Abstract
Varicella-zoster virus (VZV) glycoprotein E (gE) interacts with glycoprotein I and with insulin degrading enzyme (IDE), which is a receptor for the virus. We found that a VZV gE deletion mutant could only be grown in cells expressing gE. Expression of VZV gE on the surface of cells did not interfere with VZV infection. HSV deleted for gE is impaired for cell-to-cell spread; VZV gE could not complement this activity in an HSV gE null mutant. VZV lacking the IDE binding domain of gE grew to peak titers nearly equivalent to parental virus; however, it was impaired for cell-to-cell spread and for infectivity with cell-free virus. VZV deleted for a region of gE that binds glycoprotein I could not replicate in cell culture unless grown in cells expressing gE. We conclude that the IDE binding domain is important for efficient cell-to-cell spread and infectivity of cell-free virus.
Keywords: glycoprotein E, varicella-zoster virus, herpes simplex virus, glycoprotein I, insulin degrading enzyme
Introduction
Varicella-zoster virus (VZV) causes chickenpox and shingles. The virus expresses at least seven glycoproteins. Glycoprotein B (gB) binds to cellular heparan sulfate proteoglycans (Jacquet et al., 1998), and is likely important for entry of VZV into the cell. gC is not required for growth in virus in vitro (Cohen and Seidel, 1994), but is critical for replication in fetal human skin in the SCID-hu mouse (Moffat et al., 1998). gH facilitates cell-to-cell spread of virus (Rodriquez et al, 1993). gL acts a chaperone for transport of gH to the cell surface (Duus et al., 1995). gI forms a complex with gE, and while gI is not required for replication of VZV in some cells, gI is required for VZV replication in Vero cells, T cells, and human skin (Cohen and Nguyen, 1997; Moffat et al., 2002). gK is required for replication of VZV and may have a role in syncytia formation (Mo et al., 1999), while gM is important for cell-to-cell spread. (Yamagishi et al., 2008).
gE is the most abundant glycoprotein on the surface of VZV-infected cells (Montalvo et al., 1985). gE is also present in the cytoplasm of infected cells and on the surface of virions. gE binds to the Fc portion of human immunoglobulin (Litwin et al., 1992). Epithelial cells expressing gE show increased cell-to-cell contact with enhanced trans-epithelial resistance (Mo et al., 2000). During infection, gE is taken up by endocytosis from the plasma membrane and incorporated into virions (Maresova et al., 2005). The ORF47 protein kinase phosphorylates gE, resulting in transport of endocytosed gE to the trans-Golgi network (Kenyon et al., 2002). gE forms a heterodimer with VZV gI (Kimura et al., 1997) which enhances the ability of gE to serve as an Fc receptor (Litwin et al., 1992). The SP1 transcription factor is important for regulating expression of VZV gE (Berarducci et al., 2007).
gE binds to insulin degrading enzyme (IDE) which serves as a receptor for the virus (Li et al., 2006). IDE is important for infection of cell-free VZV, and for cell-to-cell spread of the virus in vitro. A portion of the amino terminus of gE (amino acids 32–71) is required for binding to IDE and a region within in the first third of gE (amino acids 163-208) is important for its ability to form a complex with gI (Li et al., 2007).
A gE mutant isolated from patients with varicella showed enhanced cell-to-cell spread (Santos et al., 2000). While VZV deleted for gE failed to replicate in vitro, the latter was not complemented in a cell line, and thus it was not formally shown to be essential (Mo et al., 2002). Viable VZV mutants could be constructed in a gE domain (amino acids 568 to 571- AYRV) that directs the protein to the trans-Golgi network, but not in a domain (amino acids 582 to 585, YAGL) that mediates endocytosis of the gE (Moffat et al., 2004). Deletion of amino acids 27–51 of gE resulted in a virus that was not impaired for growth in cell culture, deletion of amino acids 51 to 187 of gE yielded a virus that was impaired for replication in vitro, while deletion of amino acids 27–187 resulted in a virus that could not replicate in cell culture (Berarducci et al., 2006).
We have constructed deletion mutants of VZV lacking the IDE binding domain of gE, a gI binding domain of gE, and nearly the entire open reading frame of gE; the latter two mutants could only be grown in cells expressing gE. We also determine if VZV gE expressed in mammalian cells can complement the growth of an HSV-1 gE deletion mutant or inhibit the growth of VZV.
Results
VZV gE is required for virus replication
Cosmid VZV MstII A-68D is deleted for amino acids 1 to 469 of the 623 amino acid gE (Fig. 1). Transfection of melanoma cells with plasmid pCMV62 and cosmids NotI A, Not IB, Mst IIA, and Mst IIB resulted in infectious VZV 7 days after later, while transfection of cells with plasmid pCMV62 and cosmids NotI A, Not IB, Mst IIB, and Mst IIA-68D failed to yield CPE despite multiple transfections.
Fig. 1.
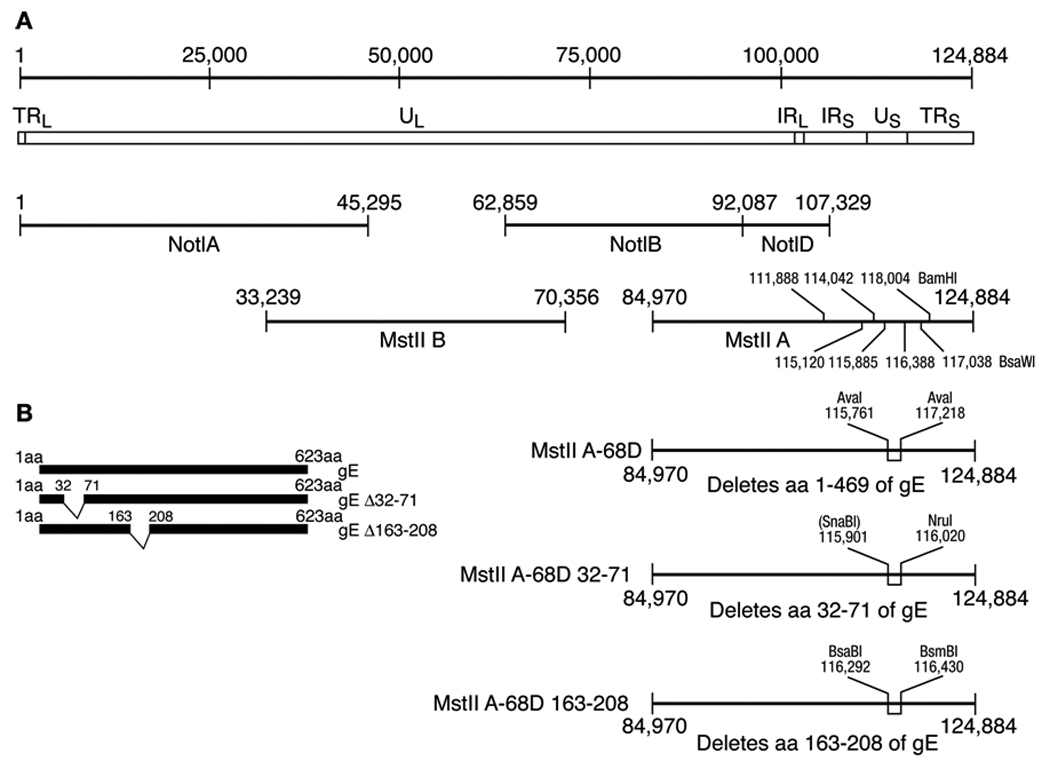
Construction of recombinant VZV deleted for gE and for the IDE and gI binding domains of gE. (A) The prototype VZV strain Dumas genome is 124,884 bp in length (line 1), with unique long (UL), unique short (US) and internal repeat (IR) and terminal repeat (TR) regions (line 2). Four cosmids encompass the VZV genome (lines 3, 4). Selected BamHI and BsaWI restriction sites used in Southern blots of virion DNA are shown in cosmid MstII A. Cosmid MstII A-68D is deleted for codons 1–469 of ORF68 which encodes glycoprotein E (line 5). Cosmid MstIIA-68D32-71 is deleted for codons 32 to 71 which contain the IDE binding domain of gE (line 6). Cosmid MstIIA-68D163-208 is deleted for codons 163-208 which encode the gI binding domain of gE (line 7). (B) The structures of VZV gE, gEΔ32-71, and gEΔ163-208 are shown.
Baculovirus expressing gE was constructed so that melanoma cells could be infected and express the glycoprotein to complement a gE deletion mutant virus. Baculo gE-RV contains gE driven by the human CMV IE promoter. Lysates of Sf9 cells infected with Baculo gE-RV and immunoblotted for gE did not show evidence of gE expression (Fig. 2A, lane 2). Lysates of melanoma cells infected with the virus also failed to show expression of gE (Fig. 2A, lane 3). Since sodium butyrate, a histone deacetylase inhibitor, enhances expression of foreign genes expressed by baculoviruses in mammalian cells (Condreay et al., 1999), we treated Baculo gE-RV-infected cells with sodium butyrate, and detected gE (Fig. 2A, lane 4). Similarly, when cells were infected with Baculo gE-RV and then infected with VZV deleted for gE (see below) the glycoprotein was detected (Fig. 2A, lane 7). The size of gE in cells infected with Baculo gE-RV was similar to that of cells infected with VZV (Fig. 2A, lane 8).
Fig. 2.
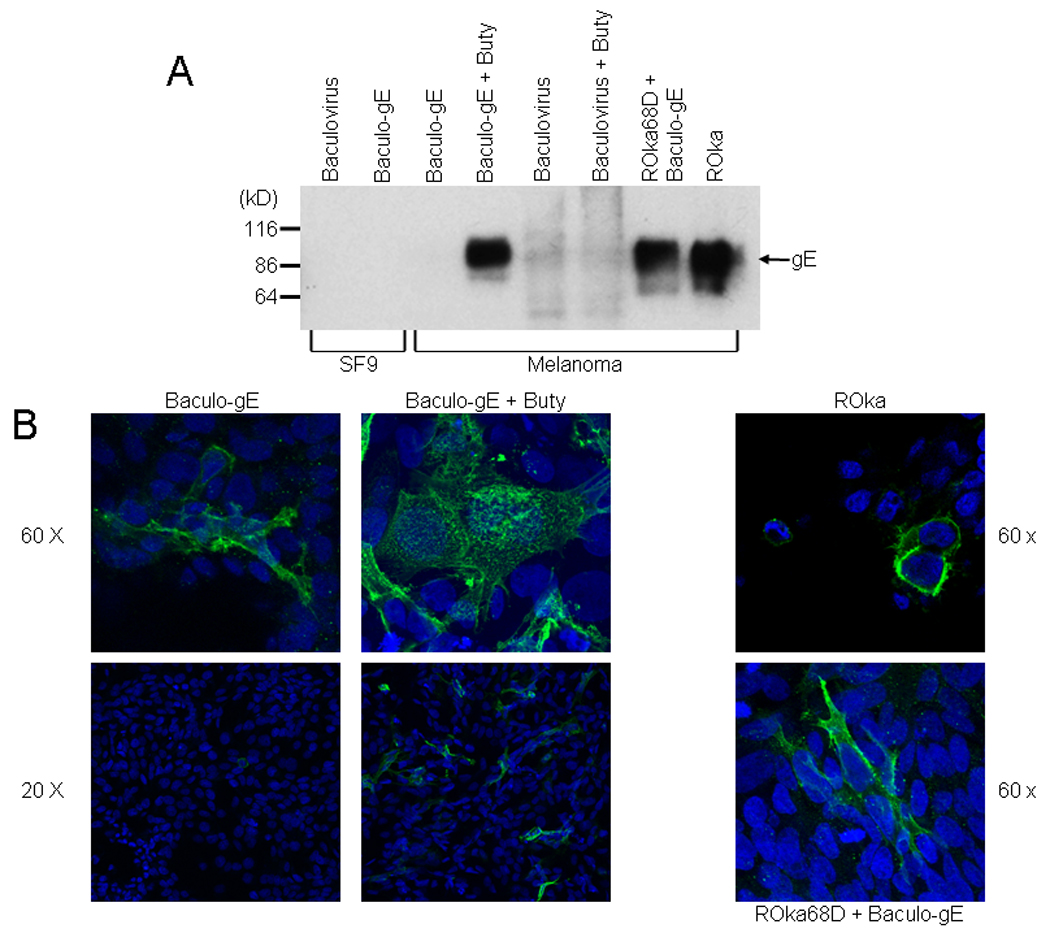
Expression of VZV gE in cells infected with a baculovirus expression vector. (A) Expression of VZV gE in Sf9 or melanoma cells. Sf9 cells were infected with parental baculovirus AcNPV (lane 1) or Baculo gE-RV (lane 2). Melanoma cells were infected with Baculo gE-RV (lanes 3,4) or parental baculovirus (lanes 5,6) in the absence (lanes 3,5) or presence (lanes 4,6) of sodium butyrate (buty). Melanoma cells were infected with Baculo gE-RV in the absence of butyrate followed by VZV ROka68D (lane 7) or with VZV ROka (lane 8). (B) Immunofluorescence microscopy for VZV gE in cells infected with VZV ROka, Baculo gE-RV alone, Baculo gE-RV and sodium butyrate, or Baculo gE-RV followed by ROka68D. Nuclei are stained with DAPI.
gE was present predominantly on the surface of melanoma cells infected with VZV ROka, and on the surface and in the cytoplasm of cells infected with Baculo gE-RV (in the absence of gI) (Fig 2B). Addition of butyrate to cells infected with Baculo gE-RV resulted in a marked increase in expression of gE. Cells infected with Baculo gE-RV and VZV deleted for gE (see below) showed gE predominantly on the surface with some gE also in the cytoplasm.
Melanoma cells were infected with baculovirus expressing gE 1 hr before transfection. Infection of cells with Baculo gE-StuI (which contains VZV gE driven by the baculovirus polyhedron promoter and the CMV IE promoter) followed by transfection with plasmid pCMV62 and cosmids NotI A, Not IB, Mst IIB, and Mst IIA-68D resulted in infectious VZV 11 days later in one experiment, and 20 days later in a second experiment. Infection of melanoma cells with Baculo gE-RV followed by transfection with pCMV62 (which expresses the IE62 protein and enhances the efficiency of the transfection) and the four cosmids (including the ORF68 deletion mutant cosmid) yielded VZV after 11 days in one experiment and 17 days in the second experiment. Virus resulting from the first infection with Baculo gE-RV was termed ROka68D and was chosen for use in subsequent experiments. The virus was passaged in cells that had been infected with Baculo gE-RV one day prior to infection with VZV.
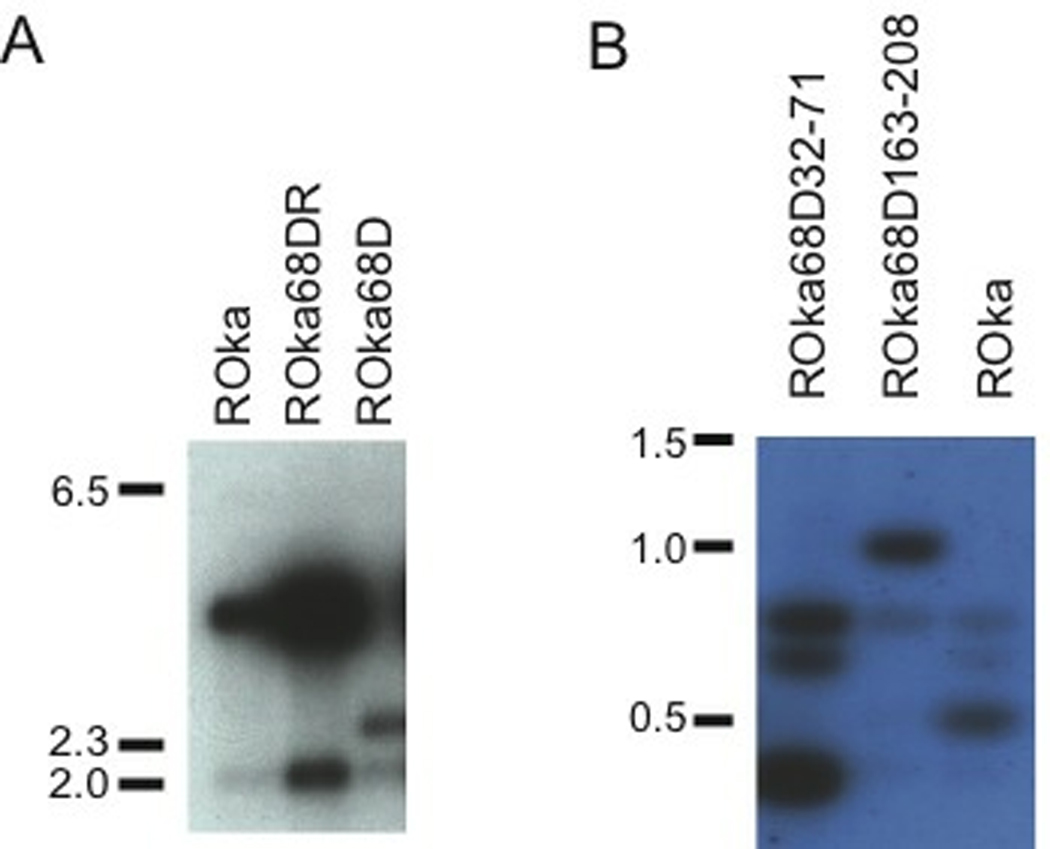
To verify that ROka68D had the expected genome structure, virion DNA was cut with BamHI and Southern blotting was performed using probes for gE. Digestion of ROka68D resulted in two bands of 2.5 and 2.1 kb, while digestion of ROka yielded bands of 4.0 and 2.1 (Fig. 3A). PCR was performed, using primers that span the gE deletion in ROka68D, and sequence analysis showed that ROka68D had the expected deletion in gE.
Fig. 3.
Southern blots of VZV virion DNAs from cells infected with gE deletion mutants. (A) Cells were infected with ROka, gE rescued mutant (ROka68DR), or ROka68D VZV DNA. Virion DNA was cut with BamHI and hybridized with a probe corresponding to gE. (B) Virion DNA from cells infected with ROka68D32-71, ROka68D163-208, or ROka were cut with BsaWI and hybridized with a probe corresponding to a portion of gE. Markers indicate the size of DNAs in kilobase pairs.
Immunoblotting of cells infected with Baculo gE-RV and ROka68D using monoclonal antibody to gE yielded bands of 60 to 90 kDa (Fig. 4A). A band of 52 kDa was detected using antibody to VZV IE4. After one passage in melanoma cells without Baculo gE-RV, the level of gE was reduced while the amount of viral IE4 was not diminished (Fig 4B).
Fig. 4.
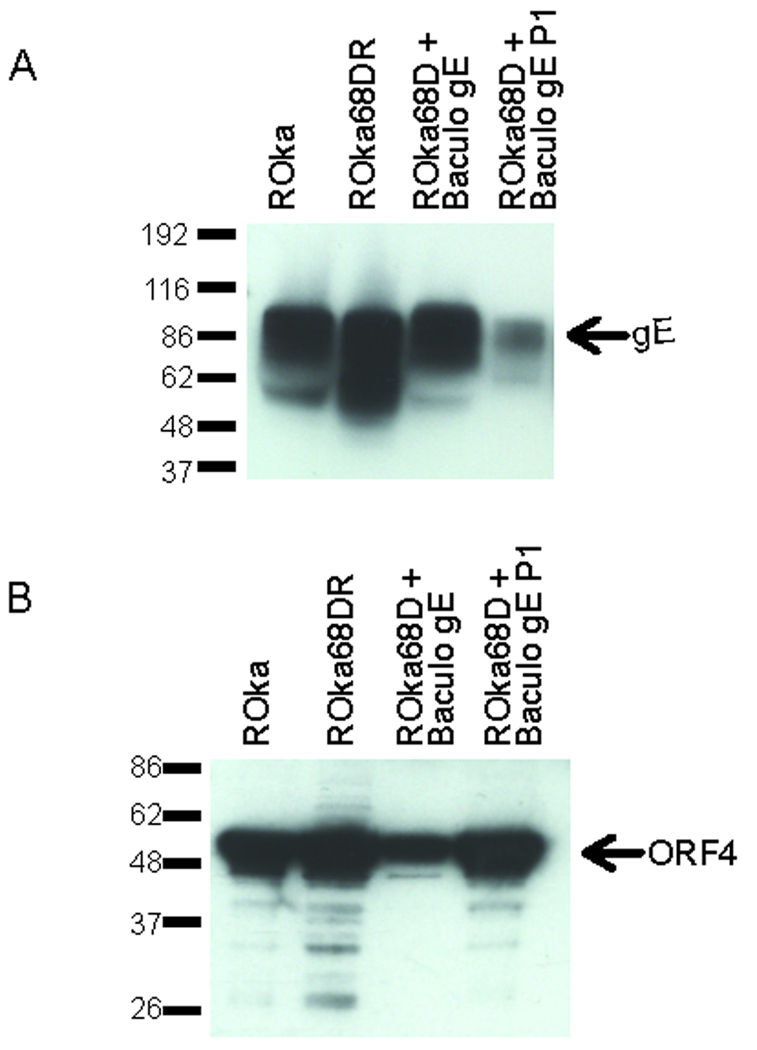
Immunoblot of gE expression in lysates from cells infected with ROka, ROka68DR, or ROka68D in the presence (lane 3) or absence of Baculo gE-RV (lane 4). (A) The same lysates in panel A were probed with antibody to VZV IE4. (B).
Rescue of the gE deletion restores infectivity to VZV
To verify that the deletion in ORF68 was responsible for the lack of infectivity of ROka68D in MeWo cells, rather than a mutation at another site in the genome, we constructed a rescued virus. MeWo cells were cotransfected with VZV ROka68D virion DNA and a 7.5 kb DNA fragment containing ORF68. Rescued virus was isolated by serial cycles of plaque purification, and monitored by PCR of viral DNA using primers that amplify the region that spans the deletion. After multiple cycles of plaque purification a virus termed ROka68DR was isolated.
Southern blotting of virion DNA from ROka68DR-infected cells showed two bands of 4.0 and 2.1 kb similar to the pattern seen with ROka (Fig. 3A). To verify that the rescued virus expressed gE, lysates of melanoma cells infected with ROka68DR were prepared and an immunoblot was performed using antibody to gE. Similar levels of gE were detected in cells infected with VZV ROka and ROka68DR (Fig. 4A).
Growth of ROka68D and ROka68DR in cell culture
Melanoma cells were infected with ROka68D, ROka68DR, and ROka and for the next 5 days, the titers of virus in infected cells were determined. ROka and ROka68DR grew to similar titers in vitro, while ROka68D was unable to replicate in melanoma cells in the absence of Baculo gE-RV (Fig. 5A).
Fig 5.
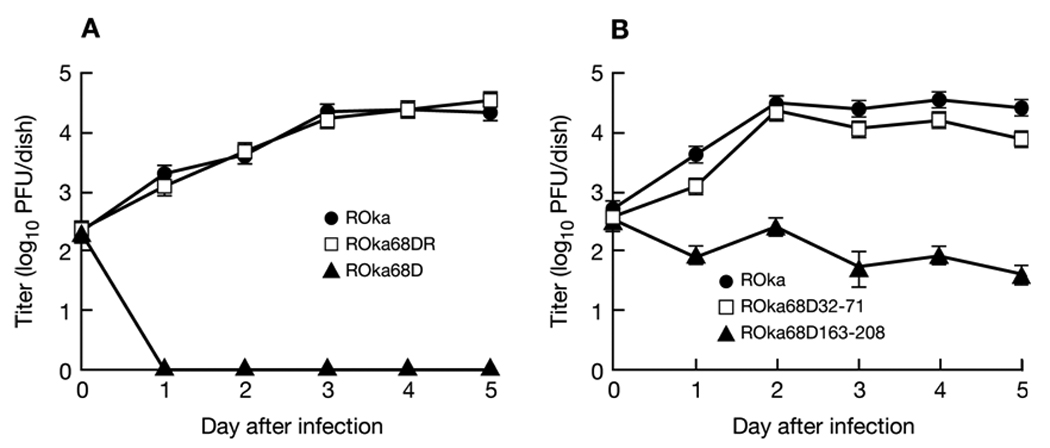
Growth of VZV gE deletion mutants. Melanoma cells were infected with VZV ROka, ROka68DR, or ROka68D (A), or ROka, ROka68D32-71, or ROkaD163-208 (B), and the titer of virus in cells was assayed on days 0 to 5 after infection. Each value represents the average number of plaques in two wells and error bars indicate standard errors. The experiment was performed twice and a representative result is shown.
Expression of VZV gE in a cell line does not inhibit infection by VZV
Since cells infected with baculovirus expressing VZV gE complemented the gE deletion mutant, we determined if overexpression of gE would inhibit the growth of wild-type VZV. HSV-1 gD has been shown to block infection with HSV-1 as evidenced by a marked decrease in plaque number in cells expressing gD versus control cells (Campadelli-Fiume et al., 1988; Johnson and Spear, 1989). VZV lacks a homolog for HSV-1 gD and gE may serve a similar role in VZV. To determine whether expression of VZV gE might block infection with VZV, melanoma cells were infected with baculovirus expressing gE, sodium butyrate was added at 24 hrs, and 1 day later the cells were infected with cell-free or cell-associated VZV ROka. Baculovirus expressing VZV ORF29 was used as a control. The number of plaques in VZV-infected cells did not differ in cells infected with baculovirus expressing VZV gE compared with cells that had been infected with baculovirus expressing VZV ORF29 or not infected with baculovirus (Table 1). While high level expression of gE did not inhibit the growth VZV, it remains possible that gE needs to be complexed with gI to interfere with VZV replication.
Table 1.
Plaque numbers for cells infected with baculovirus expressing VZV gE followed by infection with VZV ROka.
| Baculovirrus |
VZV Source |
Mean Plaque Number ± SE |
|---|---|---|
| None | Cell-associated virus | 147 ± 7.1 |
| Baculo gE-RV | Cell-associated virus | 136 ± 3.5 |
| Baculo 29 | Cell-associated virus | 146 ± 2.8 |
| None | Cell-free virus | 61 ± 6.7 |
| Baculo gE-RV | Cell-free virus | 75 ± 3.9 |
| Baculo 29 | Cell-free virus | 41 ± 3.2 |
VZV gE cannot complement the growth of an HSV gE mutant
VZV gE and HSV gE share 30% amino acid identity and 44% amino acid homology. Since some proteins of VZV (encoded by ORFs 10, 61, and 62) can complement their corresponding HSV homologs (VP16, ICP0, ICP4, respectively; Felser et al., 1988; Moriuchi et al., 1992; Moriuchi et al., 1993), we asked whether VZV gE can complement HSV gE. HSV-1 gE null mutants are impaired for growth on human keratinocyte cell lines (Wisner et al., 2000). We infected HaCAT cells, a human keratinocyte cell line, with baculovirus expressing VZV gE and treated the cells with sodium butyrate for 24 hr to induce expression of VZV gE. The cells were then infected with HSV-1 F or HSV-1 F-gEβ (a gE null mutant). The plaque size for cells infected with HSV-1 F-gEβ was not increased in the presence of baculovirus expressing VZV gE (Fig. 6). The failure of VZV gE to complement the growth of the HSV gE null mutant may have been due to the inability of VZV gE to complex with HSV gI and/or for VZV gE to reach the plasma membrane.
Fig. 6.
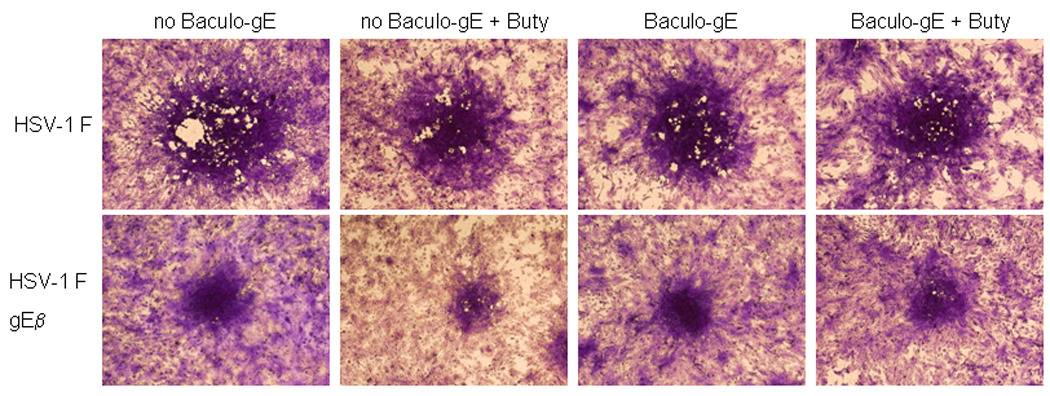
VZV gE does not complement HSV-1 lacking gE. HaCAT cells were not infected with baculovirus (columns 1,2) or infected with baculovirus expressing gE (Baculo gE-RV) (columns 3,4) and cells were not treated (columns 1,3) or treated (columns 2,4) with sodium butyrate (buty). One day later the cells were infected with HSV-1 F or HSV-1 F-gEβ and three days later the cells were stained with crystal violet. A representative focus of infected cells is shown in the center of each panel.
Amino acid 163-208 of gE, which are required for binding to gI, are essential for VZV replication
Amino acids 163-208 of gE are important for its ability to bind to VZV gI (Li et al., 2007). To construct a virus lacking these codons, melanoma cells were infected with Baculo gE-StuI and then transfected with plasmid pCMV62 and cosmids NotI A, Not IB, Mst IIB, and Mst IIA or MstIIA-68D163-208 (Fig. 1). Infectious VZV was detected 20 days after transfection with MstIIA-68D163-208, and 7 days after transfection with MstIIA. Virus resulting from the gE deletion mutant cosmid was termed ROka68D163-208. The virus was passaged in cells that had been infected with Baculo gE-RV one day prior to infection with VZV. ROka68D163-208 could not be passaged in melanoma cells in the absence of baculovirus expressing gE, and could not be produced in cells transfected with MstIIA-68D163-208 and the other 3 cosmids in the absence of the baculovirus.
To verify that ROka68D163-208 had the expected genome structure, virion DNA was cut with BsaWI and Southern blotting was performed using a gE probe. Digestion of ROka68D163-208 resulted in bands 1.0 and 0.8 kb, while digestion of ROka yielded bands 0.8, 0.7 and 0.5 kb (Fig. 3B). This difference in size of bands is due to the deletion of a BsaWI site in ROka68D163-208. PCR was performed and sequence analysis showed that ROka68D163-208 had the expected deletion in gE.
VZV lacking the IDE binding domain of gE is minimally impaired for replication in cell culture
VZE gE is predicted to be a 623 amino acid protein (Davison and Scott, 1986) with a 22 amino acid signal sequence at its amino terminus and a 537 amino acid extracellular domain. Amino acids 32–71 of gE are important for its ability to bind to IDE (Li et al., 2007). To construct a virus lacking these codons, melanoma cells were infected with Baculo gE-StuI and then transfected with plasmid pCMV62 and cosmids NotI A, Not IB, Mst IIB, and Mst IIA or MstIIA-68D32-71 (Fig. 1). Infectious VZV was detected 10 days after transfection with MstIIA-68D32-71 and 7 days after transfection with MstIIA. The resulting virus was found to replicate in the absence of baculovirus expressing gE. Therefore, melanoma cells that had not been infected with baculovirus expressing gE were transfected with plasmid pCMV62 and cosmids NotI A, Not IB, Mst IIB, and Mst IIA or MstIIA-68D32-71. Infectious VZV was detected 11 days after transfection with MstIIA-68D32-71, and 7 days after transfection with MstIIA. Virus resulting from the gE deletion mutant cosmid was termed ROka68D32-71.
To verify that ROka68D32-71 had the expected genome structure, virion DNA was cut with BsaWI and Southern blotting was performed using a gE probe. Digestion of ROka68D32-71 resulted in bands of 0.8, 0.7 and 0.4 kb, while digestion of ROka yielded bands of 0.8, 0.7, and 0.5 kb (Fig. 3B). The difference is due to a deletion in the smaller BsaWI fragment in ROka68D32-71. PCR was performed and sequence analysis showed that ROka68D32-71 had the expected deletion in gE.
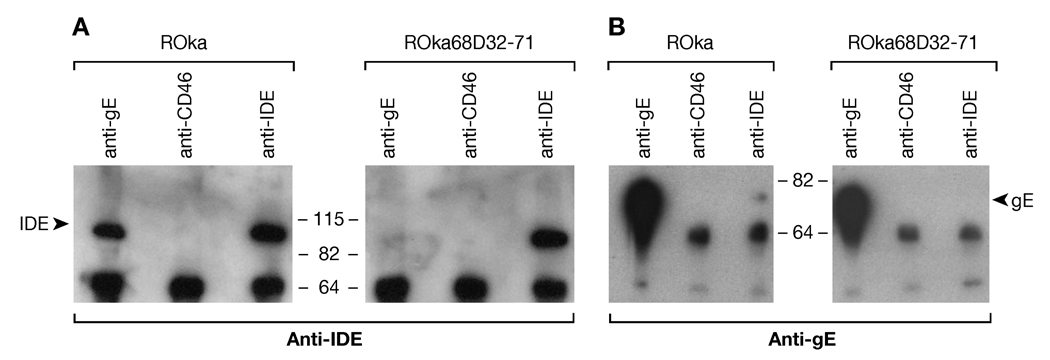
To verify that ROka68D32-71 is impaired for binding to IDE, melanoma cells were infected with ROka or ROka68D32-71, and infected cell lysates were immunoprecipitated with antibody to VZV gE, IDE, or CD46 (a control antibody). Immunoblotting with antibody to IDE showed that gE from cells infected with ROka bound to IDE, but gE from cells infected ROka68D32-71 bound little IDE (Fig. 7A, lane 1 vs. 4). Similar levels of IDE were present in the immunoprecipitates (Fig 7A, lanes 3, 6), and as expected antibody to CD46 did not immunoprecipitate IDE (Fig 7A, lanes 2, 5). Adequate levels of gE were present in cells infected with ROka68D32-71 (Fig 7B, lane 4). Immunoprecipitation of lysate from cells infected ROka using antibody to IDE showed that IDE from infected cells bound to gE (Fig. 7B, lane 3), although the amount of gE bound was only a fraction of the total gE that was immunoprecipitated with anti-gE Ab (Fig. 7B, lane 1). In contrast, gE was not detected in lysate from cells infected with ROka68D32-71 and immunoprecipitated with antibody to IDE (Fig. 7B, lane 6).
Fig. 7.
VZV deleted for the IDE binding domain of gE is impaired for binding to IDE. Melanoma cells were infected with ROka or ROka68D32-71, cells lysates were immunoprecipitated with antibodies to VZV gE (Chemicon International), CD46, or IDE (Covance), and immunoblotting was performed with antibodies to IDE-1 (Li et al 2006) (A) or VZV gE (7A7, a gift from B. Forghani) (B).
Infection of melanoma cells with cell-associated ROka68D32-71 showed that the virus grew to maximum titers that were nearly as high as those obtained for parental (ROka) VZV (Fig. 5B).
VZV lacking the IDE binding domain of gE is impaired for infectivity with cell-free virus and for cell-to-cell spread
The prior experiments studied the replication of cell-associated ROka68D32-71. To examine the infectivity of cell-free ROka68D32-71, melanoma cells were infected with VZV ROka-GFP and ROka68D32-71. Since ROka68D32-71 is partially impaired for growth in cell culture, cells were infected 1 day earlier with the gE mutant virus than with ROka-GFP. When similar CPE was noted for both viruses, cells were sonicated, the lysate was centrifuged, and the supernatant was used to infect melanoma cells, while the lysate was immunoblotted with antibodies to VZV proteins to confirm that the level of viral protein expression was comparable. Infection of melanoma cells with cell-free ROka68D32-71 resulted in significantly fewer plaques (mean 12 plaques ± 4 plaques) than infection with cell-free ROka-GFP (mean 111 plaques ± 13 plaques, p<0.001, T test, Fig. 8A). Immunoblotting of lysates from cells infected with ROka68D32-71 and ROka-GFP showed that similar levels of VZV IE4, thymidine kinase, and glycoprotein B were expressed, but that cells infected with ROka68D32-71 expressed less gE than cells infected with ROka-GFP (Fig. 8B). The antibody used to detect gE recognizes an epitope outside of the region deleted, and detects recombinant extracellular gE (gEt) or extracellular gE lacking amino acids 32–71 (gEtΔ32–71) (Li et al., 2007) to a similar extent (Fig. 7). The difference in the amount of gE expressed in cells infected with ROka or ROka68D32-71 was more apparent on immunoblot with whole cell lyste (Fig 8B) than after immunoprecipitation with antibody to gE followed by immunoblotting with antibody to gE (Fig. 7B). The difference might be due to a limiting amount of gE antibody used for the immunoprecipitation or a difference in the antibodies used for the immunoblots.
Fig. 8.
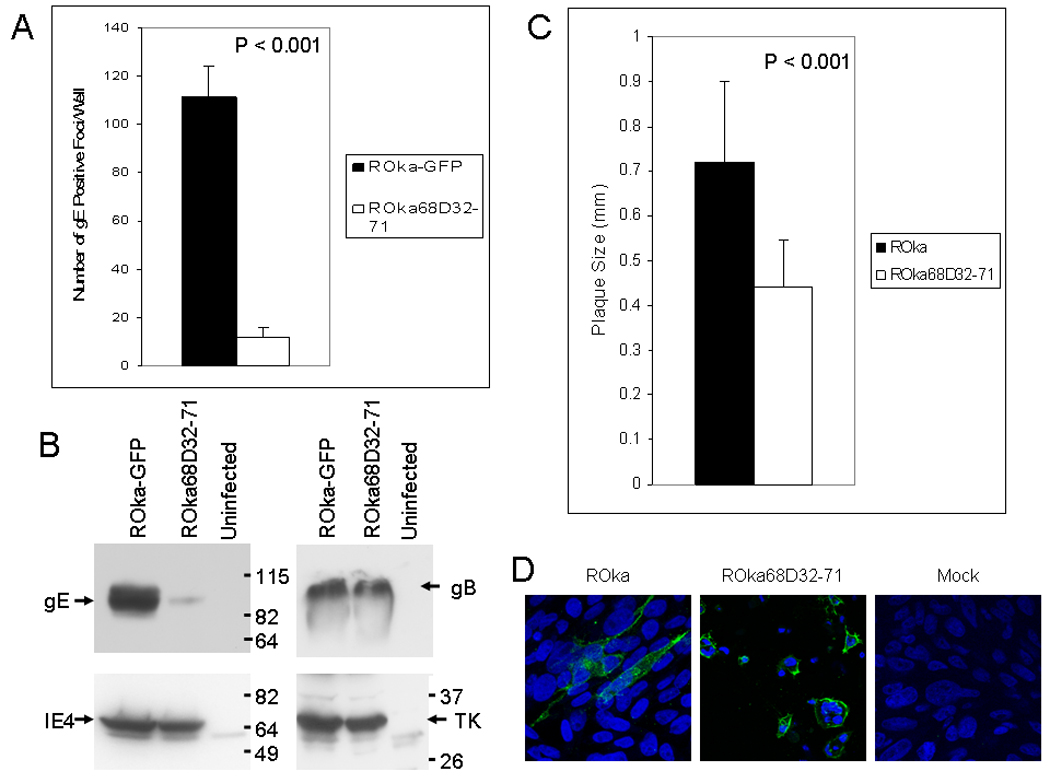
VZV deleted for the IDE binding domain of gE is partially impaired for cell-to-cell spread of virus and for infectivity. (A) Melanoma cells were infected with ROka68D32-71 or ROka-GFP and when CPE was similar for cells infected with the two viruses, the cells were sonicated and centrifuged. The supernatant, containing cell-free virus, was used to infect melanoma cells and the cells were stained with antibody to VZV gE to determine the number of virus-infected foci. (B) The pellet from the centrifugation was used for immunoblotting with antibody to VZV gE (Chemicon International), gB, IE4, or thymidine kinase (TK). (C) Melanoma cells were infected with cell-associated VZV ROka or ROka68D32-71 and plaque diameters were measured by microscopy. (D) Expression of gE in cells infected with ROka or ROka68D32-71. Infected or mock infected cells were fixed with 2% paraformaldehyde, stained with monoclonal antibody to VZV gE followed by Alexa Fluor 488 goat anti-mouse antibody with DAPI, and confocal immunofluorescence microscopy was performed.
To determine if ROka68D32-71 is impaired for cell-to-cell spread, melanoma cells were infected with ROka68D32-71 or ROka and plaque sizes were measured. Cells infected with ROka68D32-71 showed smaller plaques (mean 0.44 mm ± 0.1 mm) than cells infected with parental virus (mean 0.72 mm ± 0.18 mm, Fig. 8C). The difference in the mean plaque size was statistically significant (p<0.001, T test).
Immunofluorescence for VZV gE showed that gE was predominantly on the surface of cells infected with VZV ROka or VZV ROka68D32-71 (Fig. 8D). Thus, the impairment of ROka68D32-71 for cell-to-cell spread or for infectivity with cell-free virus was not due to the inability of gE to traffic to the cell surface.
VZV lacking the IDE binding domain of gE is not impaired for virion maturation or transport to the cell surface
Since ROka68D32-71 is impaired for cell-to-cell spread, we analyzed whether the virus could traffic to the surface of the cell. Although fewer infected cells were observed for cells infected with ROka68D32-71 than with ROka, virions in ROka68D32-71-infected cells were detected on the surface of cells by scanning electron microscopy similar to those in ROka-infected cells (Fig. 9, top panels). Transmission electron microscopy showed that VZV lacking the IDE binding domain was not impaired for virion maturation or transport to the cell surface (Fig 9, bottom panels).
Fig. 9.
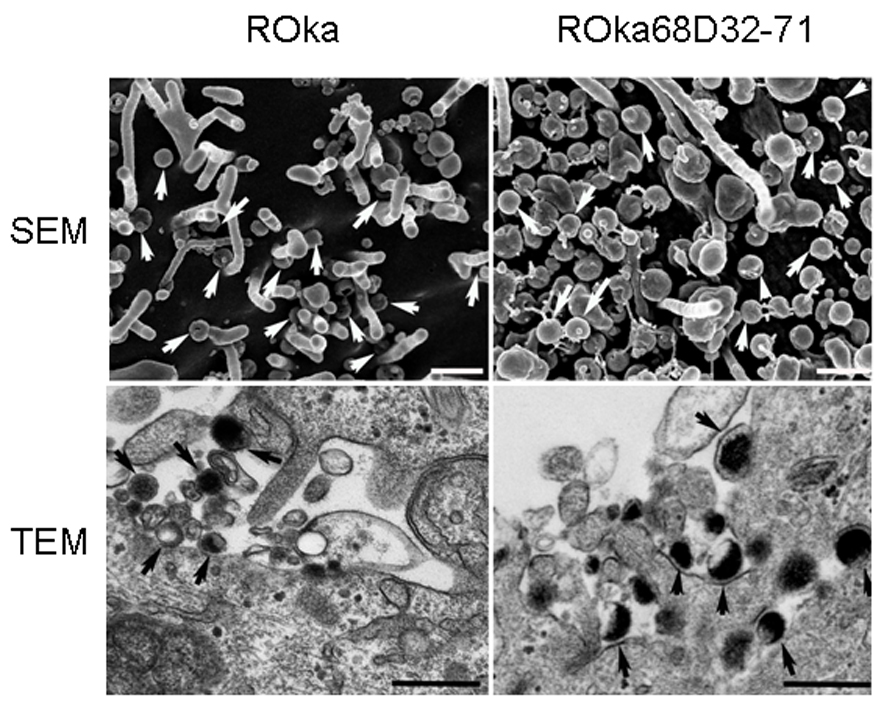
VZV deleted for the IDE binding domain of gE traffics to the cell surface. Melanoma cells were infected with VZV ROka or ROka68D32-71, cells were fixed as described in the Methods, and scanning electron microscopy (SEM) or transmission electron microscopy (TEM) was performed. Arrows indicate virions and bars are 400 nm.
Discussion
We have found that gE and its gI binding domain are required for VZV replication, while the IDE binding domain of gE is not essential. We used baculovirus expressing VZV gE to complement the growth of a VZV gE deletion mutant. While a prior experiment using a cell line expressing VZV gE from its homologous promoter resulted in complementation of a VZV point mutation (Moffat et al., 2004), the authors did not show that gE was expressed on the surface of the cells. We found that baculovirus expression of gE from the human CMV IE promoter resulted in expression of the glycoprotein in the cytoplasm and on the plasma membrane, especially in the presence of sodium butyrate or with infection by the VZV gE deletion mutant (Fig 2B). Constitutive expression of gE may be toxic to cells (Litwin et al., 1992), as has been reported for other herpesvirus glycoproteins (Friedman et al., 1989: Tikoo et al., 1990). Since gE expression was not detected in insect cells infected with baculovirus in the absence of butyrate or VZV (Fig. 2A), the baculovirus system should be useful for expression of other VZV glycoproteins in mammalian cells for complementation studies.
VZV gE shares many of the properties of HSV gD. Both proteins bind to cellular receptors for their respective viruses (Cocchi et al., 1998; Geraghty et al., 1998; Li et al., 2006; Montgomery et al., 1996), both are essential for virus replication (Feenstra et al., 1990), and both are located in the unique short region of their genomes. Prior studies showed that cell lines expressing HSV-1 gD interfere with HSV-1 infection (Campadelli-Fiume et al., 1988; Johnson and Spear, 1989). Infection of these cell lines with HSV-1 failed to induce formation of plaques even with an inoculum of 106 PFU, and HSV-1 protein synthesis was markedly diminished in the cell lines (Johnson and Spear, 1989). In contrast, we found no evidence for inhibition of VZV infection in cells that had been infected with baculovirus expressing gE (Table 1). This effect was seen for infection with either cell-associated or cell-free VZV. While a prior paper also showed that a cell line expressing VZV gE from its endogenous promoter did not inhibit the growth of VZV (Moffat et al., 2004), the authors did not show that VZV gE was expressed on the surface of the cell line. The inhibition of HSV-1 infection by cells expressing gD has been postulated to prevent superinfection of the cells, and help allow progeny virus to escape from infected cells (Johnson and Spear, 1989). It is interesting to note that virtually no infectious cell-free virus is released from VZV-infected cells in vitro; therefore, the inability of VZV gE to block VZV infection or reinfection might contribute to the inability of cultured cells to release infectious cell-free VZV into the supernatant. In addition, HSV does not result in the marked syncytia formation in vitro, while VZV-infected cells form large syncytia. Since HSV gD blocks HSV superinfection, it might limit the degree of virus-infected cell fusion and perhaps cell-to-cell fusion that occurs in vitro (Johnson and Spear, 1989). In contrast the inability of gE to block VZV infection may favor virus-cell and cell-cell fusion resulting in formation of large syncytia. Thus while VZV gE and HSV gD bind to cellular receptors, there are likely important biological differences in how the molecules interact with their receptors on cells.
VZV gE did not complement the growth of an HSV-1 gE null mutant. While VZV gE is essential for VZV replication, HSV-1 gE is not required for virus entry and replication, but is important for sorting nascent virions to cell junctions and is essential for efficient cell-to-cell spread of HSV-1 (Collins and Johnson, 2003; Dingwell and Johnson, 1998; Polcicova et al., 2005). Alignment of VZV gE with HSV-1 gE shows that amino acids 337 to 574 of VZV share 31% identity and 45% homology with amino acids 220 to 454 of HSV-1. Thus it was possible that VZV gE might complement HSV-1 gE. The failure of VZV gE to complement HSV-1 gE may be due to several mechanisms. First, VZV gE might not interact with HSV-1 gI properly. Since VZV gI is important for proper maturation and cycling of VZV gE between the Golgi apparatus and the cell surface (Mo et al., 2002; Olson and Grose, 1998), HSV-1 gI might not be able to substitute for VZV gI. Second, the VZV ORF47 protein kinase is required for phosphorylation of gE, localization of gE to the Golgi apparatus, and for normal cell-to-cell spread of the virus in infected cells (Kenyon et al., 2002). It is unknown whether the HSV UL13 protein kinase, the homolog of VZV ORF47, can phosphorylate VZV gE properly. Third, the interaction of VZV gE, but not HSV-1 gE, with IDE (24) could interfere with HSV-1 replication.
We found that amino acids 163–208 of VZV gE, which are required for its interaction with gI (Li et al., 2007), are essential for virus replication. Berarducci et al. (2006) found that amino acids 51–187 of VZV gE partially impaired the growth of the virus in cell culture, reduced cell-to-cell spread, and resulted in fewer numbers of intracellular and extracellular viral particles by electron microscopy. Taken together with our results, these experiments indicate that amino acids 188–208 of gE are likely required for VZV replication. While the loss of a gI binding domain may be important for proper gE maturation and localization, or for gE to form a complex with gI to so that gE is fully functional, the observation that gI is not essential for VZV replication in cell culture (Cohen and Nguyen, 1997; Mallory et al., 1997) suggests that this region of gE may have other activities, or that it could have a critical role for the proper conformation of the glycoprotein.
Amino acids 32–71 of VZV gE were not essential for virus replication. Deletion of this region resulted in peak titers that were nearly as high as those obtained with ROka. This is consistent with the finding from electron microscopy studies in which ROka68D32-71 showed normal maturation and transport to the cell surface. In contrast, VZV deleted in this region was partially impaired for cell-to-cell spread and for infectivity with cell-free virus. Amino acids 32–71 of gE are required for binding IDE, a cellular receptor for the virus (Li et al., 2006). Inhibition of IDE activity using anti-IDE antibody or siRNA specific for IDE reduced cell-to-cell spread of virus and blocked virus infectivity by 30–70%. The reduction in infectivity seen with cell-free ROka68D32-71 when compared with ROka was 70–90% (Fig. 8A), which suggests that this region of gE may have other activities besides interaction with IDE. For example, ROka68D32-71 displayed a reduced level of gE expression.
Transmission and scanning electron microscopy of cells infected with VZV lacking the IDE binding domain of gE showed no impairment in virion maturation or transport to the cell surface. Bacitracin inhibits IDE activity (Bennett et al., 2003), blocks IDE-gE complex formation, inhibits VZV infectivity, and reduces cell-to-cell spread of virus (Li et al., 2006). Bacitracin treatment of VZV-infected melanoma cells had no effect on nucleocapid formation, virus maturation, or transport of virions to the cell surface. Thus, the failure to observe differences in virus maturation or trafficking by electron microscopy in cells infected with VZV deleted for the IDE binding domain of gE is consistent with the earlier observations of virus-infected cells treated with bacitracin.
In summary we have shown that deletion of the IDE binding domain of gE in VZV results in a mutant that is impaired cell-to-cell spread and infectivity by cell-free virus. Unlike HSV-1 gE, VZV gE is essential for virus replication. Cells expressing VZV gE complement a VZV deletion mutant, but not an HSV-1 gE mutant. Unlike HSV-1 gD, VZV gE does not inhibit VZV infection. These studies continue to demonstrate the differences between HSV and VZV and emphasize the need for further studies of VZV.
Materials and methods
Cells and viruses
Human melanoma (Mewo) cells were used for transfections and VZV propagation. Sf9 (Spodoptera frugiperda) insect cells were used to grow baculovirus. Two baculoviruses expressing VZV gE were constructed. A 1.9 kb StuI-KpnI fragment which contains the ORF68 gene and its native polyadenylation site was amplified by PCR from the Oka strain of VZV and inserted into the corresponding sites of plasmid pAc-CMV (Zhou et al., 2000). The resulting plasmid pAc-CMV-gE-StuI contains the baculovirus polyhedrin promoter and the human immediate-early (IE) cytomegalovirus (CMV) promoter driving gE. A second plasmid was constructed by cutting plasmid pAc-CMV-gE-StuI with XhoI, blunting the ends with Klenow, cutting the plasmid with KpnI, and inserting the 2.7 kb XhoI-KpnI fragment containing the IE CMV promoter and the ORF68 gene into the EcRV-KpnI site of plasmid pAc-CMV. This plasmid, pAc-CMV-gE-RV, has only the human IE CMV promoter driving gE. Recombinant baculoviruses were constructed by cotransfecting Sf9 cells with BaculoGold-linearized baculovirus DNA (BD Biosciences, San Diego, CA) and either pAc-CMV-gE-StuI or pAc-CMV-gE-RV. The resulting viruses, Baculo gE-StuI or Baculo gE-RV, were concentrated by centrifugation at 8,800 × g for 2 hr and resuspended in phosphate-buffered saline containing 1% fetal bovine serum. Baculovirus expressing VZV ORF29 was described previously (Cohen et al., 2007).
VZV ROka was derived by transfection from cosmids corresponding to the Oka vaccine strain and ROka-GFP, expressing the green fluorescence protein, has been described previously (Li et al., 2006). HSV-1 F and HSV-1 F-gEβ, a gE-null mutant derived from strain F, were previously described (Dingwell et al., 1994).
VZV cosmids and transfections
Cosmids NotI A, Not IB, Mst IIA, and Mst IIB are derived from the vaccine Oka strain of VZV and encompass the whole genome (Fig. 1). VZV ORF68, which encodes gE, is located at VZV nucleotides 115,808 to 117,676 (Davison and Scott, 1986). To produce VZV deleted for ORF68, cosmid Mst IIA was cut with AvrII and SgrAI which cut at VZV nucleotides 112,853 and 117,355 and cloned into plasmid pGEM2 into which AvrII and SgrAI linkers had been inserted. The latter plasmid was then cut with AvaI which cuts at VZV nucleotides 115,761, 116,648, and 117,218 and the large fragment was blunted with the Klenow fragment of E. coli DNA polymerase and ligated to a double stranded oligonucleotide (TAGCTAGGCGCGCCTAGCTA). The latter contains stop codons in all three open reading frames and an AscI linker. The resulting plasmid was cut with AvrII and SgrAI and inserted in place of the wild-type sequence into cosmid Mst IIA. The resulting cosmid, Mst IIA-68D, has a deletion that begins 49 base pairs upstream of ORF68 and continues to codon 469.
Melanoma cells were transfected with plasmid pCMV62 and VZV cosmids that had been linearized using MstII or NotI as described previously (Cohen and Nguyen, 1997). Cells were treated with trypsin and passaged once a week.
To rescue the ORF68 deletion mutant virus, cosmid MstII A was cut with SnaBI which cuts at 115,808 and 117,676 and the 7.5 kb fragment was gel purified. Melanoma cells were transfected with the 7.5 kb fragment containing ORF68, virion DNA from the ORF68 deletion mutant, and plasmid pCMV62. After CPE was observed, infected cells were sonicated to prepare cell-free virus, and centrifugation was performed to isolate virus from insoluble debris. Serial dilutions of the supernatant were used to infect melanoma cells. Multiple cycles of sonication and serial dilution were performed to obtain plaque purified rescued virus.
Cosmids deleted for the IDE or the gI binding domains of gE (Li et al., 2007) were constructed. First plasmid pUC19 was linearized with Pfo I, blunt ended with the Klenow fragment of E. coli DNA polymerase I (New England BioLabs, Beverly, MA), and ligated to itself. The resulting plasmid was cut with Sma I and a double stranded oligonucleotide, derived from annealing 5’-CTAGGCGCGTCCCGGATCTAGACACCGGTG-3’ and its complementary sequence 5’-GATCCGCGCAGGGCCTAGATCTGTGGCCAC-3’ together was inserted into the Sma I site. This results in insertion of Avr II, Pfo I and SgrA I sites into pUC19. A 268 bp PfoI- Acl I fragment containing a portion of the gE gene was obtained by PCR using primers 5’-CTAGCAACGATTCGCGAAG-3’ and 5’-AATCATCGTATCGCAAGACGG-3’, VZV cosmid MstII A as a template, and PfuUltra high-fidelity DNA polymerase (Strategene, La Jolla, CA). The PCR product was digested with Pfo I and Acl I and used in a three fragment ligation with (a) the modified pUC19 plasmid described above that had been digested with Pfo I-SgrAI and treated with alkaline phosphatase, and (b) the Acl I-SgrA I fragment from plasmid gEtΔ32–71 (1662 bp fragment) or gEtΔ163-208 (1641 bp fragment) which have mutated gE sequences (Li et al., 2007). The resulting plasmids were named pPfoI-SgrAI-gEtΔ32–71 and pPfoI-SgrAI-gEtΔ163-208. The latter two plasmids were each digested with Pfo I and SgrA 1 and a 1.6 kb fragment containing the gE mutant sequences from each was used in two separate three fragment ligation reactions with (a) the modified pUC19 plasmid described above that had been digested with Avr II and SgrA I and treated with alkaline phosphatase, and (b) a 2.8kb Avr II-Pfo I fragment from VZV cosmid MstII A to yield plasmids pAvrII-SgrA-gEtΔ32–71 and pAvrII-SgrAI-gEtΔ163-208. Finally, the latter two plasmids were each digested with AvrII and SgrAI and a 4.5 kb fragment containing the gE mutant sequences from each was separately ligated to the 35.4 kb Avr II-SgrA I fragment of VZV cosmid MstII A to yield cosmids VZV MstIIA-68D32-71 and MstIIA-68D163-208 which are deleted for the IDE and gI binding domains of gE, respectively (Fig 1).
Southern blotting, sequence analysis, immunoblotting, and immunoprecipitations
Virion DNA was prepared from nucleocapsids, cut with BamHI or BsaWI, separated on 0.8% agarose gels, transferred to nylon membranes and hybridized with [32P]dCTP radiolabeled probes corresponding to ORF68.
Sequence analysis of cells infected with ORF68 deletion mutants was performed by PCR amplification of the regions surrounding the deletions. Primers 5'-AGCAACGATTCGCGAAGAATCC-3' (corresponding to VZV nucleotides 115,503–115,524) and 5'-CTTGGAGTCCCAAGAACCTAC-3' (corresponding to nucleotides 117,802 to 117,822) were used to amplify DNA in virus infected cells derived from cosmids VZV Mst IIA-68D, MstIIA-68D32-71, and MstIIA-68D163-208.
Cell lysates of virus-infected cells were separated on SDS-PAGE gels, transferred to nitrocellulose membranes, blocked with milk, and incubated with murine monoclonal antibody to VZV gE (Chemicon International, Temcula, Calif.), VZV glycoprotein B (Biodesign, Saco, ME), IE4 (Moriuchi et al., 1995), or thymidine kinase (a gift from Christine Talarico) followed by horseradish peroxidase-conjugated anti-mouse antibody and developed using enhanced chemiluminescence (Pierce Chemical Co., Rockford, Ill.).
Cells lysates were prepared from VZV-infected cells in lysis buffer (Li et al., 2006), and antibodies to VZV gE (Chemicon International, Temecula, CA and a gift from Bagher Forghani, California State Department of Health Services, Berkeley, CA), CD46 (Immunotech, Marseille Cedex, France) or IDE (Covance, Berkeley, CA) were added to the lysates with protein A. The immune complexes were fractionated on SDS polyacrylamide gels and immunoblotted with rabbit antibody to IDE-1 (Li et al., 2006) or mouse antibody to gE, followed by horseradish peroxidase-conjugated rabbit or mouse antibody, and enhanced chemiluminescence,
Virus growth analysis, cell-free virus preparation, and immunofluorescence
Virus growth was analyzed by infecting 25 cm2 flasks with about 200 PFU of virus, and each day thereafter for five consecutive days two flasks for each virus were treated with trypsin and serial dilutions were used to infect melanoma cells. One week later the cells were fixed and stained with crystal violet and plaques were counted.
Cell-free VZV was prepared by scraping flasks of VZV-infected cells in SPGC (10% fetal bovine serum, 0.1% sodium glutamate, 5% sucrose in PBS), sonicating the lysate, and centrifuging the lysate at 1,240 × g for 10 min at 4°C. The supernatant was used to infect melanoma cells and the pellet was used for immunoblotting.
Immunofluorescence studies of gE were performed by infecting cells with baculovirus expressing gE and 24 hrs later treating the cells with 5 mM sodium butyrate. One day later the cells were infected with VZV and two days later the cells were fixed with 2% paraformaldehyde for 5 min and stained with monoclonal antibody to VZV gE (Chemicon, Temecula, CA) followed by Alexa Fluor 488 goat anti-mouse antibody (Invitrogen, Carlsbad, CA), mounted with ProLong Gold antifade reagent with DAPI (Invitrogen), and visualized by confocal immunofluorescence microscopy.
Scanning electron microscopy
VZV-infected cells grown on silicon chips (Ted Pella, Redding, CA) were fixed with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer, then in 1% tannic acid, and then overnight at 4°C with 0.1% uranyl acetate and 4% paraformaldehyde. Specimens were rehydrated with a graded ethanol series, critical point dried under CO2 in a Bal-Tec model cpd 030 Drier (Balzers, Liechtenstein), mounted on aluminum studs and sputter coated with 30 angstroms of iridium in a model IBS/e ion beam sputterer (South Bay Technology, Inc, San Clemente, CA) prior to viewing at 5 kV in a Hitachi S-5200 in-lens scanning electron microscope (Hitachi, Tokyo, Japan).
Transmission electron microscopy
VZV-infected cells were grown on thermanox coverslips (Nalge Nunc International, Rochester, NY) and fixed overnight at 4°C with 2.5% glutaraldehyde, 4% paraformaldehyde, and 0.1 M sodium cacodylate buffer, pH 7.4. Samples were post fixed 1 h with 0.5% osmium tetroxide/0.8 % potassium ferricyanide followed by 1 hr in 1% tannic acid, overnight with 1% uranyl acetate at 4°C, and dehydration with a graded ethanol series, prior to embedding in Spurr’s resin. Thin sections were cut with an RMC MT-7000 ultramicrotome (Ventana, Tucson, AZ), stained with 1% uranyl acetate and Reynold’s lead citrate prior to viewing at 80 kV on a Hitachi H-7500 transmission electron microscope (Hitachi, Tokyo, Japan). Digital images were acquired with a bottom mount AMT digital camera system (AMT, Chazy, NY).
Acknowledgements
This work was supported by the intramural research program of the National Institute of Allergy and Infectious Diseases. We thank David Johnson for HSV-1 F and HSV-1-gEβ, Bernard Roizman for plasmid Ac-CMV, Christine Talarico for antibody to VZV thymidine kinase, Bagher Forghani for antibodies to VZV gE, and Yo Hoshino for assistance with artwork.
Footnotes
Note added in Proof: Since this manuscript was submitted, Berarducci et al. (J Virol. 2009;83:228-40) showed that deletion of amino acids 208–236 of VZV gE abolishes binding of gE to gI and reduces cell-to-cell spread of virus, but still allows VZV entry and replication in vitro.
References
- Bennett RG, Hamel FG, Duckworth WC. An insulin-degrading enzyme inhibitor decreases amylin degradation, increases amylin-induced cytotoxicity, and increases amyloid formation in insulinoma cell cultures. Diabetes. 2003;52:2315–2320. doi: 10.2337/diabetes.52.9.2315. [DOI] [PubMed] [Google Scholar]
- Berarducci B, Ikoma M, Stamatis S, Sommer M, Grose C, Arvin AM. Essential functions of the unique N-terminal region of the varicella-zoster virus glycoprotein E ectodomain in viral replication and in the pathogenesis of skin infection. J. Virol. 2006;80:9481–9496. doi: 10.1128/JVI.00533-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berarducci B, Sommer M, Zerboni L, Rajamani J, Arvin AM. Cellular and viral factors regulate the varicella-zoster virus gE promoter during viral replication. J. Virol. 2007;81:10258–10267. doi: 10.1128/JVI.00553-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campadelli-Fiume G, Arsenakis M, Farabegoli F, Roizman B. Entry of herpes simplex virus 1 in BJ cells that constitutively express viral glycoprotein D is by endocytosis and results in degradation of the virus. J. Virol. 1988;62:159–167. doi: 10.1128/jvi.62.1.159-167.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi F, Menotti L, Mirandola P, Lopez M, Campadelli-Fiume G. The ectodomain of a novel member of the immunoglobulin subfamily related to the poliovirus receptor has the attributes of a bona fide receptor for herpes simplex virus types 1 and 2 in human cells. J. Virol. 1998;72:9992–10002. doi: 10.1128/jvi.72.12.9992-10002.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JI, Krogmann T, Pesnicak L, Ali MA. Absence or overexpression of the Varicella-Zoster Virus (VZV) ORF29 latency-associated protein impairs late gene expression and reduces VZV latency in a rodent model. J. Virol. 2007;81:1586–1591. doi: 10.1128/JVI.01220-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JI, Nguyen H. Varicella-zoster glycoprotein I is essential for growth of virus in Vero cells. J. Virol. 1997;71:6913–6920. doi: 10.1128/jvi.71.9.6913-6920.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JI, Seidel KE. Absence of varicella-zoster virus (VZV) glycoprotein V does not alter growth of VZV in vitro or sensitivity to heparin. J. Gen. Virol. 1994;75:3087–3093. doi: 10.1099/0022-1317-75-11-3087. [DOI] [PubMed] [Google Scholar]
- Collins WJ, Johnson DC. Herpes simplex virus gE/gI expressed in epithelial cells interferes with cell-to-cell spread. J. Virol. 2003;77:2686–2695. doi: 10.1128/JVI.77.4.2686-2695.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condreay JP, Witherspoon SM, Clay WC, Kost TA. Transient and stable gene expression in mammalian cells transduced with a recombinant baculovirus vector. Proc. Natl. Acad. Sci. USA. 1999;96:127–132. doi: 10.1073/pnas.96.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison AJ, Scott JE. The complete genome of varicella-zoster virus. J. Gen. Virol. 1986;67:1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- Dingwell KS, Brunetti CR, Hendricks RL, Tang Q, Tang M, Rainbow AJ, Johnson DC. Herpes simplex virus glycoproteins E and I facilitate cell-to-cell spread in vivo and across junctions of cultured cells. J. Virol. 1994;68:834–845. doi: 10.1128/jvi.68.2.834-845.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingwell KS, Johnoson DC. The herpes simplex virus gE-gI complex facilitates cell-to-cell spread and binds to components of cell junctions. J. Virol. 1998;72:8933–8942. doi: 10.1128/jvi.72.11.8933-8942.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duus KM, Hatfield C, Grose C. Cell surface expression and fusion by the varicella-zoster virus gH:gL glycoprotein complex: Analysis by laser scanning confocal microscopy. Virology. 1995;210:429–440. doi: 10.1006/viro.1995.1359. [DOI] [PubMed] [Google Scholar]
- Feenstra V, Hodaie M, Johnson DC. Deletions in herpes simplex virus glycoprotein D define nonessential and essential domains. J. Virol. 1990;64:2096–2102. doi: 10.1128/jvi.64.5.2096-2102.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felser JM, Kinchington PR, Inchauspe G, Straus SE, Ostrove JM. Cell lines containing varicella-zoster virus open reading frame 62 and expressing the "IE" 175 protein complement ICP4 mutants of herpes simplex virus type 1. J Virol. 1988;62:2076–2082. doi: 10.1128/jvi.62.6.2076-2082.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman HM, Yee A, Diggelmann H, Hastings JC, Tal-Singer R, Seidel-Dugan CA, Eisenberg RJ, Cohen GH. Use of a glucocorticoid-inducible promoter for expression of herpes simplex virus type 1 glycoprotein gC1, a cytotoxic protein in mammalian cells. Mol. Cell. Biol. 1989;9:2303–2314. doi: 10.1128/mcb.9.6.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraghty RJ, Krummenacher C, Cohen GH, Eisenberg RJ, Spear PG. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science. 1998;280:1618–1620. doi: 10.1126/science.280.5369.1618. [DOI] [PubMed] [Google Scholar]
- Jacquet A, Haumont M, Chellun D, Massaer M, Tufaro F, Bollen A, Jacobs P. The varicella-zoster virus glycoprotein B (gB) plays a role in virus binding to cell heparan sulfate proteoglycans. Virus Res. 1998;53:197–207. doi: 10.1016/s0168-1702(97)00149-4. [DOI] [PubMed] [Google Scholar]
- Johnson RM, Spear PG. Herpes simplex virus glycoprotein D mediates interference with herpes simplex virus infection. J. Virol. 1989;63:819–827. doi: 10.1128/jvi.63.2.819-827.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon TK, Cohen JI, Grose C. Phosphorylation by the varicella-zoster virus ORF47 protein serine kinase determines whether viral gE traffics to the TGN or recycles to the cell membrane and thereby regulates viral cell-cell spread. J. Virol. 2002;76:10980–10993. doi: 10.1128/JVI.76.21.10980-10993.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H, Straus SE, Williams RK. Varicella-zoster virus glycoproteins E and I expressed in insect cells form a heterodimer that requires the N-terminal domain of glycoprotein I. Virology. 1997;233:383–391. doi: 10.1006/viro.1997.8625. [DOI] [PubMed] [Google Scholar]
- Li Q, Ali MA, Cohen JI. Insulin degrading enzyme is a cellular receptor for varicella-zoster virus infection and for cell-to-cell spread of virus. Cell. 2006;127:305–316. doi: 10.1016/j.cell.2006.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Krogmann T, Ali MA, Tang W-J, Cohen JI. The amino terminus of varicella-zoster virus (VZV) glycoprotein E is required for binding to insulin degrading enzyme, a VZV receptor. J. Virol. 2007;81:8525–8532. doi: 10.1128/JVI.00286-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwin V, Jackson, Grose C. Receptor properties of two varicella-zoster virus glycoproteins, gpI and gpIV, homologous to herpes simplex virus gE and gI. J. Virol. 1992;66:3643–3651. doi: 10.1128/jvi.66.6.3643-3651.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallory S, Sommer M, Arvin AM. Mutational analysis of the role of glycoprotein I in varicella-zoster virus replication and its effects on glycoprotein E conformation and trafficking. J. Virol. 1997;71:8279–8288. doi: 10.1128/jvi.71.11.8279-8288.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresova L, Pasieka TJ, Homan E, Gerday E, Grose C. Incorporation of three endocytosed varicella-zoster virus glycoproteins, gE, gH, and gB into the virion envelope. J. Virol. 2005;79:997–1007. doi: 10.1128/JVI.79.2.997-1007.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo C, Lee J, Sommer M, Grose C, Arvin AM. The requirement of varicella-zoster virus glycoprotein E (gE) for viral replication and effects of glycoprotein I on gE in melanoma cells. Virology. 2002;304:176–186. doi: 10.1006/viro.2002.1556. [DOI] [PubMed] [Google Scholar]
- Mo C, Schneeberger EE, Arvin AM. Glyprotein E of varicella-zoster virus enhances cell-cell contact in polarized epithelial cells. J. Virol. 2000;74:11377–11387. doi: 10.1128/jvi.74.23.11377-11387.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo C, Suen J, Sommer M, Arvin AM. Characterization of varicella-zoster virus glycoprotein K (open reading frame 5) and its role in virus growth. J. Virol. 1999;73:4197–4207. doi: 10.1128/jvi.73.5.4197-4207.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat JF, Ito H, Sommer M, Taylor S, Arvin AM. Glycoprotein I of varicella-zoster virus is required for viral replication in skin and T cells. J. Virol. 2002;76:8468–8471. doi: 10.1128/JVI.76.16.8468-8471.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat J, Mo C, Cheng JJ, Sommer M, Zerboni L, Stamatis S, Arvin AM. Functions of C-terminal domain of varicella-zoster virus glycoprotein E in viral replication in vitro and skin and T-cell tropism in vivo. J. Virol. 2004;78:12406–12415. doi: 10.1128/JVI.78.22.12406-12415.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat JF, Zerboni L, Kinchington PR, Grose C, Kaneshima H, Arvin AM. Attenuation of the vaccine Oka strain of varicella-zoster virus and role of glycoprotein C in alphaherpesvirus virulence demonstrated in the SCID-hu mouse. J. Virol. 1998;72:965–974. doi: 10.1128/jvi.72.2.965-974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalvo EA, Parmley RT, Grose C. Structural analysis of the varicella-zoster virus gp98-gp62 complex: Posttranslational addition of N-linked and O-linked oligosaccharide moieties. J. Virol. 1985;53:761–770. doi: 10.1128/jvi.53.3.761-770.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery RI, Warner MS, Lum BS, Spear PG. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- Moriuchi H, Moriuchi M, Smith HA, Straus SE, Cohen JI. Varicella-zoster virus open reading frame 61 protein is functionally homologous to herpes simplex virus type 1 ICP0. J Virol. 1992;66:7303–7308. doi: 10.1128/jvi.66.12.7303-7308.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriuchi H, Moriuchi M, Straus SE, Cohen JI. Varicella-zoster virus open reading frame 10 protein, the herpes simplex virus VP16 homolog, transactivates herpesvirus immediate-early gene promoters. J. Virol. 1993;67:2739–2746. doi: 10.1128/jvi.67.5.2739-2746.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriuchi M, Moriuchi M, Debrus S, Piette J, Cohen JI. The acidic amino-terminal region of varicella-zoster virus open reading frame 4 protein is required for transactivation and can functionally replace the corresponding region of herpes simplex virus ICP27. Virology. 1995;208:376–382. doi: 10.1006/viro.1995.1164. [DOI] [PubMed] [Google Scholar]
- Olson JK, Grose C. Complex formation facilitates endocytosis of the varicella-zoster virus gE:gI Fc receptor. J. Virol. 1998;72:1542–1551. doi: 10.1128/jvi.72.2.1542-1551.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polcicova K, Goldsmith K, Rainish BL, Wisner TW, Johnson DC. The extracellular domain of herpes simplex virus gE is indispensable for efficient cell-to-cell spread: evidence for gE/gI receptors. J. Virol. 2005;79:11990–12001. doi: 10.1128/JVI.79.18.11990-12001.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriquez JE, Moninger T, Grose C. Entry and egress of varicella-zoster virus blocked by same anti-gH monoclonal antibody. Virology. 1993;196:840–844. doi: 10.1006/viro.1993.1543. [DOI] [PubMed] [Google Scholar]
- Santos RA, Hatfield CC, Cole NL, Moffat JF, Arvin AM, Ruyechan WT, Hay J, Grose C. Varicella-zoster virus gE escape mutant VZV-MSP exhibits an accelerated cell-to-cell spread phenotype in both infected cell cultures and SCID-hu mice. Virology. 2000;275:306–317. doi: 10.1006/viro.2000.0507. [DOI] [PubMed] [Google Scholar]
- Tikoo SK, Fitzpatrick DR, Babiuk LA, Zamb TJ. Molecular cloning, sequencing, and expression of functional bovine herpesvirus 1 glycoprotein gIV in transfected bovine cells. J. Virol. 1990;64:5132–5142. doi: 10.1128/jvi.64.10.5132-5142.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisner T, Brunetti C, Dingwell K, Johnson DC. The extracellular domain of herpes simplex virus gE is sufficient for accumulation at cell junctions but not for cell-to-cell spread. J. Virol. 2000;74:2278–2287. doi: 10.1128/jvi.74.5.2278-2287.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi Y, Sadaoka T, Yoshii H, Somboonthum P, Imazawa T, Nagaike K, Ozono K, Yamanishi K, Mori Y. Varicella-zoster virus glycoprotein M homolog is glycosylated, expressed on the viral envelope, and functions in viral cell-to-cell spread. J. Virol. 2008;82:795–804. doi: 10.1128/JVI.01722-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Galvan V, Campadelli-Fiume G, Roizman B. Glycoprotein D or J delivered in trans blocks apoptosis in SK-N-SH cells induced by a herpes virus 1 mutant lacking intact genes expressing both glycoproteins. J. Virol. 2000;74:11782–11791. doi: 10.1128/jvi.74.24.11782-11791.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]