Abstract
Aims
The response of the myocardium to an ischaemic insult is regulated by two highly homologous protein kinase C (PKC) isozymes, δ and εPKC. Here, we determined the spatial and temporal relationships between these two isozymes in the context of ischaemia/reperfusion (I/R) and ischaemic preconditioning (IPC) to better understand their roles in cardioprotection.
Methods and results
Using an ex vivo rat model of myocardial infarction, we found that short bouts of ischaemia and reperfusion prior to the prolonged ischaemic event (IPC) diminished δPKC translocation by 3.8-fold and increased εPKC accumulation at mitochondria by 16-fold during reperfusion. In addition, total cellular levels of δPKC decreased by 60 ± 2.7% in response to IPC, whereas the levels of εPKC did not significantly change. Prolonged ischaemia induced a 48 ± 11% decline in the ATP-dependent proteasomal activity and increased the accumulation of misfolded proteins during reperfusion by 192 ± 32%; both of these events were completely prevented by IPC. Pharmacological inhibition of the proteasome or selective inhibition of εPKC during IPC restored δPKC levels at the mitochondria while decreasing εPKC levels, resulting in a loss of IPC-induced protection from I/R. Importantly, increased myocardial injury was the result, in part, of restoring a δPKC-mediated I/R pro-apoptotic phenotype by decreasing pro-survival signalling and increasing cytochrome c release into the cytosol.
Conclusion
Taken together, our findings indicate that IPC prevents I/R injury at reperfusion by protecting ATP-dependent 26S proteasomal function. This decreases the accumulation of the pro-apoptotic kinase, δPKC, at cardiac mitochondria, resulting in the accumulation of the pro-survival kinase, εPKC.
Keywords: Cardioprotection, Ischaemia/reperfusion, Apoptosis, Proteasome, PKC, Ischaemic preconditioning
1. Introduction
Myocardial ischaemia and reperfusion (I/R)-induced damage is associated with both apoptotic and necrotic cell death.1 We have shown this to be dependent on translocation of delta protein kinase C (δPKC) to cardiac mitochondria where it inhibits mitochondrial function.2–4 Inhibition of δPKC with the selective peptide inhibitor, δV1–1, protects the heart from ischaemic injury.4,5 Ischaemic preconditioning (IPC) observed in animals and humans6–8 protects the heart from reperfusion injury by activating pro-survival kinases,9–11 preventing apoptosis12,13 and necrosis2,4 preserving mitochondrial function,14,15 and reducing ROS generation.16 Many of these effects are afforded, at least in part, through activation and translocation of εPKC to cardiac mitochondria,17–20 resulting in diminished apoptosis and necrosis.12,21
Interestingly, activation of δPKC is also required for both opioid22 and IPC-mediated protection.23,24 We showed that activation of δPKC is cardioprotective provided there is sufficient time allowed for εPKC activation.25 Furthermore, εPKC is activated by ROS during IPC,26 whereas δPKC plays a role in ROS generation.27 Therefore, although both PKC isozymes play a role in IPC, the mechanism by which the pro-survival kinase (εPKC) and the pro-death kinase (δPKC) interact is not known. Here, we present evidence of a novel mechanism in which the proteasome alters the ratio between δPKC and εPKC, thereby regulating myocardial viability following I/R.
2. Methods
2.1. Materials
All antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Lactacystin, LLVY-AMC, epoxomycin, and MG-132 were from Biomol (Plymouth Meeting, PA). εV1–228 and ψεRACK29 conjugated to TAT were made by Anaspec, San Jose, CA. This study conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
2.2. Isolated perfused rat heart model and measurement of tissue necrosis
All procedures were carried out as described.2 All animal protocols were approved by the Institutional Animal Care and Use Committee of Stanford University.
2.3. Cellular fractionation and western blotting
Isolated hearts were homogenized in 210 mM mannitol, 70 mM sucrose, 1 mM EDTA, and 5 mM MOPS, pH = 7.4. After filtering through cheesecloth and a 5 min centrifugation at 800×g, the supernatant was centrifuged (10 000×g; 10 min) to obtain the mitochondrial pellet and the cytosolic extract (supernatant). This technique provides a mitochondrial fraction with only traces of sarcolemmal and plasma membrane contamination.30 Western blot analysis used polyclonal εPKC, δPKC, p-Akt, Akt, and cytochrome c antibodies, was normalized to ANT (mitochondria) and GAPDH (total and cytosolic homogenates) and was expressed as percent control.
2.4. Assay of proteasome activity
Chymotrypsin-like activity of the proteasome was assayed using the fluorogenic peptide Suc-Leu-Leu-Val-Tyr-7-amido-4-methylcoumarin (25 μM, LLVY-MCA) in a microtiter plate (50 μg protein) with 200 μl of 10 mM MOPS, pH 7.4. Assays were carried out in the absence and presence of 2.5 mM ATP and 5.0 mM Mg2+ with the difference attributed to ATP-dependent proteasome activity. The rate of fluorescent product formation was measured with excitation and emission wavelengths of 350 and 440 nm, respectively. In order to block proteasome activity during the experimental protocol, 2.0 μM lactacystin was perfused during the preconditioning protocol and the first 10 min of reperfusion.
2.5. Slot blot analysis of cellular misfolded proteins
Heart tissue homogenate (25 μg protein) was normalized and slot blotted onto PVDF membrane (Millipore, Bedford, MA, USA) and membranes were washed three times with 0.05% Tween 20, 10 mM Tris, pH 7.5, 100 mM NaCl (T-TBS) and blocked in T-TBS + 5% milk. After 4 h of incubation with an anti-soluble oligomer antibody (Biosource International, Camarillo, CA), an antibody that recognizes misfolded proteins,31 proteins were visualized as in the western blot analysis. Sample loading was normalized by Ponceau staining.
2.6. Analysis of cellular ATP levels
ATP determination was carried out using the Molecular Probes luciferase-based ATP determination kit (Kit# A22066). In brief, 100 μg of protein was incubated in a 96-well plate with 50 μM luciferin and 1.25 μg/mL luciferase in a Tris-based 1X reaction buffer containing DTT. ATP was measured after 5 min using a luminometer (560 nm at room temperature) using a standard curve of known ATP concentrations.
2.7. Statistics
Data are represented as the mean ± SE, and significance was determined by one-way analysis of variance with a post-hoc Tukey test or a two-tailed t-test.
3. Results
3.1. IPC diminishes δPKC at the mitochondria and increases εPKC translocation
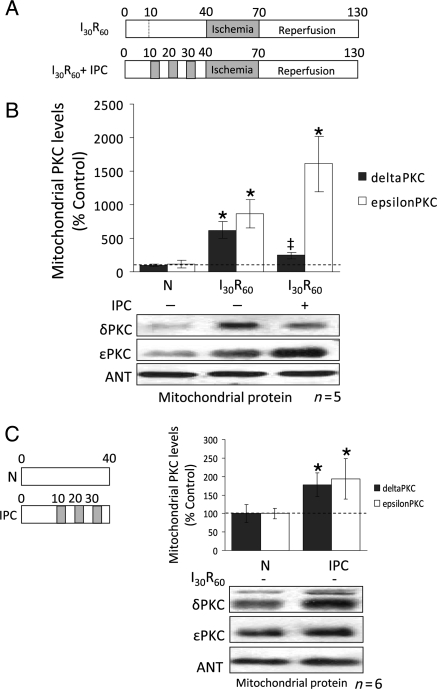
Since the mitochondria are critical sites of regulation by δ and εPKC during I/R,2–6,10,16,19–22 we first determined the levels of δ and εPKC in this fraction. Thirty minutes of ischaemia and 60 min reperfusion (I30/R60) (Figure 1A) resulted in accumulation of δPKC (∼6-fold; P < 0.001; n = 5) and εPKC (∼9-fold; P < 0.05; n = 5) at the mitochondria (Figure 1B). However, after IPC, I/R-induced δPKC accumulation at the mitochondrial fraction was largely prevented, whereas εPKC translocation increased ∼2-fold higher than hearts that were not preconditioned (P < 0.01; n = 5) (Figure 1B). Interestingly, IPC resulted in a seven-fold greater increase in εPKC at cardiac mitochondria relative to δPKC (Figure 1B). The IPC stimulus alone (without subsequent I30/R60) (Figure 1C left panel) increased the levels of both δPKC and εPKC and caused an ∼2-fold increase in their mitochondrial levels relative to normoxic hearts (Figure 1C right panel; P < 0.05; n = 6).
Figure 1.
Ischaemic preconditioning decreases δPKC and increases εPKC levels at cardiac mitochondria during reperfusion. (A) Hearts were hung in Langendorff mode and treated with the listed perfusion protocols. Hearts were then removed, homogenized, fractionated, and the mitochondrial fraction was subjected to western blot analysis with antibodies against the proteins listed in the figure. Values were normalized to adenine nucleotide translocase (ANT), a mitochondrial marker, and expressed as % of normoxia (N) control. (B) Western blot analysis of mitochondrial protein showing that 30 min of ischaemia followed by 60 min of reperfusion (I30R60) resulted in translocation of δPKC to mitochondria (six-folds increase; P < 0.05 vs. N), which was blocked when hearts were preconditioned (IPC) before the global ischaemic event (P < 0.05 vs. I30R60). Likewise, εPKC association with cardiac mitochondria increased ∼7-folds during I30R60 (P < 0.05 vs. N), but in contrast to δPKC, IPC prior to I/R further increased translocation of εPKC to 16-folds over the levels seen in normoxic hearts (P < 0.05 vs. N and I30R60). (C) Western blot analyses showing that the IPC stimulus alone (without I30R60) significantly increased translocation of δPKC (P < 0.05 vs. N) and εPKC translocation (P < 0.05 vs. N) to the mitochondria. *P < 0.05 vs. N, ‡P < 0.05 vs. I30R60) Translocation of δPKC and εPKC to the mitochondria were analysed by one-way analysis of variance with a post-hoc Tukey test. Mitochondrial PKC levels were analysed by Student's t-test.
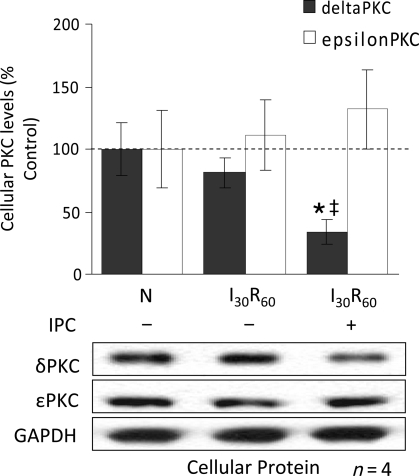
3.2. Total cellular levels of δPKC are greatly diminished by IPC
To determine whether the changes in the mitochondrial levels of δ and εPKC reflect changes in the cellular levels of these kinases, we next determined the levels of both εPKC and δPKC in total cardiac extracts. I30/R60 alone did not affect the overall levels of δPKC or εPKC vs. normoxia (N; n = 4; Figure 2). However, after IPC followed by ischaemia, δPKC levels decreased by 33% (vs. N, P < 0.05; n = 4), whereas εPKC levels did not. Therefore, the reduction in δPKC translocation to the mitochondria (Figure 1B) appears to be associated with diminished protein levels, and this effect is selective and does not seem to affect εPKC translocation.
Figure 2.
Diminished mitochondrial levels of δPKC following IPC are due to decreased cellular levels of the isozyme. Hearts were hung in Langendorff mode and treated with the listed perfusion protocols in Figure 1. Hearts were then removed, and homogenized, and the total homogenate was subjected to western blot analysis with antibodies against the proteins listed in the figure. Values were normalized to glyceraldehyde phosphate dehydrogenase (GAPDH), a cytosolic protein, and expressed as %N. I30R60 had no significant effect on total levels of either δPKC or εPKC. However, IPC before the global ischaemic event decreased the overall levels of δPKC by ∼80% (P < 0.05 vs. N), but not εPKC levels. *P < 0.05 vs. Normoxia and ‡P < 0.05 vs. I30R60. Cellular δPKC and εPKC levels were analysed by one-way analysis of variance with a post-hoc Tukey test.
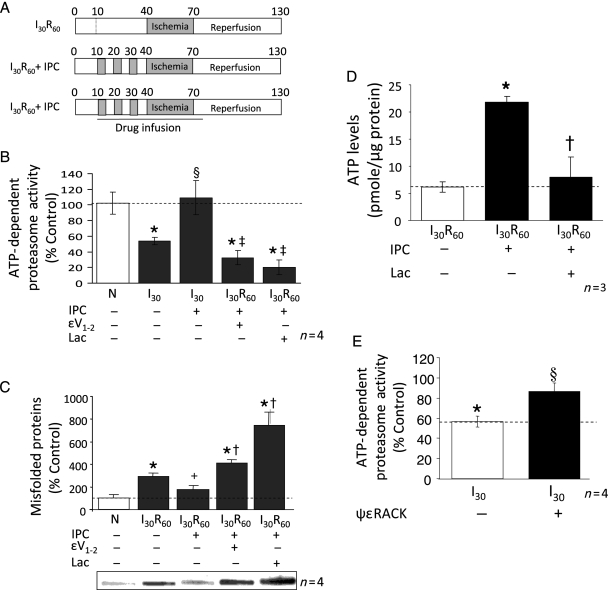
3.3. IPC-induced proteasomal degradation of δPKC diminished its translocation to cardiac mitochondria with a concurrent increase in εPKC translocation to this fraction
δPKC has been shown to be degraded by the 26S proteasome.29 Declines in ATP levels during ischaemia result in the disassembly of the 26S proteasome into the 20S form.32 In contrast, IPC reduces ischaemia-mediated declines in ATP levels.8,33 We therefore determined whether the loss in δPKC levels following IPC prior to I/R relates to preservation of the 26S proteasome activity. ATP Mg2+-stimulated peptidase activity is a reflection of the relative level of the 26S proteasome. Ischaemia induced a 45% decline in ATP-dependent proteasomal activity (Figure 3B) that was associated with an ∼3-fold increase in the accumulation of misfolded proteins during reperfusion (Figure 3C). IPC prevented the ischaemia-mediated declines in proteasomal activity and reduced the levels of misfolded proteins (Figure 3B and C; P < 0.05; n = 4). Furthermore, ATP levels correlated with proteasomal activity; ATP levels diminished during ischaemia/reperfusion and IPC significantly prevented this decline (Figure 3D).
Figure 3.
Effect of preconditioning on ischaemia-induced loss in ATP-dependent proteasome activity. (A) Cytosolic extracts were prepared from hearts exposed to 70 min of normoxic perfusion (N), 30 min of ischaemia (I30), or three cycles of preconditioning (5 min ischaemia and 5 min reperfusion) followed by 30 min of ischaemia (I30 + IPC) in the absence or presence of the proteasome inhibitor lactacystin or the specific εPKC inhibitor εV1–2. Chymotrypsin-like activity of the proteasome present in the cytoplasmic milieu was evaluated and the specific inhibitor MG-132 (20 μM) was utilized to ensure that measured activities were due to the proteasome (data not shown). The presence of unfolded proteins was evaluated using the slot blot technique with an anti-soluble oligomer antibody. Values representing ATP-dependent proteasome activity and misfolded proteins are presented as a percent of values obtained with samples from hearts exposed to 60 min of normoxic perfusion (N). Values represent the mean ± standard deviation (n = 4). (B) Ischaemia resulted in a 50% decline in ATP-dependent proteasome activity (P < 0.05 vs. N), which was completely reversed by IPC (P < 0.05 vs. I30). The proteasome inhibitor, lactacystin (2 μM) and the specific εPKC inhibitor (1μM εV1–2) both significantly decreased the activity of the proteasome (P < 0.05 vs. I30R60 + IPC; n = 4). (C) I30R60 resulted in an ∼3-fold increase in misfolded proteins which was prevented by IPC (P < 0.05; n = 4). Treatment of hearts with lactacystin or εV1–2 blocked the effect of IPC and increased the accumulation of misfolded proteins. (D) IPC elevated ATP levels by 3.5-fold in hearts that had undergone I30R60, and εV1–2 blocked these effects. (E) Treatment with the εPKC activator (ψεRACK) protected the proteasome from ischaemia-mediated inhibition (P < 0.05 vs. I30). *P < 0.05 vs. Normoxia, +P = 0.05 vs. I30R60, §P < 0.05 vs. I30, ‡P < 0.05 vs. IPC + I30, †P < 0.05 vs. IPC + I30R60; Misfolded protein accumulation and proteasome activity were analysed by one-way analysis of variance with a post-hoc Tukey test. Figure 3D, proteasome activity was analysed by Student's t-test.
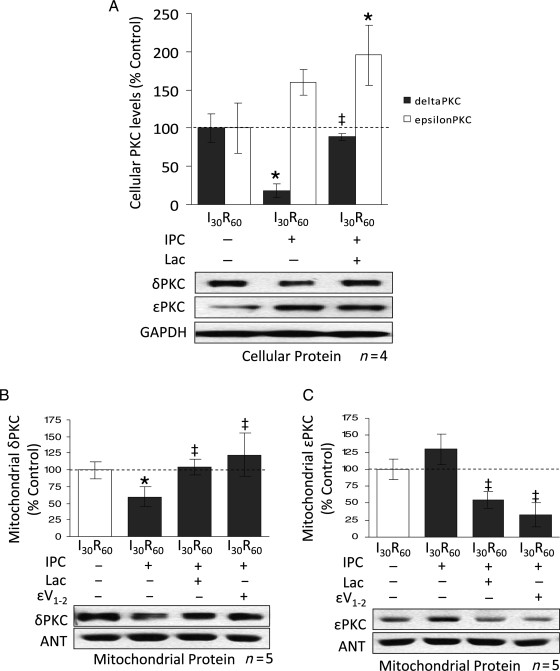
To determine if IPC increases proteasomal degradation of δPKC, we perfused the proteasome inhibitor, lactacystin (2 μM), during IPC and the first 10 min of reperfusion (Figure 3A). Lactacystin significantly blocked the activity of the proteasome (∼75%; P < 0.01; n = 4; Figure 3B) and increased the levels of misfolded proteins by ∼7-fold (Figure 3C). In agreement with our findings in Figure 2, IPC reduced post-reperfusion cellular levels of δPKC by ∼80% (P < 0.01; n = 4; Figure 4A). However, inhibition of the proteasome with lactacystin prevented the loss of δPKC after IPC and I30R60 (Figure 4A). Although lactacystin treatment did not affect the levels of εPKC relative to IPC, the ratio of εPKC to δPKC decreased due to elevated levels of δPKC.
Figure 4.
Inhibition of the proteasome restores δPKC cellular and mitochondrial levels in IPC hearts with a resultant decrease in εPKC levels. (A) Hearts were hung in Langendorff mode and treated with the above-mentioned perfusion protocols. Hearts were then removed, homogenized, and the total homogenate and mitochondrial fractions were subjected to western blot analysis with antibodies against the proteins listed in the figure. Values were normalized to GAPDH (total homogenate) or ANT (mitochondrial fraction) and expressed as % I30R60. IPC before prolonged ischaemia reduced total levels of δPKC by ∼80% (P < 0.05 vs. I30R60). Inhibition of the proteasome with 2 μM lactacystin blocked δPKC degradation (P < 0.05 vs. I30R60 + IPC). Similar to Figure 2, IPC before prolonged ischaemia did not significantly change overall levels of εPKC. However, inhibition of the proteasome increased εPKC levels by ∼2-folds (P < 0.05 vs. I30R60). Inhibition of εPKC activity with εV1–2 did not significantly change the overall levels of either δ or εPKC isozymes (data not shown). (B) As in Figure 2, IPC before I30R60 decreased levels of δPKC at mitochondria by ∼60% (P < 0.05 vs. I30R60). This was completely prevented in hearts treated with 2 μM lactacystin and 1 μM εV1–2 (P < 0.05 vs. I30R60 + IPC). (C) Although δPKC mitochondrial levels were restored, εPKC levels were diminished by 40% relative to I30R60 and by 60% relative to I30R60 + IPC (P < 0.05). Hearts that were treated with 1 μM of a peptide inhibitor of εPKC (εV1–2) during the IPC protocol showed a significant decrease in εPKC mitochondrial levels (P < 0.05 vs. I30R60 + IPC). *P < 0.05 vs. I30R60, ‡P < 0.05 vs. IPC I30R60. Cellular and mitochondrial PKC levels were analysed by the one-way analysis of variance with a post-hoc testing by Tukey.
Since lactacystin-mediated inhibition of the proteasome during IPC prevented δPKC degradation, it may promote translocation of δPKC to cardiac mitochondria. Indeed, lactacystin treatment completely blocked IPC-induced reductions in δPKC levels, restoring mitochondrial δPKC to levels obtained after I30/R60 alone (Figure 4B). Interestingly, in contrast to the effects of proteasome inhibition on total εPKC levels, mitochondrial levels of εPKC were reduced by 50% in the presence of lactacystin (Figure 4C). These data suggest that IPC inversely affects the ratios of these two PKC isozymes at cardiac mitochondria following I/R likely through the regulation of proteasome activity. Additionally, lactacystin abolished IPC-mediated protection of ATP levels following ischaemia/reperfusion (Figure 3D).
We determined whether εPKC indirectly downregulates δPKC by protecting proteasomal function during IPC. To this end, we perfused the specific εPKC inhibitor, εV1–234 (Figure 3A) and found an ∼70% inhibition of proteasomal activity, an effect that was similar to that obtained by lactacystin (Figure 3B), and resulted in a corresponding increase in cellular misfolded proteins (Figure 3C). Similar to lactacystin, εV1–2 treatment significantly decreased εPKC levels at cardiac mitochondria (71% vs. IPC), and restored δPKC levels in this fraction (P < 0.05; n = 7) (Figure 4B and C). Perfusion with a specific εPKC activator, ψεRACK, before ischaemia mimicked the IPC-mediated protective effect on proteasomal activity and prevented the loss in proteasomal activity seen during I/R (Figure 3E). This is likely an indirect effect, since εPKC was not found to associate with the proteasome following ψεRACK treatment (data not shown).
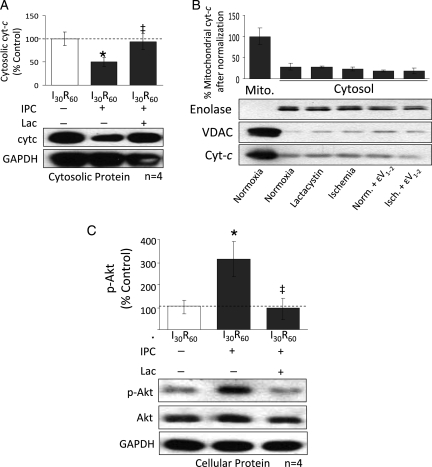
3.4. Inhibition of the proteasome prevents Akt activation and increases release of cytochrome c during IPC
IPC activates the pro-survival kinases, Akt, and ERK1/2 and blocks cytochrome c release during reperfusion.10,15,35 In contrast, δPKC decreases Akt activation and increases cytochrome c release during I/R.2 Here we found that IPC significantly blocked I30/R60 mediated release of mitochondrial cytochrome c into the cytosol (60%; P < 0.05; n = 4) (Figure 5A) and inhibition of the proteasome with lactacystin restored cytochrome c release to the levels seen during I30/R60 (P < 0.05). Treatment of non-ischaemic hearts with either lactacystin or εV1–2 did not cause significant release of cytochrome c into the cytosol (Figure 5B). Additionally, IPC significantly increased the phosphorylation of the pro-survival kinase, Akt, over I30/R60 levels (300%), and this was abolished by lactacystin treatment (Figure 5C). Li et al. showed that activation of δPKC reduces Akt phosphorylation whereas inhibition of δPKC increased Akt phosphorylation. They suggested that δPKC-mediated inhibition of Akt proceeds through increased association of protein phosphatase 2a.36 Neither IPC nor lactacystin treatments significantly changed the phosphorylation levels of ERK-1/2 (not shown), consistent with the findings of other studies.37,38
Figure 5.
Inhibition of δPKC degradation restores the apoptotic phenotype seen during reperfusion. Hearts were hung in Langendorff mode and treated with the listed perfusion protocols. Hearts were then removed, homogenized, fractionated, and the cytosolic homogenate was subjected to western blot analysis with antibodies against the proteins listed in the figure. Values were normalized to GAPDH and expressed as % I30R60. (A) IPC before prolonged ischaemia significantly decreased cytochrome c release into the cytosol (P < 0.05 vs. I30R60). Inhibition of the proteasome with 2 μM lactacystin restored cytochrome c release to levels seen during I30R60 (P < 0.05 vs. I30R60 + IPC). (B) Ischaemia alone and perfusion with lactacystin or εV1–2 alone did not result in significant release of cytochrome c into the cytosol. Additionally, as evidenced by a lack of mitochondrial VDAC in the cytosolic fraction, there was little contamination from mitochondrial cytochrome c in this fraction. Enolase was used as a cytosolic loading control. (C) IPC before prolonged ischaemia also increased phosphorylation of the pro-survival kinase, Akt, by ∼3-fold (P < 0.05 vs. I30R60). Inhibition of the proteasome with 2 μM lactacystin decreased phosphorylation back to I30R60 levels (P < 0.05 vs. I30R60 + IPC). *P < 0.05 vs. I30R60, ‡P < 0.05 vs. IPC I30R60. Cytosolic cytochrome c levels and p-Akt were analysed by one-way analysis of variance with a post-hoc Tukey test.
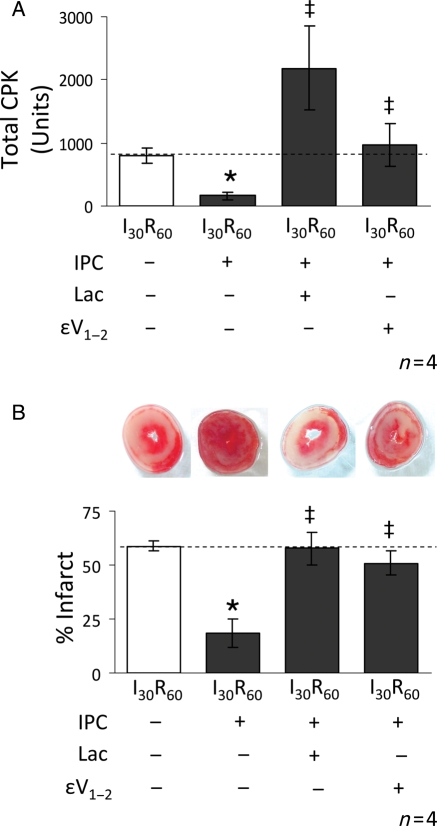
3.5. Inhibition of the proteasome during IPC increases tissue injury
Since pharmacological inhibition of the proteasome during IPC restored the apoptotic phenotype, we determined if tissue injury is altered by proteasome inhibition. As reported, IPC decreased both creatine phosphokinase (CPK) release and tetrazolium tetrachloride (TTC) staining of the myocardium by ∼70 and 60%, respectively (Figure 6A and B) and Lactacystin reversed the benefits of IPC-mediated protection. As we found before in isolated myocytes,28 in addition to the effects of εV1–2 on proteasomal function and δPKC translocation, inhibition of εPKC also completely reversed the protective effects of IPC on the myocardium (Figure 6A and B). Finally, to confirm the effects of lactacystin, we utilized another highly selective inhibitor of the proteasome, epoxomicin. Similar to lactacystin, inhibition of the proteasome with epoxomicin (2 μM) abolished the cardioprotective effects of IPC (data not shown).
Figure 6.
Inhibition of the proteasome reverses the IPC-mediated protective effects on tissue injury. Hearts were hung in Langendorff mode and treated with the listed perfusion protocols. Tissue injury was determined by measuring the release of CPK into the cardiac effluent (total CPK units). Following removal, hearts were sliced and stained with TTC to differentiate between necrotic (stained white) and viable (stained red) tissue (% infarct). (A,B) Hearts subjected to I30R60 showed an increase in both CPK release and myocardial infarction and both were blocked by IPC (reductions of ∼60% for CPK release and ∼40% for infarction, respectively). Perfusion of 2 μM lactacystin during the IPC protocol and for the first 10 min of reperfusion reversed this effect resulting in significantly higher levels of CPK release (P < 0.05 vs. I30R60 + IPC) and myocardial infarction (P < 0.05 vs. I30R60 + IPC). Similar to proteasome inhibition, εPKC inhibition (1 μM εV1–2) also significantly increased both CPK release (P < 0.05 vs. I30R60 + IPC) and myocardial infarction (P < 0.05 vs. I30R60 + IPC). *P < 0.05 vs. I30R60, ‡P < 0.05 vs. IPC I30R60. Total CPK and % infarcted area were analysed by the one-way analysis of variance with a post-hoc Tukey test.
4. Discussion
Our data suggest that IPC-induced decreases in mitochondrial δPKC levels are due to decreased total levels of δPKC. We also show that IPC prevents ischaemia-mediated declines in the 26S ATP-dependant proteasomal activity and that this is associated with diminished accumulation of cellular misfolded proteins. Ischaemia-mediated declines in forebrain ATP levels promote dissociation of the 26S proteasome (the form responsible for δPKC degradation29) to the 20S proteasome.32 During I/R, (in the absence of preconditioning) the significant decrease in ATP-dependent proteasomal activity is therefore likely due to decreased ATP levels within the cells. Indeed, as has been shown before,4,39 and here in an ex vivo model of ischaemia/reperfusion, ATP levels significantly declined during ischaemia/reperfusion and IPC significantly prevented this decline (Figure 3D). Alternatively, modifications by lipid peroxidation products and accumulation of oxidized proteins during I/R may also act as inhibitors of proteasomal function.40 Inhibition of the proteasome with lactacystin or epoxomicin blocked the protective effects of IPC. Additionally, lactacystin treatment elevated δPKC cellular and mitochondrial levels, and promoted cytochrome c release. δPKC is ubiquitinated within 30 min of activation41 and direct inhibition of the 26S proteasome with Bortezomib, a highly selective proteasome inhibitor currently in clinical use for the treatment of haematological cancers, increases mitochondrial ROS generation, cytochrome c release, and apoptosis associated with mitochondrial accumulation of δPKC.29,42 We suggest that since IPC and εPKC activation slow ATP depletion during prolonged ischaemia8,43 and δPKC is likely activated by the IPC stimulus (Figure 1C)3 the 26S proteasomal activity is maintained leading to the degradation of pro-apoptotic and pro-necrotic, δPKC,2,4 thereby conferring cardiac protection. Although the most likely explanation for the decrease in δPKC levels in the mitochondria is a decrease in total level of this isozyme in the cells (due to its increased degradation by the proteasome), we cannot exclude the possibility that decreased affinity of the binding site for δPKC in the mitochondria and post-translational modifications of the enzyme or its binding proteins also contribute to δPKC declined levels and therefore activity in the mitochondria. We have previously shown that accumulation of δPKC at cardiac mitochondria increases PDH phosphorylation and the inhibitor δV1–1 prevents this.3 Additionally, we have shown that εPKC is able to activate ALDH2 in hearts in the same in vivo model of I/R.44 Therefore, we have already provided direct evidence in this model of I/R that increased levels of PKC isozymes in the mitochondrial fraction are associated with increased phosphorylation of target substrates and hence reflect increased catalytic activity of these isozymes.
In addition to the protection afforded by degrading δPKC, increased εPKC accumulation at cardiac mitochondria is also likely to confer protection. εPKC translocates to mitochondria,16,19–22 where it prevents opening of the mitochondrial permeability transition pore,19,45 opens kATP channels,9 forms signalling complexes with MAPK,9 retards the reduction in cellular ATP levels,46 interacts with the electron transport chain,47 and augments mitochondrial function39 all of which contribute to cardioprotection. Since the relative level of εPKC at the mitochondria during reperfusion in the absence of the IPC stimulus is similar to δPKC levels, εPKC-mediated cardioprotection may be masked by the pro-apoptotic and pro-necrotic effects of δPKC during reperfusion. Administration of the εPKC activator, ψεRACK, prior to ischaemia, which mimics IPC and protects mitochondrial function39 prevented ischaemia-mediated declines in proteasome activity. Although recent studies suggest that kinases may regulate proteasome function directly,48 we did not find any physical association between εPKC and the proteasome.
In summary, activated δPKC has two potential fates that appear to depend on the metabolic state of the cell. If mitochondrial function, cellular energy status, and the integrity of the 26S proteasome are maintained, δPKC is efficiently degraded. In contrast, if mitochondrial function and ATP production are compromised, the ATP-dependent 26S proteasome activity is diminished, resulting in increased levels of activated δPKC at the mitochondria, where it participates in the induction of cell death. The proteasome can therefore be viewed as a sensor of cellular viability, determining the ratio of pro-apoptotic δPKC and pro-survival εPKC at the mitochondria and thus the ultimate fate of the cell. We propose the following mechanism. The decrease in ATP levels seen during I/R (Figure 3D) and increased generation of reactive oxygen species, will diminish 26S proteasome activity.40 δPKC is activated by ROS4 and also during the early stages of reperfusion, resulting in its accumulation at cardiac mitochondria (Figure 1).3 Because the activity of the proteasome is diminished (Figure 3), δPKC is not degraded, favouring its accumulation at cardiac mitochondria (Figure 4), where it triggers pro-apoptotic cytochrome c release and inactivation of Akt (Figure 5), leading to tissue injury (Figure 6). In contrast, IPC is associated with a small burst of mitochondrial ROS during the trigger phase of IPC, which decreases ROS generation during the effector phase49 and may also act as a stimulus for δPKC activation (Figure 1C). Diminished ROS generation and maintenance of cellular ATP levels (Figure 3D) result in protection of proteasomal function,50 which leads to degradation of δPKC (Figure 4A). Since both εPKC and δPKC accumulate at the mitochondria during I30R60 (Figure 1B) and since εPKC is not degraded during I30R60 (Figure 2A), degradation of δPKC during IPC tips the balance towards the accumulation of the pro-survival kinase, εPKC, at cardiac mitochondria, thus protecting mitochondrial function and proteasomal activity thereby diminishing I/R-mediated tissue injury.
Conflict of interest: D.M.-R. is the founder of KAI Pharmaceuticals. However, none of the work described in the study is based on or supported by the company.
Funding
NIH AA11147 to D.M.-R., Oklahoma Center for Advancement of Science and Technology (HR05-171S) to L.I.S. Funding to pay the Open Access publication charges for this article was provided by Dr Mochly-Rosen's unrestricted funding source (no grant was used).
References
- 1.Buja LM, Entman ML. Modes of myocardial cell injury and cell death in ischemic heart disease. Circulation. 1998;98:1355–1357. doi: 10.1161/01.cir.98.14.1355. [DOI] [PubMed] [Google Scholar]
- 2.Murriel CL, Churchill E, Inagaki K, Szweda LI, Mochly-Rosen D. Protein kinase Cdelta activation induces apoptosis in response to cardiac ischemia and reperfusion damage: a mechanism involving BAD and the mitochondria. J Biol Chem. 2004;279:47985–47991. doi: 10.1074/jbc.M405071200. [DOI] [PubMed] [Google Scholar]
- 3.Churchill EN, Murriel CL, Chen CH, Mochly-Rosen D, Szweda LI. Reperfusion-induced translocation of deltaPKC to cardiac mitochondria prevents pyruvate dehydrogenase reactivation. Circ Res. 2005;97:78–85. doi: 10.1161/01.RES.0000173896.32522.6e. [DOI] [PubMed] [Google Scholar]
- 4.Inagaki K, Chen L, Ikeno F, Lee FH, Imahashi K, Bouley DM, et al. Inhibition of delta-protein kinase C protects against reperfusion injury of the ischemic heart in vivo. Circulation. 2003;108:2304–2307. doi: 10.1161/01.CIR.0000101682.24138.36. [DOI] [PubMed] [Google Scholar]
- 5.Chen L, et al. Opposing cardioprotective actions and parallel hypertrophic effects of δ and εPKC. Proc Natl Acad Sci USA. 2001;98:11114–11119. doi: 10.1073/pnas.191369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yellon DM, Alkhulaifi AM, Pugsley WB. Preconditioning the human myocardium. Lancet. 1993;342:276–277. doi: 10.1016/0140-6736(93)91819-8. [DOI] [PubMed] [Google Scholar]
- 7.Liu GS, Thornton J, Van Winkle DM, Stanley AW, Olsson RA, Downey JM. Protection against infarction afforded by preconditioning is mediated by A1 adenosine receptors in rabbit heart. Circulation. 1991;84:350–356. doi: 10.1161/01.cir.84.1.350. [DOI] [PubMed] [Google Scholar]
- 8.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 9.Baines CP, Zhang J, Wang GW, Zheng YT, Xiu JX, Cardwell EM, et al. Mitochondrial PKCepsilon and MAPK form signaling modules in the murine heart: enhanced mitochondrial PKCepsilon–MAPK interactions and differential MAPK activation in PKCepsilon-induced cardioprotection. Circ Res. 2002;90:390–397. doi: 10.1161/01.res.0000012702.90501.8d. [DOI] [PubMed] [Google Scholar]
- 10.Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM. Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol Heart Circ Physiol. 2005;288:H971–H976. doi: 10.1152/ajpheart.00374.2004. [DOI] [PubMed] [Google Scholar]
- 11.Solenkova NV, Solodushko V, Cohen MV, Downey JM. Endogenous adenosine protects preconditioned heart during early minutes of reperfusion by activating Akt. Am J Physiol Heart Circ Physiol. 2006;290:H441–H449. doi: 10.1152/ajpheart.00589.2005. [DOI] [PubMed] [Google Scholar]
- 12.Liu H, McPherson BC, Yao Z. Preconditioning attenuates apoptosis and necrosis: role of protein kinase C epsilon and -delta isoforms. Am J Physiol Heart Circ Physiol. 2001;281:H404–H410. doi: 10.1152/ajpheart.2001.281.1.H404. [DOI] [PubMed] [Google Scholar]
- 13.Xu M, Wang Y, Hirai K, Ayub A, Ashraf M. Calcium preconditioning inhibits mitochondrial permeability transition and apoptosis. Am J Physiol Heart Circ Physiol. 2001;280:H899–H908. doi: 10.1152/ajpheart.2001.280.2.H899. [DOI] [PubMed] [Google Scholar]
- 14.Hausenloy D, Wynne A, Duchen M, Yellon D. Transient mitochondrial permeability transition pore opening mediates preconditioning-induced protection. Circulation. 2004;109:1714–1717. doi: 10.1161/01.CIR.0000126294.81407.7D. [DOI] [PubMed] [Google Scholar]
- 15.Lundberg KC, Szweda LI. Preconditioning prevents loss in mitochondrial function and release of cytochrome c during prolonged cardiac ischemia/reperfusion. Arch Biochem Biophys. 2006;453:130–134. doi: 10.1016/j.abb.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Park JW, Chun YS, Kim YH, Kim CH, Kim MS. Ischemic preconditioning reduces Op6 generation and prevents respiratory impairment in the mitochondria of post-ischemic reperfused heart of rat. Life Sci. 1997;60:2207–2219. doi: 10.1016/s0024-3205(97)00236-1. [DOI] [PubMed] [Google Scholar]
- 17.Jaburek M, Costa AD, Burton JR, Costa CL, Garlid KD. Mitochondrial PKC epsilon and mitochondrial ATP-sensitive K+ channel copurify and coreconstitute to form a functioning signaling module in proteoliposomes. Circ Res. 2006;99:878–883. doi: 10.1161/01.RES.0000245106.80628.d3. [DOI] [PubMed] [Google Scholar]
- 18.Ohnuma Y, Miura T, Miki T, Tanno M, Kuno A, Tsuchida A, et al. Opening of mitochondrial K(ATP) channel occurs downstream of PKC-epsilon activation in the mechanism of preconditioning. Am J Physiol Heart Circ Physiol. 2002;283:H440–H447. doi: 10.1152/ajpheart.00434.2001. [DOI] [PubMed] [Google Scholar]
- 19.Baines CP, Song CX, Zheng YT, Wang GW, Zhang J, Wang OL, et al. Protein kinase Cepsilon interacts with and inhibits the permeability transition pore in cardiac mitochondria. Circ Res. 2003;92:873–880. doi: 10.1161/01.RES.0000069215.36389.8D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogbi M, Chew CS, Pohl J, Stuchlik O, Ogbi S, Johnson JA. Cytochrome c oxidase subunit IV as a marker of protein kinase Cepsilon function in neonatal cardiac myocytes: implications for cytochrome c oxidase activity. Biochem J. 2004;382:923–932. doi: 10.1042/BJ20040468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ytrehus K, Liu Y, Downey JM. Preconditioning protects ischemic rabbit heart by protein kinase C activation. Am J Physiol. 1994;266:H1145–H1152. doi: 10.1152/ajpheart.1994.266.3.H1145. [DOI] [PubMed] [Google Scholar]
- 22.Fryer RM, Wang Y, Hsu AK, Gross GJ. Essential activation of PKC-delta in opioid-initiated cardioprotection. Am J Physiol Heart Circ Physiol. 2001;280:H1346–H1353. doi: 10.1152/ajpheart.2001.280.3.H1346. [DOI] [PubMed] [Google Scholar]
- 23.Kawamura S, Yoshida K, Miura T, Mizukami Y, Matsuzaki M. Ischemic preconditioning translocates PKC-delta and -epsilon, which mediate functional protection in isolated rat heart. Am J Physiol. 1998;275:H2266–H2271. doi: 10.1152/ajpheart.1998.275.6.H2266. [DOI] [PubMed] [Google Scholar]
- 24.Zhao J, Renner O, Wightman L, Sugden PH, Stewart L, Miller AD, et al. The expression of constitutively active isotypes of protein kinase C to investigate preconditioning. J Biol Chem. 1998;273:23072–23079. doi: 10.1074/jbc.273.36.23072. [DOI] [PubMed] [Google Scholar]
- 25.Inagaki K, Mochly-Rosen D. DeltaPKC-mediated activation of epsilonPKC in ethanol-induced cardiac protection from ischemia. J Mol Cell Cardiol. 2005;39:203–211. doi: 10.1016/j.yjmcc.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 26.Kabir AM, Clark JE, Tanno M, Cao X, Hothersall JS, Dashnyam S, et al. Cardioprotection initiated by reactive oxygen species is dependent on activation of PKC{varepsilon} Am J Physiol Heart Circ Physiol. 2006;291:H1893–H1899. doi: 10.1152/ajpheart.00798.2005. [DOI] [PubMed] [Google Scholar]
- 27.Mayr M, Metzler B, Chung YL, McGregor E, Mayr U, Troy H, et al. Ischemic preconditioning exaggerates cardiac damage in PKC-delta null mice. Am J Physiol Heart Circ Physiol. 2004;287:H946–H956. doi: 10.1152/ajpheart.00878.2003. [DOI] [PubMed] [Google Scholar]
- 28.Gray MO, Karliner JS, Mochly-Rosen D. A selective epsilon-protein kinase C antagonist inhibits protection of cardiac myocytes from hypoxia-induced cell death. J Biol Chem. 1997;272:30945–30951. doi: 10.1074/jbc.272.49.30945. [DOI] [PubMed] [Google Scholar]
- 29.Durrant D, Liu J, Yang HS, Lee RM. The bortezomib-induced mitochondrial damage is mediated by accumulation of active protein kinase C-delta. Biochem Biophys Res Commun. 2004;321:905–908. doi: 10.1016/j.bbrc.2004.07.049. [DOI] [PubMed] [Google Scholar]
- 30.Churchill EN, Disatnik MH, Mochly-Rosen D. Time-dependent and ethanol-induced cardiac protection from ischemia mediated by mitochondrial translocation of varepsilonPKC and activation of aldehyde dehydrogenase 2. J Mol Cell Cardiol. 2009;46:278–284. doi: 10.1016/j.yjmcc.2008.09.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanbe A, Osinska H, Villa C, Gulick J, Klevitsky R, Glabe CG, et al. Reversal of amyloid-induced heart disease in desmin-related cardiomyopathy. Proc Natl Acad Sci USA. 2005;102:13592–13597. doi: 10.1073/pnas.0503324102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asai A, Tanahashi N, Qiu JH, Saito N, Chi S, Kawahara N, et al. Selective proteasomal dysfunction in the hippocampal CA1 region after transient forebrain ischemia. J Cereb Blood Flow Metab. 2002;22:705–710. doi: 10.1097/00004647-200206000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Fralix TA, Murphy E, London RE, Steenbergen C. Protective effects of adenosine in the perfused rat heart: changes in metabolism and intracellular ion homeostasis. Am J Physiol. 1993;264:C986–C994. doi: 10.1152/ajpcell.1993.264.4.C986. [DOI] [PubMed] [Google Scholar]
- 34.Johnson JA, Gray MO, Chen CH, Mochly-Rosen D. A protein kinase C translocation inhibitor as an isozyme-selective antagonist of cardiac function. J Biol Chem. 1996;271:24962–24966. doi: 10.1074/jbc.271.40.24962. [DOI] [PubMed] [Google Scholar]
- 35.Uchiyama T, Engelman RM, Maulik N, Das DK. Role of Akt signaling in mitochondrial survival pathway triggered by hypoxic preconditioning. Circulation. 2004;109:3042–3049. doi: 10.1161/01.CIR.0000130647.29030.90. [DOI] [PubMed] [Google Scholar]
- 36.Li L, Sampat K, Hu N, Zakari J, Yuspa SH. Protein kinase c negatively regulates Akt activity and modifies UVC-induced apoptosis in mouse keratinocytes. J Biol Chem. 2006;281:3237–3243. doi: 10.1074/jbc.M512167200. [DOI] [PubMed] [Google Scholar]
- 37.Behrends M, Schulz R, Post H, Alexandrov A, Belosjorow S, Michel MC, et al. Inconsistent relation of MAPK activation to infarct size reduction by ischemic preconditioning in pigs. Am J Physiol Heart Circ Physiol. 2000;279:H1111–H1119. doi: 10.1152/ajpheart.2000.279.3.H1111. [DOI] [PubMed] [Google Scholar]
- 38.Mockridge JW, Punn A, Latchman DS, Marber MS, Heads RJ. PKC-dependent delayed metabolic preconditioning is independent of transient MAPK activation. Am J Physiol Heart Circ Physiol. 2000;279:H492–H501. doi: 10.1152/ajpheart.2000.279.2.H492. [DOI] [PubMed] [Google Scholar]
- 39.McCarthy J, McLeod CJ, Minners J, Essop MF, Ping P, Sack MN. PKCepsilon activation augments cardiac mitochondrial respiratory post-anoxic reserve—a putative mechanism in PKCepsilon cardioprotection. J Mol Cell Cardiol. 2005;38:697–700. doi: 10.1016/j.yjmcc.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 40.Bulteau AL, Lundberg KC, Humphries KM, Sadek HA, Szweda PA, Friguet B, et al. Oxidative modification and inactivation of the proteasome during coronary occlusion/reperfusion. J Biol Chem. 2001;276:30057–30063. doi: 10.1074/jbc.M100142200. [DOI] [PubMed] [Google Scholar]
- 41.Lu Z, Liu D, Hornia A, Devonish W, Pagano M, Foster DA. Activation of protein kinase C triggers its ubiquitination and degradation. Mol Cell Biol. 1998;18:839–845. doi: 10.1128/mcb.18.2.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ling YH, Liebes L, Zou Y, Perez-Soler R. Reactive oxygen species generation and mitochondrial dysfunction in the apoptotic response to Bortezomib, a novel proteasome inhibitor, in human H460 non-small cell lung cancer cells. J Biol Chem. 2003;278:33714–33723. doi: 10.1074/jbc.M302559200. [DOI] [PubMed] [Google Scholar]
- 43.Murry CE, Richard VJ, Reimer KA, Jennings RB. Ischemic preconditioning slows energy metabolism and delays ultrastructural damage during a sustained ischemic episode. Circ Res. 1990;66:913–931. doi: 10.1161/01.res.66.4.913. [DOI] [PubMed] [Google Scholar]
- 44.Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008;321:1493–1495. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ping P, Zhang J, Pierce WM, Jr, Bolli R. Functional proteomic analysis of protein kinase C epsilon signaling complexes in the normal heart and during cardioprotection. Circ Res. 2001;88:59–62. doi: 10.1161/01.res.88.1.59. [DOI] [PubMed] [Google Scholar]
- 46.Cross HR, Murphy E, Bolli R, Ping P, Steenbergen C. Expression of activated PKC epsilon (PKC epsilon) protects the ischemic heart, without attenuating ischemic H(+) production. J Mol Cell Cardiol. 2002;34:361–367. doi: 10.1006/jmcc.2001.1518. [DOI] [PubMed] [Google Scholar]
- 47.Yu Q, Nguyen T, Ogbi M, Caldwell RWP, Johnson JA. Differential loss of cytochrome c oxidase (CO) subunits in ischemia reperfusion injury: exacerbation of COI loss by {varepsilon}PKC inhibition. Am J Physiol Heart Circ Physiol. 2008;294:H2637–H2645. doi: 10.1152/ajpheart.91476.2007. [DOI] [PubMed] [Google Scholar]
- 48.Zong C, Gomes AV, Drews O, Li X, Young GW, Berhane B, et al. Regulation of murine cardiac 20S proteasomes: role of associating partners. Circ Res. 2006;99:372–380. doi: 10.1161/01.RES.0000237389.40000.02. [DOI] [PubMed] [Google Scholar]
- 49.Baines CP, Goto M, Downey JM. Oxygen radicals released during ischemic preconditioning contribute to cardioprotection in the rabbit myocardium. J Mol Cell Cardiol. 1997;29:207–216. doi: 10.1006/jmcc.1996.0265. [DOI] [PubMed] [Google Scholar]
- 50.Powell SR, Wang P, Katzeff H, Shringarpure R, Teoh C, Khaliulin I, Das DK, Davies KJ, Schwalb H. Oxidized and ubiquitinated proteins may predict recovery of postischemic cardiac function: essential role of the proteasome. Antioxid Redox Signal. 2005;7:538–546. doi: 10.1089/ars.2005.7.538. [DOI] [PubMed] [Google Scholar]