Abstract
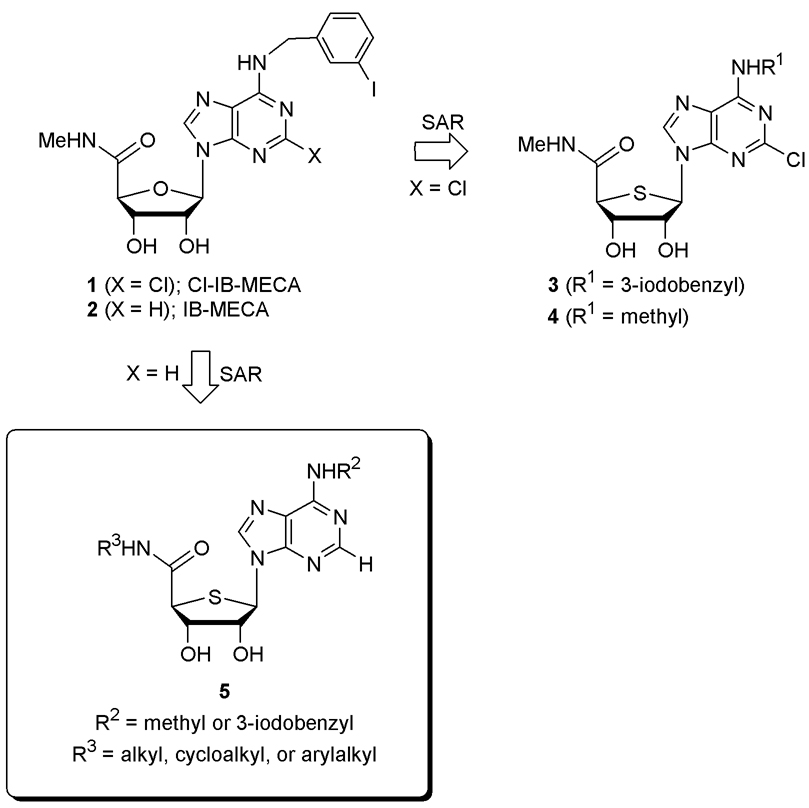
On the basis of a bioisosteric rationale, 4′-thionucleoside analogues of IB-MECA, which is a potent and selective A3 adenosine receptor agonist (AR), were synthesized from d-gulonic acid γ-lactone. The 4′-thio analogue (5h) of IB-MECA showed extremely high binding affinity (Ki = 0.25 nM) at the human A3AR and was more potent than IB-MECA (Ki = 1.4 nM). Bulky substituents at the 5′-uronamide position, such as cyclohexyl and 2- methylbenzyl, in this series of 2-H nucleoside derivatives were tolerated in A3AR binding, although small alkyl analogues were more potent.
Keywords: A3 adenosine receptor, 4’-thionucleosides, agonist, binding affinity
Introduction
The A3 adenosine receptor (AR), which belongs to the family of G-protein-coupled receptors (GPCRs), is known to be involved in cell signaling by modulating the levels of cAMP, inositol triphosphate (IP3), and diacylglycerol (DAG) through binding of the endogenous chemical messenger, adenosine.1 Thus, the A3AR has been regarded as good therapeutic target for the treatment of several diseases associated with cell signaling such as cancer, ischemia, inflammation, and glaucoma.2
Adenosine has served as a good template for the development of A3AR ligands. Extensive modification of adenosine resulted in the discovery of Cl-IB-MECA, 1 (Ki = 1.0 nM for human A3AR)3 and IB-MECA, 2 (Ki = 1.4 nM for human A3AR) 4 as potent and selective A3AR agonists and these are in clinical trials as anticancer agents5 (Figure 1). Recently, probing the structure-activity relationship (SAR) of compound 1 on the basis of a bioisosteric rationale indicated that a 4′-thionucleoside could serve as an excellent template for the development of A3AR agonists, among which compounds 3 and 4 were discovered as more potent A3AR agonists (Ki = 0.38 and 0.28 nM, respectively) than Cl-IB-MECA 1.6 Compound 3 also showed potent in vitro as well as in vivo anticancer activity.7 Because IB-MECA 2 is another representative of A3AR agonists, it would be of great interest to synthesize its 4′-thio analogue and to compare their biological activities and substituent effects (H vs Cl) at the C2 position. It would be also interesting to study SAR surrounding the 5′-uronamide moiety of the 4′-thionucleosides. The A3AR has displayed a moderate tolerance for sterically bulky substituents at this position, in contrast to the N-methylamides of the protypical agonists 1 and 2.6b Herein, we report the synthesis and binding affinity of the series of compound 5 as potent and selective A3AR agonists.
Figure 1.
Results and discussion
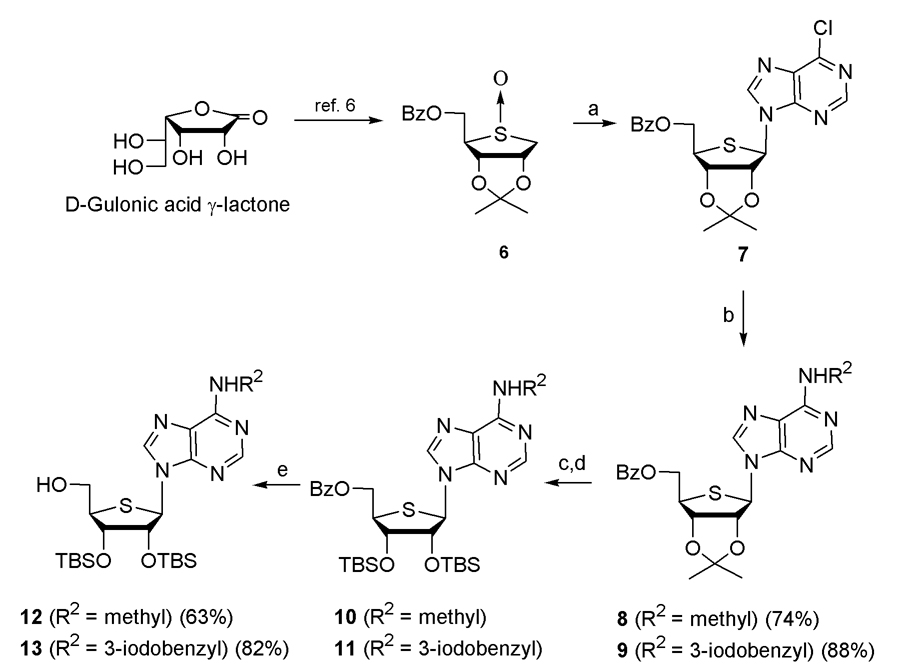
The target nucleoside 5 was synthesized from d-gulonic acid γ-lactone, as shown in Scheme 1.
Scheme 1a.
aReagents & conditions: a) 6-chloropurine, TMSOTf, ClCH2CH2Cl, rt to 80 °C; b) R2NH2, Et3N, EtOH, rt; c) 80% AcOH, 70 °C; d) TBSOTf, pyridine, 50 °C; e) NaOMe, MeOH
d-Gulonic acid γ-lactone was smoothly converted to the glycosyl donor 6 according to our previously published procedure.6 Condensation of 6 with 6-chloropurine in the presence of TMSOTf afforded the 6-chloropurine derivative 7 (53%) and its α-anomer (5.4%).8 The anomeric configuration of 7 was easily confirmed by an NOE effect between 1′-H and 4′-H. Irradiation on 1′-H of compound 6 gave NOE effect on its 4′-H, indicating β-anomer, but no NOE effect was observed on the same experiment in the case of its α-anomer.8 Treatment of 7 with methyl amine and 3-iodobenzylamine gave the N6-methyladenine derivative 8 and N6-(3-iodobenzyl)adenine derivative 9, respectively. For the conversion of 4′-hydroxymethyl group into various 5′-uronamides, the 2′,3′-isopropylidene group was first changed to the 2′,3′-di-O-TBS group, because the removal of the 2′,3′-isopropylidene group at the final step resulted in the deglycosylation. Treatment of 8 and 9 with 80% acetic acid followed by protection of the resulting diol with the TBS group yielded 10 and 11, respectively. Removal of the benzoyl group in 10 and 11 with sodium methoxide gave the 4′-hydroxymethyl derivatives 12 and 13, respectively.
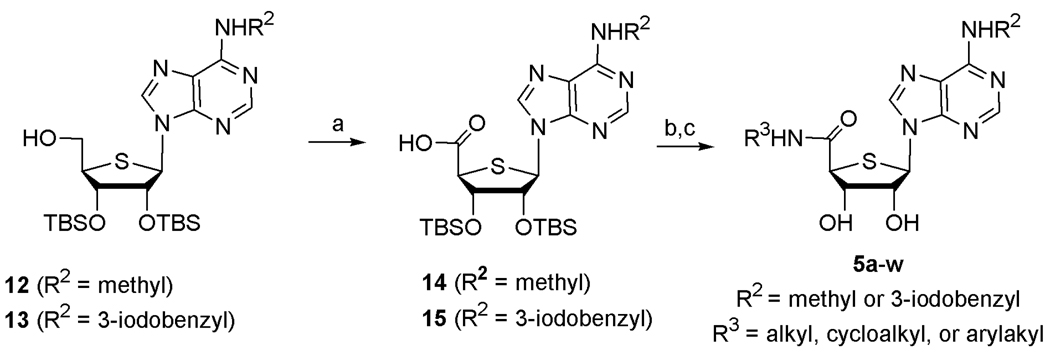
Treatment of 12 and 13 with PDC in DMF afforded the carboxylic acid derivatives 14 and 15, respectively. Coupling of the acids 14 and 15 with various primary amines in the presence of EDC and HOBt yielded various 5′-uronamides 5a–w after the removal of the TBS group.
Radioligand binding assay was performed using adherent CHO (Chinese hamster ovary) cells stably transfected with cDNA encoding the human ARs.9 Bindings at the human ARs were carried out using [3H]R-PIA for A1AR, [3H]CGS21680 for A2AAR, and [125I]I-AB-MECA for A3AR as radioligands. In cases of weak binding, the percent inhibition of radioligand binding to the human ARs was determined at 1 µM. Percent activation (inhibition of adenylate cyclase in comparison to the full agonist Cl-IB-MECA, 1) of the human A3AR was determined at 1 µM.
Most of the synthesized compounds showed very high binding affinity at the human A3AR with high selectivity in comparison to other subtypes (Table 1). When compared with the 4′-oxonucleoside, IB-MECA (2) (Ki = 1.4 nM), the corresponding 4′-thionucleoside, thio-IB-MECA (5h) exhibited higher binding affinity at the human A3AR (Ki = 0.25 nM) as well as higher selectivity over other subtypes, indicating that thio-IB-MECA (5h) has the potential to be developed as a clinical candidate as IB-MECA (2). A similar trend was observed between Cl-IB-MECA (1) (Ki = 1.0 nM) and thio-Cl-IB-MECA (3) (Ki = 0.38 nM). However, thio-IB-MECA (5h) was found to be less selective (81- vs 508-fold) for the human A3AR in comparison to the human A1AR than thio-Cl-IB-MECA (3). This result indicated that substitution of the 2-H atom with more hydrophobic 2-Cl substituent increased the A3 AR selectivity.10 It should be noted that thio-IB-MECA (5h) showed the best binding affinity at the human A3AR among 4′-thionucleosides synthesized so far. This compound also showed very high binding affinity (Ki = 1.86 ± 0.36 nM) at the rat A3AR. In the N6-methyladenine series, the binding affinity at the human A3AR of the synthesized 5′-uronamides was dependent on the 5′-N substitution in the following order: ethyl = cyclopentyl > cyclobutyl > cyclopropylmethyl > methyl > cyclopropyl > 3-iodobenzyl, indicating that alkyl and cycloalkyl derivatives 5a–f showed better binding affinity (Ki = 0.97 ~ 2.16 nM) than arylalkyl derivative 5g (Ki = 15.6 nM). A similar trend was observed in the N6-(3-iodobenzyl)adenine series, except that the N6-(3-iodobenzyl)adenine derivatives showed slightly better binding affinity at the human A3AR and generally were more selective versus the human A1AR than the N6-methyladenine derivatives.
Table 1.
Potency of 4′-thioadenosine-5′-uronamide derivatives 5a–x at human A1, A2A, and A3ARs expressed in CHO cells.a
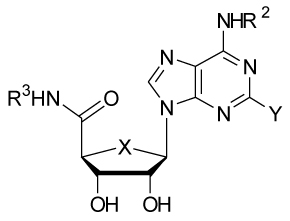 | |||||
|---|---|---|---|---|---|
| Compound No. | R2 | R3 |
Ki (hA1AR) nMa or % inhibition at 1 µM |
Ki (hA2AAR) nMa or % inhibition at 1 µM |
Ki (hA3AR) nMa or % inhibition at 1 µM |
| 1 (X = O, Y = Cl) | 3-iodobenzyl | methyl | 222 ± 22 | 5360 ± 2470 | 1.4 ± 0.3 |
| 2 (X = O, Y = H) | 3-iodobenzyl | methyl | 51 | 2900 | 1.0 |
| 3 (X = S, Y = Cl) | 3-iodobenzyl | methyl | 193 ± 46 | 223 ± 36 | 0.38 ± 0.07 |
| 4 (X = S, Y = Cl) | methyl | methyl | 1330 ± 240 | 20% | 0.28 ± 0.09 |
| 5a (X = S, Y = H) | methyl | methyl | 69.5±5.2 | 35±8% | 1.19±0.23 |
| 5b (X = S, Y = H) | methyl | ethyl | 4.83±0.20 | 20% | 0.97±0.23 |
| 5c (X = S, Y = H) | methyl | cyclopropyl | 6.58±0.60 | 161±16 | 2.16±0.24 |
| 5d (X = S, Y = H) | methyl | cyclopropylmethy l |
33.1±1.6 | 38±5% | 1.35±0.08 |
| 5e (X = S, Y = H) | methyl | cyclobutyl | 6.27±0.50 | 108±18 | 1.04±0.05 |
| 5f (X = S, Y = H) | methyl | cyclopentyl | 48.6±14.2 | 45±1% | 0.97±0.07 |
| 5g (X = S, Y = H) | methyl | 3-iodobenzyl | 24±1% | 27±10% | 15.6±5.6 |
| 5h (X = S, Y = H) | 3-iodobenzyl | methyl | 20.2±2.9 | 475±144 | 0.25±0.06 |
| 5i (X = S, Y = H) | 3-iodobenzyl | ethyl | 5.4±0.3 | 57.6±6.9 | 0.42±0.22 |
| 5j (X = S, Y = H) | 3-iodobenzyl | cyclopropyl | 9.27±0.83 | 15.2±2.6 | 3.03±0.23 |
| 5k (X = S, Y = H) | 3-iodobenzyl | cyclopropylmethy l |
159±40 | 1600±80 | 2.16±0.29 |
| 5l (X = S, Y = H) | 3-iodobenzyl | cyclobutyl | 23.6±4.2 | 122±62 | 1.17±0.16 |
| 5m (X = S, Y = H) | 3-iodobenzyl | cyclohexyl | 28±14% | 24±7% | 35.4±10.5 |
| 5n (X = S, Y = H) | 3-iodobenzyl | 3-fluorobenzyl | 25±4% | 35±8% | 61.1±17.6 |
| 5o (X = S, Y = H) | 3-iodobenzyl | 3-chlorobenzyl | 25±3% | 44±1% | 144±33 |
| 5p (X = S, Y = H) | 3-iodobenzyl | 2-methylbenzyl | 18±5% | 26±10% | 31.0±7.1 |
| 5q (X = S, Y = H) | 3-iodobenzyl | 3-methylbenzyl | 4070±560 | 31±1% | 94.9±37.3 |
| 5r (X = S, Y = H) | 3-iodobenzyl | 4-methylbenzyl | 15±3% | 37±0% | 135±55 |
| 5s (X = S, Y = H) | 3-iodobenzyl | 2-methoxybenzyl | 34±1% | 28±9% | 97.0±51.2 |
| 5t (X = S, Y = H) | 3-iodobenzyl | 2-ethoxybenzyl | 12±7% | 23±8% | 113±2å |
| 5u (X = S, Y = H) | 3-iodobenzyl | α-naphthylmethyl | 20±6% | 20±8% | 1208 |
| 5v (X = S, Y = H) | 3-iodobenzyl | 2-phenylethyl | 20% | 11% | 433±141 |
| 5w (X = S, Y = H) | 3-iodobenzyl | 1,1-diphenylethyl | 17±0% | 38±8% | 116±48 |
All AR experiments were performed using adherent CHO cells stably transfected with cDNA encoding the human or rat ARs. Percent activation of the human A3AR was determined at 1 µM. Binding was carried out as described in Methods using as radioligand [3H]R-PIA or [3H]NECA at the human A1AR and [3H]CGS 21680 at the human A2AAR. Values from the present study are expressed as mean ± s.e.m., n = 3–5.
The most potent and selective compound 5h was a full agonist in an assay of human A3 adenosine receptor-mediated inhibition of cyclic AMP in transfected CHO cells, as previously observed for compounds 1–4.
In summary, we have established SARs of bioisosteric 4′-thio analogues of potent and selective A3AR agonist, IB-MECA (2). From this study, thio-IB-MECA (5h) was discovered as being among the most potent A3 AR agonists and more potent than IB-MECA (2). It was also revealed that small alkyl or cycloalkyl substituents on the 5′-uronamide were essential for optimal binding affinity. We believe that compound 5h has promise to be developed as a clinically useful agent.
Experimental Section
1H NMR spectra (CDCl3, CD3OD, or DMSO-d6) were recorded on Varian Unity Inova 400 MHz. Chemical shifts were reported in ppm units with TMS as the internal standard. 13C NMR spectra (CDCl3, CD3OD, or DMSO-d6) were recorded on Varian Unity Inova 100 MHz. Analytical thin-layer chromatography (TLC) was performed on Merck silica gel 60F-254 glass plates. Optical rotations were determined on Jasco III in methanol. UV spectra were recorded on U-3000 made by Histachi in methanol. Elementary analyses were measured on EA1110. The crude products were purified using a silica gel 60 (230–400 mesh, Merck). Reagents were purchased from Aldrich Chemical Company. All the anhydrous solvents were distilled over CaH2 or P2O5 or Na/benzophenone prior to the reaction.
Benzoic acid (3aS,4R,6R,6aR)-6-(6-chloro-purin-9-yl)-2,2-dimethyl-tetrahydro-thieno[3,4-d][1,3]dioxol-4-ylmethyl ester (7)8
To a suspension of 6-chloropurine (3.36 g, 21.69 mmol) in a solution of dry CH3CN (20 mL) and 1,2-dichloroethane (10 mL) were added Et3N (2.19 g, 21.69 mmol) and TMSOTf (9.64 g, 43.38 mmol), and the mixture was stirred at room temperature until the solution was clear. A solution of sulfoxide 6 (3.36 g, 10.85 mmol) in dry 1,2-dichloroethane (10 mL) was added to the resulting solution in one shot at room temperature. An additional amount of Et3N (2.19 g, 21.69 mmol) was added to the reaction mixture to initiate the Pummerer reaction. The reaction mixture was stirred under reflux at 80 °C for 4 d, during which time the initially formed N-3 isomer was converted to N-9 isomer. The reaction mixture was partitioned between EtOAc and aqueous saturated NaHCO3 solution, and the organic layer was washed with brine, dried (MgSO4), filtered and evaporated. The residue was purified by flash silica gel column chromatography (Hexane:EtOAc = 5:1) to give 7 (2.57 g, 53%), whose spectral data were identical with those of authentic sample8.
General procedure for the preparation of the N6-substituted nucleosides 8 and 9
To a solution of 7 in anhydrous EtOH (20 mL per mmol) were added triethylamine (3.0 equiv) and appropriate amine (1.2 equiv). After being stirred at room temperature for 24 h, the reaction mixture was evaporated. The residue was purified by silica gel column chromatography (hexane/ethyl acetate = 1:1) to give the N6-substituted nucleosides 8 and 9.
6-Methylamino-9-[(5’-O-benzoyl-2’,3’-O-isopropylidene)-4’-thio-β-D-ribofuranosyl]purine (8)
Compound 8 was prepared using methylamine·HCl: yield 74%; white foam; UV (MeOH) λ max 271 nm (pH 7); 1H NMR (CDCl3) δ 1.40 (s, 3 H, CH3), 1.68 (s, 3 H, CH3), 3.10 (d, 3 H, J = 3.0 Hz, N-CH3), 4.12 (td, 1 H, J = 2.7, 7.8 Hz, 4’-H), 4.60 (dd, 1 H, J = 6.7, 11.4 Hz, BzOCHH), 4.63 (dd, 1 H, J = 7.8, 11.5 Hz, BzOCHH), 4.99 (dd, 1 H, J = 2.9, 5.6 Hz, 3’-H), 5.03 (dd, 1 H, J = 1.9, 5.6 Hz, 2’-H), 6.01 (d, 1 H, J = 1.9 Hz, 1’-H), 6.31 (br s, 1 H, NH), 7.37–7.88 (m, 5 H, Ph), 8.46 (s, 1 H, H-8), 8.57 (s, 1H, H-2); FAB-MS m/z 442 (M++1); Anal. Calcd for C21H23N5O4S: C, 57.13; H, 5.25; N, 15.86; S, 7.26. Found: C, 57.20; H, 5.28; N, 15.75; S, 7.32.
6-(3-Iodo-benzylamino)-9-[(5’-O-benzoyl-2’,3’-O-isopropylidene)-4’-thio-β-D-ribofuranosyl] purine (9)
Compound 9 was prepared using 3-iodo-benzylamine: yield 88%; white foam; UV (MeOH) λ max 272 nm (pH 7); 1H NMR (CDCl3) δ̣ 1.23 (s, 3 H, CH3), 1.37 (s, 3 H, CH3), 4.06 (td, 1 H, J = 2.4, 7.3 Hz, 4’-H), 4.46 (dd, 1 H, J = 6.8, 11.4 Hz, BzOCHH), 4.53 (dd, 1 H, J = 2.7, 11.4 Hz, BzOCHH), 4.73 (d, 2 H, J = 5.8 Hz, N-CH2), 4.89 (dd, 1 H, J = 2.4, 5.6 Hz, 3’-H), 5.02 (dd, 1 H, J = 2.0, 5.6 Hz, 2’-H), 5.96 (s, 1H, 1’-H), 6.59 (br s, 1 H, NH), 7.11–7.84 (m, 9 H, aromatic H), 8.52 (s, 1 H, H-8), 8.58 (s, 1 H, H-2); FAB-MS m/z 644 (M++1); Anal. Calcd for C27H26IN5O4S: C, 50.40; H, 4.07; N, 10.88; S, 4.98. Found: C, 50.33; H, 4.21; N, 10.90; S, 4.88.
General Procedure for the preparation of the 4’-hydroxymethyl analogues 12 and 13
A solution of per mmol of N6-substituted nucleosides (8 and 9) in 80% aqueous AcOH solution (30 mL) was stirred at 70 °C for 12 h. The solvent was removed under reduced pressure and the mixture was neutralized with methanolic ammonia. After evaporation, the residue was purified by silica gel column chromatography (CH2Cl2/MeOH = 10:1) to give diol as a white foam.
To a stirred solution of per mmol of diol in dry pyridine (20 mL) was added a solution of TBDMSOTf (5.0 equiv) dropwise and the reaction mixture was stirred at 50 °C for 5 h. The mixture was partitioned between CH2Cl2 and H2O and the organic layer was washed with water, aqueous NaHCO3 solution, water, brine, dried over anhydrous MgSO4, and evaporated. The crude disilyl ether was used in the next step without further purification.
To a stirred solution of per mmol of disilyl ether in anhydrous methanol (30 mL) was added sodium methoxide (1.5 equiv) and the mixture was stirred at room temperature for 4 h. After being neutralized with glacial acetic acid, the mixture was evaporated. The resulting residue was purified by silica gel column chromatography (hexane/ethyl acetate = 1:1) to gve 4’-hydroxymethyl analogues 12 and 13, respectively.
9-[(2’,3’-Bis-tert-butyl-dimethyl-silanyloxy-5’-hydroxymethyl)-6-methylamino-4’-thio-β-D-ribofuranosyl] purine (12)
yield 63%; white solid; UV (MeOH) λ max 270 nm (pH 7); 1H NMR (CDCl3) δ 0.01 (m, 12 H, 4×Si-CH3), 0.60 (s, 9 H, C(CH3)3), 0.85 (s, 9 H, C(CH3)3), 3.10 (br s, 3 H, N-CH3), 3.20 (dd, 1 H, J = 5.6, 11.7 Hz, 4’-H), 4.20 (m, 1 H, 3’-H), 4.45 (dd, 1 H, J = 5.8, 11.7 Hz, HOCHH), 4.75 (m, 1 H, 2’-H), 4.87 (dd, 1 H, J = 6.3, 11.7 Hz, HOCHH), 5.43 (d, 1 H, J = 4.6 Hz, 1’-H), 5.65 (br s, 1 H, OH), 8.00 (s, 1 H, H-2), 8.03 (br s, 1 H, NH), 8.23 (s, 1 H, H-8); FAB-MS m/z 526 (M++1); Anal. Calcd for C23H43N5O3SSi2: C, 52.53; H, 8.24; N, 13.32; S, 6.10. Found: C, 52.40; H, 8.35; N, 13.22; S, 6.14.
9-[(2’,3’-Bis-tert-butyl-dimethyl-silanyloxy-5’-hydroxymethyl)-4’-thio-β-D-ribofuranosyl]-6-(3-iodobenzylamino)purine (13)
yield 82%; white solid; UV (MeOH) λ max 270 nm (pH 7); 1H NMR (CDCl3) δ 0.01 (m, 12 H, 4×Si-CH3), 0.62 (s, 9 H, C(CH3)3), 0.83 (s, 9 H, C(CH3)3), 3.27 (dd, 1 H, J = 5.6, 11.7 Hz, 2-H), 3.74 (m, 1 H, HOCHH), 3.86 (m, 1 H, HOCHH), 4.14 (dd, 1 H, J = 5.7, 11.5 Hz, 3’-H), 4.63 (br s, 2H, N-CH2), 5.20 (dd, 1 H, J = 6.3, 11.9 Hz, 2’-H), 5.60 (d, 1 H, J = 4.6 Hz, 1’-H), 5.93 (br s, 1 H, OH), 6.93 (t, 1 H, J = 7.7 Hz, aromatic H), 7.13 (s, 1 H, NH), 7.58 (d, 1 H, J = 7.5 Hz, aromatic H), 7.60 (d, 1 H, J = 7.8 Hz, aromatic H), 7.67 (s, 1 H, aromatic H), 8.01 (s, 1 H, H-2), 8.28 (s, 1 H, H-8); FAB-MS m/z 728 (M+); Anal. Calcd for C29H46IN5O3SSi2: C, 47.86; H, 6.37; N, 9.62; S, 4.41. Found: C, 48.02; H, 6.43; N, 9.65; S, 4.39.
General Procedure for the preparation of the N6-Substituted-4′-thioadenosine-5′-uronamides 5a–w
To a stirred solution of 4′-hydroxymethyl analogue (1 mmol, 12 and 13) in dry DMF (10 mL) was added pyridinium dichromate (10.0 equiv) and the reaction mixture was stirred at room temperature for 20 h. Water (50 mL) was added to the reaction mixture, and stirred at room temperature for 1 h. The precipitate was filtered and the filter cake was washed with water many times and dried under high vacuum to give the acid (14 and 15) as a brownish solid, which was used in the next step without further purification.
To a solution of 14 and 15 (1 mmol) in CH2Cl2 (20 mL) were added EDC (1.5 equiv), HOBt (1.5 equiv), appropriate amine (1.5 equiv), and DIPEA (3.0 equiv) and the mixture was stirred at room temperature for 15 h. The reaction mixture was evaporated and the residue was purified by a silica gel column chromatography (hexane/EtOAc = 10:1-5:1) to give the corresponding silyl amide as a white foam. To a stirred solution of silyl amide (1 mmol) in THF (5 mL) was added TBAF (2.5 equiv) and the reaction mixture was stirred at room temperature for 1 h. The solvent was evaporated and the resulting residue was purified by silica gel column chromatography (CH2Cl2/MeOH = 10:1) to give 5a–w as white solids.
N6-Methyl-9-(5’-methylaminocarbonyl-4’-thio-β-D-ribofuranosyl)adenine (5a)
Compound 5a was prepared using methylamine·HCl: yield 70%; white solid; [α]25 D −24.0° (c 0.13, MeOH); UV (MeOH) λ max 270 nm (pH 7); 1H NMR (DMSO-d6) δ 2.78 (d, 3 H, J = 4.0 Hz, N-CH3), 2.90 (d, 3 H, J = 4.5 Hz, N-CH3), 3.76 (d, 1 H, J = 4.2 Hz, 4’-H), 4.35 (dd, 1 H, J = 4.6, 8.0 Hz, 3’-H), 4.52 (dd, 1 H, J = 4.8, 8.5 Hz, 2’-H), 5.42 (d, 1 H, J = 5.4 Hz, exchangeable with D2O, OH), 5.70 (d, 1 H, J = 5.1 Hz, exchangeable with D2O, OH), 5.80 (d, 1 H, J = 5.4 Hz, 1’-H), 7.98 (s, 1 H, H-2), 8.32 (br q, 2 H, exchangeable with D2O, NH, NH), 8.54 (s, 1 H, H-8); 13C NMR (DMSO-d6) δ 51.0, 55.3, 64.3, 70.2, 75.4, 78.2, 118.5, 139.9, 149.5, 150.3, 152.5, 174.4; FAB-MS m/z 325 (M++1); Anal. Calcd for C12H16N6O3S: C, 44.44; H, 4.97; N, 25.91; S, 9.89. Found: C, 44.52; H, 5.03; N, 25.98; S, 9.86.
9-(5’-Ethylaminocarbonyl-4’-thio-β-D-ribofuranosyl)-N6-methyladenine (5b)
Compound 5b was prepared using ethylamine·HCl: yield 65%; white solid; [α]25 D −48.2° (c 0.15, MeOH); UV (MeOH) λ max 270 nm (pH 7); 1H NMR (DMSO-d6) δ 1.07 (t, 3 H, J = 7.2 Hz, CH2CH3), 2.55 (d, 3 H, J = 4.5 Hz, N-CH3), 3.11 (m, 2 H, N-CH2), 3.76 (d, 1 H, J = 4.0 Hz, 4’-H), 4.04 (dd, 1 H, J = 4.0, 7.5 Hz, 3’-H), 4.48 (dd, 1 H, J = 5.5, 8.0Hz, 2’-H), 5.54 (d, 1 H, J = 5.0 Hz, exchangeable with D2O, OH), 5.68 (d, 1 H, J = 5.2 Hz, exchangeable with D2O, OH), 5.80 (d, 1 H, J = 5.5 Hz, 1’-H), 8.05 (s, 1 H, H-2), 8.30 (br s, 2 H, exchangeable with D2O, NH, NH), 8.53 (s, 1 H, H-8); 13C NMR (DMSO-d6) δ 24.3, 48.9, 50.2, 61.3, 73.2, 74.4, 78.5, 116.3, 140.5, 150.8, 151.5, 152.5, 173.4; FAB-MS m/z 339 (M++1); Anal. Calcd for C13H18N6O3S: C, 46.14; H, 5.36; N, 24.84; S, 9.48. Found: C, 46.25; H, 5.30; N, 25.00; S, 9.56.
9-(5’-Cyclopropylaminocarbonyl-4’-thio-β-D-ribofuranosyl)-N6-methyladenine (5c)
Compound 5c was prepared using cyclopropyl amine: yield 61%; white solid; [α]25 D −15.8° (c 0.15, MeOH); UV (MeOH) λ max 269.0 nm (pH 7); 1H NMR (DMSO-d6) δ 0.04 (m, 2 H, cyclopropyl-CH2), 0.27 (m, 2 H, cyclopropyl-CH2), 2.65 (m, 1 H, NCH), 2.93 (d, 3 H, J = 4.5 Hz, N-CH3), 3.65 (d, 1 H, J = 4.0 Hz, 4’-H), 4.45 (dd, 1 H, J = 4.5, 8.0 Hz, 3’-H), 4.52 (dd, 1 H, J = 5.0, 8.5 Hz, 2’-H), 5.40 (d, 1 H, J = 4.0 Hz, exchangeable with D2O, OH), 5.59 (d, 1 H, J = 4.8 Hz, exchangeable with D2O, OH), 5.85 (d, 1 H, J = 7.8 Hz, 1’-H), 8.05 (s, 1 H, H-2), 8.35 (br s, 1 H, exchangeable with D2O, NH), 8.38 (br s, 1 H, exchangeable with D2O, NH), 8.57 (s, 1 H, H-8); 13C NMR (DMSO-d6) δ 6.1, 6.8, 24.0, 48.5, 50.6, 58.4, 75.4, 78.3, 114.8, 148.3, 150.2, 152.5, 155.3, 174.5; FAB-MS m/z 351 (M++1); Anal. Calcd for C14H18N6O3S: C, 47.99; H, 5.18; N, 23.98; S, 9.15. Found: C, 48.12; H, 5.25; N, 24.05; S, 9.23.
9-(5’-Cyclopropylmethylaminocarbonyl-4’-thio-β-D-ribofuranosyl)-N6-methyladenine (5d)
Compound 5d was prepared using cyclopropyl methylamine·HCl: yield 68%; white solid; [α]25 D −15.8° (c 0.15, MeOH); UV (MeOH) λ max 270 nm (pH 7); 1H NMR (DMSO-d6) δ 0.05 (m, 2 H, cyclopropyl-CH2), 0.35 (m, 2 H, cyclopropyl-CH2), 0.78 (m, 1 H, cyclopropyl-CH), 2.93 (d, 3 H, J = 4.0 Hz, N-CH3), 3.03 (t, 2 H, J = 4.5, 7.8 Hz, N-CH2), 3.77 (d, 1 H, J = 4.0 Hz, 4’-H), 4.27 (dd, 1 H, J = 4.5, 9.0 Hz, 3’-H), 4.45 (dd, 1 H, J = 4.6, 8.8 Hz, 2’-H), 5.46 (d, 1 H, J = 4.3 Hz, exchangeable with D2O, OH), 5.68 (d, 1 H, J = 4.8 Hz, exchangeable with D2O, OH), 5.77 (d, 1 H, J = 7.8 Hz, 1’-H), 8.12 (s, 1 H, H-2), 8.34 (br s, 1 H, exchangeable with D2O, NH), 8.43 (br s, 1 H, exchangeable with D2O, NH), 8.53 (s, 1 H, H-8); 13C NMR (CD3OD) δ̣ 2.5, 2.7, 10.8, 43.2, 46.3, 58.3, 60.5, 72.3, 75.6, 120.5, 148.7, 150.1, 153.4, 156.5, 173.5; FAB-MS m/z 365 (M++1); Anal. Calcd for C15H20N6O3S: C, 49.44; H, 5.53; N, 23.06; S, 8.80. Found: C, 49.40; H, 5.55; N, 22.98; S, 8.87.
9-(5’-Cyclobutylaminocarbonyl-4’-thio-β-D-ribofuranosyl)-N6-methyladenine (5e)
Compound 5e was prepared using cyclobutylamine: yield 59%; white solid; [α]25 D −24.0° (c 0.10, MeOH); UV (MeOH) λ max 270 nm (pH 7); 1H NMR (DMSO-d6) δ̣ 1.71 (m, 2 H, cyclobutyl-CH2), 1.96 (m, 2 H, cyclobutyl-CH2), 2.25 (m, 2 H, cyclobutyl-CH2), 2.98 (d, 3 H, J = 4.5 Hz, N-CH3), 3.84 (m, 1 H, cyclopropyl-CH), 4.01 (d, 1 H, J = 4.0 Hz, 4’-H), 4.47 (dd, 1 H, J = 4.7, 8.8 Hz, 3’-H), 4.54 (dd, 1 H, J = 4.5, 8.0 Hz, 2’-H), 5.63 (d, 1 H, J = 4.5 Hz, exchangeable with D2O, OH), 5.83 (d, 1 H, J = 4.8 Hz, exchangeable with D2O, OH), 5.90 (d, 1 H, J = 8.0 Hz, 1’-H), 8.21 (s, 1 H, H-2), 8.34 (br s, 1 H, exchangeable with D2O, NH), 8.43 (br s, 1H, exchangeable with D2O, NH), 8.53 (s, 1 H, H-8); 13C NMR (CD3OD) δ̣ 16.3, 32.5, 33.2, 35.3, 40.3, 42.8, 65.4, 75.3, 76.5, 135.4, 145.3, 147.8, 153.4, 155.0, 175.4; FAB-MS m/z 365 (M++1); Anal. Calcd for C15H20N6O3S: C, 49.44; H, 5.53; N, 23.06; S, 8.80. Found: C, 49.51; H, 5.49; N, 23.10; S, 8.78.
9-(5’-Cyclopentylaminocarbonyl-4’-thio-β-D-ribofuranosyl)-N6-methyladenine (5f)
Compound 5f was prepared using cyclopentylamine: yield 65%; white solid; [α]25 D −13.8° (c 0.13, MeOH); UV (MeOH) λ max 269 nm (pH 7); 1H NMR (DMSO-d6) δ̣ 1.30 (m, 4 H, cyclopentyl-CH2×2), 1.59 (m, 4 H, cyclopentyl-CH2×2), 2.95 (d, 3 H, J = 4.0 Hz, N-CH3), 3.53 (m, 1 H, NCH), 3.87 (d, 1 H, J = 4.0 Hz, 4’-H), 4.07 (dd, 1 H, J = 4.5, 8.0 Hz, 3’-H), 4.54 (dd, 1 H, J = 5.0, 8.8 Hz, 2’-H), 5.55 (d, 1 H, J = 4.0 Hz, exchangeable with D2O, OH), 5.78 (d, 1 H, J = 4.8 Hz, exchangeable with D2O, OH), 5.82 (d, 1 H, J = 7.8 Hz, 1’-H), 8.25 (s, 1 H, H-2), 8.33 (br s, 1H, exchangeable with D2O, NH), 8.43 (br s, 1 H, J = 4.5 Hz, exchangeable with D2O, NH), 8.53 (s, 1 H, H-8); 13C NMR (CD3OD) δ̣ 23.4, 24.5, 31.7, 32.5, 38.3, 48.5, 48.6, 65.3, 74.5, 80.4, 129.3, 145.3, 148.5, 153.0, 155.4, 175.2; FAB-MS m/z 379(M++1); Anal. Calcd for C16H22N6O3S: C, 50.78; H, 5.86; N, 22.21; S, 8.47. Found: C, 50.75; H, 5.53; N, 22.45; S, 8.68.
9-[(5’-(3-Iodo-benzylaminocarbonyl)-4’-thio-β-D-ribofuranosyl)]-N6-methyladenine (5g)
Compound 5g was prepared using 3-iodo-benzylamine: yield 54%; white solid; [α]25 D −23.0° (c 0.10, MeOH); UV (MeOH) λ max 270 nm (pH 7); 1H NMR (DMSO-d6) δ̣ 2.94 (d, 3 H, J = 4.5 Hz, N-CH3), 3.87 (d, 1 H, J = 4.3 Hz, 4’-H), 4.25 (dd, 1 H, J = 4.4, 8.8 Hz, 3’-H), 4.56 (dd, 1 H, J = 4.9, 8.8 Hz, 2’-H), 4.68 (d, 2 H, J = 3.5 Hz, NH2), 5.56 (d, 1 H, J = 4.0 Hz, exchangeable with D2O, OH), 5.80 (d, 1 H, J = 4.8 Hz, exchangeable with D2O, OH), 5.82 (d, 1 H, J = 7.8 Hz, 1’-H), 7.14–7.76 (m, 4 H, aromatic H), 7.98 (br s, 1H, exchangeable with D2O, NH), 8.05 (br s, 1 H, J = 4.5 Hz, exchangeable with D2O, NH), 8.25 (s, 1 H, H-2), 8.53 (s, 1 H, H-8); 13C NMR (CD3OD) δ 45.3, 50.1, 53.3, 65.4, 78.8, 85.4, 99.4, 125.3, 127.5, 128.4, 130.2, 135.0, 144.2, 145.3, 148.5, 152.3, 156.8, 173.5; FAB-MS m/z 527 (M++1); Anal. Calcd for C18H19IN6O3S: C, 41.07; H, 3.64; N, 15.97; S, 6.09. Found: C, 41.35; H, 3.66; N, 16.12; S, 6.24.
N6-(3-Iodo-benzyl)-9-(5’-methylaminocarbonyl-4’-thio-β-D-ribofuranosyl)adenine (5h)
Compound 5h was prepared using methylamine·HCl: yield 53%; white solid; [α]25 D −20.5° (c 0.15, MeOH); UV (MeOH) λ max 270 nm (pH 7); 1H NMR (DMSO-d6) δ̣ 2.70 (d, 3 H, J = 4.0 Hz, N-CH3), 3.82 (d, 1 H, J = 4.5 Hz, 4’-H), 4.37 (br dd, 1 H, J = 4.5, 8.4 Hz, 3’-H), 4.57 (m, 1 H, 2’-H), 4.65 (d, 2 H, J = 5.7 Hz, N-CH2), 5.62 (d, 1 H, J = 5.5 Hz, exchangeable with D2O, OH), 5.80 (d, 1 H, J = 5.1 Hz, exchangeable with D2O, OH), 5.88 (d, 1 H, J = 5.4 Hz, 1’-H), 7.13 (t, 1 H, J = 7.8 Hz, aromatic H), 7.35 (d, 1 H, J = 7.6 Hz, aromatic H), 7.60 (d, 1 H, J = 7.8 Hz, aromatic H), 7.73 (s, 1 H, aromatic H), 8.26 (br s, 1 H, H-2), 8.54 (s, 1 H, H-8), 8.55 (br s, 1 H, exchangeable with D2O, NH); 13C NMR (DMSO-d6) δ̣ 42.0, 51.5, 60.3, 64.5, 76.0, 78.2, 118.8, 126.4, 133.5, 135.3, 136.0, 140.3, 141.5, 149.9 , 150.4, 153.0, 154.7, 170.3; FAB-MS m/z 527 (M++1); Anal. Calcd for C18H19IN6O3S: C, 41.07; H, 3.64; N, 15.97; S, 6.09. Found: C, 41.35; H, 3.66; N, 16.12; S, 6.24.
9-(5’-Ethylaminocarbonyl-4-thio-β-D-ribofuranosyl)-N6-(3-iodo-benzyl)adenine (5i)
Compound 5i was prepared using ethylamine·HCl: yield 66%; white solid; [α]20 D −45.6° (c 0.15, MeOH); UV (MeOH) λ max 273.0 nm (pH 7); 1H NMR (DMSO-d6) δ̣ 1.09 (t, 3 H, J = 7.0 Hz, CH2CH3), 3.19 (q, 2 H, J = 6.0 Hz, CH2CH3), 3.82 (d, 1 H, J = 4.0 Hz, 4’-H), 4.38 (d, 1 H, J = 4.5 Hz, 3’-H), 4.59 (d, 1 H, J = 3.6 Hz, 2’-H), 4.67 (d, 2 H, J = 4.5 Hz, N-CH2), 5.60 (d, 1 H, J = 5.0 Hz, exchangeable with D2O, OH), 5.77 (d, 1 H, J = 4.8 Hz, exchangeable with D2O, OH), 5.88 (d, 1 H, J = 5.0 Hz, 1’-H), 7.12 (t, 1 H, J = 8.0 Hz, aromatic H), 7.38 (d, 1 H, J = 7.6 Hz, aromatic H), 7.60 (d, 1 H, J = 7.6 Hz, aromatic H), 7.73 (s, 1 H, aromatic H), 8.25 (s, 1H, H-2), 8.50 (br s, 1 H, exchangeable with D2O, NH), 8.55 (s, 1 H, H-8); 13C NMR (CD3OD) δ̣ 15.4, 35.3, 44.5, 54.4, 68.0, 79.1, 80.1, 94.5, 120.3, 125.8, 128.5, 130.5, 135.3, 140.4, 142.7, 151.4, 155.5, 172.3, 174.5; FAB-MS m/z 541 (M++1); Anal. Calcd for C19H21IN6O3S: C, 42.23; H, 3.92; N, 15.55; S, 5.93. Found: C, 42.51; H, 3.95; N, 15.73; S, 5.95.
9-(5’-Cyclopropylaminocarbonyl-4’-thio-β-D-ribofuranosyl)-N6-(3-iodo-benzyl)adenine (5j)
Compound 5j was prepared using cyclopropyl amine: yield 62%; white solid; [α]20 D −35.8° (c 0.15, MeOH); UV (MeOH) λ max 272.0 nm (pH 7); 1H NMR (DMSO-d6) δ̣ 0.48 (br s, 2 H, cyclopropyl-CH2), 0.72 (m, 2 H, cyclopropyl-CH2), 2.54 (m, 1 H, NH), 3.80 (d, 1 H, J = 4.3 Hz, 4’-H), 4.18 (dd, 1 H, J = 4.0, 8.5 Hz, 3’-H), 4.42 (m, 1 H, 2’-H), 4.70 (br s, 2 H, N-CH2), 5.63 (d, 1 H, J = 5.5 Hz, exchangeable with D2O, OH), 5.83 (d, 1 H, J = 5.0 Hz, exchangeable with D2O, OH), 5.90 (d, 1 H, J = 5.4 Hz, 1’-H), 7.13 (t, 1 H, J = 7.8 Hz, aromatic H), 7.35 (d, 1 H, J = 7.6 Hz, aromatic H), 7.60 (d, 1 H, J = 7.8 Hz, aromatic H), 7.73 (s, 1 H, aromatic H), 8.27 (br s, 1 H, H-2), 8.58 (s, 1 H, H-8), 8.59 (br s, 1 H, exchangeable with D2O, NH); 13C NMR (DMSO-d6) δ 8.4, 10.5, 24.5, 50.5, 64.3, 66.8, 77.0, 80.4, 116.5, 127.4, 133.5, 135.4, 135.9, 140.3, 141.5, 148.5 , 150.3, 152.9, 153.5, 171.4; FAB-MS m/z 553 (M++1); Anal. Calcd for C20H21IN6O3S: C, 43.49; H, 3.83; N, 15.21; S, 5.80. Found: C, 43.54; H, 3.92; N, 15.28; S, 5.85.
9-(5’-Cyclopropylmethylaminocarbonyl-4’-thio-β-D-ribofuranosyl)-N6-(3-iodobenzyl)adenine (5k)
Compound 5k was prepared using cyclopropylmethyl amine·HCl: yield 61%; white solid; [α]20 D −14.8° (c 0.15, MeOH); UV (MeOH) λ max 274.0 nm (pH 7); 1H NMR (DMSO-d6) δ̣ 0.18 (m, 2 H, cyclopropyl-CH2), 0.23 (m, 2 H, cyclopropyl-CH2), 0.75 (m, 1 H, cyclopropyl-CH), 2.54 (m, 1 H, NH), 2.87 (t, 2 H, J = 3.8 Hz, N-CH2), 3.67 (d, 1 H, J = 4.2 Hz, 4’-H), 4.19 (dd, 1 H, J = 4.0, 8.7 Hz, 3’-H), 4.42 (m, 1 H, 2’-H), 4.47 (br s, 2 H, N-CH2-cyclopropyl), 5.41 (d, 1 H, J = 5.0 Hz, exchangeable with D2O, OH), 5.59 (d, 1 H, J = 4.8 Hz, exchangeable with D2O, OH), 5.70 (d, 1 H, J = 5.3 Hz, 1’-H), 6.92 (t, 1 H, J = 7.6 Hz, aromatic H), 7.18 (d, 1 H, J = 7.6 Hz, aromatic H), 7.40 (d, 1 H, J = 7.8 Hz, aromatic H), 7.53 (s, 1 H, aromatic H), 8.05 (br s, 1 H, H-2), 8.33 (s, 1 H, H-8), 8.43 (br s, 1 H, exchangeable with D2O, NH); 13C NMR (DMSO-d6) δ̣ 2.3, 3.2, 13.4, 23.8, 54.3, 63.5, 67.0, 77.4, 81.0, 116.4, 126.3, 132.1, 133.4, 135.2, 139.8, 140.2, 147.5 , 150.1, 151.9, 153.4, 171.8; FAB-MS m/z 567 (M++1); Anal. Calcd for C21H23IN6O3S: C, 44.53; H, 4.09; N, 14.84; S, 5.66. Found: C, 44.60; H, 4.12; N, 14.95; S, 5.62.
9-(5’-Cyclobutylaminocarbonyl-4’-thio-β-D-ribofuranosyl)-N6-(3-iodobenzyl)adenine (5l)
Compound 5l was prepared using cyclobutyl amine: yield 53%; white solid; [α]20 D −15.3° (c 0.10, MeOH); UV (MeOH) λ max 270 nm (pH 7); 1H NMR (DMSO-d6) δ 1.71 (m, 2 H, cyclobutyl-CH2), 1.96 (m, 2 H, cyclobutyl-CH2), 2.25 (m, 2 H, cyclobutyl-CH2), 3.84 (d, 1 H, J = 4.0 Hz, 4’-H), 4.30 (m, 1 H, NCH), 4.41 (dd, 1 H, J = 4.5, 8.7 Hz, 3’-H), 4.61 (m, 1 H, 2’-H), 4.70 (br s, 2 H, N-CH2), 5.63 (d, 1 H, J = 5.5 Hz, exchangeable with D2O, OH), 5.83 (d, 1 H, J = 5.0 Hz, exchangeable with D2O, OH), 5.90 (d, 1 H, J = 5.4 Hz, 1’-H), 7.15 (t, 1 H, J = 8.0 Hz, aromatic H), 7.41 (d, 1 H, J = 7.6 Hz, aromatic H), 7.62 (d, 1 H, J = 7.8 Hz, aromatic H), 7.76 (s, 1 H, aromatic H), 8.28 (br s, 1 H, H-2), 8.57 (s, 1 H, H-8), 8.72 (br s, 1 H, exchangeable with D2O, NH); 13C NMR (DMSO-d6) δ 20.4, 35.3, 37.2, 50.5, 54.8, 64.2, 66.5, 78.0, 79.5, 116.4, 126.5, 132.7, 135.3, 135.8, 141.7, 142.3, 148.4, 151.3, 153.0, 154.5, 172.0; FAB-MS m/z 567 (M++1); Anal. Calcd for C21H23IN6O3S: C, 44.53; H, 4.09; N, 14.84; S, 5.66. Found: C, 44.55; H, 4.12; N, 14.96; S, 5.70.
9-(5’-Cyclohexylaminocarbonyl-4’-thio-β-D-ribofuranosyl)-N6-(3-iodobenzyl)adenine (5m)
Compound 5m was prepared using cyclohexyl amine: yield 68%; white solid; [α]20 D −25.4° (c 0.13, MeOH); UV (MeOH) λ max 272 nm (pH 7); 1H NMR (DMSO-d6) δ 1.41–1.82 (m, 10 H, cyclohexyl-CH2), 3.65 (m, 1 H, cyclohexyl-CH), 3.83 (d, 1 H, J = 4.5 Hz, 4’-H), 4.45 (dd, 1 H, J = 4.4, 9.0 Hz, 3’-H), 4.75 (m, 1 H, 2’-H), 4.82 (br s, 2 H, N-CH2), 5.65 (d, 1 H, J = 5.5 Hz, exchangeable with D2O, OH), 5.85 (d, 1 H, J = 5.0 Hz, exchangeable with D2O, OH), 5.95 (d, 1 H, J = 5.4 Hz, 1’-H), 7.25 (t, 1 H, J = 7.5 Hz, aromatic H), 7.43 (d, 1 H, J = 8.0 Hz, aromatic H), 7.65 (d, 1 H, J = 7.8 Hz, aromatic H), 7.79 (s, 1 H, aromatic H), 8.25 (br s, 1 H, H-2), 8.54 (s, 1 H, H-8), 8.56 (br s, 1 H, exchangeable with D2O, NH); 13C NMR (DMSO-d6) δ 20.5, 22.4, 30.5, 35.4, 36.8, 46.3, 55.6, 64.4, 66.9, 77.5, 79.4, 117.5, 128.4, 133.5, 134.0, 135.6, 141.6, 142.1, 148.5, 152.1, 153.5, 154.4, 172.8; FAB-MS m/z 595 (M++1); Anal. Calcd for C23H27IN6O3S: C, 46.47; H, 4.58; N, 14.14; S, 5.39. Found: C, 46.55; H, 4.46; N, 14.25; S, 5.50.
9-[5’-(3-Fluoro-benzylaminocarbonyl-4’-thio-β-D-ribofuranosyl)]-N6-(3-iodobenzyl)adenine (5n)
Compound 5n was prepared using 3-fluoro-benzylamine: yield 59%; white solid; [α]20 D −40.1° (c 0.10, MeOH); UV (MeOH) λ max 272 nm (pH 7); 1H NMR (DMSO-d6) δ̣ 3.79 (d, 1 H, J = 4.0 Hz, 4’-H), 4.28 (m, 3 H, 3’-H, N-CH2), 4.50 (d, 1 H, J = 4.0 Hz, 2’-H), 4.62 (d, 2 H, J = 4.4 Hz, N-CH2), 5.54 (d, 1 H, J = 5.6 Hz, exchangeable with D2O, OH), 5.67 (d, 1 H, J = 4.8 Hz, exchangeable with D2O, OH), 5.78 (d, 1 H, J = 5.2 Hz, 1’-H), 6.92–7.58 (m, 8 H, aromatic H), 8.34 (s, 1 H, H-2), 8.53 (s, 1 H, H-8), 8.91 (br s, 1 H, exchangeable with D2O, NH), 9.03 (br s, 1 H, exchangeable with D2O, NH); 13C NMR (CD3OD) δ 50.3, 52.4, 54.3, 60.4, 77.4, 78.5, 101.3, 114.3, 115.4, 116.8, 125.7, 125.9, 128.4, 130.5, 135.0, 140.3, 142.1, 145.8, 150.2, 155.31, 158.2, 160.5, 164.8, 173.5; FAB-MS m/z 621 (M++1); Anal. Calcd for C24H22FIN6O3S: C, 46.46; H, 3.57; N, 13.55; S, 5.17. Found: C, 46.55; H, 3.46; N, 13.65; S, 5.26.
9-[5’-(3-Chloro-benzylaminocarbonyl-4’-thio-β-D-ribofuranosyl)]-N6-(3-iodobenzyl)adenine (5o)
Compound 5o was prepared using 3-chloro-benzylamine: yield 68%; white solid; [α]20 D −25.4° (c 0.13, MeOH); UV (MeOH) λ max 273 nm (pH 7); 1H NMR (DMSO-d6) δ̣ 3.78 (d, 1 H, J = 3.8 Hz, 4’-H), 4.26 (m, 3 H, 3’-H, N-CH2), 4.49 (m, 3 H, N-CH2), 5.52 (d, 1 H, J = 5.0 Hz, exchangeable with D2O, OH), 5.67 (d, 1 H, J = 4.8 Hz, exchangeable with D2O, OH), 5.77 (d, 1 H, J = 5.2 Hz, 1’-H), 6.94–7.58 (m, 8 H, aromatic H), 8.03 (s, 1 H, H-2), 8.37 (s, 1 H, H-8), 8.38 (br s, 1 H, exchangeable with D2O, NH), 9.02 (br s, 1H, exchangeable with D2O, NH); 13C NMR (CD3OD) δ̣ 51.4, 52.5, 54.0, 61.3, 78.1, 79.8, 102.3, 114.8, 115.6, 117.2, 125.9, 128.0, 136.3, 138.4, 140.0, 141.2, 142.0, 148.3, 149.5, 150.2, 153.4, 157.2, 158.4, 174.0; FAB-MS m/z 637 (M++1); Anal. Calcd for C24H22ClIN6O3S: C, 45.26; H, 3.48; N, 13.20; S, 5.03. Found: C, 45.46; H, 3.46; N, 13.25; S, 5.26.
N6-(3-Iodo-benzyl)-9-[5’-(2-methyl-benzylaminocarbonyl-4’-thio-β-D-ribofuranosyl)]adenine (5p)
Compound 5p was prepared using 2-methyl-benzylamine: yield 60%; white solid; [α]20 D −43.0° (c 0.10, MeOH); UV (MeOH) λ max 274 nm (pH 7); 1H NMR (DMSO-d6) δ 2.32 (s, 3 H, CH3), 3.98 (d, 1 H, J = 2.7 Hz, 4’-H), 4.40 (m, 3 H, 3’-H, N-CH2), 4.69 (m, 3 H, 2’-H, N-CH2), 5.68 (d, 1 H, J = 5.0 Hz, exchangeable with D2O, OH), 5.85 (d, 1 H, J = 4.8 Hz, exchangeable with D2O, OH), 5.94 (d, 1 H, J = 5.0 Hz, 1’-H), 7.13–7.76 (m, 8 H, aromatic H), 8.11 (s, 1 H, H-2), 8.54 (s, 1 H, H-8), 8.96 (br s, 1 H, exchangeable with D2O, NH); 13C NMR (CD3OD) δ̣ 24.8, 49.8, 50.3, 52.8, 56.3, 68.4, 78.0, 80.3, 98.6, 102.4, 115.8, 120.3, 125.6, 135.4, 137.6, 139.8, 140.4, 142.5, 146.4, 148.1, 151.3, 154.5, 156.7, 158.4, 173.8; FAB-MS m/z 617 (M++1); Anal. Calcd for C25H25IN6O3S: C, 48.71; H, 4.09; N, 13.63; S, 5.20. Found: C, 48.88; H, 4.14; N, 13.82; S, 5.26.
N6-(3-Iodo-benzyl)-9-[5’-(3-methyl-benzylaminocarbonyl-4’-thio-β-D-ribofuranosyl)]adenine (5q)
Compound 5q was prepared using 3-methyl-benzylamine: yield 58%; white solid; [α]20 D −33.5° (c 0.13, MeOH); UV (MeOH) λ max 274 nm (pH 7); 1H NMR (DMSO-d6) δ̣ 2.31 (s, 3 H, CH3), 3.96 (d, 1 H, J = 2.0 Hz, 4’-H), 4.40 (d, 2 H, J = 8.0 Hz, N-CH2), 4.45 (dd, 1 H, J = 5.2, 7.8 Hz, 3’-H), 4.68 (m, 3 H, 2’-H, N-CH2), 5.69 (d, 1 H, J = 5.0 Hz, exchangeable with D2O, OH), 5.84 (d, 1 H, J = 4.8 Hz, exchangeable with D2O, OH), 5.94 (d, 1 H, J = 5.0 Hz, 1’-H), 7.09–7.76 (m, 8 H, aromatic H), 8.17 (s, 1 H, H-2), 8.53 (s, 1 H, H-8), 8.54 (br s, 1 H, exchangeable with D2O, NH), 8.96 (br s, 1 H, exchangeable with D2O, NH); 13C NMR (CD3OD) δ 23.5, 50.0, 50.4, 52.8, 56.4, 68.4, 78.1, 80.3, 98.6, 102.4, 115.9, 120.3, 125.6, 135.4, 136.5, 140.0, 140.5, 142.5, 146.5, 148.2, 151.3, 154.6, 156.7, 158.5, 174.0; FAB-MS m/z 617 (M++1); Anal. Calcd for C25H25IN6O3S: C, 48.71; H, 4.09; N, 13.63; S, 5.20. Found: C, 48.85; H, 4.13; N, 13.62; S, 5.15.
N6-(3-Iodo-benzyl)-9-[5’-(4-methyl-benzylaminocarbonyl-4’-thio-β-D-ribofuranosyl)]adenine (5r)
Compound 5r was prepared using 4-methyl-benzylamine: yield 60%; white solid; [α]20 D −38.1° (c 0.15, MeOH); UV (MeOH) λ max 273 nm (pH 7); 1H NMR (DMSO-d6) δ̣ 2.31 (s, 3 H, CH3), 3.95 (d, 1 H, J = 2.5 Hz, 4’-H), 4.39 (d, 2 H, J = 8.0 Hz, N-CH2), 4.44 (br s, 1 H, 3’-H), 4.68 (br s, 3 H, 2’-H, N-CH2), 5.69 (br s, 1 H, exchangeable with D2O, OH), 5.82 (br s, 1 H, exchangeable with D2O, OH), 5.95 (d, 1 H, J = 4.8 Hz, 1’-H), 7.13–7.76 (m, 8 H, aromatic H), 8.16 (s, 1 H, H-2), 8.53 (s, 1 H, H-8), 8.54 (br s, 1 H, exchangeable with D2O, NH), 9.13 (br s, 1 H, exchangeable with D2O, NH); 13C NMR (CD3OD) δ̣ 20.9, 49.8, 50.5, 53.4, 56.5, 67.9, 78.4, 80.5, 98.9, 102.7, 116.3, 120.4, 125.6, 135.5, 136.7, 140.5, 140.9, 142.5, 146.4, 148.2, 151.4, 154.6, 156.8, 158.5, 173.9; FAB-MS m/z 617 (M++1); Anal. Calcd for C25H25IN6O3S: C, 48.71; H, 4.09; N, 13.63; S, 5.20. Found: C, 48.79; H, 4.15; N, 13.55; S, 5.18.
N6-(3-Iodo-benzyl)-9-[5’-(2-methoxy-benzylaminocarbonyl-4’-thio-β-D-ribofuranosyl)]adenine (5s)
Compound 5s was prepared using 2-methoxy-benzylamine: yield 72%; white solid; [α]20 D −30.2° (c 0.10, MeOH); UV (MeOH) λ max 270 nm (pH 7); 1H NMR (DMSO-d6) δ 3.83 (s, 3 H, OCH3), 3.98 (d, 1 H, J = 2.5 Hz, 4’-H), 4.38 (m, 3 H, 3’-H, N-CH2), 4.69 (m, 3 H, 2’-H, N-CH2), 5.69 (d, 1 H, J = 5.5 Hz, exchangeable with D2O, OH), 5.82 (d, 1 H, J = 4.8 Hz, exchangeable with D2O, OH), 5.94 (d, 1 H, J = 5.0 Hz, 1’-H), 6.94–7.61 (m, 8 H, aromatic H), 8.15 (s, 1 H, H-2), 8.53 (br s, 1 H, exchangeable with D2O, NH), 8.54 (s, 1 H, H-8), 8.97 (br s, 1 H, exchangeable with D2O, NH); 13C NMR (CD3OD) δ̣ 49.8, 50.4, 52.8, 56.3, 57.8, 68.0, 78.4, 80.9, 98.5, 102.8, 115.8, 120.5, 125.6, 135.8, 137.6, 139.8, 140.5, 142.7, 146.4, 148.1, 151.8, 153.5, 156.9, 158.3, 173.9; FAB-MS m/z 633 (M++1); Anal. Calcd for C25H25IN6O4S: C, 48.48; H, 3.98; N, 13.29; S, 5.07. Found: C, 48.65; H, 4.04; N, 13.42; S, 5.01.
9-[5’-(2-Ethoxy-benzylaminocarbonyl-4’-thio-β-D-ribofuranosyl)]-N6-(3-iodobenzyl)adenine (5t)
Compound 5t was prepared using 2-ethoxy-benzylamine: yield 63%; white solid; [α]20 D −35.8° (c 0.10, MeOH); UV (MeOH) λ max 270 nm (pH 7); 1H NMR (DMSO-d6) δ̣ 1.36 (t, 3 H, J = 4.2 Hz, OCH2CH3), 4.09 (m, 2 H, OCH2CH3), 3.98 (d, 1 H, J = 2.5 Hz, 4’-H), 4.38 (m, 3 H, 3’-H, N-CH2), 4.70 (m, 3 H, 2’-H, N-CH2), 5.69 (d, 1 H, J = 5.5 Hz, exchangeable with D2O, OH), 5.83 (d, 1 H, J = 4.8 Hz, exchangeable with D2O, OH), 5.95 (d, 1 H, J = 4.8 Hz, 1’-H), 6.93–7.76 (m, 8 H, aromatic H), 8.14 (s, 1 H, H-2), 8.53 (br s, 1 H, exchangeable with D2O, NH), 8.54 (s, 1 H, H-8), 8.93 (br s, 1 H, exchangeable with D2O, NH); 13C NMR (CD3OD) δ̣ 23.8, 48.2, 51.3, 52.8, 57.8, 65.2, 67.3, 78.4, 81.0, 98.6, 103.4, 116.4, 120.1, 124.5, 135.7, 137.8, 139.8, 141.3, 142.9, 146.5, 148.3, 152.1, 153.6, 155.4, 158.4, 170.1; FAB-MS m/z 647 (M++1); Anal. Calcd for C26H27IN6O4S: C, 48.30; H, 4.21; N, 13.00; S, 4.96. Found: C, 48.35; H, 4.24; N, 13.13; S, 5.04.
N6-(3-Iodo-benzyl)-9-[5’-(1-naphthylmethylaminocarbonyl-4’-thio-β-D-ribofuranosyl)]adenine (5u)
Compound 5u was prepared using 1-naphthyl-methylamine: yield 57%; white solid; [α]20 D −13.5° (c 0.15, MeOH); UV (MeOH) λ max 273 nm (pH 7); 1H NMR (DMSO-d6) δ 3.97 (d, 1 H, J = 2.5 Hz, 4’-H), 4.47 (d, 2 H, J = 8.4 Hz, N-CH2), 4.68 (br s, 1 H, 3’-H), 4.92 (br s, 3 H, 2’-H, N-CH2), 5.66 (br s, 1 H, exchangeable with D2O, OH), 5.82 (br s, 1 H, exchangeable with D2O, OH), 5.93 (d, 1 H, J = 4.8Hz, 1’-H), 7.10–7.98 (m, 11 H, aromatic H), 8.10 (s, 1 H, H-2), 8.53 (s, 1 H, H-8), 8.54 (br s, 1 H, exchangeable with D2O, NH), 9.09 (br s, 1 H, exchangeable with D2O, NH); 13C NMR (CD3OD) δ̣ 45.3, 46.5, 55.3, 60.4, 73.8, 89.1, 98.4, 121.2, 122.4, 122.5, 124.1, 124.8, 126.3, 127.0, 127.8, 128.1, 129.2, 132.4, 133.5, 135.4, 136.0, 144.8, 145.5, 147.8, 152.0, 154.4, 156.8, 174.4; FAB-MS m/z 653 (M++1); Anal. Calcd for C28H25IN6O3S: C, 51.54; H, 3.86; N, 12.88; S, 4.91. Found: C, 51.68; H, 3.92; N, 12.92; S, 4.90.
N6-(3-Iodo-benzyl)-9-[5’-(2-phenetylaminocarbonyl-4’-thio-β-D-ribofuranosyl)]adenine (5v)
Compound 5v was prepared using 2-phenylethylamine: yield 59%; white solid; [α]20 D −45.0° (c 0.15, MeOH); UV (MeOH) λ max 270 nm (pH 7); 1H NMR (DMSO-d6) δ̣ 2.65 (t, 2 H, J = 2.5 Hz, NCH2CH2), 3.54 (m, 2 H, NCH2CH2), 3.71 (d, 1 H, J = 4.0 Hz, 4’-H), 4.23 (br s, 1 H, 3’-H), 4.48 (br s, 1 H, 2’-H), 4.55 (d, 2 H, J = 8.0 Hz, N-CH2), 5.51 (br s, 1 H, exchangeable with D2O, OH), 5.65 (br s, 1 H, exchangeable with D2O, OH), 5.77 (d, 1 H, J = 5.2 Hz, 1’-H), 6.98–7.61 (m, 9 H, aromatic H), 8.09 (s, 1 H, H-2), 8.38 (s, 1 H, H-8), 8.39 (br s, 1 H, exchangeable with D2O, NH), 8.57 (br s, 1 H, exchangeable with D2O, NH); 13C NMR (CD3OD) δ̣ 37.3, 44.5, 45.3, 56.0, 60.3, 74.2, 79.8, 97.3, 123.1, 123.4, 124.5, 125.3, 126.4, 126.8, 127.1, 128.4, 129.4, 136.2, 137.1, 144.3, 145.2, 147.1, 147.8, 154.2, 174.5; FAB-MS m/z 617 (M++1); Anal. Calcd for C25H25IN6O3S: C, 48.71; H, 4.09; N, 13.63; S, 5.20. Found: C, 48.79; H, 4.15; N, 13.55; S, 5.18.
9-[5’-(3,3-Diphenylpropylaminocarbonyl-4’-thio-β-D-ribofuranosyl)]-N6-(3-iodobenzyl)adenine (5w)
Compound 5w was prepared using 3,3-diphenylpropylamine: yield 72%; white solid; [α]20 D −25.6° (c 0.32, MeOH); UV (MeOH) λ max 274 nm (pH 7); 1H NMR (DMSO-d6) δ̣ 2.27 (m, 2 H, NCH2CH2CH), 3.10 (m, 2 H, NCH2CH2CH), 3.87 (d, 1 H, J = 4.0 Hz, 4’-H), 4.07 (t, 1 H, J = 8.0 Hz, NCH2CH2CH), 4.41 (dd, 1 H, J = 3.6, 7.4 Hz, 3’-H), 4.64 (m, 1 H, 2’-H), 4.69 (d, 2 H, J = 5.7 Hz, N-CH2), 5.64 (d, 1 H, J = 5.6 Hz, exchangeable with D2O, OH), 5.83 (d, 1 H, J = 5.6 Hz, exchangeable with D2O, OH), 5.93 (d, 2 H, J = 4.2 Hz, 1’-H), 7.13–7.76 (m, 14 H, aromatic H), 8.20 (s, 1 H, H-2), 8.54 (s, 1 H, H-8), 8.55 (br s, 1 H, exchangeable with D2O, NH), 8.62 (br d, 1 H, J = 6.1 Hz, exchangeable with D2O, NH); 13C NMR (CD3OD) δ 34.5, 35.3, 40.6, 45.5, 60.1, 73.4, 79.1, 80.4, 97.1, 112.3, 120.5, 126.0, 128.4, 129.3, 129.5, 129.7, 129.9, 132.7, 135.3, 141.5, 143.0, 144.3, 147.8, 152.4, 155.7, 174.8; FAB-MS m/z 707 (M++1); Anal. Calcd for C32H31IN6O3S: C, 54.39; H, 4.42; N, 11.89; S, 4.54. Found: C, 54.48; H, 4.45; N, 11.75; S, 4.64.
Scheme 2a.
aReagents & conditions: a) PDC, DMF; b) R3NH2, EDC, HOBt, DIPEA, CH2Cl2, c) TBAF, THF
Acknowledgement
This work was supported by the grant from the World Class University (WCU) Project from Ministry of Education and Science, Korea (R31-2008-000-10010-0). KAJ and ZG acknowledge support from the NIDDK Intramural Research Program of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.(a) Olah ME, Stiles GL. Pharmacol. Ther. 2000;85:55. doi: 10.1016/s0163-7258(99)00051-0. [DOI] [PubMed] [Google Scholar]; (b) Liang BT, Jacobson KA. Proc. Natl. Acad. Sci. 1998;95:6995. doi: 10.1073/pnas.95.12.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Jacobson KA, Gao Z-G. Nature Rev. Drug Disc. 2006;5:247. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Klotz K-N. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2000;362:382. doi: 10.1007/s002100000315. [DOI] [PubMed] [Google Scholar]; (c) Baraldi PG, Cacciari B, Romagnoli R, Merighi S, Varani K, Borea PA, Spalluto G. Med. Res. Rev. 2000;20:103. doi: 10.1002/(sici)1098-1128(200003)20:2<103::aid-med1>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 3.Kim HO, Ji X-d, Siddiqi SM, Olah ME, Stiles GL, Jacobson KA. J. Med. Chem. 1994;37:3614. doi: 10.1021/jm00047a018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallo-Rodriguez C, Ji X-d, Melman N, Siegman BD, Sanders LH, Orlina J, Fischer B, Pu Q, Olah ME. J. Med. Chem. 1994;37:636. doi: 10.1021/jm00031a014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fishman P, Madi L, Bar-Yehuda S, Barer F, Del Valle L, Khalili K. Oncogene. 2002;21:4060. doi: 10.1038/sj.onc.1205531. [DOI] [PubMed] [Google Scholar]
- 6.(a) Jeong LS, Jin DZ, Kim HO, Shin DH, Moon HR, Gunaga P, Chun MW, Kim Y-C, Melman N, Gao Z-G, Jacobson KA. J. Med. Chem. 2003;46:3775. doi: 10.1021/jm034098e. [DOI] [PubMed] [Google Scholar]; (b) Jeong LS, Lee HW, Jacobson KA, Kim HO, Shin DH, Lee JA, Gao Z-G, Lu C, Duong HT, Gunaga P, Lee SK, Jin DZ, Chun MW, Moon HR. J. Med. Chem. 2006;49:273. doi: 10.1021/jm050595e. [DOI] [PubMed] [Google Scholar]
- 7.(a) Lee EJ, Min HY, Chung HJ, Park EJ, Shin DH, Jeong LS, Lee SK. Biochem. Pharmacol. 2005;70:918. doi: 10.1016/j.bcp.2005.06.017. [DOI] [PubMed] [Google Scholar]; (b) Chung H, Jung J-Y, Cho S-D, Hong K-A, Kim H-J, Shin D-H, Kim H, Kim HO, Lee HW, Jeong LS, Gong K. Mol. Cancer Ther. 2006;5:685. doi: 10.1158/1535-7163.MCT-05-0245. [DOI] [PubMed] [Google Scholar]
- 8.Jeong LS, Lee HW, Kim HO, Jung JY, Gao Z-G, Duong HT, Rao S, Jacobson KA, Shin DH, Lee JA, Gunaga P, Lee SK, Jin DZ, Chun MW. Bioorg. Med. Chem. 2006;14:4718. doi: 10.1016/j.bmc.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 9.(a) Gao Z-G, Kim SK, Biadatti T, Chen W, Lee K, Barak D, Kim SG, Johnson CR, Jacobson KA. J. Med. Chem. 2002;45:4471. doi: 10.1021/jm020211+. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Gao Z-G, Blaustein JB, Gross AS, Melman N, Jacobson KA. Biochem. Pharmacol. 2003;65:1675. doi: 10.1016/s0006-2952(03)00153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Gao Z-G, Jeong LS, Moon HR, Kim HO, Choi WJ, Shin DH, Elhalem E, Comin MJ, Melman N, Mamedova L, Gross AS, Rodriguez JB, Jacobson KA. Biochem. Pharmacol. 2004;67:893. doi: 10.1016/j.bcp.2003.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melman A, Gao Z-G, Kumar D, Wan TC, Gizewski E, Auchampach JA, Jacobson KA. Bioorg. Med. Chem.Lett. 2008;18:2813. doi: 10.1016/j.bmcl.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]