Abstract
The ubiquitin-proteasome system (UPS) represents the major pathway for degradation of intracellular proteins. This article reviews the major components and configurations of the UPS including the 26S proteasome and 11S activated proteasome relevant to myocardial ischaemia. We then present the evidence that the UPS is dysfunctional during myocardial ischaemia as well as potential consequences of this, including dysregulation of target substrates, many of them active signalling proteins, and accumulation of oxidized proteins. As part of this discussion, potential mechanisms, including ATP depletion, inhibition by insoluble protein aggregates, and oxidation of proteasome and regulatory particle subunits, are discussed. Finally, the evidence suggesting a role for the UPS in ischaemic preconditioning is presented. Much of this is inferential but clearly indicates the need for additional research.
Keywords: Ubiquitin-proteasome system, Immunoproteasome, Myocardial ischaemia, Ischaemic preconditioning
1. Introduction
The ubiquitin-proteasome system (UPS) is the major non-lysosomal pathway for intracellular degradation of proteins and plays a major role in regulating many cellular processes. These include the cell cycle,1,2 cell signalling,3–6 apoptosis,7–9 immune response and antigen presentation via the immunoproteasome,10–12 and protein turnover under normal and pathologic conditions.13–17 Regulation of cell functions through degradation of proteins prompted Ciechanover et al.3 to term this ‘biological regulation via destruction’. Moreover, the UPS plays key roles in protein quality control by removal of damaged, oxidized, and/or misfolded proteins.18–22 Following is a relatively brief discussion of the forms of the proteasome thought to be involved in myocardial ischaemia.
1.1. Structure of the ubiquitin-proteasome system
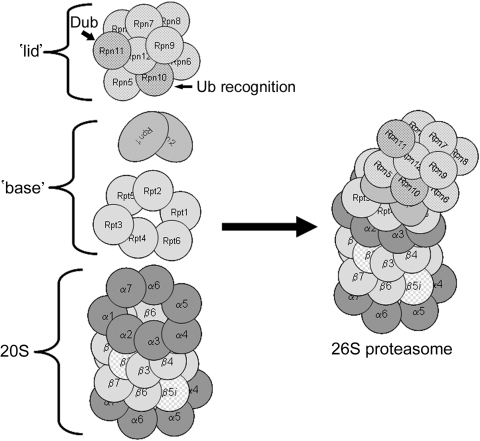
The key components of the UPS are the 26S proteasome and ubiquitin. The 26S-proteasome is a macromolecular structure consisting of two subcomplexes, the 20S-proteasome, and one (mushroom configuration) or two (dumbbell configuration; actually 30S proteasome) 19S-regulatory particles (Figure 1). The 20S-proteasome is the proteolytic core and is a barrel-shaped structure consisting of two pairs of homologous rings each containing seven subunits. The proteolytic activity resides in the inner two rings which contain the β-type subunits, designated β1 through β7. The proteasome has three main proteolytic activities: ‘trypsin-like’, assigned to the β2 subunit; ‘chymotrypsin-like’, assigned to the β5 subunit; and ‘caspase-like’ activity assigned to the β1 subunit. Under certain conditions, the β1, 2, and 5 subunits can be replaced by immunoforms and are designated β1i, β2i, and β5i and the transformed 20S-proteasome called the immunoproteasome.23 Replacement with the immunoforms has been associated with additional proteolytic activities, including BrAAP (cleavage after branched chain amino acids) and SNAAP activities (cleavage after small neutral amino acids) favouring formation of peptides consistent with the MHC class I antigens.24 While this can occur in response to exposure to γ-interferon, in depth proteomic analysis of the cardiac proteasome has indicated that the presence of these immunoforms is more prevalent than previously thought and rather than being homogeneous, the proteasome is quite heterogeneous, and exists as a dynamic mixture of both constitutive and induced (immuno) catalytic β-type subunits.25,26 Subunit heterogeneity is thought to account for the multitude of catalytic activities displayed by the proteasome allowing for cleavage of diverse substrates and production of diverse peptides. The outer rings contain the α-type subunits, designated α1 through α7. In the eukaryotic proteasome, these subunits have no direct proteolytic activity but play an important gating role in preventing access of folded and unfolded proteins to the central proteolytic chamber when proteasome is in the non-activated state.27 X-ray crystallography indicates that the N-termini of subunits α1, α2, α3, α6, and α7 project into the openings at either end of the proteasome effectively sealing it and preventing access to the central chamber. Docking of the 19S regulatory particle to the 20S proteasome core activates the proteasome causing these subunits to rearrange allowing access to the proteolytic core.28,29
Figure 1.
Structure of the 26S proteasome. The 26S-proteasome is composed of the 20S proteasome which is a barrel-shaped structure composed of four rings each consisting of seven subunits. Note that this 20S proteasome has immunoforms of the β2 (β2i) subunit in the upper β ring and the β5 (β5i) subunit in the lower β ring illustrating the heterogeneity of this structure. Docked at one or both ends is the 19S regulatory particle consisting of an additional 18 proteins. For the sake of clarity, the illustrated structure has only one 19S regulatory particle docked. This is the mushroom configuration and is actually 26S. When two 19S regulatory particles are docked at either end this is called the dumbbell configuration and is actually 30S but is also commonly called the 26S proteasome.
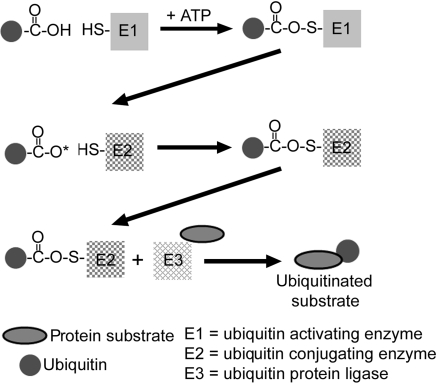
Docked at either end of the 20S-proteasome are the 19S regulatory particles containing an additional 18 subunits arranged in two distinct subcomplexes, the ‘base’ and the ‘lid’. The base consists of the six ATPase subunits, Rpt1 through Rpt6, plus the two largest of the non-ATPase subunits, Rpn1 and Rpn2. The base activates the 20S-core by inducing a conformational change in the α-subunits opening up the entrance channel to the catalytic chamber, and also unfolds the substrate in an ATP requiring process.22 The Rpt2, Rpt5, and Rpn2 subunits also appear to play pivotal roles in attachment of the ‘base’ to 20S proteasome α-rings and binding of the 19S particle ‘lid’ to the ‘base’.30–32 The ‘lid’ contains the remaining non-ATPase subunits, Rpn3 through Rpn12 whose functions are somewhat obscure. Rpn10 contains the main ubiquitin binding (or recognition) domain, and Rpn11 is one of the intrinsic deubiquitinating enzymes. The 26S-proteasome recognizes and cleaves polyubiquitinated substrates into peptides of 5–12 residues in length. The multi-ubiquitination of a protein by sequential addition of ubiquitins to the ε-NH2 of a lysine residue is an energy requiring process involving: (i) activation of ubiquitin by a ubiquitin activating enzyme (E1); (ii) transfer of the activated ubiquitin to a protein substrate by a ubiquitin carrying or conjugating enzyme (E2); and (iii) addition of the ubiquitin to the substrate by a ubiquitin protein ligase (E3) which is the rate-limiting step (Figure 2). Specificity of the UPS resides in the multitude of E3s which number in the 100s (possibly 1000s) and recognize specific motifs on target proteins.24,30,33–37
Figure 2.
Ubiquitination of a protein. Ubiquitin is activated by the ubiquitin activating enzyme, E1, which requires ATP. This is then transferred to a ubiquitin conjugating protein, E2, producing a high-energy E2–ubiquitin thiol ester intermediate. The intermediate is then ligated to a protein substrate bound to a specific ubiquitin protein ligase, E3.
1.2. The 11S activated proteasome
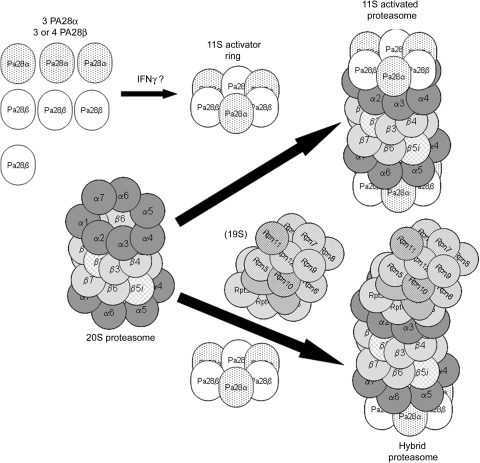
The 11S activated proteasome is an alternate form of the proteasome (‘zomes’) that is regulated by a complex other than the 19S regulatory particle. The regulatory particle controls recognition and unfolding of the protein substrate with the possible exception being direct recognition of oxidized proteins by the 20S proteasome.38 The regulatory particle for the 11S activatedproteasome is the 11S activator ring which is a heterohexamer or heteroheptamer consisting of three PA28α and three PA28β subunits or three PA28α and four PA28β subunits, respectively (Figure 3).39,40 The 11S activated proteasome consists of a 20S proteasome that is docked with one or two 11S activator rings, or one 19S regulatory particle on one end and an 11S activator ring on the other end, called the hybrid proteasome. Docking of an 11S activator ring with the 20S proteasome appears to increase its proteolytic capacity without affecting overall catalytic subunit activity. This is thought to occur as a result of insertion of the carboxy-terminus tails of the PA28 subunits into pockets of the 20S proteasome resulting in conformational changes in its α-subunits. This in turn opens the access channel to a greater degree enhancing access to the catalytic chamber.41,42 PA28 subunits are induced by interferon γ, and despite the fact that knockdowns have very little effect, the 11S activator ring is thought to play a role in antigen presentation, thus this ‘zome’ has been referred to as the immunoproteasome.43–45 All of these subunits and regulatory particles can exist simultaneously and a study of Hela cells suggests that the free 20S proteasome predominates (31%), followed by the hybrid (18%), the double 11S activator ring configuration (15%), the 26S proteasome (11%), and the remainder a mixture of free regulatory subunits.46 An in vitro study suggests dynamic interplay between the capping regulatory particles such that when function of the 19S regulatory particle is impaired, the 20S proteasome may actively recruit and exchange PA28 subunits.47 Even though the function of the 11S activated proteasome is somewhat obscure, it is relevant to any discussion of the role of the proteasome in myocardial ischaemia as studies have suggested a role in removal of damaged or senescent proteins.48,49 Indeed, we50 have shown that this ‘zome’ may be upregulated in experimental hyperglycaemia associated with increased oxidative stress. An intriguing hypothesis is that the 11S activated proteasome, in one form or another, may be responsible for degradation of proteins oxidized during myocardial ischaemia.
Figure 3.
The 11S activated proteasome and the related hybrid proteasome. The 11S activated proteasome is formed when an 11S activator ring docks at one or both ends of a 20S proteasome. The 11S activator ring is composed of either three PA28α and three PA28β subunits (shown) or three PA28α and four PA28β subunits and may be induced by interferon γ. This form of the proteasome or ‘zome’ has also been called the immunoproteasome and invariably contains one or more of the immunoforms of the catalytic β-type subunits. Another ‘zome’ is the hybrid proteasome which has an 11S activator ring docked at one end of the 20S proteasome and a 19S regulatory particle docked at the end. This ‘zome’ has also been called the immunoproteasome.
2. The UPS in ischaemia/reperfusion
Ischaemia is defined as the condition of oxygen deprivation accompanied by inadequate removal of metabolites consequent to reduced perfusion. Since this spotlight issue is focused on the various pathophysiologic roles of the proteasome in cardiovascular disease, any discussion of the role of the UPS in ischaemia/reperfusion should be limited to the heart. However, not to include a discussion of UPS in brain ischaemia or stroke would be a serious omission from a historical perspective as all of the heart studies derive from the earlier brain studies demonstrating dysfunctional proteasome.
2.1. Brain ischaemia/reperfusion
Perhaps the earliest suggestion that the UPS may be dysfunctional following ischaemia/reperfusion was in 1992 with a report of an increase in insoluble ubiquitin-conjugates in the mitochondrial fraction of gerbil cortex and hippocampus following 5 min of transient forebrain ischaemia.51 In 1996, this same group reported reversible decreased 26S proteasome activity in gerbil cortex following 10 min bilateral common carotid artery occlusion.52 This was followed by a report in 2000 of reperfusion time-dependent proteasome dysfunction in both cortex and hippocampus of mice subjected to 1 h middle cerebral artery occlusion, also the first report to suggest that dysfunction may be secondary to oxidative stress.53 Subsequent reports have confirmed and extended these earlier reports.54 The first report55 suggesting proteasome dysfunction as a result of myocardial ischaemia/reperfusion was not until 2001.
2.2. Myocardial ischaemia/reperfusion
In the following discussion, we examine reports indicating that proteasome is dysfunctional during myocardial ischaemia/reperfusion. These reports have raised several questions in regards to mechanism and consequences, if any. Further, some investigators have advocated the rather counter-intuitive use of proteasome inhibitors as a treatment modality for the ischaemic myocardium, a somewhat controversial issue described briefly further on.
The earliest report of decreased proteasome function in myocardial ischaemia/reperfusion was presented by Bulteau et al.55 who showed loss of chymotrypsin-, caspase- and trypsin-like activity following 30 min of in vivo LAD artery occlusion. Following purification, only the decrease in trypsin-like activity was observable, yet ubiquitinated proteins were increased suggesting a functional loss of proteasome activity. Subsequently, we56,57 confirmed this observation in the isolated perfused heart and also demonstrated that the ATP-dependent proteasome activity is decreased and preferentially affected suggesting defects in 26S-proteasome function consistent with increases in myocardial ubiquitinated proteins. While the Bulteau et al.55 study and our studies56,57 may suggest global cardiac proteasome dysfunction, a more recent study58 suggests that at least short-term ischaemia/reperfusion may be more associated with selective dysfunction affecting degradation of specific proteins. However, this study58 did not directly assess proteasome activity but rather took an indirect approach examining degradation (or lack thereof) of signalling proteins known to be subject to UPS-mediated degradation and also examined effects of proteasome inhibitors on these proteins as well. While this type of approach can yield some useful information, the lack of studies of ubiquitinated homologues of these signalling proteins makes it difficult to determine the role of the proteasome. Why proteasome becomes dysfunctional is not clear but could be the result of multiple processes which are discussed in the next section.
2.3. Possible mechanisms for proteasome dysfunction
Three possible mechanisms may explain dysfunction of the UPS during ischaemia/reperfusion: (i) ATP depletion; (ii) direct inhibition by protein aggregates; and (iii) oxidative damage to proteasome and/or regulatory subunits.
2.3.1. ATP depletion during ischaemia
The hypothesis that ATP depletion could be partially responsible for decreased proteasome activity in the ischaemic heart is based on the requirement for ATP by the 19S regulatory particle to unfold protein substrates for presentation to the 20S proteasome.59 It is known that ATP is depleted during ischaemia60 and is likely a contributing factor to proteasome dysfunction during the ischaemic period; however, this is difficult to prove thus remains purely conjecture at this point.
2.3.2. Direct inhibition by protein aggregates
This hypothesis is based on the earlier studies of Sitte et al.61 demonstrating that the incubation of fibroblasts with a synthetic lipofuscin-like material results in proteasome inhibition. These authors theorized that due to their inherent ‘stickiness’, lipofuscin or other crossed linked aggregates of oxidized proteins can physically ‘plug’ the chamber preventing substrate access.62 Subsequently, we63 showed that incubation of cardiomyocytes with a synthetic lipofuscin-like material derived from peroxidized liver mitochondria results in cell death secondary to proteasome inhibition and generalized accumulation of UPS degraded pro-apoptotic proteins. Later studies by several groups have confirmed that accumulation of misfolded or mutated proteins can inhibit the cardiac UPS and result in cardiomyopathy.64–66 These cardiomyopathies are now grouped together in a class known as ‘surplus mutant protein cardiomyopathies’ and is the subject of other reviews in this issue (see Su and Wang67). During myocardial ischaemia/reperfusion, proteins are subject to oxidation66 and these do accumulate in the heart through what is likely a combination of enhanced production56 and decreased proteasome-mediated degradation.68 An attractive hypothesis is that these modified proteins accumulate to a level favouring formation of insoluble aggregates capable of inhibiting the proteasome. In fact, accumulation of aggregates of oxidized proteins leading to impairment of proteasome function has been shown to occur following brain ischaemia/reperfusion.69 To these authors’ knowledge, the only example of such a process reported in heart was an increase in lipofuscin granules in atrial appendage following atrial fibrillation associated with cardiopulmonary bypass.70 However, this was not linked to changes in proteasome activity. Whether or not protein aggregates accumulate as a general consequence of myocardial ischaemia/reperfusion is as of yet unknown.
2.3.3. Oxidation of proteasome and/or regulatory subunits
Oxidative modification of proteins affects their secondary and tertiary structures resulting in unfolding and exposure of hydrophobic patches leading to loss of function and enhanced degradation.71,72 We and others have shown that during myocardial ischaemia/reperfusion, many cytosolic, myofibrillar, and mitochondrial proteins are subject to various oxidative modifications.73–76 Since the proteasome is a macromolecular structure composed of multiple protein subunits, it stands to reason that it could be a potential target of the oxidative species produced during ischaemia/reperfusion. In fact, both 20S and 26S proteasome have been shown to be subject to oxidative inactivation. In vitro studies have shown that exposure of purified proteasome to oxidants, including 4-hydroxynonenal,77,78 peroxynitrite,79,80 hypochlorite, and hydrogen peroxide81 leads to inactivation with the 26S configuration approximately 10-fold more sensitive.81 Consistent with the view that proteasome can be damaged by oxidative phenomena, we82 have shown that pre-treatment of isolated hearts with α-tocotrienol, a vitamin E analogue, preserves post-ischaemic proteasome function. Few studies have examined oxidative damage to proteasome subunits. In their original study, Bulteau et al.55 observed 4-hydroxynonelation of several α-type subunits of the 20S proteasome following ischaemia/reperfusion, though these modifications could not be related to the decreases in proteasome activity. Subsequently, this group reported that proteasome purified from rat heart seems to be somewhat resistant to inactivation by 4-hydroxynonenal requiring concentrations in excess of 100 µM to observe loss of chymotryptic- and caspase-like activities possibly explained by the lack of modification of the catalytic β-type subunits although several α-type units were modified.83 The higher vulnerability of the 26S proteasome to oxidative inactivation84 and our study suggesting that the ATP-dependent activity of the proteasome is most affected by ischaemia/reperfusion,57 has led to an intriguing hypothesis that perhaps subunits of the 19S regulatory particle are more sensitive to oxidative inactivation than subunits of the catalytic 20S proteasome. In fact, this has been demonstrated in SH-SY5Y cells exposed to an oxidative environment in which the Rpt3 (S6 ATPase) subunit, of all 26S proteasome subunits, was found to be uniquely sensitive to carbonylation reactions and that suppressing this subunit using RNAi diminishes 26S proteasome activity.85 In ongoing studies in our laboratory, we have tentatively identified Rpt3/Rpt5 as the only 26S proteasome subunits significantly carbonylated during myocardial ischaemia/reperfusion.86 While much work needs to be done on this promising line of research, we have come to the conclusion that proteasome dysfunction is probably not due to any one factor but rather a combination of multiple processes.
2.4. Consequences of UPS dysfunction
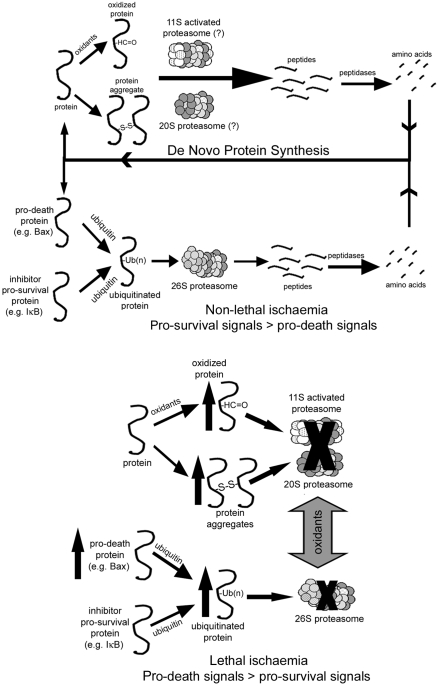
Since the UPS degrades numerous proteins and regulates multiple signalling pathways, it is reasonable to suggest that dysfunction of this complex during ischaemia/reperfusion could have profound effects on myocardial function. We56 have observed that the degree of proteasome dysfunction during reperfusion is dependent on length of ischaemia and correlates with levels of oxidized and ubiquitinated proteins which tend to increase as proteasome activity decreases. Based on this study, we have proposed that dysregulation is occurring in which dysfunctional proteasome fails to degrade normal substrates. Keeping in mind that under normal circumstances, degradation by proteasome is not the rate-limiting step, dysregulation would occur when there is insufficient proteasome activity to degrade ubiquitinated substrates allowing them to accumulate. At what level this occurs or whether dysregulation applies equally to all proteins degraded by the UPS is not known. Our initial study56 suggests that a minimum of 50% dysfunction is necessary, but this was under conditions of high stress. There are several reports that support this hypothesis. For example, phosphorylation of c-SRC signals for ubiquitination and degradation by proteasome.87 Phosphorylated c-SRC is increased during ischaemia and associated with poor outcomes.88 We89 have shown that tocotrienol pre-treatment preserves proteasome function and decreases post-ischaemic levels of phosphorylated c-SRC suggesting that the increase was related to UPS dysfunction. Other studies have suggested that post-ischaemic accumulation of phosphorylated-IκB is related to proteasome dysfunction58 or that dysfunction in some way interferes with UPS regulation of substrate availability for interaction with lipid rafts.90 With respect to non-signalling proteins, we68 have demonstrated that pre-treatment of isolated hearts with the proteasome inhibitor, lactacystin, results in a greater accumulation of oxidized proteins and diminished degradation of oxidized actin in the post-ischaemic heart, thus implying a role in their removal. Several studies report that pre-ischaemic treatment of the heart with a proteasome inhibitor either diminishes post-ischaemic function or has little effect,56,68,91 yet others report just the opposite thus this is controversial (presented in the next section). In our original review on proteasome in the heart,92 we proposed a model to illustrate the possible consequences of UPS dysfunction in the ischaemic heart. According to this model (Figure 4), in the non-ischaemic heart, the UPS functions to degrade oxidized, misfolded, and ubiquitinated proteins thus recycling the constituent amino acids, and maintaining a dynamic balance between pro-survival and pro-death signalling proteins. During ischaemia/reperfusion resulting in cell death or dysfunction, UPS function is inhibited leading to accumulation of oxidized and ubiquitinated proteins. Dysregulation may occur in which normal UPS-mediated degradation of pro-death proteins is depressed. Over the past few years, little has changed that would cause us to alter this model.
Figure 4.
Potential roles for the UPS and 11S-activated proteasome in short and long duration ischaemia. In the non-ischaemic heart, oxidized, misfolded, and ubiquitinated proteins are degraded through both ubiquitin- and non-ubiquitin-mediated pathways, recycling the constituent amino acids, and maintaining a dynamic balance between pro-survival and pro-death signals. During an ischaemic insult resulting in cell death or dysfunction, UPS function is inhibited leading to accumulation of oxidized and ubiquitinated proteins. In addition, a condition known as dysregulation may occur in which normal UPS-mediated degradation of pro-death proteins is depressed.
2.5. Use of proteasome inhibitors to mitigate myocardial ischaemic injury
A handful of studies in the literature suggest that this strategy may be beneficial. Most of these studies examined the proteasome inhibitor, PS-519 (Millennium Pharmaceuticals), with the rationale that the inhibitor would either decrease leukocyte adhesion to endothelial cells or diminish nuclear translocation of NFκB thus limiting the inflammatory response.93–95 The most recent of these studies95 prompted us to respond with a letter96 expressing concerns in light of several studies97–99 reporting cardiac toxicity associated with administration of the proteasome inhibitor, bortizomeb (Velcade®), for the treatment of multiple myeloma. As stated previously,92,96 under certain conditions, it is conceivable that a proteasome inhibitor may be of some value due to their potential anti-inflammatory properties. However, when proteasome may already be significantly dysfunctional, caution is advisable as additional inhibition may push the cell towards death. We do not envision global proteasome inhibitors as the future for this type of therapy, but rather foresee altering degradation of specific proteins through targeting of specific E3 ligases as a future viable therapeutic intervention. The use of proteasome inhibitors in myocardial ischaemia is discussed in depth in this issue by Yu and Kem.100
2.6. UPS dysfunction during ischaemia of other organs
Proteasome activity has been examined in one other organ, the kidney. Long-term renal ischaemia induced by permanent clipping of one renal artery101 and reversible renal ischaemia followed by reperfusion102 are associated with diminished proteasome activity. The observance of similar findings in multiple organ systems suggests broad relevance of these findings.
2.7. Autophagy and the UPS
Autophagy represents an additional cellular pathway for protein degradation. Studies have indicated that in certain pathological states, including some cardiac proteinopathies, autophagy and the UPS may be activated in parallel or alone to compensate if one or the other is inhibited (Su and Wang,67 this issue). Although studies have indicated a role for autophagy in ischaemia/reperfusion103 and adenosine-mediated preconditioning,104 evidence for a similar link to the UPS under these conditions is lacking at this time.
3. Potential role for the UPS in ischaemic preconditioning
Ischaemic preconditioning (IPC) decreases vulnerability of the myocardium to longer durations of ischaemia as a result of pre-ischaemic exposure to short ischaemic bursts resulting in improved post-ischaemic haemodynamic function and reduced markers of myocardial injury.105 The mechanisms by which IPC exert its protective effects appear to involve signalling changes resulting in opening of the inward mitochondrial KATP channels106 and prevention of opening of the mitochondrial permeability transition pore.107 Early effects of IPC include decreased release of cytochrome C, diminished cellular apoptosis,107,108 and decreased production of oxidative species during the early phases of reperfusion.109 One of the later effects of IPC is decreased levels of certain pro-apoptotic proteins, such as Bax.110 For a more complete review of the signalling changes associated with IPC see Murphy and Steenbergen.111 Given that the UPS degrades up to 70% of all intracellular proteins, including many signalling proteins,92 a hypothesis has emerged that perhaps the UPS plays a role in IPC whereby this system facilitates some of the signalling changes associated with this protective manoeuvre. As discussed earlier, the UPS may become dysfunctional as a result of ischaemia so by necessity IPC must in some way preserve post-ischaemic proteasome function. In the following discussion, we review what is primarily inferential evidence in support of this hypothesis.
Like ischaemia/reperfusion, much of the inferential evidence can be derived from the ischaemic brain literature. For example, the pro-apoptotic protein, Bim, and its ubiquitinated homologues accumulate following simulated ischaemia (hypoxia and glucose deprivation) of cultured neurons, yet are rapidly degraded if ischaemia is preceded by a preconditioning stimulus112 suggesting preservation of functional UPS. Other studies report that IPC prior to transient focal brain ischaemia results in diminished production of protein aggregates113 or that prior administration of a proteasome inhibitor prevents IPC induced translocation of NFκB and diminishes protective effects.114
To date, most of the studies in heart parallel observations in the brain. At least two studies indicate that prior treatment with a proteasome inhibitor can prevent protective effects of myocardial IPC that include degradation of PTEN91 and IκB;58 and one study115 that suggests a similar effect on postconditioning where the intermittent ischaemia is initiated within the first 10 min of reperfusion. A recent study presented the rather intriguing hypothesis that the immunoproteasome is in some way involved in IPC based on the observation that protection is lost in mice deficient in the β1i subunit (LMP2) and implicated pre-ischaemic changes in PTEN.116 Given the diverse pathways regulated by the UPS, that while important it is unlikely that only changes in PTEN can account for the loss of IPC protection in this transgene. Further, this study focused on the immunoproteasome with no analysis of constitutive proteasome, did not assess global function or possible defective assembly of proteasome in the presence of the subunit knockout, and did not assess the effect of IPC on proteasome function, thus leaving many questions unanswered. The only data that IPC might actually preserve post-ischaemic function of the proteasome is presented in our original study56 where preliminary results suggest that pharmacologic preconditioning with nicorandil may have some protective effects. Nicorandil is an anti-anginal drug thought to open the inward mitochondrial KATP channels and mimic the protective effects of IPC.117 While these studies may suggest a role for the UPS in IPC, there have been no definitive studies clearly demonstrating a protective effect of IPC on post-ischaemic proteasome activity and the underlying ischaemia-induced defect or on UPS regulated signalling events.
4. Summary
This review has presented evidence that the UPS plays a role in myocardial ischaemia and IPC. The evidence that the UPS is dysfunctional during myocardial ischaemia/reperfusion was examined. Potential mechanisms for the dysfunction were discussed but it is clear that the actual mechanism(s) is not known indicating the need for more studies. Also, potential consequences of proteasome dysfunction were presented with the evidence suggesting that the process of dysregulation is occurring. However, even with this topic, many of the studies were inferential at best and none addressed the critical question as to the level of dysfunction necessary before regulation of a protein becomes dysregulated. Lastly, we examined the evidence that the UPS plays some role in IPC possibly by facilitating degradation of pro-apoptotic proteins in the post-ischaemic period, yet the evidence here is even more inferential. Clearly, additional studies are required to improve our understanding of UPS function during ischaemia/reperfusion so that viable therapeutic modalities can be developed that target specific proteins.
Conflict of interest: none declared.
Funding
This work was supported by National Institutes of Health (HL68936 to S.R.P.).
References
- 1.Castro A, Bernis C, Vigneron S, Labbe JC, Lorca T. The anaphase-promoting complex: a key factor in the regulation of cell cycle. Oncogene. 2005;24:314–325. doi: 10.1038/sj.onc.1207973. [DOI] [PubMed] [Google Scholar]
- 2.Hershko A. Roles of ubiquitin-mediated proteolysis in cell cycle control. Curr Opin Cell Biol. 1997;9:788–799. doi: 10.1016/s0955-0674(97)80079-8. [DOI] [PubMed] [Google Scholar]
- 3.Ciechanover A, Orian A, Schwartz AL. Ubiquitin-mediated proteolysis: biological regulation via destruction. BioEssays. 2000;22:442–451. doi: 10.1002/(SICI)1521-1878(200005)22:5<442::AID-BIES6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 4.Desterro JM, Rodriguez MS, Hay RT. Regulation of transcription factors by protein degradation. Cell Mol Life Sci. 2000;57:1207–1219. doi: 10.1007/PL00000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuchs SY, Fried VA, Ronai Z. Stress-activated kinases regulate protein stability. Oncogene. 1998;17:1483–1490. doi: 10.1038/sj.onc.1202184. [DOI] [PubMed] [Google Scholar]
- 6.Haglund K, Di Fiore PP, Dikic I. Distinct monoubiquitin signals in receptor endocytosis. Trends Biochem Sci. 2003;28:598–603. doi: 10.1016/j.tibs.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Grimm LM, Osborne BA. Apoptosis and the proteasome. Results Probl Cell Differ. 1999;23:209–228. doi: 10.1007/978-3-540-69184-6_10. [DOI] [PubMed] [Google Scholar]
- 8.Jesenberger V, Jentsch S. Deadly encounter: ubiquitin meets apoptosis. Nat Rev Mol Cell Biol. 2002;3:112–121. doi: 10.1038/nrm731. [DOI] [PubMed] [Google Scholar]
- 9.Orlowski RA. The role of the ubiquitin-proteasome pathway in apoptosis. Cell Death Differentiat. 1999;6:303–313. doi: 10.1038/sj.cdd.4400505. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg AL, Gaczynska M, Grant E, Michalek M, Rock KL. Functions of the proteasome in antigen presentation. Cold Spring Harb Symp Quant Biol. 1995;60:479–490. doi: 10.1101/sqb.1995.060.01.052. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg AL, Cascio P, Saric T, Rock KL. The importance of the proteasome and subsequent proteolytic steps in the generation of antigenic peptides. Mol Immunol. 2002;39:147–164. doi: 10.1016/s0161-5890(02)00098-6. [DOI] [PubMed] [Google Scholar]
- 12.Kloetzel PM. Antigen processing by the proteasome. Nat Rev Mol Cell Biol. 2001;2:179–187. doi: 10.1038/35056572. [DOI] [PubMed] [Google Scholar]
- 13.Ciechanover A. Proteolysis: from the lysosome to ubiquitin and the proteasome. Nat Rev Mol Cell Biol. 2005;6:79–87. doi: 10.1038/nrm1552. [DOI] [PubMed] [Google Scholar]
- 14.Price SR. Increased transcription of ubiquitin-proteasome system components: molecular responses associated with muscle atrophy. Int J Biochem Cell Biol. 2003;35:617–628. doi: 10.1016/s1357-2725(02)00385-0. [DOI] [PubMed] [Google Scholar]
- 15.Tisdale MJ. Pathogenesis of cancer cachexia. J Support Oncol. 2003;1:159–168. [PubMed] [Google Scholar]
- 16.Hasselgren PO, Fischer JE. Muscle cachexia: current concepts of intracellular mechanisms and molecular regulation. Ann Surg. 2001;233:9–17. doi: 10.1097/00000658-200101000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor RG, Tassy C, Briand M, Robert N, Briand Y, Ouali A. Proteolytic activity of proteasome on myofibrillar structures. Mol Biol Rep. 1995;21:71–73. doi: 10.1007/BF00990974. [DOI] [PubMed] [Google Scholar]
- 18.Brodsky JL, McCracken AA. ER protein quality control and proteasome-mediated protein degradation. Semin Cell Dev Biol. 1999;10:507–513. doi: 10.1006/scdb.1999.0321. [DOI] [PubMed] [Google Scholar]
- 19.Davies KJ. Degradation of oxidized proteins by the 20S proteasome. Biochimie. 2001;83:301–310. doi: 10.1016/s0300-9084(01)01250-0. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–899. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- 21.Hirsch C, Jarosch E, Sommer T, Wolf DH. Endoplasmic reticulum-associated protein degradation—one model fits all? Biochim Biophys Acta. 2004;1695:215–223. doi: 10.1016/j.bbamcr.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Rabl J, Smith DM, Yu Y, Chang SC, Goldberg AL, Cheng Y. Mechanism of gate opening in the 20S proteasome by the proteasomal ATPases. Mol Cell. 2008;30:360–368. doi: 10.1016/j.molcel.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaczynska M, Rock KL, Goldberg AL. Role of proteasomes in antigen presentation. Enzyme Protein. 1993;47:354–369. doi: 10.1159/000468693. [DOI] [PubMed] [Google Scholar]
- 24.Orlowski M, Wilk S. Catalytic activities of the 20 S proteasome, a multicatalytic proteinase complex. Arch Biochem Biophys. 2000;383:1–16. doi: 10.1006/abbi.2000.2036. [DOI] [PubMed] [Google Scholar]
- 25.Drews O, Zong C, Ping P. Exploring proteasome complexes by proteomic approaches. Proteomics. 2007;7:1047–1058. doi: 10.1002/pmic.200600574. [DOI] [PubMed] [Google Scholar]
- 26.Gomes AV, Zong C, Edmondson RD, Li X, Stefani E, Zhang J, et al. Mapping the murine cardiac 26S Proteasome complexes. Circ Res. 2006;99:362–371. doi: 10.1161/01.RES.0000237386.98506.f7. [DOI] [PubMed] [Google Scholar]
- 27.Groll M, Huber R. Substrate access and processing by the 20S proteasome core particle. Int J Biochem Cell Biol. 2003;35:606–616. doi: 10.1016/s1357-2725(02)00390-4. [DOI] [PubMed] [Google Scholar]
- 28.Moore DJ, Dawson VL, Dawson TM. Role for the ubiquitin-proteasome system in Parkinson's disease and other neurodegenerative brain amyloidoses. Neuromolecular Med. 2003;4:95–108. doi: 10.1385/NMM:4:1-2:95. [DOI] [PubMed] [Google Scholar]
- 29.Groll M, Bajorek M, Kohler A, Moroder L, Rubin DM, Huber R, et al. A gated channel into the proteasome core particle. Nat Struct Biol. 2000;7:1062–1067. doi: 10.1038/80992. [DOI] [PubMed] [Google Scholar]
- 30.DeMartino GN. Purification of PA700, the 19S regulatory complex of the 26S proteasome. Methods Enzymol. 2005;398:295–306. doi: 10.1016/S0076-6879(05)98024-5. [DOI] [PubMed] [Google Scholar]
- 31.Gorbea C, Taillandier D, Rechsteiner M. Assembly of the regulatory complex of the 26S proteasome. Mol Biol Rep. 1999;26:15–19. doi: 10.1023/a:1006957802028. [DOI] [PubMed] [Google Scholar]
- 32.Gillette TG, Kumar B, Thompson D, Slaughter CA, DeMartino GN. Differential roles of the COOH termini of AAA subunits of PA700 (19 S regulator) in asymmetric assembly and activation of the 26 S proteasome. J Biol Chem. 2008;283:31813–31822. doi: 10.1074/jbc.M805935200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sawada H, Muto K, Fujimuro M, Akaishi T, Sawada MT, Yokosawa H, et al. Different ratios in 20 S proteasomes and regulatory subunit complexes in two isoforms of the 26 S proteasome purified from rabbit skeletal muscle. FEBS Lett. 1993;335:207–212. doi: 10.1016/0014-5793(93)80731-9. [DOI] [PubMed] [Google Scholar]
- 34.Zwickl P, Voges D, Baumeister W. The proteasome: a macromolecular assembly designed for controlled proteolysis. Philos Trans R Soc Lond B Biol Sci. 1999;354:1501–1511. doi: 10.1098/rstb.1999.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heinemeyer W, Ramos PC, Dohmen RJ. The ultimate nanoscale mincer: assembly, structure and active sites of the 20S proteasome core. Cell Mol Life Sci. 2004;61:1562–1578. doi: 10.1007/s00018-004-4130-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baumeister W, Walz J, Zuhl F, Seemuller E. The proteasome: paradigm of a self-compartmentalizing protease. Cell. 1998;92:367–380. doi: 10.1016/s0092-8674(00)80929-0. [DOI] [PubMed] [Google Scholar]
- 37.Glickman MH, Rubin DM, Coux O, Wefes I, Pfeifer G, Cjeka Z, et al. A subcomplex of the proteasome regulatory particle required for ubiquitin-conjugate degradation and related to the COP9-signalosome and eIF3. Cell. 1998;94:615–623. doi: 10.1016/s0092-8674(00)81603-7. [DOI] [PubMed] [Google Scholar]
- 38.DeMartino GN, Slaughter CA. Regulatory proteins of the proteasome. Enzyme Protein. 1993;47:314–324. doi: 10.1159/000468689. [DOI] [PubMed] [Google Scholar]
- 39.Song X, von Kampen J, Slaughter CA, DeMartino GN. Relative functions of the alpha and beta subunits of the proteasome activator, PA28. J Biol Chem. 1997;272:27994–28000. doi: 10.1074/jbc.272.44.27994. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Z, Krutchinsky A, Endicott S, Realini C, Rechsteiner M, Standing KG. Proteasome activator 11S REG or PA28: recombinant REG alpha/REG beta hetero-oligomers are heptamers. Biochemistry. 1999;38:5651–5658. doi: 10.1021/bi990056+. [DOI] [PubMed] [Google Scholar]
- 41.Stohwasser R, Salzmann U, Giesebrecht J, Kloetzel PM, Holzhutter HG. Kinetic evidences for facilitation of peptide channelling by the proteasome activator PA28. Eur J Biochem. 2000;267:6221–6230. doi: 10.1046/j.1432-1327.2000.01706.x. [DOI] [PubMed] [Google Scholar]
- 42.Whitby FG, Masters EI, Kramer L, Knowlton JR, Yao Y, Wang CC, et al. Structural basis for the activation of 20S proteasomes by 11S regulators. Nature. 2000;408:115–120. doi: 10.1038/35040607. [DOI] [PubMed] [Google Scholar]
- 43.Groettrup M, Soza A, Eggers M, Kuehn L, Dick TP, Schild H, et al. A role for the proteasome regulator PA28alpha in antigen presentation. Nature. 1996;381:166–168. doi: 10.1038/381166a0. [DOI] [PubMed] [Google Scholar]
- 44.Kloetzel PM, Soza A, Stohwasser R. The role of the proteasome system and the proteasome activator PA28 complex in the cellular immune response. Biol Chem. 1999;380:293–297. doi: 10.1515/BC.1999.040. [DOI] [PubMed] [Google Scholar]
- 45.Murata S, Kawahara H, Tohma S, Yamamoto K, Kasahara M, Nabeshima Y, et al. Growth retardation in mice lacking the proteasome activator PA28gamma. J Biol Chem. 1999;274:38211–38215. doi: 10.1074/jbc.274.53.38211. [DOI] [PubMed] [Google Scholar]
- 46.Tanahashi N, Murakami Y, Minami Y, Shimbara N, Hendil KB, Tanaka K. Hybrid proteasomes. Induction by interferon-gamma and contribution to ATP-dependent proteolysis. J Biol Chem. 2000;275:14336–14345. doi: 10.1074/jbc.275.19.14336. [DOI] [PubMed] [Google Scholar]
- 47.Shibatani T, Carlson EJ, Larabee F, McCormack AL, Fruh K, Skach WR. Global organization and function of mammalian cytosolic proteasome pools: implications for PA28 and 19S regulatory complexes. Mol Biol Cell. 2006;17:4962–4971. doi: 10.1091/mbc.E06-04-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferrington DA, Husom AD, Thompson LV. Altered proteasome structure, function, and oxidation in aged muscle. FASEB J. 2005;19:644–646. doi: 10.1096/fj.04-2578fje. [DOI] [PubMed] [Google Scholar]
- 49.Husom AD, Peters EA, Kolling EA, Fugere NA, Thompson LV, Ferrington DA. Altered proteasome function and subunit composition in aged muscle. Arch Biochem Biophys. 2004;421:67–76. doi: 10.1016/j.abb.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 50.Powell SR, Samuel SM, Wang P, Divald A, Thirunavukkarasu M, Koneru S, et al. Upregulation of myocardial 11S-activated proteasome in experimental hyperglycemia. J Mol Cell Cardiol. 2008;44:618–621. doi: 10.1016/j.yjmcc.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 51.Hayashi T, Takada K, Matsuda M. Post-transient ischemia increase in ubiquitin conjugates in the early reperfusion. NeuroReport. 1992;3:519–520. doi: 10.1097/00001756-199206000-00016. [DOI] [PubMed] [Google Scholar]
- 52.Kamikubo T, Hayashi T. Changes in proteasome activity following transient ischemia. Neurochem Int. 1996;28:209–212. doi: 10.1016/0197-0186(95)00071-2. [DOI] [PubMed] [Google Scholar]
- 53.Keller JN, Huang FF, Zhu H, Yu J, Ho YS, Kindy TS. Oxidative stress-associated impairment of proteasome activity during ischemia-reperfusion injury. J Cereb Blood Flow Metab. 2000;20:1467–1473. doi: 10.1097/00004647-200010000-00008. [DOI] [PubMed] [Google Scholar]
- 54.Asai A, Tanahashi N, Qiu JH, Saito N, Chi S, Kawahara N, et al. Selective proteasomal dysfunction in the hippocampal CA1 region after transient forebrain ischemia. J Cereb Blood Flow Metab. 2002;22:705–710. doi: 10.1097/00004647-200206000-00009. [DOI] [PubMed] [Google Scholar]
- 55.Bulteau AL, Lundberg KC, Humphries KM, Sadek HA, Szweda PA, Friguet B, et al. Oxidative modification and inactivation of the proteasome during coronary occlusion/reperfusion. J Biol Chem. 2001;276:30057–30063. doi: 10.1074/jbc.M100142200. [DOI] [PubMed] [Google Scholar]
- 56.Powell SR, Wang P, Katzeff HL, Shringarpure R, Teoh C, Khaliulin I, et al. Oxidized and ubiquitinated proteins may predict recovery of postischemic cardiac function. Essential role of the proteasome. Antioxid Redox Signal. 2005;7:538–535. doi: 10.1089/ars.2005.7.538. [DOI] [PubMed] [Google Scholar]
- 57.Powell SR, Davies KJA, Divald A. Optimal determination of heart tissue 26S Proteasome activity requires maximal stimulating concentrations of ATP. J Mol Cell Cardiol. 2007;42:265–269. doi: 10.1016/j.yjmcc.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gurusamy N, Goswami S, Malik G, Das DK. Oxidative injury induces selective rather than global inhibition of proteasomal activity. J Mol Cell Cardiol. 2008;44:419–428. doi: 10.1016/j.yjmcc.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 59.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 60.Jennings RB, Steenbergen C., Jr Nucleotide metabolism and cellular damage in myocardial ischemia. Annu Rev Physiol. 1985;47:727–749. doi: 10.1146/annurev.ph.47.030185.003455. [DOI] [PubMed] [Google Scholar]
- 61.Sitte N, Huber M, Grune T, Ladhoff A, Doecke WD, von Zglinicki T, et al. Proteasome inhibition by lipofuscin/ceroid during postmitotic aging of fibroblasts. FASEB J. 2000;14:1490–1498. doi: 10.1096/fj.14.11.1490. [DOI] [PubMed] [Google Scholar]
- 62.Grune T, Jung T, Merker K, Davies KJ. Decreased proteolysis caused by protein aggregates, inclusion bodies, plaques, lipofuscin, ceroid, and 'aggresomes' during oxidative stress, aging, and disease. Int J Biochem Cell Biol. 2004;36:2519–2530. doi: 10.1016/j.biocel.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 63.Powell SR, Wang P, Divald A, Teichberg S, Haridas V, McCloskey TW, et al. Aggregates of oxidized proteins (lipofuscins) induce apoptosis through proteasome inhibition and dysregulation of pro-apoptotic proteins. Free Radic Biol Med. 2005;38:1093–1101. doi: 10.1016/j.freeradbiomed.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 64.Liu J, Tang M, Mestril R, Wang X. Aberrant protein aggregation is essential for a mutant desmin to impair the proteolytic function of the ubiquitin-proteasome system in cardiomyocytes. J Mol Cell Cardiol. 2006;40:451–454. doi: 10.1016/j.yjmcc.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 65.Liu J, Chen Q, Huang W, Horak KM, Zheng H, Mestril R, et al. Impairment of the ubiquitin-proteasome system in desminopathy mouse hearts. FASEB J. 2006;20:362–364. doi: 10.1096/fj.05-4869fje. [DOI] [PubMed] [Google Scholar]
- 66.Sarikas A, Carrier L, Schenke C, Doll D, Flavigny J, Lindenberg KS, et al. Impairment of the ubiquitin-proteasome system by truncated cardiac myosin binding protein C mutants. Cardiovasc Res. 2005;66:33–44. doi: 10.1016/j.cardiores.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 67.Su H, Wang X. The ubiquitin-proteasome system in cardiac proteinopathy: a quality control perspective. Cardiovasc Res. 2010;85:253–262. doi: 10.1093/cvr/cvp287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Divald A, Powell SR. Proteasome mediates removal of proteins oxidized during myocardial ischemia. Free Radic Biol Med. 2006;40:156–164. doi: 10.1016/j.freeradbiomed.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 69.Ge P, Luo Y, Liu CL, Hu B. Protein aggregation and proteasome dysfunction after brain ischemia. Stroke. 2007;38:3230–3236. doi: 10.1161/STROKEAHA.107.487108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ad N, Snir E, Vidne BA, Golomb E. Histologic atrial myolysis is associated with atrial fibrillation after cardiac operation. Ann Thorac Surg. 2001;72:688–693. doi: 10.1016/s0003-4975(01)02882-x. [DOI] [PubMed] [Google Scholar]
- 71.Davies KJ, Lin SW, Pacifici RE. Protein damage and degradation by oxygen radicals. IV. Degradation of denatured protein. J Biol Chem. 1987;262:9914–9920. [PubMed] [Google Scholar]
- 72.Davies KJ, Delsignore ME. Protein damage and degradation by oxygen radicals. III. Modification of secondary and tertiary structure. J Biol Chem. 1987;262:9908–9913. [PubMed] [Google Scholar]
- 73.Canton M, Neverova I, Menabo R, Van Eyk J, Di Lisa F. Evidence of myofibrillar protein oxidation induced by postischemic reperfusion in isolated rat hearts. Am J Physiol Heart Circ Physiol. 2004;286:H870–H877. doi: 10.1152/ajpheart.00714.2003. [DOI] [PubMed] [Google Scholar]
- 74.Khaliulin I, Schwalb H, Wang P, Houminer E, Grinberg L, Katzeff H, et al. Preconditioning improves postischemic mitochondrial function and diminishes oxidation of mitochondrial proteins. Free Radic Biol Med. 2004;37:1–9. doi: 10.1016/j.freeradbiomed.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 75.Park Y, Kanekal S, Kehrer JP. Oxidative changes in hypoxic rat heart tissue. Am J Physiol Heart Circ Physiol. 1991;260:H1395–H1405. doi: 10.1152/ajpheart.1991.260.5.H1395. [DOI] [PubMed] [Google Scholar]
- 76.Powell SR, Gurzenda EM, Wahezi SE. Actin is oxidized during myocardial ischemia. Free Radic Biol Med. 2001;30:1171–1176. doi: 10.1016/s0891-5849(01)00514-7. [DOI] [PubMed] [Google Scholar]
- 77.Friguet B, Szweda LI. Inhibition of the multicatalytic proteinase (proteasome) by 4-hydroxy-2-nonenal cross-linked protein. FEBS Lett. 1997;405:21–25. doi: 10.1016/s0014-5793(97)00148-8. [DOI] [PubMed] [Google Scholar]
- 78.Okada K, Wangpoengtrakul C, Osawa T, Toyokuni S, Tanaka K, Uchida K. 4-Hydroxy-2-nonenal-mediated impairment of intracellular proteolysis during oxidative stress. Identification of proteasomes as target molecules. J Biol Chem. 1999;274:23787–23793. doi: 10.1074/jbc.274.34.23787. [DOI] [PubMed] [Google Scholar]
- 79.Amici M, Lupidi G, Angeletti M, Fioretti E, Eleuteri AM. Peroxynitrite-induced oxidation and its effects on isolated proteasomal systems. Free Radic Biol Med. 2003;34:987–996. doi: 10.1016/s0891-5849(02)01369-2. [DOI] [PubMed] [Google Scholar]
- 80.Osna NA, Haorah J, Krutik VM, Donohue TM., Jr Peroxynitrite alters the catalytic activity of rodent liver proteasome in vitro and in vivo. Hepatology. 2004;40:574–582. doi: 10.1002/hep.20352. [DOI] [PubMed] [Google Scholar]
- 81.Reinheckel T, Sitte N, Ullrich O, Kuckelkorn U, Davies KJ, Grune T. Comparative resistance of the 20S and 26S proteasome to oxidative stress. Biochem J. 1998;335:637–642. doi: 10.1042/bj3350637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Das S, Powell SR, Wang P, Divald A, Nesaretnam K, Tosaki A, et al. Cardioprotection with palm tocotrienol: antioxidant activity of tocotrienol is linked with its ability to stabilize proteasomes. Am J Physiol Heart Circ Physiol. 2005;289:361–367. doi: 10.1152/ajpheart.01285.2004. [DOI] [PubMed] [Google Scholar]
- 83.Farout L, Mary J, Vinh J, Szweda LI, Friguet B. Inactivation of the proteasome by 4-hydroxy-2-nonenal is site specific and dependant on 20S proteasome subtypes. Arch Biochem Biophys. 2006;453:135–142. doi: 10.1016/j.abb.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 84.Reinheckel T, Ullrich O, Sitte N, Grune T. Differential impairment of 20S and 26S proteasome activities in human hematopoietic K562 cells during oxidative stress. Arch Biochem Biophys. 2000;377:65–68. doi: 10.1006/abbi.2000.1717. [DOI] [PubMed] [Google Scholar]
- 85.Ishii T, Sakurai T, Usami H, Uchida K. Oxidative modification of proteasome: identification of an oxidation-sensitive subunit in 26 s proteasome. Biochemistry. 2005;44:13893–13901. doi: 10.1021/bi051336u. [DOI] [PubMed] [Google Scholar]
- 86.Powell SR, Divald A, Wang P, Roberts B, Teichberg S. Myocardial ischemic preconditioning preserves postischemic function through diminished oxidative damage to 19S regulatory particle subunits. (Abstract) Free Radic Biol Med. 2008;45:S148–S149. doi: 10.1161/CIRCRESAHA.110.219485. [DOI] [PubMed] [Google Scholar]
- 87.Harris KF, Shoji I, Cooper EM, Kumar S, Oda H, Howley PM. Ubiquitin-mediated degradation of active Src tyrosine kinase. Proc Natl Acad Sci USA. 1999;96:13738–13743. doi: 10.1073/pnas.96.24.13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hattori R, Otani H, Uchiyama T, Imamura H, Cui J, Maulik N, et al. Src tyrosine kinase is the trigger but not the mediator of ischemic preconditioning. Am J Physiol. 2001;281:H1066–H1074. doi: 10.1152/ajpheart.2001.281.3.H1066. [DOI] [PubMed] [Google Scholar]
- 89.Bence NF, Sampat RM, Kopito RR. Impairment of the ubiquitin-proteasome system by protein aggregation. Science. 2001;292:1552–1555. doi: 10.1126/science.292.5521.1552. [DOI] [PubMed] [Google Scholar]
- 90.Das M, Das S, Wang P, Powell SR, Das DK. Caveolin and proteasome in tocotrienol mediated cellular protection. Cell Physiol Biochem. 2008;22:287–294. doi: 10.1159/000149807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cai Z, Semenza GL. PTEN activity is modulated during ischemia and reperfusion: involvement in the induction and decay of preconditioning. Circ Res. 2005;97:1351–1359. doi: 10.1161/01.RES.0000195656.52760.30. [DOI] [PubMed] [Google Scholar]
- 92.Powell SR. The ubiquitin proteasome system in cardiac physiology and pathology. Am J Physiol Heart Circ Physiol. 2006;291:1–19. doi: 10.1152/ajpheart.00062.2006. [DOI] [PubMed] [Google Scholar]
- 93.Campbell B, Adams J, Shin YK, Lefer AM. Cardioprotective effects of a novel proteasome inhibitor following ischemia and reperfusion in the isolated perfused rat heart. J Mol Cell Cardiol. 1999;31:467–476. doi: 10.1006/jmcc.1998.0880. [DOI] [PubMed] [Google Scholar]
- 94.Pye J, Ardeshirpour F, McCain A, Bellinger DA, Merricks E, Adams J, et al. Proteasome inhibition ablates activation of NF-kappa B in myocardial reperfusion and reduces reperfusion injury. Am J Physiol Heart Circ Physiol. 2003;284:H919–H926. doi: 10.1152/ajpheart.00851.2002. [DOI] [PubMed] [Google Scholar]
- 95.Stansfield WE, Moss NC, Willis MS, Tang R, Selzman C. Proteasome inhibition attenuates infarct size and preserves cardiac function in a murine model of myocardial ischemia-reperfusion injury. Ann Thorac Surg. 2007;84:120–125. doi: 10.1016/j.athoracsur.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 96.Powell SR. Proteasome inhibitors in myocardial ischemia, some concerns. Ann Thorac Surg. 2008;85:1503–1504. doi: 10.1016/j.athoracsur.2007.10.049. [DOI] [PubMed] [Google Scholar]
- 97.Voortman J, Giaccone G. Severe reversible cardiac failure after bortezomib treatment combined with chemotherapy in a non-small cell lung cancer patient: a case report. BMC Cancer. 2006;6:129. doi: 10.1186/1471-2407-6-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Enrico O, Gabriele B, Nadia C, Sara G, Daniele V, Giulia C, et al. Unexpected cardiotoxicity in haematological bortezomib treated patients. Br J Haematol. 2007;138:396–397. doi: 10.1111/j.1365-2141.2007.06659.x. [DOI] [PubMed] [Google Scholar]
- 99.Hacihanefioglu A, Tarkun P, Gonullu E. Acute severe cardiac failure in a myeloma patient due to proteasome inhibitor bortezomib. Int J Hematol. 2008;88:219–222. doi: 10.1007/s12185-008-0139-7. [DOI] [PubMed] [Google Scholar]
- 100.Yu X, Kem DC. Proteasome inhibition during myocardial infarction. Cardiovasc Res. 2010;85:312–320. doi: 10.1093/cvr/cvp309. [DOI] [PubMed] [Google Scholar]
- 101.Kruszewski K, Kalinowska J, Chabielska E, Kasacka I, Ostrowska H. Changes in proteasome activity in the ischemic kidney of rat with experimental renovascular hypertension. Rocz Akad Med Bialymst. 2004;49(Suppl. 1):252–254. [PubMed] [Google Scholar]
- 102.Yeboah MM, Xue X, Javdan M, Susin M, Metz CN. Nicotinic acetylcholine receptor expression and regulation in the rat kidney after ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2008;295:F654–F661. doi: 10.1152/ajprenal.90255.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hamacher-Brady A, Brady NR, Gottlieb RA. The interplay between pro-death and pro-survival signaling pathways in myocardial ischemia/reperfusion injury: apoptosis meets autophagy. Cardiovasc Drugs Ther. 2006;20:445–462. doi: 10.1007/s10557-006-0583-7. [DOI] [PubMed] [Google Scholar]
- 104.Yitzhaki S, Huang C, Liu W, Lee Y, Gustafsson AB, Mentzer RM, et al. Autophagy is required for preconditioning by the adenosine A1 receptor-selective agonist CCPA. Basic Res Cardiol. 2009;104:157–167. doi: 10.1007/s00395-009-0006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Murry CE, Jennings RB, Reimer KA. New insights into potential mechanisms of ischemic preconditioning. Circulation. 1991;84:442–445. doi: 10.1161/01.cir.84.1.442. [DOI] [PubMed] [Google Scholar]
- 106.Gross GJ, Fryer RM. Sarcolemmal versus mitochondrial ATP-sensitive K+ channels and myocardial preconditioning. Circ Res. 1999;84:973–979. doi: 10.1161/01.res.84.9.973. [DOI] [PubMed] [Google Scholar]
- 107.Korge P, Honda HM, Weiss JN. Protection of cardiac mitochondria by diazoxide and protein kinase C: implications for ischemic preconditioning. Proc Natl Acad Sci USA. 2002;99:3312–3317. doi: 10.1073/pnas.052713199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Maulik N, Yoshida T, Engelman RM, Deaton D, Flack JE, III, Rousou JA, et al. Ischemic preconditioning attenuates apoptotic cell death associated with ischemia/reperfusion. Mol Cell Biochem. 1998;186:139–145. [PubMed] [Google Scholar]
- 109.Vanden Hoek TL, Becker LB, Shao ZH, Li CQ, Schumacker PT. Preconditioning in cardiomyocytes protects by attenuating oxidant stress at reperfusion. Circ Res. 2000;86:541–548. doi: 10.1161/01.res.86.5.541. [DOI] [PubMed] [Google Scholar]
- 110.Nakamura M, Wang NP, Zhao ZQ, Wilcox JN, Thourani V, Guyton RA, et al. Preconditioning decreases Bax expression, PMN accumulation and apoptosis in reperfused rat heart. Cardiovasc Res. 2000;45:661–670. doi: 10.1016/s0008-6363(99)00393-4. [DOI] [PubMed] [Google Scholar]
- 111.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Meller R, Cameron JA, Torrey DJ, Clayton CE, Ordonez AN, Henshall DC, et al. Rapid degradation of Bim by the ubiquitin-proteasome pathway mediates short-term ischemic tolerance in cultured neurons. J Biol Chem. 2006;281:7429–7436. doi: 10.1074/jbc.M512138200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu C, Chen S, Kamme F, Hu BR. Ischemic preconditioning prevents protein aggregation after transient cerebral ischemia. Neuroscience. 2005;134:69–80. doi: 10.1016/j.neuroscience.2005.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pradillo JM, Romera C, Hurtado O, Cardenas A, Moro MA, Leza JC, et al. TNFR1 upregulation mediates tolerance after brain ischemic preconditioning. J Cereb Blood Flow Metab. 2005;25:193–203. doi: 10.1038/sj.jcbfm.9600019. [DOI] [PubMed] [Google Scholar]
- 115.Dosenko VE, Nagibin VS, Tumanovskaya LV, Zagoriy VY, Moibenko AA, Vaage J. Proteasome inhibitors eliminate protective effect of postconditioning in cultured neonatal cardiomyocytes. Fiziol Zh. 2006;52:15–24. [PubMed] [Google Scholar]
- 116.Cai Z, Shen Z, Kaer LV, Becker LC. Ischemic preconditioning-induced cardioprotection is lost in mice with immunoproteasome subunit low molecular mass polypeptide-2 deficiency. FASEB J. 2008;22:4248–4257. doi: 10.1096/fj.08-105940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sato T, Sasaki N, O'Rourke B, Marban E. Nicorandil, a potent cardioprotective agent, acts by opening mitochondrial ATP-dependent potassium channels. J Am Coll Cardiol. 2000;35:514–518. doi: 10.1016/s0735-1097(99)00552-5. [DOI] [PubMed] [Google Scholar]