Abstract
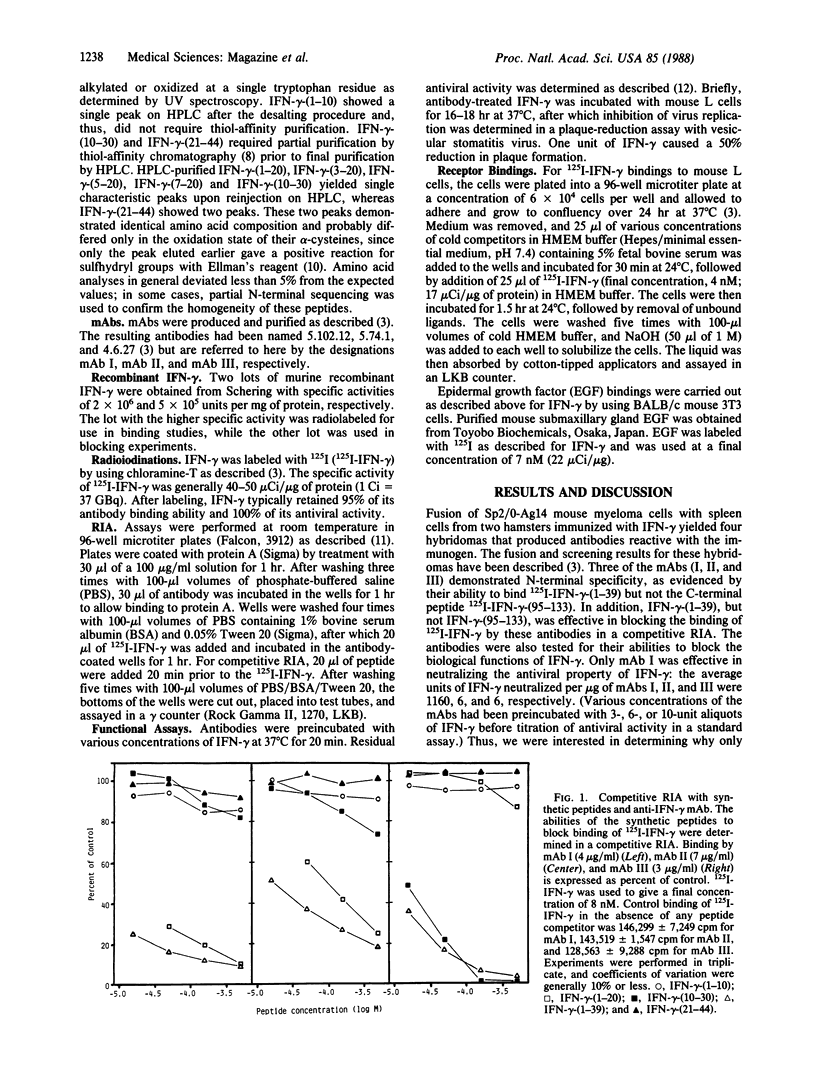
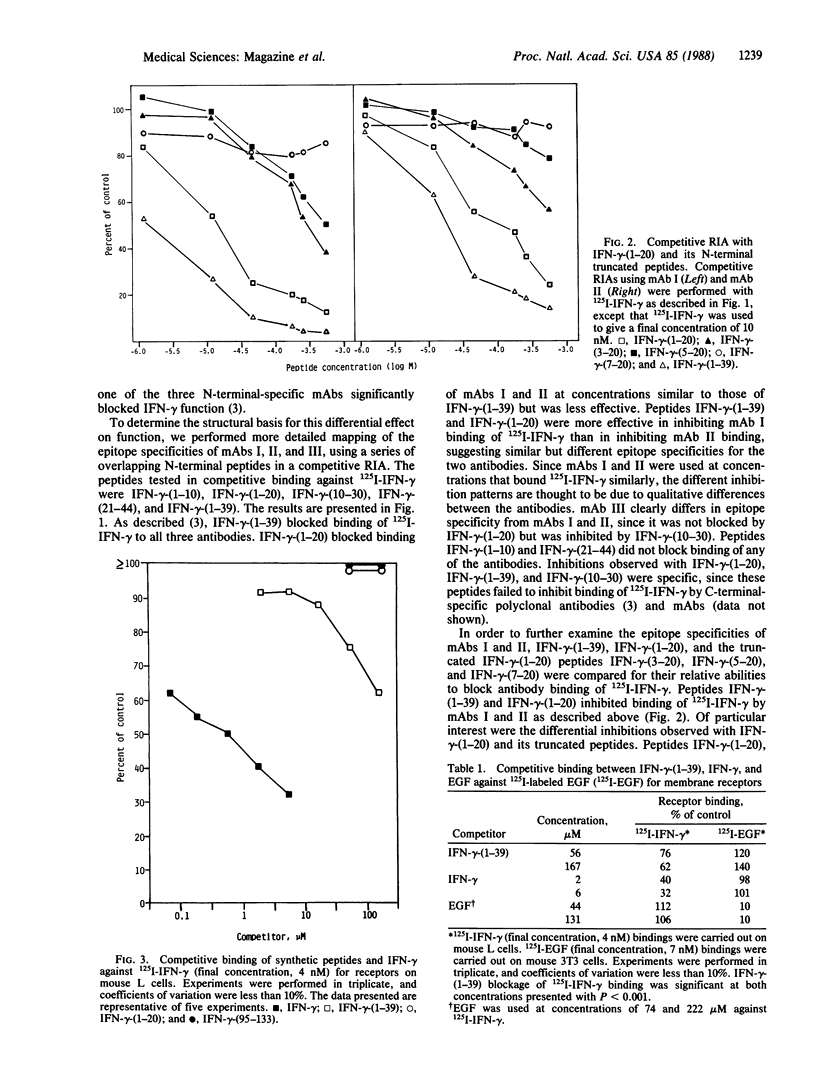
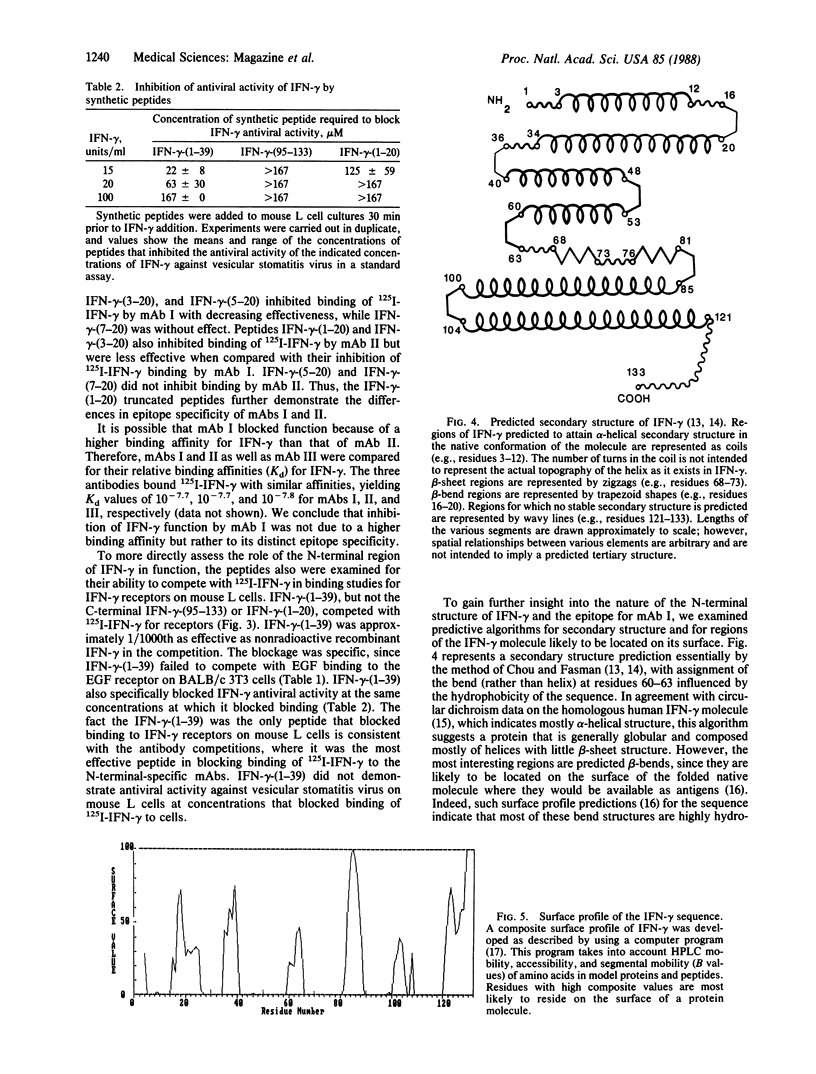
We previously have assigned N-terminal specificity to three hamster monoclonal antibodies (mAbs I, II, and III) produced to mouse recombinant gamma interferon (IFN-gamma), based on the ability of the N-terminal peptide IFN-gamma-(1-39) to block binding of 125I-labeled IFN-gamma (125I-IFN-gamma) and on the ability of these antibodies to bind 125I-IFN-gamma-(1-39). Only mAb I blocked function and binding to the IFN-gamma receptor, suggesting that it may bind to a region of the molecule involved in interaction with the receptor. To further define the epitope specificities of the antibodies, a series of N-terminal peptides were synthesized and tested for their ability to block antibody binding of 125I-IFN-gamma. Peptides IFN-gamma-(1-39), IFN-gamma-(1-20), IFN-gamma-(3-20), and IFN-gamma-(5-20) inhibited binding of 125I-IFN-gamma by mAb I in order of decreasing effectiveness, while peptide IFN-gamma-(7-20) was without effect. Peptides IFN-gamma-(1-39), IFN-gamma-(1-20), and IFN-gamma-(3-20) also inhibited binding of 125I-IFN-gamma by mAb II but were less effective when compared with their inhibition of mAb I. IFN-gamma-(5-20) and IFN-gamma-(7-20) did not inhibit binding by mAb II. Peptides IFN-gamma-(1-10), IFN-gamma-(10-30), and IFN-gamma-(21-44) did not inhibit either mAb I or mAb II. While IFN-gamma-(1-39) and IFN-gamma-(10-30) inhibited binding by mAb III, neither IFN-gamma-(1-20) nor any of its truncated forms were inhibitory. All three antibodies had similar Kd values for 125I-IFN-gamma. A prediction of the secondary structure of the molecule and the peptide inhibition data suggest that the epitope (possible receptor binding region) for mAb I involves a loop in the area containing residues 12-20, with sequences N-terminal to these residues possibly stabilizing the loop conformation. Direct evidence that the N-terminal 1-39 region of IFN-gamma is important in receptor binding was the observation that IFN-gamma-(1-39), but not the C-terminal IFN-gamma-(95-133), competed with 125I-IFN-gamma for the receptor on mouse L cells. IFN-gamma-(1-39) also specifically blocked IFN-gamma antiviral activity at concentrations that blocked binding to the receptor.The fact that IFN-gamma-(1-39) was the only peptide that blocked both IFN-gamma binding to receptor and function is consistent with the antibody competition data, where it was the most effective peptide in blocking binding of 125I-IFN-gamma by the N-terminal-specific mAbs. The combination of peptide mapping of epitope specificities and receptor competition should further help define the structural basis for IFN-gamma action.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiyer R. A., Serrano L. E., Jones P. P. Interferon-gamma binds to high and low affinity receptor components on murine macrophages. J Immunol. 1986 May 1;136(9):3329–3334. [PubMed] [Google Scholar]
- Amit A. G., Mariuzza R. A., Phillips S. E., Poljak R. J. Three-dimensional structure of an antigen-antibody complex at 2.8 A resolution. Science. 1986 Aug 15;233(4765):747–753. doi: 10.1126/science.2426778. [DOI] [PubMed] [Google Scholar]
- Arakawa T., Hsu Y. R., Parker C. G., Lai P. H. Role of polycationic C-terminal portion in the structure and activity of recombinant human interferon-gamma. J Biol Chem. 1986 Jun 25;261(18):8534–8539. [PubMed] [Google Scholar]
- Chandler C. E., Parsons L. M., Hosang M., Shooter E. M. A monoclonal antibody modulates the interaction of nerve growth factor with PC12 cells. J Biol Chem. 1984 Jun 10;259(11):6882–6889. [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Beta-turns in proteins. J Mol Biol. 1977 Sep 15;115(2):135–175. doi: 10.1016/0022-2836(77)90094-8. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Hsu Y. R., Arakawa T. Structural studies on acid unfolding and refolding of recombinant human interferon gamma. Biochemistry. 1985 Dec 31;24(27):7959–7963. doi: 10.1021/bi00348a018. [DOI] [PubMed] [Google Scholar]
- Krieger D. E., Erickson B. W., Merrifield R. B. Affinity purification of synthetic peptides. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3160–3164. doi: 10.1073/pnas.73.9.3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford M. P., Weigent D. A., Chan T. S., Johnson H. M., Stanton G. J. Antibodies to the carboxyl terminus of mouse interferon-gamma neutralize its immunoregulatory and antiviral activities. J Interferon Res. 1987 Feb;7(1):95–101. doi: 10.1089/jir.1987.7.95. [DOI] [PubMed] [Google Scholar]
- Langford M. P., Weigent D. A., Stanton G. J., Baron S. Virus plaque-reduction assay for interferon: microplaque and regular macroplaque reduction assays. Methods Enzymol. 1981;78(Pt A):339–346. doi: 10.1016/0076-6879(81)78139-4. [DOI] [PubMed] [Google Scholar]
- Leszczynski J. F., Rose G. D. Loops in globular proteins: a novel category of secondary structure. Science. 1986 Nov 14;234(4778):849–855. doi: 10.1126/science.3775366. [DOI] [PubMed] [Google Scholar]
- Merrifield B. Solid phase synthesis. Science. 1986 Apr 18;232(4748):341–347. doi: 10.1126/science.3961484. [DOI] [PubMed] [Google Scholar]
- Parker J. M., Guo D., Hodges R. S. New hydrophilicity scale derived from high-performance liquid chromatography peptide retention data: correlation of predicted surface residues with antigenicity and X-ray-derived accessible sites. Biochemistry. 1986 Sep 23;25(19):5425–5432. doi: 10.1021/bi00367a013. [DOI] [PubMed] [Google Scholar]
- Russell J. K., Hayes M. P., Carter J. M., Torres B. A., Dunn B. M., Russell S. W., Johnson H. M. Epitope and functional specificity of monoclonal antibodies to mouse interferon-gamma: the synthetic peptide approach. J Immunol. 1986 May 1;136(9):3324–3328. [PubMed] [Google Scholar]
- Schreiber R. D., Hicks L. J., Celada A., Buchmeier N. A., Gray P. W. Monoclonal antibodies to murine gamma-interferon which differentially modulate macrophage activation and antiviral activity. J Immunol. 1985 Mar;134(3):1609–1618. [PubMed] [Google Scholar]
- Strong D. D., Laudano A. P., Hawiger J., Doolittle R. F. Isolation, characterization, and synthesis of peptides from human fibrinogen that block the staphylococcal clumping reaction and construction of a synthetic clumping particle. Biochemistry. 1982 Mar 16;21(6):1414–1420. doi: 10.1021/bi00535a048. [DOI] [PubMed] [Google Scholar]


