Abstract
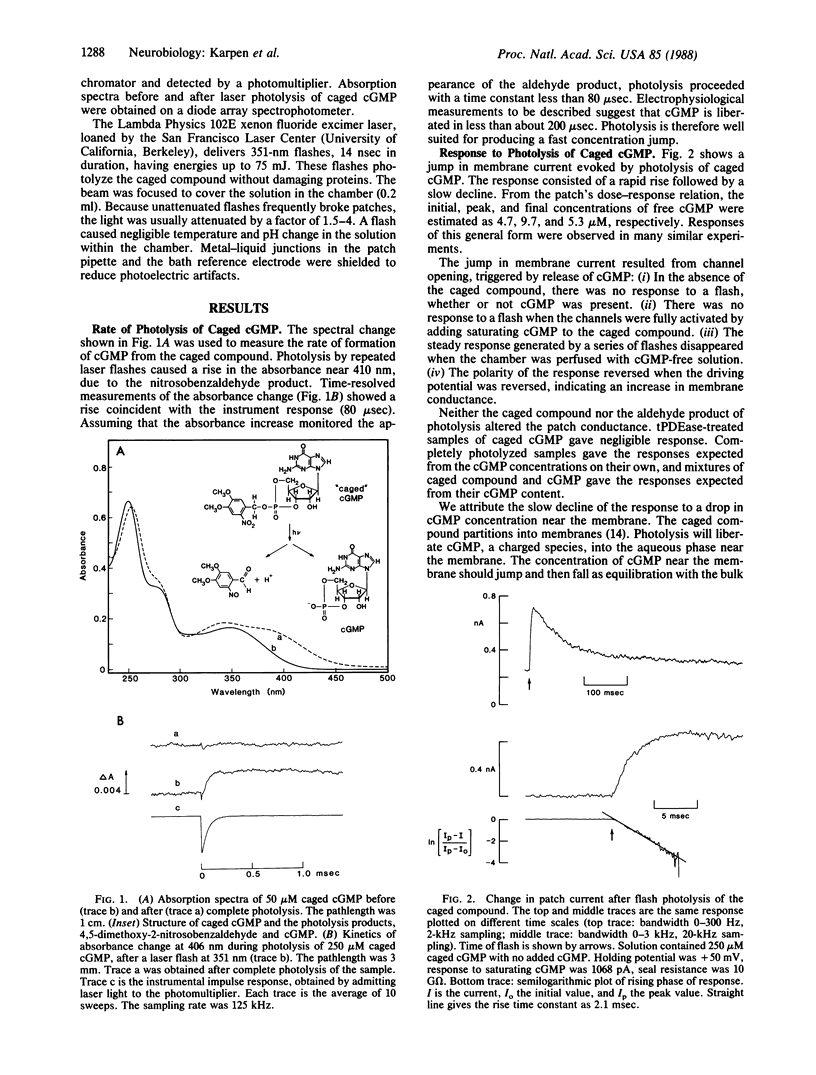
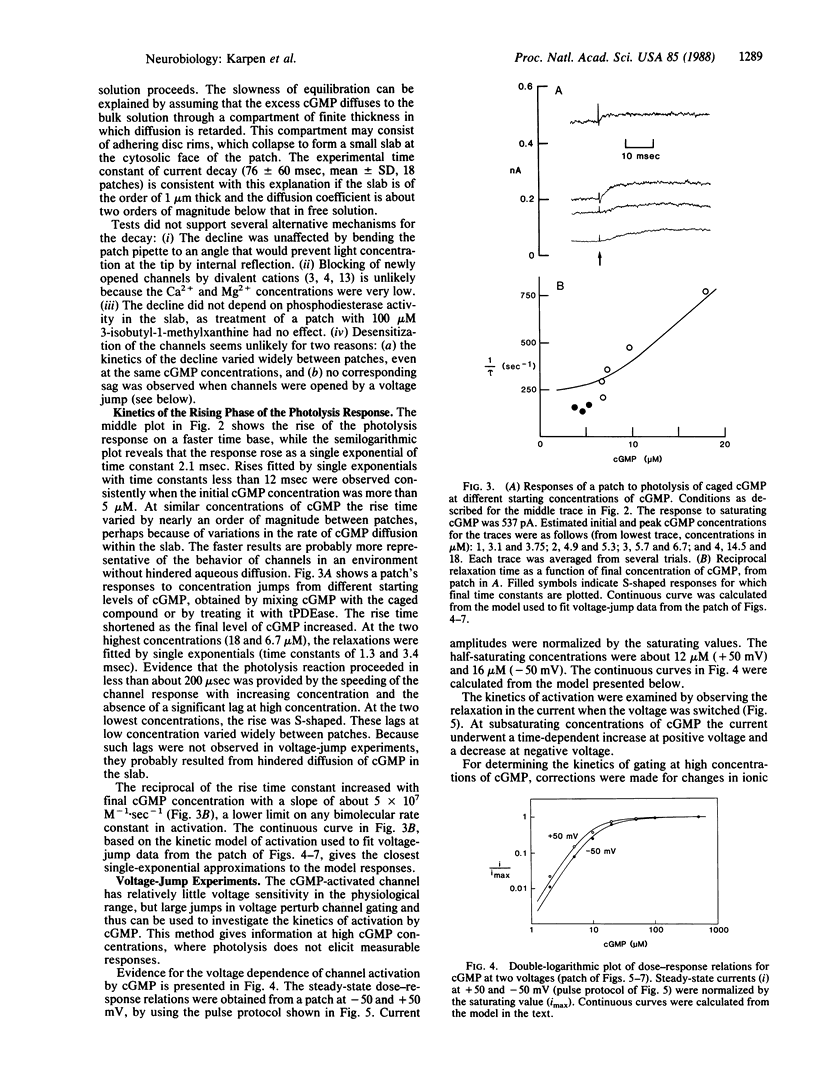
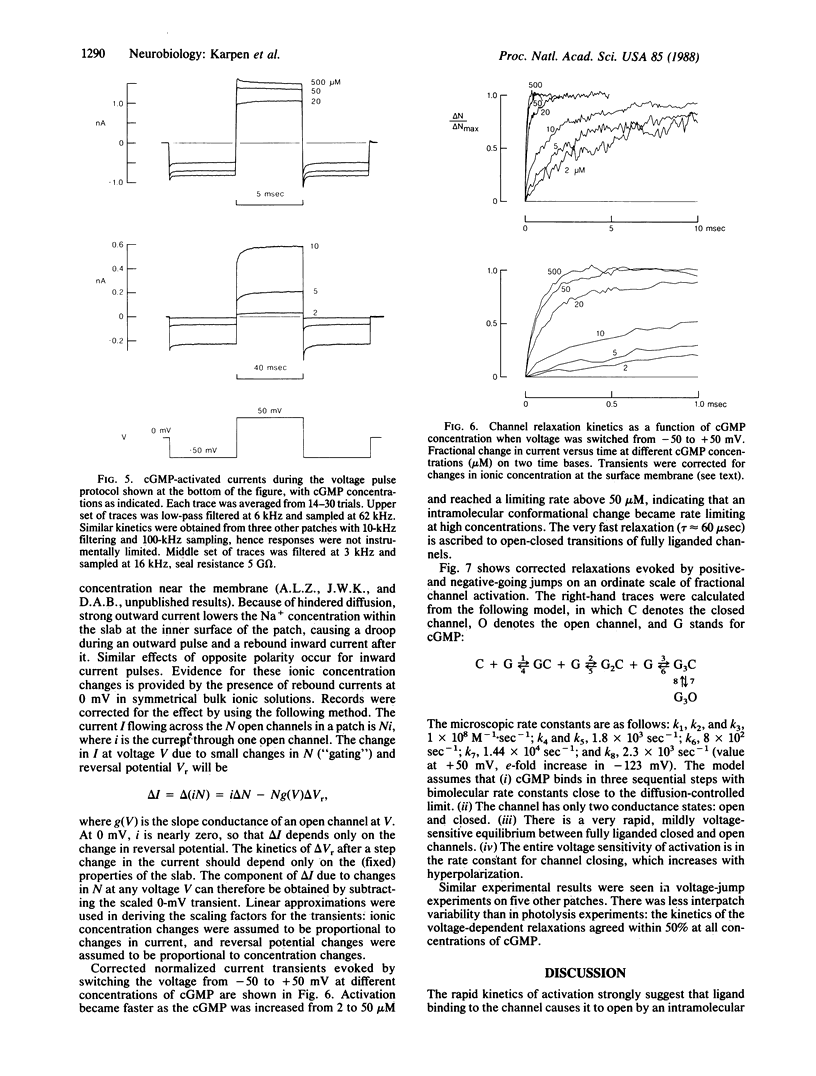
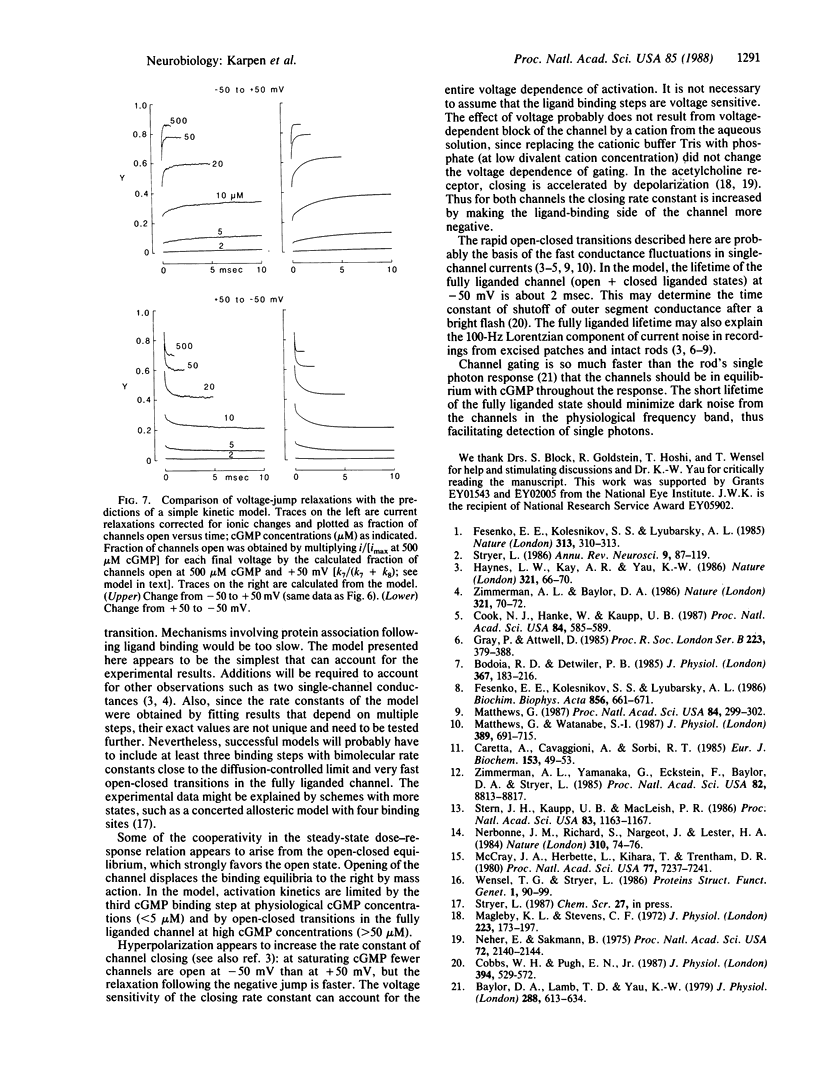
The gating kinetics of the cGMP-activated cation channel of salamander retinal rods have been studied in excised membrane patches. Relaxations in patch current were observed after two kinds of perturbation: (i) fast jumps of cGMP concentration, generated by laser flash photolysis of a cGMP ester ("caged" cGMP), and (ii) membrane voltage jumps, which perturb activation of the channel by cGMP. In both methods the speed of activation increased with the final cGMP concentration. The results are explained by a simple kinetic model in which activation involves three sequential cGMP binding steps with bimolecular rate constants close to the diffusion-controlled limit; fully liganded channels undergo rapid open-closed transitions. Voltage perturbs activation by changing the rate constant for channel closing, which increases with hyperpolarization. Intramolecular transitions of the fully liganded channel limit the kinetics of activation at high cGMP concentrations (greater than 50 microM), whereas at physiological cGMP concentrations (less than 5 microM), the kinetics of activation are limited by the third cGMP binding step. The channel appears to be optimized for rapid responses to changes in cytoplasmic cGMP concentration.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor D. A., Lamb T. D., Yau K. W. Responses of retinal rods to single photons. J Physiol. 1979 Mar;288:613–634. [PMC free article] [PubMed] [Google Scholar]
- Bodoia R. D., Detwiler P. B. Patch-clamp recordings of the light-sensitive dark noise in retinal rods from the lizard and frog. J Physiol. 1985 Oct;367:183–216. doi: 10.1113/jphysiol.1985.sp015820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caretta A., Cavaggioni A., Sorbi R. T. Binding stoichiometry of a fluorescent cGMP analogue to membranes of retinal rod outer segments. Eur J Biochem. 1985 Nov 15;153(1):49–53. doi: 10.1111/j.1432-1033.1985.tb09265.x. [DOI] [PubMed] [Google Scholar]
- Cobbs W. H., Pugh E. N., Jr Kinetics and components of the flash photocurrent of isolated retinal rods of the larval salamander, Ambystoma tigrinum. J Physiol. 1987 Dec;394:529–572. doi: 10.1113/jphysiol.1987.sp016884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook N. J., Hanke W., Kaupp U. B. Identification, purification, and functional reconstitution of the cyclic GMP-dependent channel from rod photoreceptors. Proc Natl Acad Sci U S A. 1987 Jan;84(2):585–589. doi: 10.1073/pnas.84.2.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesenko E. E., Kolesnikov S. S., Lyubarsky A. L. Direct action of cGMP on the conductance of retinal rod plasma membrane. Biochim Biophys Acta. 1986 Apr 25;856(3):661–671. doi: 10.1016/0005-2736(86)90162-8. [DOI] [PubMed] [Google Scholar]
- Fesenko E. E., Kolesnikov S. S., Lyubarsky A. L. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985 Jan 24;313(6000):310–313. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- Gray P., Attwell D. Kinetics of light-sensitive channels in vertebrate photoreceptors. Proc R Soc Lond B Biol Sci. 1985 Jan 22;223(1232):379–388. doi: 10.1098/rspb.1985.0007. [DOI] [PubMed] [Google Scholar]
- Haynes L. W., Kay A. R., Yau K. W. Single cyclic GMP-activated channel activity in excised patches of rod outer segment membrane. Nature. 1986 May 1;321(6065):66–70. doi: 10.1038/321066a0. [DOI] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. A quantitative description of end-plate currents. J Physiol. 1972 May;223(1):173–197. doi: 10.1113/jphysiol.1972.sp009840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G. Single-channel recordings demonstrate that cGMP opens the light-sensitive ion channel of the rod photoreceptor. Proc Natl Acad Sci U S A. 1987 Jan;84(1):299–302. doi: 10.1073/pnas.84.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G., Watanabe S. Properties of ion channels closed by light and opened by guanosine 3',5'-cyclic monophosphate in toad retinal rods. J Physiol. 1987 Aug;389:691–715. doi: 10.1113/jphysiol.1987.sp016678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCray J. A., Herbette L., Kihara T., Trentham D. R. A new approach to time-resolved studies of ATP-requiring biological systems; laser flash photolysis of caged ATP. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7237–7241. doi: 10.1073/pnas.77.12.7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Voltage-dependence of drug-induced conductance in frog neuromuscular junction. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2140–2144. doi: 10.1073/pnas.72.6.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerbonne J. M., Richard S., Nargeot J., Lester H. A. New photoactivatable cyclic nucleotides produce intracellular jumps in cyclic AMP and cyclic GMP concentrations. Nature. 1984 Jul 5;310(5972):74–76. doi: 10.1038/310074a0. [DOI] [PubMed] [Google Scholar]
- Stern J. H., Kaupp U. B., MacLeish P. R. Control of the light-regulated current in rod photoreceptors by cyclic GMP, calcium, and l-cis-diltiazem. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1163–1167. doi: 10.1073/pnas.83.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryer L. Cyclic GMP cascade of vision. Annu Rev Neurosci. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]
- Wensel T. G., Stryer L. Reciprocal control of retinal rod cyclic GMP phosphodiesterase by its gamma subunit and transducin. Proteins. 1986 Sep;1(1):90–99. doi: 10.1002/prot.340010114. [DOI] [PubMed] [Google Scholar]
- Zimmerman A. L., Baylor D. A. Cyclic GMP-sensitive conductance of retinal rods consists of aqueous pores. Nature. 1986 May 1;321(6065):70–72. doi: 10.1038/321070a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman A. L., Yamanaka G., Eckstein F., Baylor D. A., Stryer L. Interaction of hydrolysis-resistant analogs of cyclic GMP with the phosphodiesterase and light-sensitive channel of retinal rod outer segments. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8813–8817. doi: 10.1073/pnas.82.24.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]



