Abstract
Oncology patients often experience urgent or emergent medical complications that are a direct or indirect result of the underlying malignant condition and are first identified or clarified on radiologic imaging studies. The aim of this review is to identify, discuss, and illustrate some of the major thoracic complications in patients with primary intrathoracic or extrathoracic neoplasms; particular focus is placed on issues in which radiologic imaging may have a significant impact on patient management, including superior vena cava (SVC) syndrome, post-obstructive pneumonia, diaphragmatic paralysis, pleural effusions, pericardial disease, tracheo-esophageal fistula, deep venous thrombosis, and pulmonary embolism.
Keywords: Esophageal cancer, lung cancer, complications, thoracic neoplasms
Introduction
Oncology patients often experience urgent or emergent medical complications that are a direct or indirect result of the underlying malignant condition. These complications are frequently first identified or clarified on radiologic imaging studies. The aim of this review is to identify, discuss, and illustrate some of the major thoracic complications in patients with primary intrathoracic or extrathoracic neoplasms; particular focus is placed on issues in which radiologic imaging may have a significant impact on patient management. Neurologic complications are not included in this review, because they are the subject of another paper. In addition, post-surgical complications are not addressed in this article.
Complications may be divided into direct and indirect effects of the malignancy. Direct effects include mechanical compression or obstruction of adjacent structures by tumor mass or large fluid volumes, including superior vena cava (SVC) syndrome, tracheal compression, post-obstructive pneumonia, diaphragmatic and vocal cord paralysis, compression atelectasis due to large pleural effusion, and cardiac dysfunction due to pericardial disease. In addition, tumors may directly invade an adjacent structure, with damage to the integrity of that structure. Examples include vascular invasion resulting in hemorrhage and thoracic duct involvement leading to chylothorax. Neoplastic involvement of the pleura may cause a pneumothorax or malignant pleural effusion, and invasion through the walls of the central airways and esophagus may give rise to a tracheo-esophageal or broncho-esophageal fistula.
Indirect complications of malignancy include systemic manifestations of disease, such as a hypercoagulable state, leading to deep venous thrombosis and pulmonary embolism, and an immunocompromised state, leading to various infections, as well as paraneoplastic syndromes with a variety of manifestations.
SVC syndrome
SVC obstruction related to malignancy is generally due to compression by a bulky, adjacent tumor, although it may be caused by frank invasion and/or intraluminal tumor thrombus (Fig. 1). Clinical findings include erythema and swelling of the head and neck, dilated superficial veins in the neck, thorax, abdomen and upper extremities, and dyspnea, stridor and/or dysphagia [1]. The clinical presentation may be acute or chronic in onset. The most common types of tumors to involve the SVC are non-small cell and small cell lung cancer, lymphoma, thymic neoplasms and occasionally esophageal cancer; metastatic disease from an extrathoracic primary neoplasm is also possible.
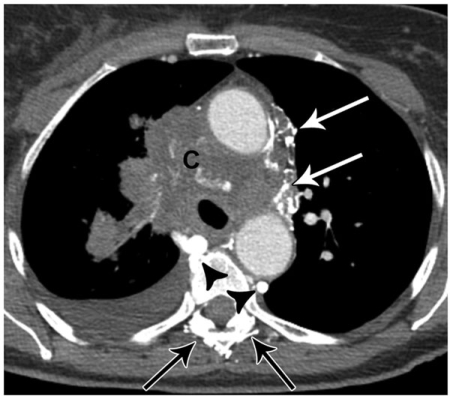
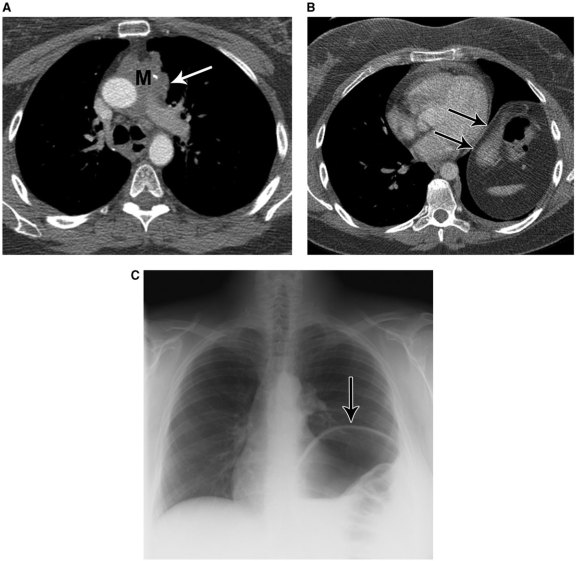
Figure 1.
A 72-year-old female with obliteration of the superior vena cava due to tumor involvement by small cell lung cancer (C). Note contrast filled collateral veins in the mediastinum (white arrows) and paraspinal regions (black arrows), as well as collateral flow in the azygous and hemiazygous veins (arrowheads).
Collateral blood flow occurs in the following venous systems: azygous, intercostal, mediastinal, paravertebral, hemiazygous, thoracoepigastric, internal mammary, thoracoacromioclavicular and anterior chest wall. Transhepatic collateral flow may develop, leading to a systemic-portal shunt. These collateral pathways may be shown to best advantage using multiplanar and three-dimensional image reconstructions[2].
SVC obstruction is often best evaluated using delayed computed tomography (CT) scanning after completion of a bolus injection of contrast material through veins in one or both arms, followed by injection of a dilute contrast/saline chaser mixture. The delay and the chaser assure optimal contrast opacification in both the right and left brachiocephalic veins, and eliminate streak artifact from dense, undiluted contrast material. Multiplanar reconstructions of the CT data may be helpful in evaluating for caval patency. Alternatively, magnetic resonance imaging (MRI) may be used in this setting.
Treatment options include anticoagulation, thrombolysis, endovascular stenting, surgical bypass, radiation therapy and/or chemotherapy.
Central airway compromise and post-obstructive pneumonia
Mediastinal and hilar tumor masses may compress or invade the central airways, resulting in respiratory compromise and post-obstructive atelectasis or pneumonia (Figs. 2 and 3). In patients with pneumonia, relief of the central obstruction is an important factor in treating the lung infection. Most commonly, these masses are due to non-small cell or small cell lung cancers, or occasionally carcinoid tumors or metastatic disease. CT with bolus administration of intravenous contrast material and fluorodeoxyglucose (FDG) positron emission tomography (PET) scanning can be helpful in distinguishing a central tumor mass from adjacent post-obstructive lung disease (Fig. 4). An obstructing airway mass may be treated with conventional radiation therapy, intraluminal brachytherapy, endobronchial laser resection, electrocautery and/or airway stenting[3–5].
Figure 2.
A 74-year-old female with encasement and compression of the trachea due to recurrent non-small cell lung cancer (arrow). The patient had undergone a right pneumonectomy for cancer 2 years previously.
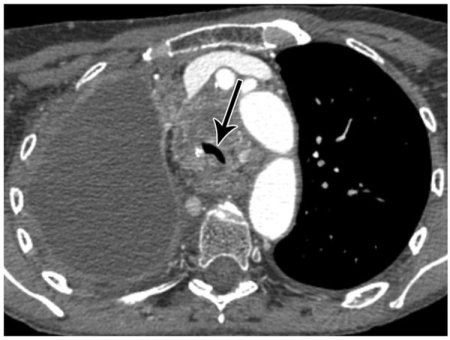
Figure 3.
A 75-year-old female with a right hilar non-small cell lung cancer (C) and post-obstructive pneumonia in the right middle lobe (*). Also note pleural effusions (black arrows) and pericardial effusion (white arrow).
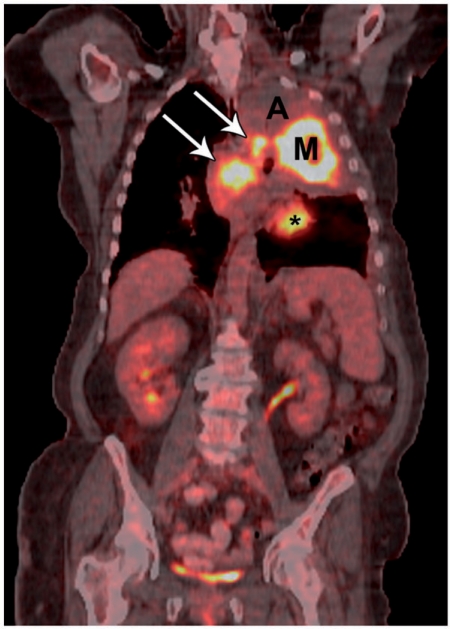
Figure 4.
A 57-year-old female with a central left upper lobe non-small cell lung cancer and post-obstructive atelectasis. CT-PET fusion image shows FDG uptake in the central mass (M) and in mediastinal lymph nodes (arrows); there is no uptake in the post-obstructive atelectasis (A). * indicates normal cardiac uptake.
Diaphragmatic paralysis
Diaphragmatic paralysis may be caused by tumor compression upon or invasion into the phrenic nerve or the C3–5 nerve roots. Patients are often relatively asymptomatic from the diaphragmatic paralysis, although there may be mild signs and symptoms of respiratory dysfunction, including dyspnea on exertion, dyspnea at rest, and orthopnea. Occasionally unilateral diaphragmatic paralysis leads to substantial decrease in pO2 and breathing capacity[6].
Common tumor types resulting in diaphragmatic paralysis include lung cancer and thymic neoplasms; other neoplasms that involve the mediastinum, neck, or cervical spine may also compromise the phrenic nerve or contributing nerve roots. Occasionally phrenic nerve damage may be induced by radiation therapy[6] or percutaneous lung tumor ablation[7].
The major imaging finding in diaphragmatic paralysis is elevation of the hemidiaphragm. Generally, a tumor mass is visualized in the expected region of the phrenic nerve, alongside the lateral aspects of the mediastinum[7] (Fig. 5), or the in region of the C3–5 nerve roots. The diagnosis may be confirmed by use of the ‘sniff test’ in which fluoroscopy or ultrasonography is used to examine movement of the hemidiaphragm during sniffing; paradoxical elevation of the hemidiaphragm during rapid inhalation is diagnostic of paralysis.
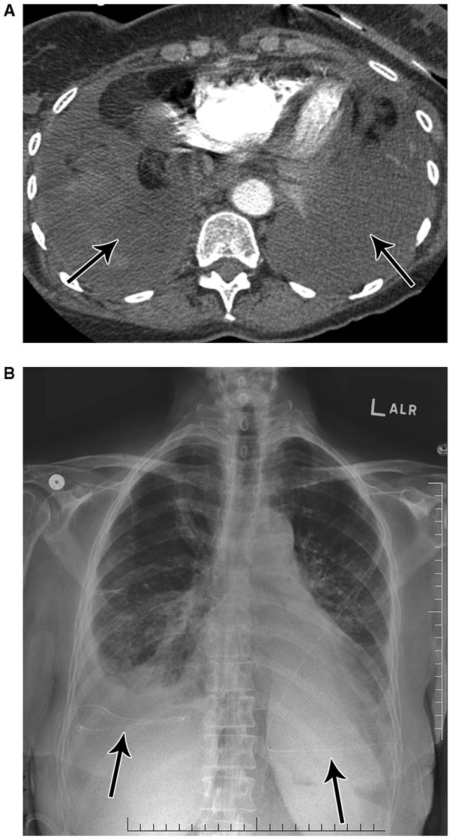
Figure 5.
A 47-year-old female with left hemidiaphragmatic paralysis due to phrenic nerve involvement by thymoma. Mediastinal soft tissue mass (M) is seen at CT (A), encroaching on the expected region of the left phrenic nerve (white arrow). Note elevated left hemidiaphragm (black arrows) at CT (B) and chest radiography (C).
Pleural effusion
Large benign and malignant pleural effusions often develop in oncology patients, leading to compression of adjacent pulmonary parenchyma, with resultant respiratory compromise (Fig. 6). Patients may present with dyspnea, cough, chest pain, weight loss, anorexia, fatigue, and/or malaise.
Figure 6.
A 74-year-old female with dyspnea due to malignant bilateral pleural effusions from metastatic small cell lung cancer. CT (A) shows bilateral, simple appearing pleural effusions (arrows). Subsequent chest radiograph (B) demonstrates bilateral pleural drainage catheters (arrows).
Benign pleural effusions may result from impaired normal pleural fluid resorption when there is lymphatic obstruction by tumor. Other mechanisms for benign effusions include sympathetic fluid accumulation in the setting of post-obstructive pneumonia or related to decreased oncotic pressure. Malignant effusions are typically due to direct pleural involvement by the neoplasm. Common neoplasms with malignant pleural effusions include tumors of the lung, breast or ovary and lymphoma. Less common tumors that cause metastatic, malignant effusions include carcinomas of the gastrointestinal and genitourinary tract. Adenocarcinoma is the most common histological type seen in malignant pleural effusions. Malignant mesotheliomas generally show a malignant effusion in association with solid pleural neoplasm.
Although a large proportion of malignant effusions appear simple on CT (water attenuation, without pleural thickening), the hallmarks of a malignant effusion include circumferential and multinodular pleural thickening. PET has been reported to show high sensitivity (86–97%) and high specificity (80–90%) for diagnosing malignancy within a pleural effusion based on FDG uptake in areas of pleural thickening and in the effusion itself[8,9]. In clinical practice, PET is useful in identifying the most suspicious region of pleural thickening, and thereby directing needle biopsy to that area.
Malignant pleural effusions may be treated using repeated thoracentesis, indwelling catheter drainage, or pleuroperitoneal shunting[10]. Pleurodesis, using talc or another type of sclerosing agent, is a commonly performed procedure for persistent effusions. This procedure is contraindicated, however, if there is trapped lung, preventing lung expansion, or multiloculation of the effusion.
Chylothorax
Sometimes a pleural effusion develops due to damage to the thoracic duct, with resultant spillage of chyle into pleural space. The most common neoplasms associated with this complication are lymphoma and metastatic carcinoma. Chylous effusions generally cannot be differentiated from simple pleural effusions based on CT attenuation values. Treatment options include pleural drainage, pleurodesis, pleuroperitoneal shunting, and ligation of thoracic duct[11].
Pericardial disease
Similar to the situation with pleural effusions, both benign and malignant pericardial effusions may be seen in oncology patients (Fig. 7). The major complication is restriction of cardiac filling with hemodynamic compromise leading to cardiac tamponade. In addition, pericardial thickening may result in constrictive pericarditis. Affected patients may be asymptomatic or may show mild symptoms such as dyspnea, fatigue, cough and chest pain. More severe symptoms include profound dyspnea that worsens with exertion, unexplained tachycardia, jugular venous distension, pulsus paradoxus, pulsus alternans and diminished heart sounds[12].
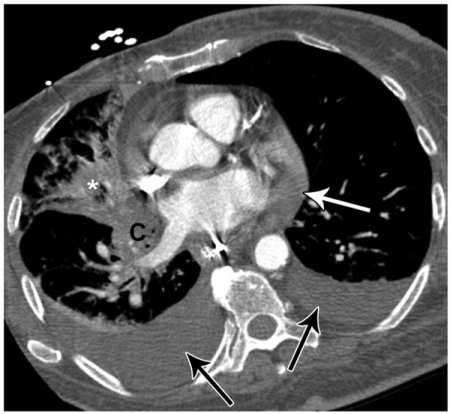
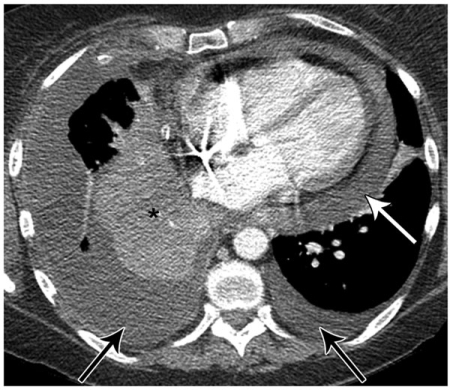
Figure 7.
A 50-year-old female with non-small cell lung cancer and shortness of breath. CT shows bilateral pleural effusions (black arrows) and a moderate sized pericardial effusion (white arrow). Subsequent echocardiogram confirmed a moderate sized pericardial effusion and showed evidence of early hemodynamic compromise. Note right lower lobe collapse (*) due to a more superior, central, obstructing bronchial mass (not shown).
Benign, inflammatory pericardial thickening or effusion may arise due to radiation therapy or chemotherapy or due to impaired lymphatic drainage, particularly in patients with lymphomatous involvement of mediastinal lymph nodes. Benign thickening or fluid may also be seen in the setting of graft versus host disease, in patients who have had previous bone marrow transplantation, and due to infectious causes in immunosuppressed patients, including bacterial, viral and fungal organisms.
There are multiple possible mechanisms for development of malignant pericardial thickening or effusion including direct pericardial extension from an adjacent neoplasm; lymphatic spread of tumor from nearby lymph nodes to the pericardium; and hematogenous spread, especially for lymphoma and leukemia. Malignant pericardial effusions may be hemorrhagic and may accumulate slowly or rapidly.
The most common tumor types leading to pericardial involvement include lung cancer, breast cancer, lymphoma and leukemia; however, almost any kind of cancer can spread to the pericardium. Occasionally mesotheliomas and primary cardiac tumors, such as sarcomas, will show pericardial disease.
Echocardiography is the test of choice to confirm a suspected pericardial effusion and to guide sampling and drainage. Generally, the aspirated fluid is sent for cytologic analysis, in order to confirm a malignant etiology. Occasionally, right heart catheterization is performed to assess for hemodynamic compromise. Both MR and cardiac CT can be performed to assess cardiac chamber volumes during different phases of the cardiac cycle.
Similar to the situation with malignant pleural effusions, many malignant pericardial effusions appear simple on CT; however, thickening and nodularity of the pericardium are suggestive of malignancy. The effusion may be high in attenuation due to debris and/or hemorrhage. Signs of right heart failure may be seen, such as such as hepatic congestion and contrast reflux into the inferior vena cava and hepatic veins.
Treatment of malignant pericardial disease generally consists of chemotherapy and/or radiation therapy for the underlying neoplasm. Pericardiocentesis, placement of an indwelling pericardial drain, and/or instillation of sclerosing agents into the pericardium may occasionally be performed for refractory effusions. Additional surgical options include construction of a pericardial window and pericardiectomy.
Tracheo-esophageal fistula
Neoplasms involving the mediastinum may erode into adjacent structures, and rarely lead to development of a fistula between the central airways and the esophagus. Generally the fistula is seen involving the trachea; however, sometimes the mainstem bronchi are involved, more commonly on the left compared with the right (where the left mainstem bronchus crosses over the esophagus). Uncommonly, a fistula may develop after radiation therapy for a tumor that involves both the esophagus and the central airway. Patients typically present with pulmonary infections, increased secretions, aspiration pneumonitis, coughing after swallowing, and/or poor nutrition[13].
The most common tumor type to cause this complication is esophageal cancer, although occasionally lung cancer may be the culprit. The diagnosis is usually clinically suspected, and it may be confirmed using barium esophagography. The fistula location and size may be further evaluated via bronchoscopy and esophagoscopy. These patients typically have a very poor prognosis and are difficult to manage clinically.
A direct communication between the esophageal and airway lumens, usually surrounded by a thick soft tissue rind of tumor, may be visualized on CT (Fig. 8). Sometimes it is helpful to view the suspicious area using wide windows, in order to confirm the communication.
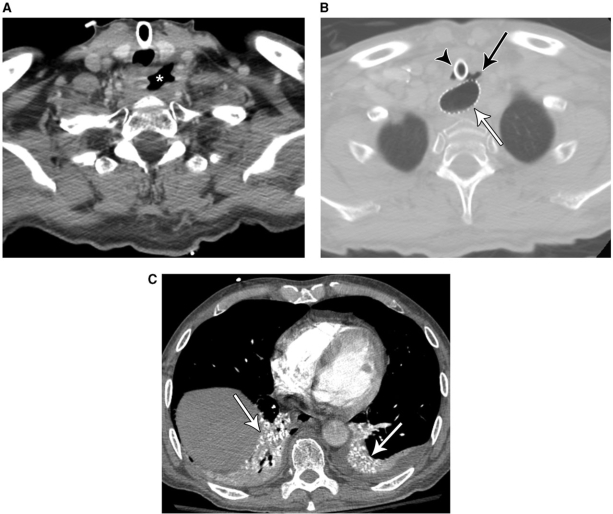
Figure 8.
A 58-year-old man with tracheoesophageal fistula due to metastatic non-small cell lung cancer treated with chemoradiation 2 months previously. CT (A) shows a cavitary mass (*) in the low neck due to metastatic disease involving the esophagus. More inferiorly (B), there is an extraluminal gas collection (black arrow) that communicates with both the trachea and the esophagus; note tracheostomy tube (arrowhead) and esophageal stent (white arrow). Image at the lung bases (C) reveals aspirated barium related to a previous fluoroscopy study that was performed before placement of the esophageal stent (arrows).
Treatment options include surgical esophageal bypass and palliative stenting of the esophagus and/or the trachea with covered stents in order to avoid aspiration pneumonia[14–16]. After stenting, CT can be used to assess for complications such as stent migration, fracture, and compression with stenosis[17].
DVT and PE
Patients with malignancies not infrequently develop a hypercoagulable state leading to deep venous thrombosis and sometimes pulmonary embolism (PE) (Fig. 9). In one CT study of 1168 patients, incidental (previously unsuspected) PE was found in 3.3% (11/332) of patients with progressive cancer, 2.5% (4/162) of patients with stable cancer, and 0.7% (2/271) of patients with no evidence of cancer post-treatment, compared to 1% (4/403) of non-oncological patients; 4.7% (9/193) of cancer patients who were receiving chemotherapy had a coincidental PE, compared with 1.4% (8/572) who were not receiving chemotherapy. The study concluded that the prevalence of previously unsuspected PE was significantly higher in patients with progressive cancer and in those receiving chemotherapy[18]. Another study found a prevalence of incidental PE on CT scanning of 4% (16/403) among oncology patients[19]. The highest prevalences were in patients with gynecologic malignancies (2/13, 15%) and melanoma (4/41, 10%). Emboli were prospectively identified on the initial CT reading in only 25% (4/16) of patients. Missed emboli were usually solitary and involved small arteries (beyond the lobar level). An additional investigation found that the use of wide window settings (width 600, level 100–150 HU) was helpful in detecting incidental PE on routine chest CT[20].
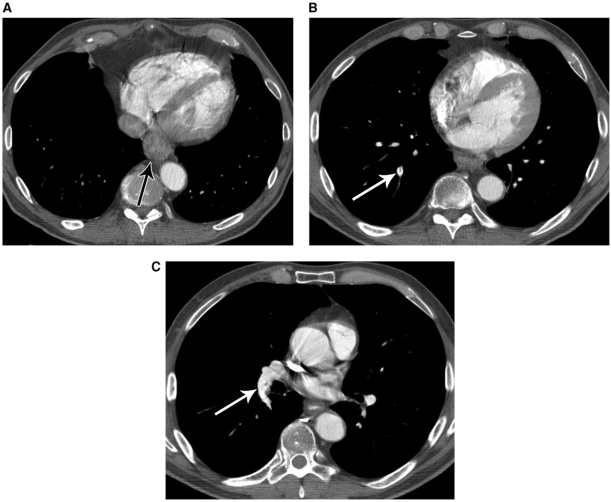
Figure 9.
A 63-year-old male with esophageal cancer and incidental pulmonary embolism. CT shows distal esophageal thickening (A), corresponding to the primary cancer (arrow). More superior images (B and C) reveal filling defects in the pulmonary arteries, representing pulmonary emboli (arrows).
Conclusion
In summary, radiological imaging plays a major role in evaluating oncologic patients with known or suspected complications and emergencies related to the underlying tumor. In addition, imaging not infrequently reveals clinically occult (but important) complications, such as silent pulmonary embolism. Knowledge of the spectrum of expected complications is important in order to optimize patient care.
References
- 1.Wilson LD, Detterbeck FC, Yahalom J. Clinical practice. Superior vena cava syndrome with malignant causes. N Engl J Med. 2007;356:1862–9. doi: 10.1056/NEJMcp067190. doi:10.1056/NEJMcp067190. PMid:17476012. [DOI] [PubMed] [Google Scholar]
- 2.Eren S, Karaman A, Okur A. The superior vena cava syndrome caused by malignant disease. Imaging with multi-detector row CT. Eur J Radiol. 2006;59:93–103. doi: 10.1016/j.ejrad.2006.01.003. doi:10.1016/j.ejrad.2006.01.003. PMid:16476534. [DOI] [PubMed] [Google Scholar]
- 3.Celebioglu B, Gurkan OU, Erdogan S, et al. High dose rate endobronchial brachytherapy effectively palliates symptoms due to inoperable lung cancer. Jpn J Clin Oncol. 2002;32:443–8. doi: 10.1093/jjco/hyf102. doi:10.1093/jjco/hyf102. PMid:12499415. [DOI] [PubMed] [Google Scholar]
- 4.Thomas S, Mathew A. Therapeutic bronchoscopy: endobronchial laser resection and airway stenting. Gastroenterol Nurs. 2002;25:60–6. doi: 10.1097/00001610-200203000-00006. doi:10.1097/00001610-200203000-00006. PMid:11984166. [DOI] [PubMed] [Google Scholar]
- 5.Lee P, Kupeli E, Mehta AC. Therapeutic bronchoscopy in lung cancer. Laser therapy, electrocautery, brachytherapy, stents, and photodynamic therapy. Clin Chest Med. 2002;23:241–56. doi: 10.1016/s0272-5231(03)00075-3. doi:10.1016/S0272-5231(03)00075-3. PMid:11901914. [DOI] [PubMed] [Google Scholar]
- 6.Elefteriades J, Singh M, Tang P, et al. Unilateral diaphragm paralysis: etiology, impact, and natural history. J Cardiovasc Surg (Torino) 2008;49:289–95. [PubMed] [Google Scholar]
- 7.Thornton RH, Solomon SB, Dupuy DE, Bains MS. Phrenic nerve injury resulting from percutaneous ablation of lung malignancy. AJR Am J Roentgenol. 2008;191:565–8. doi: 10.2214/AJR.07.3507. doi:10.2214/AJR.07.3507. PMid:18647933. [DOI] [PubMed] [Google Scholar]
- 8.Toaff JS, Metser U, Gottfried M, et al. Differentiation between malignant and benign pleural effusion in patients with extra-pleural primary malignancies: assessment with positron emission tomography-computed tomography. Invest Radiol. 2005;40:204–9. doi: 10.1097/01.rli.0000154217.71461.b4. doi:10.1097/01.rli.0000154217.71461.b4. PMid:15770138. [DOI] [PubMed] [Google Scholar]
- 9.Duysinx B, Nguyen D, Louis R, et al. Evaluation of pleural disease with 18-fluorodeoxyglucose positron emission tomography imaging. Chest. 2004;125:489–93. doi: 10.1378/chest.125.2.489. doi:10.1378/chest.125.2.489. PMid:14769729. [DOI] [PubMed] [Google Scholar]
- 10.Heffner JE. Diagnosis and management of malignant pleural effusions. Respirology. 2008;13:5–20. doi: 10.1111/j.1440-1843.2007.01154.x. [DOI] [PubMed] [Google Scholar]
- 11.Romero S. Nontraumatic chylothorax. Curr Opin Pulm Med. 2000;6:287–91. doi: 10.1097/00063198-200007000-00006. doi:10.1097/00063198-200007000-00006. PMid:10912634. [DOI] [PubMed] [Google Scholar]
- 12.Retter AS. Pericardial disease in the oncology patient. Heart Dis. 2002;4:387–91. doi: 10.1097/00132580-200211000-00008. doi:10.1097/00132580-200211000-00008. PMid:12441016. [DOI] [PubMed] [Google Scholar]
- 13.Reed MF, Mathisen DJ. Tracheoesophageal fistula. Chest Surg Clin N Am. 2003;13:271–89. doi: 10.1016/s1052-3359(03)00030-9. doi:10.1016/S1052-3359(03)00030-9. PMid:12755313. [DOI] [PubMed] [Google Scholar]
- 14.Nathwani RA, Kowalski T. Endoscopic stenting of esophageal cancer: the clinical impact. Curr Opin Gastroenterol. 2007;23:535–8. doi: 10.1097/MOG.0b013e3282a56968. doi:10.1097/MOG.0b013e3282a56968. PMid:17762559. [DOI] [PubMed] [Google Scholar]
- 15.Tomaselli F, Maier A, Sankin O, Pinter H, Smolle J, Smolle-Juttner FM. Ultraflex stent–benefits and risks in ultimate palliation of advanced, malignant stenosis in the esophagus. Hepatogastroenterology. 2004;51:1021–6. [PubMed] [Google Scholar]
- 16.Shin JH, Kim SW, Shim TS, et al. Malignant tracheobronchial strictures: palliation with covered retrievable expandable nitinol stent. J Vasc Interv Radiol. 2003;14:1525–34. doi: 10.1097/01.rvi.0000099525.29957.34. [DOI] [PubMed] [Google Scholar]
- 17.Ferretti GR, Kocier M, Calaque O, et al. Follow-up after stent insertion in the tracheobronchial tree: role of helical computed tomography in comparison with fiberoptic bronchoscopy. Eur Radiol. 2003;13:1172–8. doi: 10.1007/s00330-003-1820-0. [DOI] [PubMed] [Google Scholar]
- 18.Hui GC, Legasto A, Wittram C. The prevalence of symptomatic and coincidental pulmonary embolism on computed tomography. J Comput Assist Tomogr. 2008;32:783–7. doi: 10.1097/RCT.0b013e31815a7aea. doi:10.1097/RCT.0b013e31815a7aea. PMid:18830112. [DOI] [PubMed] [Google Scholar]
- 19.Gladish GW, Choe DH, Marom EM, Sabloff BS, Broemeling LD, Munden RF. Incidental pulmonary emboli in oncology patients: prevalence, CT evaluation, and natural history. Radiology. 2006;240:246–55. doi: 10.1148/radiol.2401051129. doi:10.1148/radiol.2401051129. PMid:16684921. [DOI] [PubMed] [Google Scholar]
- 20.Storto ML, Di Credico A, Guido F, Larici AR, Bonomo L. Incidental detection of pulmonary emboli on routine MDCT of the chest. AJR Am J Roentgenol. 2005;184:264–67. doi: 10.2214/ajr.184.1.01840264. [DOI] [PubMed] [Google Scholar]