Abstract
Primary malignant tumours arising from the meninges are distinctly uncommon, and when they occur, they are usually sarcomas. In contrast, metastatic meningeal involvement is increasingly seen as advances in cancer therapy have changed the natural history of malignant disease and prolonged the life span of cancer patients. The meninges can either be infiltrated by contiguous extension of primary tumours of the central nervous system, paranasal sinuses and skull base origin or can be diffusely infiltrated from haematogenous dissemination from distant primary malignancies. Imaging in these patients provides crucial information in planning management. This article reviews the pertinent anatomy that underlies imaging findings, discusses the mechanism of meningeal metastasis and highlights different imaging patterns of meningeal carcinomatosis and the pitfalls.
Keywords: Carcinomatosis, leptomeningeal, dura
Introduction
Advances in chemotherapy and radiotherapy have altered the natural course of disease in patients with systemic cancer, who now live longer than ever before. On the downside, prolonged survival has led to increased incidence of distant spread of disease, leptomeningeal metastasis being one such dreaded complication. However, prompt diagnosis and treatment in these patients can not only provide symptomatic relief and improve the quality of life but can also increase survival. Contrast-enhanced MR has not only increased the sensitivity and specificity to detect meningeal disease but can act as a guide to biopsy and assess response to therapy. Advances in cytological techniques and tumour markers have further increased the sensitivity in detection of malignant cells in the cerebrospinal fluid.
Anatomy
Three layers of meninges that envelope the brain are the duramater, the arachnoid and the pia mater. The dura, also called the pachymeninx, is composed of two layers, the outer periosteal and the inner meningeal layer that are fused except where they enclose the venous sinuses. The outer layer is a tough fibrous structure that firmly attaches to the inner surface of the cranium forming the periosteum of the inner table and blends imperceptibly with the pericranium on the outer table at the various sutures and the skull base foramina. One exception is its continuity across the optic foramen and superior orbital fissures to form the periosteal lining of the orbital walls. Referred to as the periorbita, it is of significance when deciding the approach to orbital surgery.
The inner true meningeal layer forms various reflections that compartmentalize and stabilize the brain parenchyma. It differs from the outer periosteal layer in that it continues into the spinal canal through the foramen magnum and extends across the various smaller skull base and intervertebral foramina as the dural sheath of the various cranial and spinal nerves before forming the perineurium. This anatomic arrangement is of clinical significance as disease processes involving the exiting nerves can spread retrogradely along the nerves and their dural sheaths. In the globe, ‘tram-track’ enhancement of the perioptic dural sheath is suggestive of a meningioma.
The arachnoid and pia are together referred to as the leptomeninges. The arachnoid is the middle meningeal layer that closely abuts the dura externally with the potential subdural space between them. The pia invests the sulci and gyri whereas the arachnoid jumps from one gyrus to another. These anatomic correlates help in understanding the various patterns of enhancement in leptomeningeal carcinomatosis.
The pia forms the tela choroidea and the choroid plexus that is central to cerebrospinal fluid (CSF) production whereas the arachnoid aids in CSF absorption by forming the arachnoid granulations. Thus leptomeninges are central to CSF physiology and it is not difficult to comprehend why disease processes that affect the meninges inadvertently affect CSF production or resorption, sometimes heralding the onset of meningeal disease.
The CSF fills in the subarachnoid space between the arachnoid and pia, which is narrow over the cerebral convexities but widens to form large cisternal spaces in the skull base. A superficial cerebral focus can rupture into the subarachnoid space and spread along the convexities into the basal cisterns. This is widely believed to the origin of basal exudates in tuberculosis and one of the possible causes of spread of malignant disease. The pia arachnoid space complex continues around the small vessels at the skull base as the perivascular Virchow Robin spaces. Metastatic disease and cryptococcosis are known to have an affinity for this pathway.
Malignant meningeal disease
Primary malignant tumours of the meninges are extremely rare and mostly sarcomas; it is believed they have an association with previous radiation therapy[1,2]. There are anecdotal case reports of primary diffuse leptomeningeal gliomatosis[3,4], primary leptomeningeal lymphoma[5] and melanoma[6].
The most common malignant involvement of the meninges is by secondary involvement referred to as meningeal carcinomatosis. Primary central nervous system (CNS) tumours that commonly metastasize to the meninges include medulloblastoma and ependymoma in children and glioblastoma multiforme in adults. Non-CNS malignancies that commonly spread to the meninges include breast, lung, melanoma, gastrointestinal and genitourinary malignancies and haematological malignancies such as leukaemias and lymphomas.
Patients typically present with signs of raised intracranial pressure as a result of hydrocephalus or cranial neuropathies due to effects of tumours cells on cranial nerves in the subarachnoid space.
It is important to understand the various probable mechanisms of tumour spread to understand the various imaging findings in such patients.
Spread of tumour cells to the meninges can occur by haematogenous spread or direct contiguous infiltration. Haematogenous dissemination to the dura can be in form of single or multiple focal deposits or diffuse infiltration. Dura is involved by contiguous extension from malignancies of the nasopharynx and paranasal sinuses (Fig. 1) or from calvarial deposits from breast, lung and even the prostate.
Figure 1.
Contiguous dural involvement from ethmoid carcinoma. T1W staging MRI (a) shows a mass (asterisk) in the left ethmoid sinus with orbital extension and involvement of the periorbita. Contrast-enhanced follow-up MRI (b) shows tumour recurrence with involvement of the dura. Note the enhancing dural thickening in the left temporal region (black arrows).
Leptomeningeal involvement by malignant cells is more common and can be haematogenous or contiguous. Haematogenous spread to the leptomeninges can potentially occur via seeding of the choroid plexus, along perivascular spaces or the Bateson's vertebral venous plexus. A primary brain tumour like a glioblastoma multiforme or parenchymal deposit in proximity to the brain surface can contiguously involve the adjoining pial lining and subsequently disseminate into the subarachnoid space.
Some tumours as medulloblastoma and ependymoma have a propensity to dissemination and meningeal involvement due to their intraventricular location and thus close proximity to the CSF pathway (Fig. 2).
Figure 2.
Diffuse subarachnoid dissemination in a case of medulloblastoma. Post-contrast sagittal (a) and axial (b,c) fat-saturated T1W images of the thoracic spine shows multiple focal enhancing nodules (arrows) in the spinal subarachnoid space. Cranial and leptomeningeal subarachnoid spaces are continuous and imaging and treatment protocols should include the entire neuraxis.
Imaging in meningeal disease
Imaging protocol
Cerebrospinal subarachnoid is continuous and thus disease processes affecting the brain can spread to the spine and vice versa. Thus despite focal neurologic signs and symptoms, imaging and treatment protocols for leptomeningeal disease should include the entire neuraxis.
Contrast-enhanced magnetic resonance imaging (MRI) has unequivocal increased sensitivity and has replaced contrast computed tomography (CT) in evaluation of patients with suspected leptomeningeal carcinomatosis[7,9,12]. This is not only due to the inherent better contrast resolution with MRI but also because a beam hardening artefact beneath the inner table limits the visualization of meningeal enhancement with CT.
It is important to understand the pattern of meningeal enhancement in normal patients so as to predict pathologic meningeal enhancement with some degree of certainty and reduce the false positive rates. The dura lacks the blood–brain barrier and thus normally enhances following administration of intravenous contrast. The normal enhancement is typically thin, smooth and discontinuous and seen more over the cerebral convexities than in the basal regions[7,8] (Fig. 3).
Figure 3.
Normal meningeal enhancement. Post-contrast coronal T1W (a) and axial gradient recalled echo (GRE) (b) images. Note the thin discontinuous enhancement (small white arrows) of the dura over the cerebral convexities. Normal enhancing meningeal vessels in the sulci (small black arrowheads) should not be confused with pial enhancement.
The enhancement pattern is dependent on several factors including the strength of the magnet, type of pulse sequence and amount of contrast. More extensive and long segments of enhancements are seen with greater field strengths and high dose of paramagnetic contrast[14,15]. The long repetition time (TR) and large flip angles used in gradient echo imaging results in decreased saturation of the contrast-enhancing meninges in contrast to the markedly saturated background tissues. Thus contrast-enhanced gradient echo sequence typically demonstrates diffuse meningeal enhancement in normal subjects compared with the thin and discontinuous enhancement in the spin echo sequences[4].
Contrast-enhanced T1-weighted (T1W) images in 3 orthogonal planes is considered the most sensitive in detection of meningeal disease of any cause. It is better to acquire post-contrast images with fat saturation as they are more sensitive in detecting calvarial deposits[5,6]. Fluid attenuated inversion recovery (FLAIR) due to suppression of the CSF signal is highly sensitive to subarachnoid disease and post-contrast FLAIR has been shown to be a useful adjunct to fat-saturated T1W images in detection of meningeal disease.
Imaging findings
Leptomeningeal carcinomatosis can cause focal or diffuse enhancement of the meninges, ependymal lining and perivascular spaces and secondary complications of hydrocephalus and cranial neuropathies. Hydrocephalus may the first and only sign of leptomeningeal spread of disease.
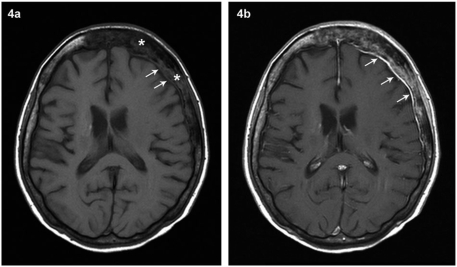
Tumour cells can involve any of the 3 meningeal layers in varying combinations. Neoplastic involvement of the dura leads to diffuse enhancement along the inner table of the skull vault (Fig. 4) or a focal extra-axial mass (Fig. 5). As expected, pial involvement leads to wavy enhancement that closely follows the brain surface along the depths of the sulci[8,11] (Fig. 6).
Figure 4.
Contiguous dural infiltration from calvarial deposit in a known case of carcinoma breast. Pre-contrast (a) and post-contrast (b) axial T1W images at the level of lateral ventricles demonstrate a calvarial deposit (asterisk) in the frontoparietal region associated with enhancing thickening of the underlying dura (white arrows) that could be reactive or due to contiguous dural involvement as in this case.
Figure 5.
Biopsy proven dural deposit in the falx in a case of breast carcinoma. Contrast-enhanced axial (a), coronal (b) and diffusion weighted (c) images shows an enhancing dural based mass in relation to the falx in the posterior interhemispheric fissure that demonstrates restricted diffusion.
Figure 6.
Pial-subarachnoid pattern of enhancement. A known case of non-small cell carcinoma of the lung with signs of raised intracranial pressure. Axial T2W image (a) shows hydrocephalus with transependymal seepage of CSF. Post-contrast fat-saturated axial (b) and coronal (b) T1W images demonstrate diffuse pial enhancement in the depths of the sulci and over the cerebellar folia (black arrowheads). CSF examination was positive for malignant cells.
It is conceivable that the arachnoid gets involved subsequently due to its close anatomic proximity to the dura on the outside and the communication with the pia via the subarachnoid space on the inside and eventually all the meningeal layers become involved (Fig. 7). This is the basis of two patterns of meningeal enhancement: the dura–arachnoid type and the pia–subarachnoid pattern described by Meltzer et al.[7].
Figure 7.
Combined dural and leptomeningeal enhancement. Contrast-enhanced axial (a)) and coronal (b) demonstrates diffuse dural (yellow arrows) and leptomeningeal enhancement (black arrows) in a known case of melanoma.
The presence of tumour cells in the CSF implies involvement of the subarachnoid space. The pial pattern of enhancement shows a higher incidence of CSF positive cytology presumably due to contiguity with the subarachnoid space. There are cases of isolated dural involvement that are repeatedly negative for tumour cells on CSF and MR imaging can guide biopsy and assess response to therapy in such cases.
Alteration of CSF physiology leads to hydrocephalus that maybe associated with transependymal seepage of CSF, referred to as interstitial oedema (Fig. 6). Enhancement of various cranial nerves in the subarachnoid cisterns maybe associated with cranial neuropathies.
Imaging pitfalls
There are many entities that may mimic leptomeningeal carcinomatosis and the radiologist must be familiar with them to reduce false positive rates and prevent unnecessary meningeal biopsies.
Focal or diffuse meningeal enhancement may persist long after a craniotomy and has been postulated to be reactive to bleeding into the subarachnoid space at the time of surgery and does not necessarily indicated infection or tumour involvement[10,11]. Patients suspected of meningeal carcinomatosis frequently undergo ventricular shunt placement for hydrocephalus that can cause focal/diffuse meningeal enhancement as a result of meningeal fibrosis[19,20] (Fig. 8).
Figure 8.
Reactive dural enhancement after ventriculoperitoneal shunt for hydrocephalus. Contrast-enhanced fat-saturated T1W axial (a) and coronal (b) images demonstrates diffuse dural enhancement over the frontoparietal convexities (black arrows). CSF examination was negative for infection or malignant cells and the patient was clinically well on long-term follow-up. Note the right-sided ventriculoperitoneal shunt on the coronal image (small white arrowhead).
In patients who have undergone spinal puncture for CSF cytology, diffuse pachymeningeal enhancement when associated with subdural collections and tonsillar herniation should alert the clinician for possible intracranial hypotension[21]. Awareness of these iatrogenic causes prevents diagnostic delay and prevents unnecessary meningeal biopsy.
Some conditions are commonly found in the general population and can cause focal/diffuse meningeal enhancement. A territorial sub-acute infarct shows transient leptomeningeal enhancement as can subarachnoid haemorrhage. Resolved subdural hematoma/empyema may show persistent thickening and enhancement of the dura adjacent to the skull vault.
Various infectious/inflammatory disorders such as tuberculosis and fungal infections and granulomatous disorders such as sarcoidosis could be difficult to differentiate radiologically from neoplastic meningitis and may require meningeal biopsy for differentiation.
Idiopathic cranial pachymeningitis is a rare disorder that causes diffuse thickening and enhancement of the dura and requires biopsy for definitive diagnosis (Fig. 9). Other relatively rare and anecdotal pathologic entities that can cause meningeal involvement are Langherhan cell histiocytosis, neurocutaneous syndromes such as meningiomatosis and Sturge–Weber syndrome. The interested reader is referred to specific articles on these pathologies.
Figure 9.
Contrast-enhanced axial T1W images at the level of third (a) and fourth ventricles (b) demonstrates diffuse dural enhancement over the cerebral convexities including the falx and the tentorium (black arrows). CSF was repeatedly negative for infection or malignant cells. Dural biopsy was consistent with idiopathic hypertrophic pachymeningitis.
Conclusion
Meningeal carcinomatosis is the single most common cause of malignant involvement of the meninges and is increasingly seen due to advances in cancer therapy and prolonged life span of cancer patients. Tumour cells can involve any of the 3 meningeal layers in a diffuse/nodular form and CSF dissemination of these cells can lead to cranial neuropathies and hydrocephalus. Contrast MR imaging not only acts as an important adjunct to CSF cytology in diagnosis but can also guide in biopsy and assessing response to therapy. It is important for the clinician to be aware of imaging findings of meningitis carcinomatosis and the various mimics, thereby instituting definitive treatment in positive cases and avoiding unnecessary biopsy in others.
References
- 1.Lee YY, Van Tassel P, Raymond A. Intracranial dural chondrosarcoma. AJNR. 1988;9:1189–93. [PMC free article] [PubMed] [Google Scholar]
- 2.Malat J, Virapongse C, Palestro C, Richman A. Primary intraspinal fibrosarcoma. Neurosurgery. 1986;19:434–6. doi: 10.1227/00006123-198609000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Ho KL, Hoschner J, Wolfe D. Primary leptomeningeal gliomatosis: symptoms suggestive of meningitis. Arch Neurol. 1981;38:662–6. doi: 10.1001/archneur.1981.00510100090018. [DOI] [PubMed] [Google Scholar]
- 4.Deitrich PY, Aapro M, Pizzolato G. Primary diffuse leptomeningeal gliomatosis. [(PDLG): a neoplastic cause of chronic meningitis. J Neurooncol. 1993;15:275–83. doi: 10.1007/BF01050075. doi:10.1007/BF01050075. PMid:8360714. [DOI] [PubMed] [Google Scholar]
- 5.Lachance D, O’Neill B, Macdonald D, et al. Primary leptomeningeal lymphoma: report of 9 cases, diagnosis immunocytochemical analysis and review of literature. Neurology. 1991;41:95–100. doi: 10.1212/wnl.41.1.95. [DOI] [PubMed] [Google Scholar]
- 6.Allcutt D, Michowitz S, Weitzman S, et al. Primary leptomeningeal melanoma: an unusual aggressive tumour in childhood. Neurosurgery. 1993;32:721–29. doi: 10.1227/00006123-199305000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Cidis Meltzer C, Fukui MB, Kanal E, Smrinniopoulos JG. MR imaging of the meninges. Part I. Normal anatomic features and non-neoplastic disease. Radiology. 1996;201:297–308. doi: 10.1148/radiology.201.2.8888215. [DOI] [PubMed] [Google Scholar]
- 8.Fukui MB, Cidis Meltzer C, Kanal E, Smrinniopoulos JG. MR imaging of the meninges. Part II. Neoplastic disease. Radiology. 1996;201:605–612. doi: 10.1148/radiology.201.3.8939203. [DOI] [PubMed] [Google Scholar]
- 9.Chamberlain MC, Sandy AC, Press GA. Leptomeningeal metastasis: a comparison of contrast-enhanced MR and contrast-enhanced CT of the brain. Neurology. 1990;40:435–8. doi: 10.1212/wnl.40.3_part_1.435. [DOI] [PubMed] [Google Scholar]
- 10.Burke JW, Podrasky AE, Bradley WG., Jr. Meninges: benign post operative enhancement on MR images. Radiology. 1990;174:99–102. doi: 10.1148/radiology.174.1.2294579. [DOI] [PubMed] [Google Scholar]
- 11.Sze G. Disease of intracranial meninges: MR imaging features. AJR. 1993;160:727–33. doi: 10.2214/ajr.160.4.8456653. [DOI] [PubMed] [Google Scholar]
- 12.Sze G, Soletsky S, Bronen R, Krol G. MR imaging of the cranial meninges with emphasis on contrast enhancement and meningeal carcinomatosis. AJNR. 1989;10:965–75. [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen J, Quint D, Eldevik O. Patterns of normal contrast enhancement on 1.5 T MR imaging. American Roentgen Ray Society Proceedings. 1995:127. [Google Scholar]
- 14.Kallmes D, Gray LD, Brown M, Glass J. riple dose gadolinium in diagnosis of leptomeningeal metastasis. American Society of Neuroradiology Proceedings. 1994:208. (abstract) [Google Scholar]
- 15.Runge V, Wells J, Nelson K, Linville P. MR imaging detection of cerebral metastasis with single injection of high dose gadoteridol. JMRI. 1994;4:669–73. doi: 10.1002/jmri.1880040509. doi:10.1002/jmri.1880040509. PMid:7981511. [DOI] [PubMed] [Google Scholar]
- 16.Farm J, Mirowitz S. MR imaging of normal meninges: comparison of contrast enhanced patterns on 3D gradient-echo and spin-echo sequences. AJR. 1994;162:131–5. doi: 10.2214/ajr.162.1.8273651. [DOI] [PubMed] [Google Scholar]
- 17.Galassi W, Phuttharak W, Hasselink JR, Healy JF, Dietrich RB, Imbesi SG. Intracranial meningeal disease: comparison of contrast-enhanced MR imaging with fluid attenuated inversion recovery and fat-suppressed T1W images. AJNR. 2005;26:553–9. [PMC free article] [PubMed] [Google Scholar]
- 18.Tsuchiya K, Katase S, Yoshino A, Hachiya J. FLAIR MR imaging for diagnosis of intracranial meningeal carcinomatosis. AJR. 2001;176:1585–8. doi: 10.2214/ajr.176.6.1761585. [DOI] [PubMed] [Google Scholar]
- 19.Burke JW, Podrasky AE, Bradley WG. Meninges: benign postoperative enhancement on MR imaging. Radiology. 1990;174:99–102. doi: 10.1148/radiology.174.1.2294579. [DOI] [PubMed] [Google Scholar]
- 20.Heier L, Zimmerman RD, Deck MDF. Meningeal fibrosis appearing shortly after ventricular shunting (reply) Radiology. 1990;174:99–102. [Google Scholar]
- 21.Panullo SC, Reich JB, Kroll G, Deck MDF, Posner JB. MRI changes in intracranial hypotension. Neurology. 1993;43:919–24. doi: 10.1212/wnl.43.5.919. [DOI] [PubMed] [Google Scholar]