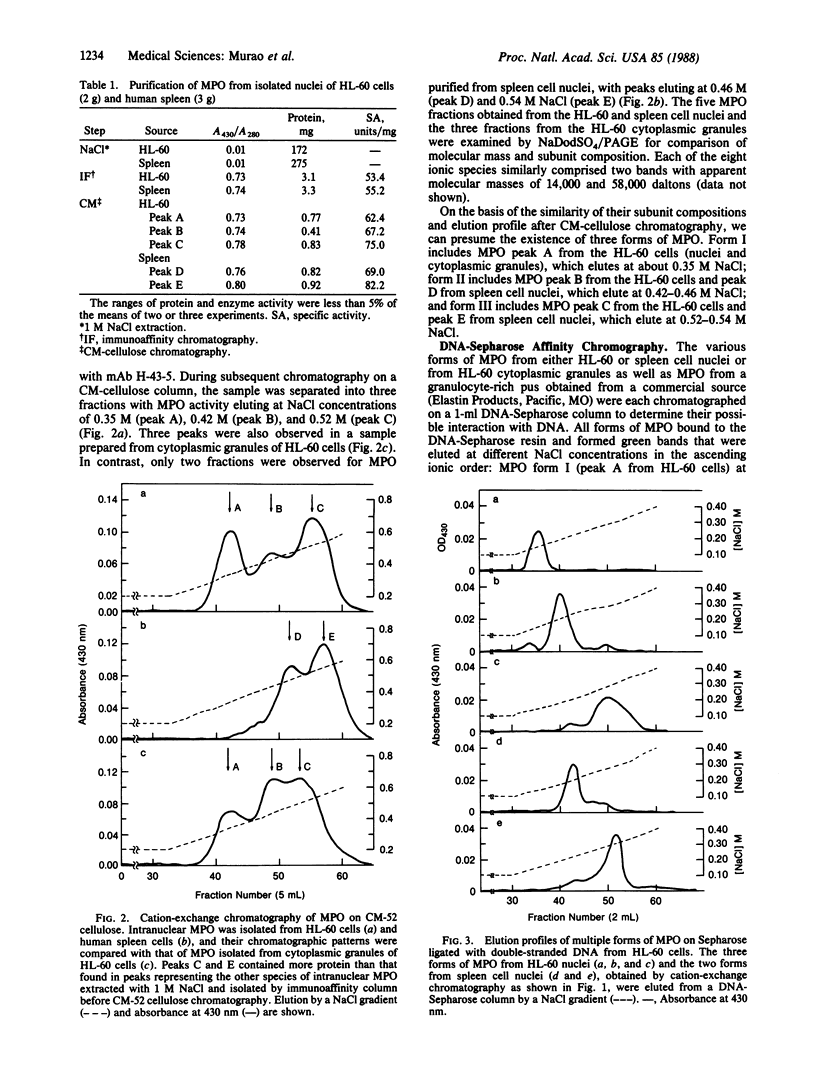
Abstract
An antigen histochemically localized in the nuclei and cytoplasmic granules of normal and leukemic human myeloid cells has been identified as myeloperoxidase (MPO; EC 1.11.1.7). The localization and amount of the enzyme was determined by using a murine monoclonal antibody designated H-43-5 raised against nuclear proteins derived from human promyelocytic HL-60 leukemia cells. The highest amount of nuclear MPO (3.5 micrograms per 10(6) nuclei) was found in granulocytes; less than half of this amount was detected in nuclei from HL-60 cells. Still lower levels were found in nuclei from monocytes and a series of human monomyelocytic leukemia cells. MPO from HL-60 cells was purified by immunoaffinity chromatography and fractionated into three components (forms I, II, and III) by CM-cellulose chromatography. Chromatography of these MPO forms on DNA-Sepharose columns confirmed that all three forms of MPO were tightly bound to DNA with apparent relative affinities in the order of form III greater than form II greater than form I. The affinity of MPO form III for DNA was sufficient to enable the formation and elution of DNA-MPO complexes during size-exclusion chromatography at high ionic strength and neutral pH. This form of MPO was also able to shield DNA from strand scission induced by active oxygen species generated by xanthine oxidase acting aerobically on xanthine. These data suggest that intranuclear MPO may help to protect DNA against damage resulting from oxygen radicals produced during myeloid cell maturation and function.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arndt-Jovin D. J., Jovin T. M., Bähr W., Frischauf A. M., Marquardt M. Covalent attachment of DNA to agarose. Improved synthesis and use in affinity chromatography. Eur J Biochem. 1975 Jun;54(2):411–418. doi: 10.1111/j.1432-1033.1975.tb04151.x. [DOI] [PubMed] [Google Scholar]
- Babior B. M. The respiratory burst of phagocytes. J Clin Invest. 1984 Mar;73(3):599–601. doi: 10.1172/JCI111249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Boxer L. A., Allen J. M., Davis J. Autooxidation as a basis for altered function by polymorphonuclear leukocytes. Blood. 1977 Aug;50(2):327–335. [PubMed] [Google Scholar]
- Baggiolini M., Hirsch J. G., De Duve C. Resolution of granules from rabbit heterophil leukocytes into distinct populations by zonal sedimentation. J Cell Biol. 1969 Feb;40(2):529–541. doi: 10.1083/jcb.40.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton D. F., Farquhar M. G. Differences in enzyme content of azurophil and specific granules of polymorphonuclear leukocytes. I. Histochemical staining of bone marrow smears. J Cell Biol. 1968 Nov;39(2):286–298. doi: 10.1083/jcb.39.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C. DNA strand breakage in human leukocytes exposed to a tumor promoter, phorbol myristate acetate. Science. 1982 Mar 5;215(4537):1247–1249. doi: 10.1126/science.6276978. [DOI] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawn K., Fridovich I. DNA strand scission by enzymically generated oxygen radicals. Arch Biochem Biophys. 1981 Feb;206(2):414–419. doi: 10.1016/0003-9861(81)90108-9. [DOI] [PubMed] [Google Scholar]
- Deimann W., Seitz M., Gemsa D., Fahimi H. D. Endogenous peroxidase in the nuclear envelope and endoplasmic reticulum of human monocytes in vitro: association with arachidonic acid metabolism. Blood. 1984 Aug;64(2):491–498. [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E., Jarvis H. M. Nitrocellulose-based assays for the detection of glycolipids and other antigens: mechanism of binding to nitrocellulose. J Immunol Methods. 1985 Oct 24;83(1):113–123. doi: 10.1016/0022-1759(85)90064-x. [DOI] [PubMed] [Google Scholar]
- Homma Y., Henning-Chubb C. B., Huberman E. Translocation of protein kinase C in human leukemia cells susceptible or resistant to differentiation induced by phorbol 12-myristate 13-acetate. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7316–7319. doi: 10.1073/pnas.83.19.7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Oxygen metabolism and the toxic properties of phagocytes. Ann Intern Med. 1980 Sep;93(3):480–489. doi: 10.7326/0003-4819-93-3-480. [DOI] [PubMed] [Google Scholar]
- Koeffler H. P., Ranyard J., Pertcheck M. Myeloperoxidase: its structure and expression during myeloid differentiation. Blood. 1985 Feb;65(2):484–491. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Murao S. I., Fukumoto Y., Katayama M., Maeda H., Sugiyama T., Huberman E. Recombinant gamma-interferon and lipopolysaccharide enhance 1,25-dihydroxyvitamin D3-induced cell differentiation in human promyelocytic leukemia (HL-60) cells. Jpn J Cancer Res. 1985 Jul;76(7):596–602. [PubMed] [Google Scholar]
- Murao S., Epstein A. L., Clevenger C. V., Huberman E. Expression of maturation-specific nuclear antigens in differentiating human myeloid leukemia cells. Cancer Res. 1985 Feb;45(2):791–795. [PubMed] [Google Scholar]
- Newburger P. E., Chovaniec M. E., Cohen H. J. Activity and activation of the granulocyte superoxide-generating system. Blood. 1980 Jan;55(1):85–92. [PubMed] [Google Scholar]
- O'Brien P. J. Free-radical-mediated DNA binding. Environ Health Perspect. 1985 Dec;64:219–232. doi: 10.1289/ehp.8564219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pember S. O., Shapira R., Kinkade J. M., Jr Multiple forms of myeloperoxidase from human neutrophilic granulocytes: evidence for differences in compartmentalization, enzymatic activity, and subunit structure. Arch Biochem Biophys. 1983 Mar;221(2):391–403. doi: 10.1016/0003-9861(83)90158-3. [DOI] [PubMed] [Google Scholar]
- Pryzwansky K. B., Rausch P. G., Spitznagel J. K., Herion J. C. Immunocytochemical distinction between primary and secondary granule formation in developing human neutrophils: correlations with Romanowsky stains. Blood. 1979 Feb;53(2):179–185. [PubMed] [Google Scholar]
- Roth J., Bendayan M., Orci L. Ultrastructural localization of intracellular antigens by the use of protein A-gold complex. J Histochem Cytochem. 1978 Dec;26(12):1074–1081. doi: 10.1177/26.12.366014. [DOI] [PubMed] [Google Scholar]
- Stevens F. J. Analysis of protein-protein interaction by simulation of small-zone size-exclusion chromatography: application to an antibody-antigen association. Biochemistry. 1986 Mar 11;25(5):981–993. doi: 10.1021/bi00353a006. [DOI] [PubMed] [Google Scholar]
- Svensson B. E., Domeij K., Lindvall S., Rydell G. Peroxidase and peroxidase-oxidase activities of isolated human myeloperoxidases. Biochem J. 1987 Mar 15;242(3):673–680. doi: 10.1042/bj2420673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsan M. F. Phorbol myristate acetate induced neutrophil autotoxicity. J Cell Physiol. 1980 Nov;105(2):327–334. doi: 10.1002/jcp.1041050215. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., LoBuglio A. F. Phagocyte-generated oxygen metabolites and cellular injury. Lab Invest. 1982 Jul;47(1):5–18. [PubMed] [Google Scholar]
- Weitzman S. A., Stossel T. P. Mutation caused by human phagocytes. Science. 1981 May 1;212(4494):546–547. doi: 10.1126/science.6259738. [DOI] [PubMed] [Google Scholar]
- Yamada M., Mori M., Sugimura T. Purification and characterization of small molecular weight myeloperoxidase from human promyelocytic leukemia HL-60 cells. Biochemistry. 1981 Feb 17;20(4):766–771. doi: 10.1021/bi00507a018. [DOI] [PubMed] [Google Scholar]





