Abstract
Ultrasound is a rapidly evolving technique that is gaining an increasing success in the assessment of psoriatic arthritis. Most of the studies have been aimed at investigating its ability in the assessment of joints, tendons, and entheses in psoriatic arthritis patients. Less attention has been paid to demonstrate the potential of ultrasound in the evaluation of skin and nail. The aim of this pictorial essay was to show the main high-frequency grayscale and power Doppler ultrasound findings in patients with psoriatic arthritis at joint, tendon, enthesis, skin, and nail level.
Keywords: Psoriatic arthritis, Ultrasound, Power Doppler, Joint, Tendon, Enthesis, Skin, Nail
Introduction
Psoriatic arthritis (PsA) is a chronic and heterogeneous inflammatory joint disease that occurs in 6–42% of patients with psoriasis [1]. A variable spectrum of pathologic condition can be found in PsA patients including joint and tendon inflammation, enthesitis, new bone formation, severe osteolysis, and overlap of all of these [2]. A common denominator is the skin psoriasis [3]. Recently, the definition “psoriatic disease” has been proposed to encompass the involvement at different tissue and organ levels [4].
The continuous technological advances in the field of ultrasound (US) allowed the development of equipments provided with high and variable frequency probes and very sensitive power Doppler (PD), which permit both the detailed study (with resolution power of 0.1 mm) of morphostructural changes and the sensitive detection of blood flow even in small vessels of superficial tissues [5–8]. Most of the studies have been aimed at investigating the ability of US in the assessment of joints, tendons, and entheses [9–15] in patients with PsA. Less attention has been paid to demonstrate the potential of US in the evaluation of skin and nail.
The aim of this pictorial essay was to show the main high-frequency grayscale US and PD findings in patients with PsA at joint, tendon, enthesis, skin, and nail level.
Methods
The US images illustrated in the present pictorial essay were obtained in a cohort of 30 patients with diagnosis of PsA, made by an experienced rheumatologist (RDA) according to the international criteria [16]. Clinical examination aimed to detect tenderness and/or swelling at joints, tendons, and entheses level was performed by the same rheumatologist. Twenty of the 30 patients presented a skin involvement and eight patients an onychopathy, diagnosed clinically by an experienced dermatologist (GF), who moreover scored both Psoriasis Area and Severity Index (range between 8 and 20, median 12.4) and Nail Psoriasis Severity index (range between 2 and 8, median 3.5). The US examinations were performed by two experienced sonographers (MG and EF), using the following US systems: MyLab 70 XVG (Esaote Biomedica Genoa, Italy) equipped with 6–18 MHz broadband multifrequency linear transducer (axial resolution = 30 μm and lateral resolution = 60 μm) and Doppler frequency ranging from 7.1 to 14.3 MHz; Technos “Partner” System (Esaote Biomedica Genoa, Italy) equipped with 8–14 MHz multifrequency linear band transducer (axial resolution = 50 μm, lateral resolution = 80 μm) and Doppler frequency ranging from 8.3 to 12.5 MHz and Logiq 9 (General Electric Medical Systems, Milwaukee, WI, USA) equipped with 8–15 MHz multifrequency linear transducer (axial resolution = 10 μm, lateral resolution = 25 μm). The most representative images showing the main pathological findings were selected from the database of the three US machines used in this study.
The US examinations of musculoskeletal system were performed with multiplanar technique, at the clinically involved sites adopting the indications provided by the European League Against Rheumatism guidelines for musculoskeletal ultrasound in rheumatology [17].
During US examination of skin, representative US images were acquired at both the center and the margins of the psoriatic lesion and at the surrounding normal skin. Skin thickness varies among healthy subjects and depends on several aspects including the different areas of the body. Thus, the thickness of the normal skin surrounding the psoriatic lesion was used as reference for detecting the thickening of the epidermis and/or the dermis. The totality of US evaluations was insonated on both longitudinal and transverse scans and perpendicularly using an amount of gel, which avoids compression of the tissues under examination. All examinations were performed in both grayscale and PD technique in order to detect the morphostructural changes and the presence of abnormal blood flow, respectively. The PD settings for all examinations were standardized with a pulse repetition frequency of 750 Hz and a Doppler frequency between 7.5 and 14.3 MHz. In order to confirm that the PD signal represented real blood flow and not an artifact, the spectral Doppler was used. The study was conducted according to the Declaration of Helsinki, and informed consent was obtained from all patients.
Results
Joint
The joint involvement is variable during PsA. The US findings in this condition are nonspecific as they may occur also in patients with other inflammatory conditions such as rheumatoid arthritis. The main joint grayscale US pathological findings with the corresponding definitions are reported in Table 1. US can be used to assess joint cavity widening (differentiation between joint effusion and synovial proliferation; Fig. 1a, b), erosions, and the hyperemia which may give indirect information about the activity of the disease. The dynamic examination of soft tissues made, by compression with the probe, results helpful for the differentiation between synovial effusion (easily moved by compression) and synovial proliferation (unchanged by compression). In the initial stages of the disease, it is possible to identify minimal US signs such as modest exudative synovitis associated with periarticular oedema. In this phase, the PD signal can be more or less present (in some cases, it can be distributed exclusively within the “fat pad in absence of other abnormalities”; Fig. 1c, d). In the late stages, US can find the typical alterations such as the presence of diffused synovial proliferation (with various degrees of vascularization) and bone erosions, which can be focal or multifocal (Fig. 1e, f).
Table 1.
Grayscale US pathological findings in the joints of patients with PsA
| Joint effusion | Homogeneous anechoic joint space widening [45] |
|---|---|
| Proliferative synovitis | Joint space widening with clusters of soft echoes (bushy and villous appearance) and/or homogeneous synovial thickening [46] |
| Bone erosion | An intra-articular discontinuity of the bone surface that is visible in two perpendicular planes [47] |
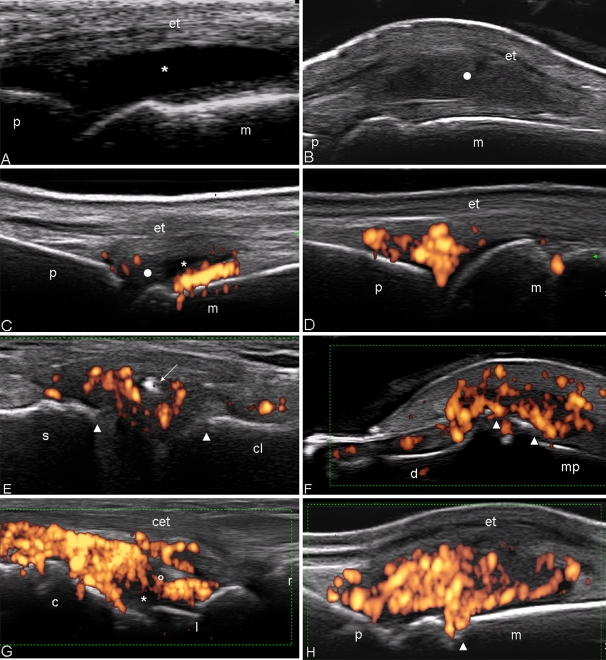
Fig. 1.
Psoriatic arthritis, metacarpophalangeal joint on dorsal longitudinal scan. a Exudative synovitis (asterisk). b Proliferative synovitis (white circle). Note why the marked joint cavity widening pushes up the extensor tendon of the finger (et). c Mild joint cavity widening of the metacarpophalangeal joint with mix content. The synovial fluid echotexture appears prevalently hypoanechoic (asterisk) whereas the synovial hypertrophy with echoic branches (white circle). Power Doppler assessment detected intense intra-articular signal distributed at the areas of synovial proliferation near to the bone profile. d Presence of diffuse power Doppler signal distributed only in the intra-articular “fat pad” of the metacarpophalangeal joint in absence of joint cavity widening. e Sternoclavicular joint on longitudinal scan. Joint cavity widening with intra-articular calcification (white arrow) and power Doppler signal. Note the irregularity of the bone profile of the two articular heads (white arrowheads). f Distal interphalangeal joint on dorsal longitudinal scan. Moderate enlargement of the joint capsule with evident sings of synovial proliferation and presence of intra-articular power Doppler generating bone erosion at the distal and middle phalanx level (white arrowheads). g Wrist on dorsal longitudinal scan. Marked joint cavity widening of both radiocarpal and intercarpal joints with intense power Doppler signal distributed exclusively in the inter-carpal joint. h Metacarpophalangeal joint on dorsal longitudinal scan. Active synovitis. Note both the marked synovial proliferation and the intense hyperaemia, detected by power Doppler, and the bone erosion in the neck of the metacarpal bone (white arrowheads). m metacarpal bone, p proximal phalanx, d distal phalanx, mp middle phalanx, c capitate bone, l lunate bone, r radius bone, et extensor digitorium tendon, cet common extensor digitorium tendons
In the great majority of joint examined, a high degree of intra-articular PD signal may be found at the level of synovial proliferation (Fig. 1g, h). This finding is more evident at small joints level where very high-frequency PD can be used. At large joint level, a relatively lower intra-articular PD signal can be found because a lower frequency must be used to investigate deeper structure with consequent reduction of the PD sensitivity (Fig. 2a, b).
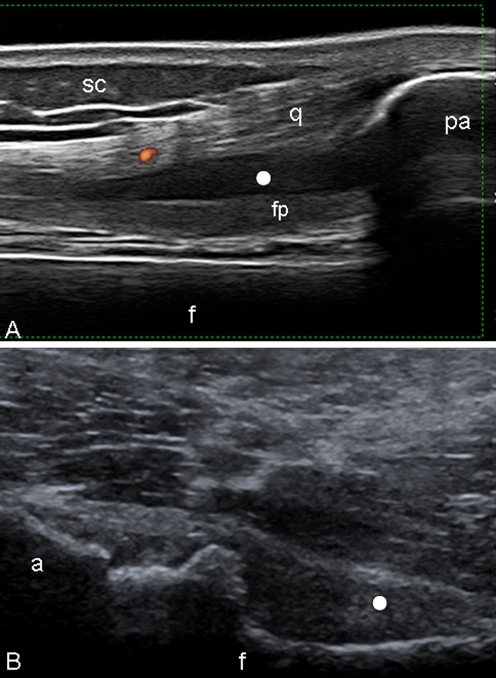
Fig. 2.
Psoriatic arthritis. Knee a Anterior longitudinal scan. Proliferative synovitis. Marked suprapatellar pouch enlargement with signs of synovial hypertrophy (white circle). Note the scarce PD signal within the synovial proliferation areas. b Hip. Anterior longitudinal scan. Marked joint cavity widening with presence of synovial proliferation (white circle). f femur, pa upper pole of the patella, q quadriceps tendon, fp intra-articular fat pad, sc subcutaneous adipose tissue, a acetabulum
Tendon
The spectrum of pathological conditions affecting tendon surrounded by synovial sheath is wide and includes: exudative or proliferative tenosynovitis, loss of “fibrillar” echotexture, and partial or complete tear (Fig. 3a–f). The “Dactylitis” is a common feature of PsA [18]. During the US examination of these patients, it is possible to detect a variable combination of the following pathological conditions: tenosynovitis of the finger or toe flexor tendons, synovitis (mainly distal and proximal interphalangeal joints), and diffuse soft tissue oedema [19–21] (Fig. 3g).
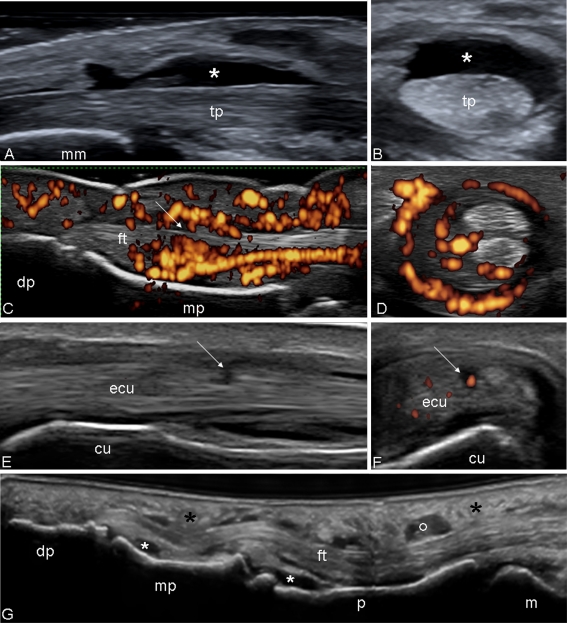
Fig. 3.
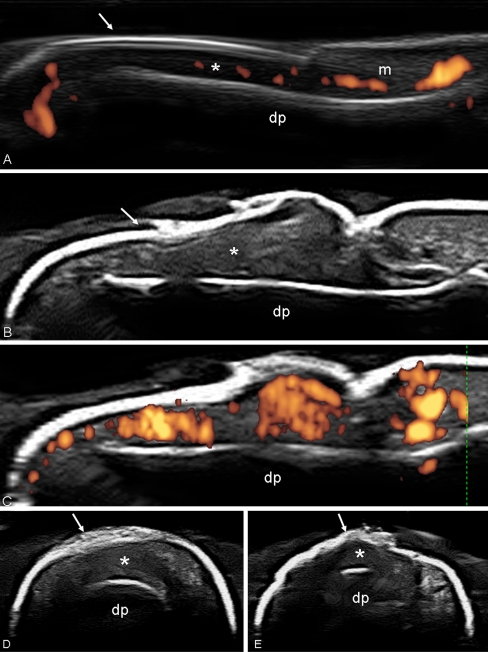
Psoriatic arthritis. Ankle. Grayscale examination. Posterior tibial tendon on longitudinal (a) and transverse (b) scan. Marked tendon sheath widening with homogeneous anechoic aspect (asterisks) of the content indicating an exudative tenosynovitis. Note as the normal fibrillar echotexture is conserved. Hand. Flexor tendons of second finger. The volar longitudinal (c) and transverse scan (d) shows a tendon sheath widening with signs of synovial proliferation, presence of intense power Doppler signal surrounding the tendon, and microinterruption of the margin (arrow). e Wrist. Extensor carpi ulnaris tendon (sixth compartment of the extensor tendons; ecu) on lateral longitudinal (e) and transverse (f) scan. Chronic tenosynovitis with clear areas of low of echogenicity and loss of the continuity of tendon fibrils indicative of partial tendon tear (arrows). Moreover, note the presence of power Doppler signal within the interruption indicating still activity of the inflammatory process. g Dactylitis. Volar longitudinal scan using the “extended view” technique, showing proliferative tenosynovitis of the finger flexor tendon (circle), exudative synovitis of both proximal and distal interphalangeal joint (white asterisks) and oedema of the peritendineous tissue (black asterisks). tp posterior tibial tendon, mm medial malleolus, dp distal phalanx, mp middle phalanx, cu cubital bone, ft flexor tendons
In the tendons without synovial sheath, inflammatory changes detectable by US include tendon thickening (that can adopt a fusiform appearance) and echotexture hypoechogenicity due to tendon oedema, with or without intratendineous PD signal (Fig. 4a). Moreover, a peritenon inflammation typically may appear as a hypoechoic swelling of the soft tissue surrounding the tendon with a usually intense PD signal (Fig. 4b).
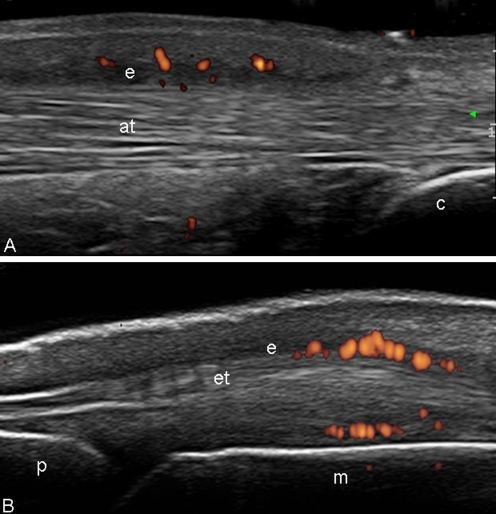
Fig. 4.
Psoriatic arthritis. a Achilles tendon. Longitudinal scan. Tendonitis. Note the hypoechogenicity of the structure of the tendon and the peritendineous oedema (e) with power Doppler signal. b Early psoriatic arthritis. Metacarpophalangeal joint. Dorsal longitudinal scan. Peritendinitis. Note the hypoechogenicity at the level of peritenon of the extensor digitorium tendons with associated oedema of the surrounding soft tissues (e) and power Doppler signal. p proximal phalanx, m metacarpal bone, at Achilles tendon, c calcaneous bone, et extensor digitorium tendon
Enthesis
Last generation US equipment provides a detailed assessment of the entheseal morphostructural features. Thanks to the superficial location of the most frequently involved entheses, probes with high-frequency PD can be used, allowing for a sensitive assessment of the entheseal perfusion status.
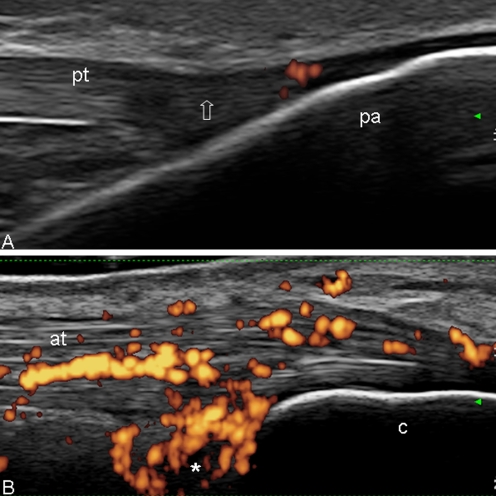
In the early stages of the disease, the enthesis and the adjacent structures may show several morphostructural changes as entheseal thickening, hypoechogenicity, and fibrillar separation due to intratendineous oedema, with or without associated bursitis and different patterns of PD signal distribution. In this stage, the bone profile usually does not show relevant changes (Fig. 5a, b).
Fig. 5.
Psoriatic arthritis. a Distal patellar enthesis. Longitudinal scan. The echotexture of the enthesis is normal. The only pathological abnormality is represented by a mild power Doppler signal within the enthesis. Note the entheseal hypoechogenicity generated by the anisotropy (arrow). b Achilles tendon. Longitudinal scan. Presence of hypoechogenicity of the tendon structure (due to intrafibrillar oedema) and retrocalcanear bursitis (asterisk), with intense power Doppler signal situated of both. Note the integrity of the bone profile. pt patellar tendon, pa patella, at Achilles tendon, c calcaneous bone
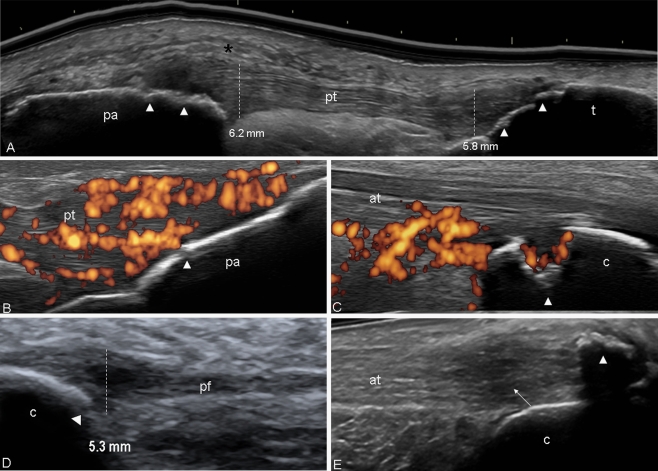
In the late stages, bony cortex changes may be related to the presence of enthesophytes and/or bone erosions (Fig. 6a–d). Enthesophytes large in size may generate acoustic shadowing, which may impair partially or completely the visualization of adjacent bone erosions (Fig. 6e).
Fig. 6.
Psoriatic arthritis. a Patelar tendon using “extended view” technique. Longitudinal view. Thickening of both proximal (6.2 mm) and distal (5.8 mm) entheses of patellar tendon (pt) with evident echotexture disomogeneity, oedema of the peritendineous tissue (black asterisk), and irregularities of the bone profile (white arrowheads). b Distal patellar enthesis. Longitudinal scan. Marked hypoechogenicity and fibrillar separation (due to intratendioneous oedema), generating an increase of the thickness of the enthesis. Note the intense power Doppler signal and the erosion of the cortical bone (white arrowhead) indicating the severity of the inflammatory process. c Achilles tendon. Longitudinal scan. Large erosion of the calcaneous bone (arrowhead) with presence of power Doppler within the tendon and inside the erosion. d Plantar fascia. Longitudinal scan. Thickening of the insertion part of the plantar fascia with associated bone erosions (white arrowhead). e Large enthesophyte generating acoustic shadow which obstructs the complete visualization of the calcaneous bone (white arrowhead). Moreover, note the evident unhomogeneity of the structure of the tendon (arrow). The vertical white line indicates where measurements were taken. The normal value of the both patellar entheses and plantar fascia according the Glasgow Ultrasound Enthesitis Scoring System is 4.1 and 4.4 mm, respectively. pt patellar tendon, pa patella, t tibia, at Achilles tendon, c calcaneous bone, pf plantar fascia
Psoriatic plaque
US features of psoriatic plaque include a wide spectrum of morphostructural changes of both epidermis and dermis and a blood flow increase within the dermis detected by PD technique. The thickening of both epidermis and dermis respect to the surrounding normal skin, and the hypoechoic band under the psoriatic area represent the most common grayscale US findings (Fig. 7a–d). Sometimes, a marked increase of the thickness of the epidermis may generate an evident acoustic shadow limiting the assessment of the underlying dermis (Fig. 8a–c). Different degrees of blood flow within the dermis can be detected by PD.
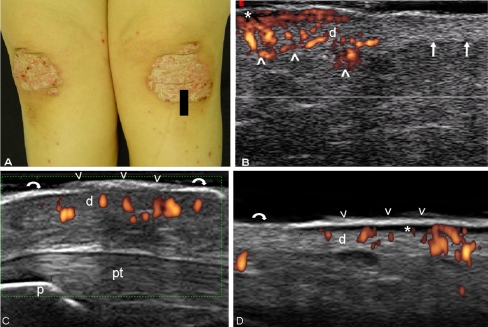
Fig. 7.
a Psoriatic arthritis. Photo of psoriatic plaques at knee level with a black footprint indicating the exact point where the probe was placed. b US image obtained with a Technos partner system equipped with an 8–14 MHz linear transducer. Power Doppler imaging with a Doppler frequency of 12.5 MHz allowed the detection of an increased blood flow at dermis (d) of the psoriatic lesion (arrowheads), not detectable in the surrounding normal skin (arrows). Psoriatic plaque at the anterior (c) knee and abdominal (d) level. Epidermal layer at psoriatic lesion appears thickened and inhomogeneous (arrowheads), whereas in the normal skin, surrounding the plaque, is thin and relatively more homogeneously hyperechoic band (curved arrows). Beneath it is evident a focal hypoechoic thickening of the dermis (d) with evident power Doppler signal revealing increase of blood perfusion in the dermis. Note the hypoechoic band in the upper dermis in correspondence of the psoriatic plaque (asterisk). p lower pole of the patella, pt patellar tendon
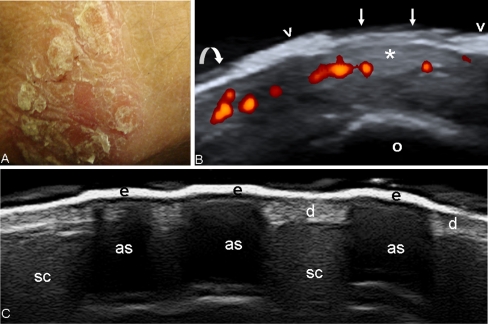
Fig. 8.
Psoriatic arthritis. a Photo of psoriatic plaque at elbow level. b Sonographic image obtained using Logiq 9 US system with a 8–15 MHz linear transducer. Note the marked thickening of the epidermis (arrowheads). Abnormal epidermis is three times thicker than in the normal surrounding skin (curved arrow). Arrows indicate the gap of epidermal layer left by the detachment of a scar. Power Doppler technique allowed the detection of an increased blood perfusion at dermis of the psoriatic lesion. c Psoriatic plaque. Note the acoustic shadows (as) generated by the marked thickening of the epidermis (e). d dermis, sc subcutaneous fat, o olecranon
Onychopathy
The pathological US findings in psoriatic onychopathy include both nail plate and nail bed. In the early stages, a minimal loss of the hyperechoic definition involving only the ventral plate may be observed, whereas the thickening and the fusion of both plates (with loss of the intermediate anechoic layer) are more frequent in the later stages. The nail bed (distance between the ventral plate and the bone margin of the distal phalanx) is usually thickened (>2.5 mm). Contrarily to other anatomical sites, a minimal quantity of blood flow can be detected occasionally in normal conditions within the nail bed (due to presence of thin arterial and venous vessels; Fig. 9a). It increases excessively (easily detectable by PD) when is presence an onychopathy (Fig. 9b–e).
Fig. 9.
a Healthy subject. Note both the typical trilaminar aspect of the nail plate (arrow) and the presence of a physiological amount of power Doppler signal within the nail bed (asterisks). b Psoriatic arthritis. Onychopathy. Grayscale US in the longitudinal scan showing the loss of the normal trilaminar aspect of the nail plate, which appears as a single hyperechoic layer with inhomogeneous thickness (arrow). Nail bed is clearly thickened (asterisk). c Power Doppler US revealing marked signal indicative of an increase of blood flow at the nail bed level. d, e Nail plate (arrows) and nail bed (asterisks) involvement is detectable also on transverse scan. dp distal phalanx, nm nail matrix. US image obtained using a MyLab 70 XVG US system with a 6–18 MHz linear transducer (B-mode frequency of 18 MHz and Doppler frequency of 9.1 MHz)
Discussion
The joint, tendon, enthesis, skin, and nail involvement has been described by the different subsets criteria as aspects to be considered in PsA [2, 22–25]. Recently, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) underlined the value of imaging findings in PsA, from both a dermatologic and rheumatic perspective [26].
In this way, there is a consistent body of evidence supporting both the role of US and its higher sensitivity over clinical examination in the diagnosis of synovitis, enthesitis, and tenosynovitis in PsA [9–15, 20, 21, 27–32]. Relatively uncertain remains its potential in the assessment of skin and nail involvement in these patients.
To date, most of the studies assessing the role of US in psoriatic skin and nail have not used the latest generation of US equipment and have concentrate mainly on pathological findings using only the grayscale technique [7, 33–40]. The images shown in this paper were acquired with “last generation” top quality US equipment provided with high and variable frequency probes and very sensitive PD.
Our results demonstrated that the increase of blood flow in psoriatic plaque and onychopathy, which is due to several dermovascularity changes such as elongation, dilatation, and twisting of the microvessels [41], can be easily detected by high PD frequency.
Considering the common pathogenesis between the angiogenesis of psoriatic plaque and synovial membrane [26], the US could be considered as a powerful method able to provide a widespread and more complete assessment of morphostructural changes and disease activity at different locations such as joint, tendons, entheses, skin, and nail in patients with PsA. Another interesting utility could be the monitoring of treatment at multiple targets, that despite the availability of “new generation” US machines, remains under investigated for this condition [42–44]. Recently, our group demonstrated the ability of US in monitoring of the psoriatic plaque in patients treated with tumor necrosis factor alpha antagonist therapy [6].
Additionally, from the joint US assessment point of view, we noted that the synovial pannus of PsA appears highly hyperemic respect to other chronic inflammatory conditions. It can easily be detected at the small joints level (distal and proximal interphalangeal joints, metacarpophalangeal and metatarsophalangeal joints) using probes with high PD frequency (>10 MHz). Its study results relatively difficult at the large joints level (such as shoulder, knee, and hip), and these, due to their anatomical depth (especially in obese patients), require low frequency probes which decrease the PD sensitivity.
In conclusion, the present report provides update pictorial evidence that high-resolution grayscale US and high-frequency PD allow a detailed assessment of the morphostructural changes and a sensitive detection of abnormal blood flow at multiple sites in patients with PsA. Studies aiming at investigating diagnostic value, validity issues including accuracy, and reproducibility are required to define the impact of these US findings in daily clinical practice.
Acknowledgments
Disclosures
None
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Glad man DD, Antoni C, Mease P, Clegg DO, Nash P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005;64(Suppl 2):ii14–ii17. doi: 10.1136/ard.2004.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGonagle D, Conaghan P, Emery P. Psoriatic arthritis—a unified concept 20 years on. Arthritis Rheum. 1999;42:1080–1086. doi: 10.1002/1529-0131(199906)42:6<1080::AID-ANR2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 3.Mease PJ. Recent advances in the management of psoriatic arthritis. Curr Opin Rheumatol. 2004;16:366–370. doi: 10.1097/01.bor.0000129720.21563.b8. [DOI] [PubMed] [Google Scholar]
- 4.Ritchlin CT. From skin to bone: translational perspectives on psoriatic disease. J Rheumatol. 2008;35:1434–1437. [PubMed] [Google Scholar]
- 5.Grassi W, Filippucci E. Is power Doppler sonography the new frontier in therapy monitoring? Clin Exp Rheumatol. 2003;21:424–428. [PubMed] [Google Scholar]
- 6.Gutierrez M, Filippucci E, Bertolazzi C, Grassi W. Sonographic monitoring of psoriatic plaque. J Rheumatol. 2009;36:850–851. doi: 10.3899/jrheum.080739. [DOI] [PubMed] [Google Scholar]
- 7.Wortsman X, Jemec GB. Ultrasound imaging of nails. Dermatol Clin. 2006;24:323–328. doi: 10.1016/j.det.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 8.Kane D. The role of ultrasound in the diagnosis and management of psoriatic arthritis. Curr Rheumatol Rep. 2005;7:319–324. doi: 10.1007/s11926-005-0043-6. [DOI] [PubMed] [Google Scholar]
- 9.Ory PA, Gladman DD, Mease PJ. Psoriatic arthritis and imaging. Ann Rheum Dis. 2005;64:55–57. doi: 10.1136/ard.2004.033928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan AL, McGonagle D. Imaging of seronegative spondyloarthritis. Best Pract Res Clin Rheumatol. 2008;22:1045–1059. doi: 10.1016/j.berh.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 11.McGonagle D. Imaging the joint and enthesis: insights into pathogenesis of psoriatic arthritis. Ann Rheum Dis. 2005;64(suppl 2):58–60. doi: 10.1136/ard.2004.034264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evangelisto A, Wakefield R, Emery P. Imaging in early arthritis. Best Pract Res Clin Rheumatol. 2004;18:927–943. doi: 10.1016/j.berh.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Weiner SM, Jurenz S, Uhl M, Lange-Nolde A, Warnatz K, Peter HH, Walker UA. Ultrasonography in the assessment of peripheral joint involvement in psoriatic arthritis: a comparison with radiography, MRI and scintigraphy. Clin Rheumatol. 2008;27:983–989. doi: 10.1007/s10067-008-0835-y. [DOI] [PubMed] [Google Scholar]
- 14.Wiell C, Szkudlarek M, Hasselquist M, Møller JM, Vestergaard A, Nørregaard J, Terslev L, Østergaard M. Ultrasonography, magnetic resonance imaging, radiography, and clinical assessment of inflammatory and destructive changes in fingers and toes of patients with psoriatic arthritis. Arthritis Res Ther. 2008;10:402. doi: 10.1186/ar2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fournié B, Margarit-Coll N, Champetier de Ribes TL, Zabraniecki L, Jouan A, Vincent V, Chiavassa H, Sans N, Railhac JJ. Extrasynovial ultrasound abnormalities in the psoriatic finger. Prospective comparative power Doppler study versus rheumatoid arthritis. Joint Bone Spine. 2006;73:527–531. doi: 10.1016/j.jbspin.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 16.Moll JM, Wright V. Psoriatic arthritis. Semin Arthritis Rheum. 1973;3:55–78. doi: 10.1016/0049-0172(73)90035-8. [DOI] [PubMed] [Google Scholar]
- 17.Backhaus M, Burmester GR, Gerber T, Grassi W, Machold KP, Swen WA, Wakefield RJ, Manger B. Working Group for Musculoskeletal Ultrasound in the EULAR Standing Committee on International Clinical Studies including Therapeutic Trials. Guidelines for musculoskeletal ultrasound in rheumatology. Ann Rheum Dis. 2001;60:641–649. doi: 10.1136/ard.60.7.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kane D, Stafford L, Bresnihan B, FitzGerald O. A prospective, clinical and radiological study of early psoriatic arthritis: an early synovitis clinic experience. Rheumatology. 2003;42:1460–1468. doi: 10.1093/rheumatology/keg384. [DOI] [PubMed] [Google Scholar]
- 19.Grassi W, Filippucci E, Farina A, Cervini C. Sonographic imaging of tendons. Arthritis Rheum. 2000;43:969–976. doi: 10.1002/1529-0131(200005)43:5<969::AID-ANR2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 20.Kane D, Greaney T, Bresnihan B, Gibney R, FitzGerald O. Ultrasonography in the diagnosis and management of psoriatic dactylitis. J Rheumatol. 1999;26:1746–1751. [PubMed] [Google Scholar]
- 21.Olivieri I, Barozzi L, Favaro L, Pierro A, de Matteis M, Borghi C, Padula A, Ferri S, Pavlica P. Dactylitis in patients with seronegative spondyloarthropathy. Assessment by ultrasonography and magnetic resonance imaging. Arthritis and Rheumatism. 1996;39:1524–1528. doi: 10.1002/art.1780390912. [DOI] [PubMed] [Google Scholar]
- 22.Bennett RM. Psoriatic arthritis (1979). In: McCarty DJ (ed), Arthritis and related conditions. Philadelphia: Lea & Febiger, p. 645
- 23.Vasey FB, Espinoza LR. Psoriatic arthritis. In: Calin A, editor. Spondyloarthropathies. Orlando: Grune; 1984. pp. 151–185. [Google Scholar]
- 24.Fournie B, Crognier L, Arnaud C, Zabraniecki L, Lascaux-Lefebvre V, Marc V. Proposed classification criteria of psoriatic arthritis. A preliminary study in 260 patients. Rev Rhum Engl Ed. 1999;66:446–456. [PubMed] [Google Scholar]
- 25.Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H, CASPAR Study Group Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2007;56:699–700. doi: 10.1002/art.22412. [DOI] [PubMed] [Google Scholar]
- 26.Coates LC, Anderson RR, Fitzgerald O, Gottlieb AB, Kelly SG, Lubrano E, McGonagle DG, Olivieri I, Ritchlin CT, Tan AL, De Vlam K, Helliwell PS. Clues to the pathogenesis of psoriasis and psoriatic arthritis from imaging: a literature review. J Rheumatol. 2008;35:1438–1442. [PubMed] [Google Scholar]
- 27.D’Agostino MA, Said-Nahal R, Hacquard-Bouder C, Brasseur JL, Dougados M, Breban M. Assessment of peripheral enthesitis in the spondylarthropathies by ultrasonography combined with power Doppler: a cross-sectional study. Arthritis Rheum. 2003;48:523–533. doi: 10.1002/art.10812. [DOI] [PubMed] [Google Scholar]
- 28.Galluzzo E, Lischi DM, Taglione ELF, Pasero G, Perri G. Sonographic analysis of the ankle in patients with psoriatic arthritis. Scan J Rheumatol. 2000;29:52–55. doi: 10.1080/030097400750001806. [DOI] [PubMed] [Google Scholar]
- 29.Gisondi P, Tinazzi I, El-Dalati G, Gallo M, Biasi D, Barbara LM, Girolomoni G. Lower limb enthesopathy in patients with psoriasis without clinical signs of arthropathy: a hospital-based case-control study. Ann Rheum Dis. 2008;67:26–30. doi: 10.1136/ard.2007.075101. [DOI] [PubMed] [Google Scholar]
- 30.Balint PV, Kane D, Wilson H, McInnes IB, Sturrock RD. Ultrasonography of entheseal insertions in the lower limb in spondyloarthropathy. Ann Rheum Dis. 2002;61:905–910. doi: 10.1136/ard.61.10.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Miguel E, Cobo T, Muñoz-Fernández S, Naredo E, Usón J, Acebes JC, Andréu JL, Martín-Mola E. Validity of enthesis ultrasound assessment in spondylarthropathy. Ann Rheum Dis. 2009;68:169–174. doi: 10.1136/ard.2007.084251. [DOI] [PubMed] [Google Scholar]
- 32.Filippucci E, Aydin SZ, Karadag O, Salaffi F, Gutierrez M, Direskeneli H, Grassi W. Reliability of high-resolution ultrasonography in the assessment of Achilles tendon enthesopathy in seronegative spondyloarthropathies (2009). Ann Rheum Dis (Epub ahead of print) [DOI] [PubMed]
- 33.Fornage BD. Sonography of the skin and subcutaneous tissues. Radiol Med. 1993;85:149–155. [PubMed] [Google Scholar]
- 34.Gupta AK, Turnbull DH, Harasiewicz KA. The use of high-frequency ultrasound as a method of assessing the severity of a plaque of psoriasis. Arch Dermatol. 1996;132:658–662. doi: 10.1001/archderm.132.6.658. [DOI] [PubMed] [Google Scholar]
- 35.Vaillant L, Berson M, Machet L, Callens A, Pourcelot L, Lorette G. Ultrasound imaging of psoriatic skin: a non-invasive technique to evaluate treatment of psoriasis. Int J Dermatol. 1994;33:786–790. doi: 10.1111/j.1365-4362.1994.tb00994.x. [DOI] [PubMed] [Google Scholar]
- 36.El Gammal S, El Gammal C, Kaspar K. Sonography of the skin at 100 MHz enables in vivo visualization of stratum corneum and viable epidermis in palmar skin and psoriatic plaques. J Invest Dermatol. 1999;13:821–829. doi: 10.1046/j.1523-1747.1999.00795.x. [DOI] [PubMed] [Google Scholar]
- 37.Olsen LO, Serup J. High-frequency ultrasound scan for non-invasive cross-sectional imaging of psoriasis. Acta Derm Venereol. 1993;73:185–187. doi: 10.2340/0001555573185187. [DOI] [PubMed] [Google Scholar]
- 38.Di Nardo A, Seidenari S, Giannetti A. B-scanning evaluation with image analysis of psoriatic skin. Exp Dermatology. 1992;1:121–125. doi: 10.1111/j.1600-0625.1992.tb00002.x. [DOI] [PubMed] [Google Scholar]
- 39.Murray AK, Herrick AL, Moore TL, King TA, Griffiths CE. Dual wavelength (532 and 633 nm) laser Doppler imaging of plaque psoriasis. Br J Dermatol. 2005;152:1182–1186. doi: 10.1111/j.1365-2133.2005.06479.x. [DOI] [PubMed] [Google Scholar]
- 40.Wortsman XC, Holm EA, Wulf HC, Jemec GB. Real-time spatial compound ultrasound imaging of skin. Skin Res Technol. 2004;10:23–31. doi: 10.1111/j.1600-0846.2004.00048.x. [DOI] [PubMed] [Google Scholar]
- 41.Creamer D, Allen MH, Sousa A, Poston R, Barker JN. Localization of endothelial proliferation and microvascular expansion in active plaque psoriasis. Br J Dermatol. 1997;136:859–865. doi: 10.1111/j.1365-2133.1997.tb03925.x. [DOI] [PubMed] [Google Scholar]
- 42.Fiocco U, Cozzi L, Rubaltelli L, Rigon C, De Candia A, Tregnaghi A, Gallo C, Favaro MA, Chieco-Bianchi F, Baldovin M, Todesco S. Long-term sonographic follow-up of rheumatoid and psoriatic proliferative knee joint synovitis. Br J Rheumatol. 1996;35:155–163. doi: 10.1093/rheumatology/35.2.155. [DOI] [PubMed] [Google Scholar]
- 43.Fiocco U, Cozzi L, Chieco-Bianchi F, Rigon C, Vezzù M, Favero E, Ferro F, Sfriso P, Rubaltelli L, Nardacchione R, Todesco S. Vascular changes in psoriatic knee synovitis. J Rheumatol. 2001;28:2480–2486. [PubMed] [Google Scholar]
- 44.Fiocco U, Ferro F, Cozzi L, Vezzù M, Sfriso P, Checchetto C, Bianchi FC, Nardacchione R, Piccoli A, Todesco S, Rubaltelli L. Contrast medium in power Doppler ultrasound for assessment of synovial vascularity: comparision with arthoscopy. J Rheumatol. 2003;30:2170–2176. [PubMed] [Google Scholar]
- 45.Grassi W, Cervini C. Ultrasonography in rheumatology: an evolving technique. Ann Rheum Dis. 1998;57:268–271. doi: 10.1136/ard.57.5.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grassi W, Filippucci E, Carotti M, Salaffi F. Imaging modalities for identifying the origin of regional musculoskeletal pain. Best Pract Res Clin Rheumatol. 2003;17:17–32. doi: 10.1016/S1521-6942(02)00099-2. [DOI] [PubMed] [Google Scholar]
- 47.Wakefield RJ, Balint P, Szkudlarek M, Filippucci E, Backhaus M, D’Agostino MA, Sanchez EN, Iagnocco A, Schmidt WA, Bruyn GA, Kane D, O’Connor PJ, Manger B, Joshua F, Koski J, Grassi W, Lassere MN, Swen N, Kainberger F, Klauser A, Ostergaard M, Brown AK, Machold KP, Conaghan PG. OMERACT 7 Special Interest Group. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol. 2005;32:2485–2487. [PubMed] [Google Scholar]