The increasingly recognized notion that conditions during fetal life program alter susceptibility to adult disease represents a prototype of how the environment can influence biological processes. Several conditions including stress exposure, maternal nutrition and environmental temperature have been shown to affect the health of offspring.
The fast growing epidemic of obesity has witnessed mounting interest in the effect of maternal obesity on disease development in the offspring. In humans, epidemiological observations consistently noted the close relationship between maternal obesity and increased adiposity in the offspring. Offspring born to overweight or obese mothers were found to be at higher risk of developing greater weight gain or obesity during childhood, adolescence or adulthood suggesting that these disorders may have a developmental origin.1,2
These studies also raised the possibility of neonatal origins of the diseases commonly associated with obesity including hypertension. Prospective cohort studies have demonstrated that maternal obesity promotes higher blood pressure in the offspring. Systolic blood pressure in the offspring was found to be on average 0.2 mmHg higher for each 0.1 kg excessive weight gained by the mother during the pregnancy.2 A causal relationship between exposure to obesity in the perinatal period and increased adiposity in the offspring may contribute to the current epidemic of obesity through a continuous intergenerational cycle (Figure).
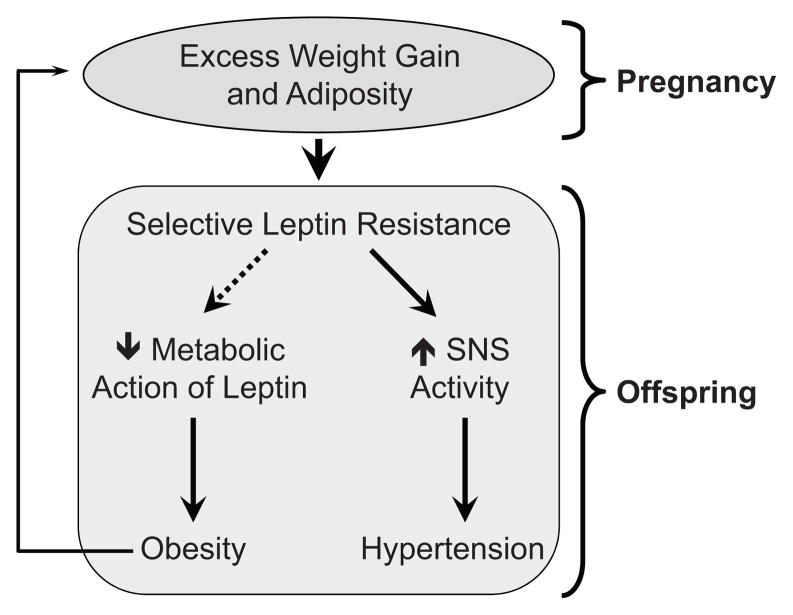
Figure.
Schematic representation of the mechanisms involved in fetal programming of obesity and hypertension. Excessive weight gain and obesity during pregnancy program selectivity in leptin resistance in the offspring: loss of metabolic action of leptin promotes obesity while preservation of sympathetic nerve activation to leptin causes higher sympathetic tone leading to hypertension. The developmental origin of excess weight gain and adiposity may contribute to epidemic of obesity through an intergenerational cycle.
Studies across animal species have confirmed the epidemiological findings and shown that rodents or sheep gestated in obese dams develop greater adiposity and excess weight gain.1 These studies also demonstrated that inducing obesity in pregnant rodents predisposes the offspring to develop hypertension.3 This confirms a close relationship between maternal obesity and hypertension in the offspring.
In the current issue of Hypertension, Samuelsson and colleagues4 further explore and dissect the mechanisms involved in maternal obesity-induced hypertension in the offspring. Arterial pressure and autonomic function were assessed in the offspring born to obese rats as compared to lean controls. In line with their previous findings3, rats born to obese dams exhibited higher arterial pressure. Strikingly, the increase in arterial pressure in the offspring occurs at early age (30 days), before the rats become obese suggesting that the development of hypertension in the offspring of obese dams is independent of obesity. However, the increase in fat mass and plasma leptin in 90 and 180 days old offspring seems to enhance the arterial pressure increase indicating that obesity may exacerbate the hypertension.
Activity of the autonomic nervous system is a key determinant of blood pressure and mounting evidence indicates that many forms of hypertension are initiated or maintained by autonomic dysfunction including sympathetic overdrive.5 To test for potential defects in the autonomic function that may account for the increase in arterial pressure associated with the offspring of obese rats, Samuelsson et al. 4 performed several experiments. First, the arterial pressure response to restraint stress, which is thought to be mediated by the sympathetic nervous system, was analyzed. Restraint stress caused a greater increase in arterial pressure in the offspring of obese dams, a clue suggesting sympathetic overdrive in these animals. The sympathetic overdrive was confirmed by the demonstration of increased renal levels of catecholamines; an enhanced fall in arterial pressure following adrenergic receptor blockade; and an elevation in low frequency oscillations of blood pressure. The enhanced sympathetic outflow in the offspring of the obese dams was associated with increased renal renin gene expression. The offspring of obese rats exhibited lower parasympathetic drive as indicated by the spectral analysis of heart rate and impairment in baroreflex sensitivity. These alterations may contribute to maternal obesity-induced hypertension in the offspring. However, these changes in parasympathetic outflow as well as baroreflex sensitivity are observed only in the 90 days fatter offspring indicating that these alterations may be due to obesity rather than fetal programming.
These findings provide an important mechanistic insight into the developmental origins of hypertension by implicating heightened sympathetic drive. These studies also suggest that activity of the sympathetic nervous system is programmed during fetal life. This is supported by the demonstration that sympathetic overdrive occurs in non-obese young offspring. Additional evidence supporting fetal programming of the sympathetic nervous system derives from the studies demonstrating alterations in sympathetic tone in the offspring of animals exposed in their early life to various cues such as temperature, litter size and undernutrition6. However, it is unclear how exposing fetuses to such different environments could lead to similar disease in the offspring. It is particularly striking that neonatal exposure to contrasting nutritional conditions such as undernutrition and obesity (a state of overnutrition) produces comparable disorders in the offspring including obesity and hypertension. It is possible that fetal exposure to various conditions leads to parallel developmental modifications aimed at maintaining physiological homeostasis of the fetus to ensure survival, but with adverse consequences in later life.
In addition to hypertensive effects, perturbations in the sympathetic nervous system may be a factor for the development of obesity in adult life. It may seem paradoxical that sympathetic overdrive could be considered as a predisposing factor for obesity, but longitudinal studies have shown that heightened sympathetic nerveactivity at entry predicts the future onset of hypertension as well as obesity.7 Increased sympathetic drive may also contribute to the insulin resistance associated with the offspring of obese animals.3 Sympathetically-mediated effects on glucose metabolism in skeletal muscle and lipolysis in adipose tissue have been proposed as potential mechanisms linking sympathetic activation and insulin resistance.7 This would imply a regional increase in the sympathetic nerve activity in the offspring of obese animals. However, it is not clear yet whether the increase in sympathetic nerve activity is specific to the cardiovascular system or also includes increased sympathetic activity to metabolically active tissues such as brown and white adipose tissue.
Although the mechanisms underlying the heightened sympathetic drive in the offspring of obese rats are not established, data on leptin effects on arterial pressure offer a potential clue. Leptin which circulates in proportion to body fat is considered a critical signal for the long-term control of energy homeostasis and body weight. In addition, leptin exerts pleiotropic effects including sympathetic nerve activation and arterial pressure elevation. Samuelsson et al.4 found that systemic leptin injection increased arterial pressure in the offspring of lean and obese rats. Strikingly, the arterial pressure effect of leptin treatment was more pronounced in the 30 and 90 days old offspring of obese rats as compared to lean controls. Of note, these authors have previously demonstrated that offspring of obese rats had a blunted effect of leptin on food intake and body weight.8 Unexpectedly, this decreased sensitivity to metabolic actions of leptin was found to be present in the 30 days old offspring rats that have normal circulating leptin levels. Together, these data highlight the importance of leptin for the metabolic as well as cardiovascular disorders in the offspring of obese animals.
Impaired metabolic action of leptin in the offspring of obese rats suggests a fetal programming of leptin sensitivity. On the other hand, the enhanced leptin-induced arterial pressure increase in the offspring is in agreement with the selectivity in leptin resistance and the emerging evidence for a pathophysiological role for leptin in the hypertension associated with obesity. The ability of leptin to increase the renal sympathetic nerve activity and arterial pressure is preserved in obesity despite the resistance to the metabolic actions of leptin9;10. In the context of high circulating levels of leptin associated with obesity such selective leptin resistance could predispose to obesity-related hypertension and other cardiovascular diseases (Figure). The findings in the offspring of obese rats extend and enhance the potential pathophysiologic significance of the phenomenon of selective leptin resistance. Studies addressing the mechanisms that account for the selectivity in leptin resistance are urgently needed.
Acknowledgments
Source of Funding
K.R. is supported by National Heart, Lung, and Blood Institute Grant HL084207.
Footnotes
Disclosure
None.
References
- 1.McMillen IC, Rattanatray L, Duffield JA, Morrison JL, MacLaughlin SM, Gentili S, Muhlhausler BS. The early origins of later obesity: pathways and Mmechanisms. Adv Exp Med Biol. 2009;646:71–81. doi: 10.1007/978-1-4020-9173-5_8. [DOI] [PubMed] [Google Scholar]
- 2.Mamun AA, O’Callaghan M, Callaway L, Williams G, Najman J, Lawlor DA. Associations of gestational weight gain with offspring body mass index and blood pressure at 21 years of age: evidence from a birth cohort study. Circulation. 2009;119:1720–1727. doi: 10.1161/CIRCULATIONAHA.108.813436. [DOI] [PubMed] [Google Scholar]
- 3.Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EHJM, Piersma AH, Ozanne SE, Twinn DF, Remacle C, Rowlerson A, Poston L, Taylor PD. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance - A novel murine model of developmental programming. Hypertension. 2008;51:383–392. doi: 10.1161/HYPERTENSIONAHA.107.101477. [DOI] [PubMed] [Google Scholar]
- 4.Samuelsson AM, Morris A, Igosheva N, Kirk SL, Pombo JMC, Coen CW, Poston L, Taylor PD. Evidence for sympathetic origins of hypertension in juvenille offspring of obese rats. Hypertension. 2009 doi: 10.1161/HYPERTENSIONAHA.109.139402. [DOI] [PubMed] [Google Scholar]
- 5.Grassi G. Assessment of sympathetic cardiovascular drive in human hypertension: achievements and perspectives. Hypertension. 2009;54:690–697. doi: 10.1161/HYPERTENSIONAHA.108.119883. [DOI] [PubMed] [Google Scholar]
- 6.Young JB. Developmental origins of obesity: a sympathoadrenal perspective. Int J Obes. 2006;30:S41–S49. doi: 10.1038/sj.ijo.0803518. [DOI] [PubMed] [Google Scholar]
- 7.Esler M, Straznicky N, Eikelis N, Masuo K, Lambert G, Lambert E. Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension. 2006;48:787–796. doi: 10.1161/01.HYP.0000242642.42177.49. [DOI] [PubMed] [Google Scholar]
- 8.Kirk SL, Samuelsson AM, Argenton M, Dhonye H, Kalamatianos T, Poston L, Taylor PD, Coen CW. Maternal obesity induced by diet in rats permanently influences central processes regulating food intake in offspring. PLoS One. 2009;4:e5870. doi: 10.1371/journal.pone.0005870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Correia ML, Haynes WG, Rahmouni K, Morgan DA, Sivitz WI, Mark AL. The concept of selective leptin resistance: evidence from agouti yellow obese mice. Diabetes. 2002;51:439–442. doi: 10.2337/diabetes.51.2.439. [DOI] [PubMed] [Google Scholar]
- 10.Rahmouni K, Morgan DA, Morgan GM, Mark AL, Haynes WG. Role of selective leptin resistance in diet-induced obesity hypertension. Diabetes. 2005;54:2012–2018. doi: 10.2337/diabetes.54.7.2012. [DOI] [PubMed] [Google Scholar]