI] [PubMed] [Google Scholar]
- Islam M. S.; Leibiger I.; Leibiger B.; Rossi D.; Sorrentino V.; Ekstrom T. J.; Westerblad H.; Andrade F. H.; Berggren P.-O. (1998) In situ activation of the type 2 ryanodine receptor in pancreatic β cells requires cAMP-dependent phosphorylation. Proc. Natl. Acad. Sci. U.S.A. 95, 6145–6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton J. D.; Lemmens R.; Shi C.-L.; Persson-Sjögren S.; Westerblad H.; Ahmed M.; Pyne N. J.; Frame M.; Furman B. L.; Islam M. S. (2003) Ryanodine receptors of pancreatic β-cells mediate a distinct context-dependent signal for insulin secretion. FASEB J. 17, 301–303. [DOI] [PubMed] [Google Scholar]
- Janjic D.; Wollheim C. B.; Sharp G. W. (1982) Selective inhibition of glucose-stimulated insulin release by dantrolene. Am. J. Physiol. 243, E59–E67. [DOI] [PubMed] [Google Scholar]
- Liu G.; Jacobo S. M.; Hilliard N.; Hockerman G. H. (2006) Differential modulation of Cav1.2 and Cav1.3-mediated glucose-stimulated insulin secretion by cAMP in INS-1 cells: Distinct roles for exchange protein directly activated by cAMP 2 (Epac2) and protein kinase A. J. Pharmacol. Exp. Ther. 318, 152–160. [DOI] [PubMed] [Google Scholar]
- Zhang Q.; Bengtsson M.; Partridge C.; Salehi A.; Braun M.; Cox R.; Eliasson L.; Johnson P. R.; Renstrom E.; Schneider T.; Berggren P. O.; Gopel S.; Ashcroft F. M.; Rorsman P. (2007) R-type Ca2+-channel-evoked CICR regulates glucose-induced somatostatin secretion. Nat. Cell Biol. 9, 453–460. [DOI] [PubMed] [Google Scholar]
- Dror V.; Kalynyak T. B.; Bychkivska Y.; Frey M. H.; Tee M.; Jeffrey K. D.; Nguyen V.; Luciani D. S.; Johnson J. D. (2008) Glucose and ER-calcium channels regulate HIF-1β via presenilin in pancreatic β-cells. J. Biol. Chem. 283, 9909–9916. [DOI] [PubMed] [Google Scholar]
- Takasawa S.; Akiyama T.; Nata K.; Kuroki M.; Tohgo A.; Noguchi N.; Kobayashi S.; Kato I.; Katada T.; Okamoto H. (1998) Cyclic ADP-ribose and inositol 1,4,5-trisphosphate as alternate second messengers for intracellular Ca2+ mobilization in normal and diabetic β-cells. J. Biol. Chem. 273, 2497–2500. [DOI] [PubMed] [Google Scholar]
- Islam M. S.; Berggren P. O. (1997) Cyclic ADP-ribose and the pancreatic β cell: Where do we stand?. Diabetologia 40, 1480–1484. [DOI] [PubMed] [Google Scholar]
- Holz G. G.; Leech C. A.; Heller R. S.; Castonguay M.; Habener J. F. (1999) cAMP-dependent mobilization of intracellular Ca2+ stores by activation of ryanodine receptors in pancreatic ss-cells. J. Biol. Chem. 274, 14147–14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang G.; Chepurny O. G.; Holz G. G. (2001) cAMP-regulated guanine nucleotide exchange factor II (Epac2) mediates Ca2+-induced Ca2+ release in INS-1 pancreatic β-cells. J. Physiol. 536, 375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromada J.; Dissing S.; Bokvist K.; Renström E.; Fro̷kjaer-Jensen J.; Wulff B. S.; Rorsman P. (1995) Glucagon-like peptide I increases cytoplasmic calcium in insulin-secreting βTC3-cells by enhancement of intracellular calcium mobilization. Diabetes 44, 767–774. [DOI] [PubMed] [Google Scholar]
- Laver D. R.; Curtis B. A. (1996) Response of ryanodine receptor channels to Ca2+ steps produced by rapid solution exchange. Biophys. J. 71, 732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadi A.; Rutter G. A. (2002) Dynamic imaging of endoplasmic reticulum Ca2+ concentration in insulin-secreting MIN6 cells using recombinant targeted cameleons: Roles of sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA)-2 and ryanodine receptors. Diabetes 51, Suppl. 1S190–S201. [DOI] [PubMed] [Google Scholar]
- MacDonald P. E.; Rorsman P. (2007) The ins and outs of secretion from pancreatic β-cells: Control of single-vesicle exo- and endocytosis. Physiology 22, 113–121. [DOI] [PubMed] [Google Scholar]
- Betzenhauser M. J.; Wagner L. E. II; Iwai M.; Michikawa T.; Mikoshiba K.; Yule D. I. (2008) ATP modulation of Ca2+ release by type-2 and type-3 InsP3R: Differing ATP sensitivities and molecular determinants of action. J. Biol. Chem. 283, 21579–21587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifach A.; Lewis R. S. (1993) Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc. Natl. Acad. Sci. U.S.A. 90, 6295–6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelttari A.; Helminen H. J. (1979) The relative thickness of intracellular membranes in epithelial cells of the ventral lobe of the rat prostate. Histochem. J. 11, 613–624. [DOI] [PubMed] [Google Scholar]
- Colbeau A.; Nachbaur J.; Vignais P. M. (1971) Enzymic characterization and lipid composition of rat liver subcellular membranes. Biochim. Biophys. Acta 249, 462–492. [DOI] [PubMed] [Google Scholar]
- Hanson G. T.; Aggeler R.; Oglesbee D.; Cannon M.; Capaldi R. A.; Tsien R. Y.; Remington S. J. (2004) Investigating mitochondrial redox potential with redox-sensitive green fluorescent protein indicators. J. Biol. Chem. 279, 13044–13053. [DOI] [PubMed] [Google Scholar]
- Mitra K.; Ubarretxena-Belandia I.; Taguchi T.; Warren G.; Engelman D. M. (2004) Modulation of the bilayer thickness of exocytic pathway membranes by membrane proteins rather than cholesterol. Proc. Natl. Acad. Sci. U.S.A. 101, 4083–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paroutis P.; Touret N.; Grinstein S. (2004) The pH of the secretory pathway: Measurement, determinants, and regulation. Physiology 19, 207–215. [DOI] [PubMed] [Google Scholar]
- Hwang C.; Sinskey A. J.; Lodish H. F. (1992) Oxidized redox state of glutathione in the endoplasmic reticulum. Science 257, 1496–1502. [DOI] [PubMed] [Google Scholar]
- Evans W. H.; Hardison W. G. (1985) Phospholipid, cholesterol, polypeptide and glycoprotein composition of hepatic endosome subfractions. Biochem. J. 232, 33–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go Y. M.; Jones D. P. (2008) Redox compartmentalization in eukaryotic cells. Biochim. Biophys. Acta 1780, 1273–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saroussi S.; Nelson N. (2009) Vacuolar H+-ATPase-an enzyme for all seasons. Pfluegers Arch. 457, 581–587. [DOI] [PubMed] [Google Scholar]
- Austin C. D.; Wen X.; Gazzard L.; Nelson C.; Scheller R. H.; Scales S. J. (2005) Oxidizing potential of endosomes and lysosomes limits intracellular cleavage of disulfide-based antibody-drug conjugates. Proc. Natl. Acad. Sci. U.S.A. 102, 17987–17992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harikumar P.; Reeves J. P. (1983) The lysosomal proton pump is electrogenic. J. Biol. Chem. 258, 10403–10410. [PubMed] [Google Scholar]
- Schoer J. K.; Gallegos A. M.; McIntosh A. L.; Starodub O.; Kier A. B.; Billheimer J. T.; Schroeder F. (2000) Lysosomal membrane cholesterol dynamics. Biochemistry 39, 7662–7677. [DOI] [PubMed] [Google Scholar]
- Steinberg B. E.; Touret N.; Vargas-Caballero M.; Grinstein S. (2007) In situ measurement of the electrical potential across the phagosomal membrane using FRET and its contribution to the proton-motive force. Proc. Natl. Acad. Sci. U.S.A. 104, 9523–9528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley C. T.; Dore T. M.; Hanson G. T.; Jackson W. C.; Remington S. J.; Tsien R. Y. (2004) Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J. Biol. Chem. 279, 22284–22293. [DOI] [PubMed] [Google Scholar]
- Moccia F.; Billington R. A.; Santella L. (2006) Pharmacological characterization of NAADP-induced Ca2+ signals in starfish oocytes. Biochem. Biophys. Res. Commun. 348, 329–336. [DOI] [PubMed] [Google Scholar]
- Witzgall R. (2007) TRPP2 channel regulation. Handb. Exp. Pharmacol., 363–375. [DOI] [PubMed] [Google Scholar]
- Peier A. M.; Moqrich A.; Hergarden A. C.; Reeve A. J.; Andersson D. A.; Story G. M.; Earley T. J.; Dragoni I.; McIntyre P.; Bevan S.; Patapoutian A. (2002) A TRP channel that senses cold stimuli and menthol. Cell 108, 705–715. [DOI] [PubMed] [Google Scholar]
- McKemy D. D.; Neuhausser W. M.; Julius D. (2002) Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416, 52–58. [DOI] [PubMed] [Google Scholar]
- Thebault S.; Lemonnier L.; Bidaux G.; Flourakis M.; Bavencoffe A.; Gordienko D.; Roudbaraki M.; Delcourt P.; Panchin Y.; Shuba Y.; Skryma R.; Prevarskaya N. (2005) Novel role of cold/menthol-sensitive transient receptor potential melastatine family member 8 (TRPM8) in the activation of store-operated channels in LNCaP human prostate cancer epithelial cells. J. Biol. Chem. 280, 39423–39435. [DOI] [PubMed] [Google Scholar]
- Latorre R.; Brauchi S.; Orta G.; Zaelzer C.; Vargas G. (2007) ThermoTRP channels as modular proteins with allosteric gating. Cell Calcium 42, 427–438. [DOI] [PubMed] [Google Scholar]
- Kraft R.; Harteneck C. (2005) The mammalian melastatin-related transient receptor potential cation channels: An overview. Pfluegers Arch. 451, 204–211. [DOI] [PubMed] [Google Scholar]
- Okada T.; Inoue R.; Yamazaki K.; Maeda A.; Kurosaki T.; Yamakuni T.; Tanaka I.; Shimizu S.; Ikenaka K.; Imoto K.; Mori Y. (1999) Molecular and functional characterization of a novel mouse transient receptor potential protein homologue TRP7. Ca2+-permeable cation channel that is constitutively activated and enhanced by stimulation of G protein-coupled receptor. J. Biol. Chem. 274, 27359–27370. [DOI] [PubMed] [Google Scholar]
- Lavender V.; Chong S.; Ralphs K.; Wolstenholme A. J.; Reaves B. J. (2008) Increasing the expression of calcium-permeable TRPC3 and TRPC7 channels enhances constitutive secretion. Biochem. J. 413, 437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramowitz J.; Birnbaumer L. (2009) Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J. 23, 297–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J.; Mori E.; Mori Y.; Mori M.; Li J.; Ito Y.; Inoue R. (2004) Multiple regulation by calcium of murine homologues of transient receptor potential proteins TRPC6 and TRPC7 expressed in HEK293 cells. J. Physiol. 561, 415–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina M. J.; Rosen T. A.; Tominaga M.; Brake A. J.; Julius D. (1999) A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 398, 436–441. [DOI] [PubMed] [Google Scholar]
- Tominaga M.; Tominaga T. (2005) Structure and function of TRPV1. Pfluegers Arch. 451, 143–150. [DOI] [PubMed] [Google Scholar]
- Story G. M.; Peier A. M.; Reeve A. J.; Eid S. R.; Mosbacher J.; Hricik T. R.; Earley T. J.; Hergarden A. C.; Andersson D. A.; Hwang S. W.; McIntyre P.; Jegla T.; Bevan S.; Patapoutian A. (2003) ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112, 819–829. [DOI] [PubMed] [Google Scholar]
- Karashima Y.; Prenen J.; Meseguer V.; Owsianik G.; Voets T.; Nilius B. (2008) Modulation of the transient receptor potential channel TRPA1 by phosphatidylinositol 4,5-biphosphate manipulators. Pfluegers Arch. 457, 77–89. [DOI] [PubMed] [Google Scholar]
- Qian F.; Noben-Trauth K. (2005) Cellular and molecular function of mucolipins (TRPML) and polycystin 2 (TRPP2). Pfluegers Arch. 451, 277–285. [DOI] [PubMed] [Google Scholar]
- Dong X. P.; Wang X.; Shen D.; Chen S.; Liu M.; Wang Y.; Mills E.; Cheng X.; Delling M.; Xu H. (2009) Activating mutations of the TRPML1 channel revealed by proline scanning mutagenesis. J. Biol. Chem. 284, 32040–32052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouin H.; Hermann A. (1994) Intracellular action of spermine on neuronal Ca2+ and K+ currents. Eur. J. Neurosci. 6, 412–419. [DOI] [PubMed] [Google Scholar]
- Roberts-Crowley M. L.; Mitra-Ganguli T.; Liu L.; Rittenhouse A. R. (2009) Regulation of voltage-gated Ca2+ channels by lipids. Cell Calcium 45, 589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R.; Borges K.; Bowie D.; Traynelis S. F. (1999) The glutamate receptor ion channels. Pharm. Rev. 51, 7–61. [PubMed] [Google Scholar]
- Mandal M.; Yan Z. (2009) PIP2 regulation of NMDA receptor channels in cortical neurons. Mol. Pharmacol. 76, 1349–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis M. F.; Khakh B. S. (2009) ATP-gated P2X cation-channels. Neuropharmacology 56, 208–215. [DOI] [PubMed] [Google Scholar]
- Vanden Abeele F.; Bidaux G.; Gordienko D.; Beck B.; Panchin Y. V.; Baranova A. V.; Ivanov D. V.; Skryma R.; Prevarskaya N. (2006) Functional implications of calcium permeability of the channel formed by pannexin 1. J. Cell Biol. 174, 535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Hondt C.; Ponsaerts R.; De Smedt H.; Bultynck G.; Himpens B. (2009) Pannexins, distant relatives of the connexin family with specific cellular functions?. BioEssays 31, 953–974. [DOI] [PubMed] [Google Scholar]