Abstract
Nociceptin/orphanin FQ (N/OFQ) is the endogenous peptide for the NOP receptors. Depending on the doses, intrathecal administration of N/OFQ has dual actions (ie, hyperalgesia and antinociception) in rodents. However, the pharmacological profile of intrathecal N/OFQ is not fully known in primates. The aim of this study was to investigate behavioral effects of intrathecal N/OFQ over a wide dose range and to compare its effects with ligands known to produce hyperalgesia or antinociception in monkeys. Intrathecal N/OFQ from 1 fmol to 1 nmol did not produce any hyperalgesic or scratching responses. In contrast, intrathecal substance P 100 nmol produced hyperalgesia, and intrathecal DAMGO 10 nmol produced antinociception. At the dose range between 10 nmol and 1 µmol, intrathecal N/OFQ dose-dependently produced thermal antinociception against a noxious stimulus in 2 intensities. More importantly, N/OFQ in combined with intrathecal morphine dose-dependently potentiated morphine-induced antinociception without inhibiting morphine-induced itch/scratching. Taken together, this study is the first to provide a unique functional profile of intrathecal N/OFQ over a wide dose range in primates. Intrathecal N/OFQ produces thermal antinociception without anti-morphine actions or scratching responses, indicating that N/OFQ or NOP receptor agonists represent a promising target as spinal analgesics.
Perspective: Intrathecal administration of N/OFQ only produced thermal antinociception, not hyperalgesia, in monkeys. In addition, intrathecal N/OFQ does not have anti-morphine actions or itch/scratching responses. This study strongly supports the therapeutic potential of N/OFQ or NOP receptor agonists as spinal analgesics for clinical trials.
Keywords: Spinal cord, analgesia, NOP receptors, substance P, thermal hyperalgesia
Spinal administration of µ-opioid receptor agonists is an important method for pain management, and it is widely used for obstetric analgesia.8,10 However, itch/pruritus is the most common side effect derived from spinal opioids, and it reduces the value of pain relief afforded by spinal opioids.8,14 Previously, we have established an experimental model of spinal opioid-induced itch/scratching in monkeys.18,21 Intrathecal administration of morphine dose-dependently produces antinociception with simultaneous scratching responses in monkeys,18 and this observation parallels closely with the behavioral effects of spinal morphine in humans.1,34 This experimental model using the intrathecal route for drug delivery in primates provides a valuable tool for identifying a novel, viable target as spinal analgesics.
Interestingly, a recent study found that intrathecal administration of an endogenous peptide, nociceptin/orphanin FQ (N/OFQ),28,36 in the dose range of nanomoles produced antinociceptive effects without itch/scratching responses in monkeys.22 Such naltrexone-insensitive effects could be blocked by the selective N/OFQ peptide receptor (NOP) antagonist J-113397 indicating that activation of spinal NOP receptors may be a promising target for spinal analgesia.22,24 However, ultra low doses of N/OFQ administered intrathecally at the dose range of femtomoles produced spontaneous agitation and pain manifested by biting, scratching, and licking behavioral responses in mice, suggesting that spinal N/OFQ has biphasic actions in rodents.15,40 Anatomical studies indicated that species differences may exist in the distribution of N/OFQ and NOP receptors.2,4 Nevertheless, most studies report that there is a high expression of N/OFQ and NOP receptors in the spinal cord of both rodents and humans.31,44 It is worth investigating whether spinal N/OFQ has both antinociceptive and pronociceptive/hyperalgesic actions and further characterizing the physiological functions of spinal N/OFQ in primates.
Therefore, the aim of this study was to extensively investigate and directly compare the behavioral effects of intrathecally administered N/OFQ over a wide dose range in monkeys. As noted, rodent studies have shown that intrathecal DAMGO and substance P produced antinociceptive and pronociceptive effects, respectively. 29,30,41 By using both behavioral end points (ie, antinociception/hyperalgesia and scratching responses), effects of intrathecal DAMGO and substance P were compared with those of intrathecal N/OFQ. Antinociceptive effects of intrathecal N/OFQ were further studied against a noxious stimulus in 2 intensities. In addition, the potential interaction between intrathecal N/OFQ and morphine was determined to explore whether N/OFQ modulated intrathecal morphine-induced antinociception and scratching responses.
Materials and Methods
Subjects
Eighteen adult intact male and female rhesus monkeys (Macacamulatta) with body weights ranging between 6.7 and 12.2 kg were used. The monkeys were housed individually with free access to water and were fed approximately 25 to 30 biscuits (Purina Monkey Chow; Ralston Purina, St. Louis, MO) and fresh fruit daily. No monkey had exposure to any opioid 1 month before the present study. The monkeys were housed in facilities accredited by the American Association for the Accreditation of Laboratory Animal Care. The studies were conducted in accordance with the University Committee on the Use and Care of Animals in the University of Michigan (Ann Arbor, MI) and the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the US National Institutes of Health (Bethesda, MD).
Procedures
Nociceptive Responses
The warm water tail-withdrawal assay was used to evaluate thermal antinociceptive or hyperalgesic effects of the test compound.19,22 Briefly, monkeys were seated in primate restraint chairs, and the lower part of their shaved tails (approximately 15 cm) were immersed in a thermal flask containing water maintained at either 42°, 46°, 50°, or 54°C. Tail-withdrawal latencies were measured using a computerized timer by an experimenter who did not know dosing conditions. In each test session, monkeys were evaluated once with 4 temperatures given in a random order. If the monkeys did not remove their tails within 20 seconds (cutoff), the flask was removed and a maximum time of 20 seconds was recorded. Test sessions began with determining a control value at each temperature. Subsequent tail-withdrawal latencies were determined at multiple time points after intrathecal administration.
Itch/Scratching Responses
Scratching behavior, inferred to be a response to itch sensation,18,21 was recorded on videotape while the monkeys were in their home cages. Each recording session was conducted for 15 minutes per test session. A scratch was defined as 1 short-duration (<1 second) episode of scraping contact of the forepaw or hind paw on the skin surface of other body parts. Scratches occurred repetitively at the same location. Scratching responses were scored by trained individuals who were blinded to experimental conditions. In addition, monkeys were rated for sedation and muscle relaxation according to 2 behavioral rating scales6 while in their home cages. The monitoring of potential side effects was conducted by an observer at the last minute of each test session.
Experimental Designs
The first part of the study was to determine behavioral responses of intrathecally administered N/OFQ over a wide range of ultra-low doses (ie, from 1 fmol to 1 nmol). In addition, effects of DAMGO and substance P were used as control conditions to compare with those of intrathecal N/OFQ. The doses of intrathecal DAMGO and substance P were selected based on a previous monkey study and our pilot study.21 The tail-withdrawal latency in the temperatures 46°C (non-noxious) and 50°C (noxious) of warm water was used to detect potential hyperalgesic/pronociceptive and antinociceptive effects, respectively, in monkeys.19,22 The second part of the study was to determine the degree of antinociception produced by intrathecal N/OFQ. The temperature 54°C of warm water represents a higher intensity of the nociceptive stimulus. The tail-withdrawal latency in both 50 and 54°C of warm water were used to characterize the antinociceptive effectiveness of intrathecal N/OFQ with increasing doses from 10 nmol to 1 µmol. The third part of the study was to investigate how behaviorally active doses of N/OFQ modulated intrathecal morphine-induced antinociception and scratching responses. The dose of intrathecal morphine 50 nmol was selected based on previous studies,20,26 showing that it produced maximal scratching responses and antinociception, and it could be used to detect whether intrathecal N/OFQ could interfere with morphine-mediated actions.
Statistical Analysis
Mean values (mean ± SEM) were calculated from individual values for all behavioral end points. Comparisons were made for the same monkeys across all test sessions in the same experiment. Data were analyzed by a 2-way analysis of variance (ANOVA) followed by the Newman-Keuls test for multiple (post hoc) comparisons. For comparison of data at a single time point, data were analyzed by 1-way ANOVA followed by the Dunnett test for multiple comparisons. The criterion for significance was set at P < .05.
Drugs
N/OFQ, morphine sulfate (National Institute on Drug Abuse, Bethesda, MD), DAMGO, and substance P (Sigma-Aldrich, St. Louis, MO) were dissolved in sterile water. Doses are presented in the compound forms listed above. For intrathecal administration, N/OFQ, morphine, or the mixture of N/OFQ and morphine was administered at a total volume of 1mL.Thedetaileddescriptionfor intrathecal drug delivery can be referred to previous studies.20,21 All experiments using intrathecal administration were conducted with a 10-day inter-injection interval.
Results
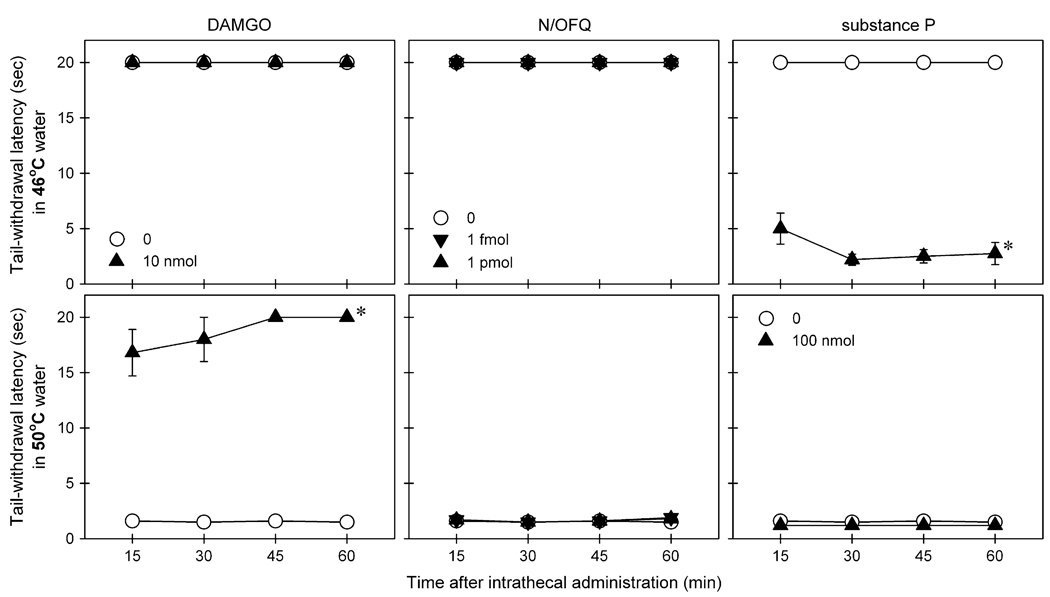
Fig 1 illustrates distinct responses to nociceptive stimuli of monkeys receiving intrathecal administration of N/OFQ, DAMGO, and substance P. Intrathecal N/OFQ over a wide range of ultra-low doses (ie, from 1 fmol to 1 nmol) did not produce either hyperalgesic or antinociceptive responses (Table 1). In contrast, intrathecal substance P 100 nmol produced hyperalgesic responses in 46°C water [F(1,5) = 1025.2; P < .05] and intrathecal DAMGO 10 nmol produced antinociceptive responses in 50°C water [F(1,5) = 335.9; P< .05].
Figure 1.
Comparison of warm water tail-withdrawal responses of intrathecally administered N/OFQ, DAMGO, and substance P. Top panels: Tail-withdrawal latency in 46°C water. Bottom panels: Tail-withdrawal latency in 50°C water. Behavioral responses were measured at 15-, 30-, 45-, and 60-minute time points after intrathecal administration of test compound, using a single dosing procedure. Each value represents mean ± SEM (n = 6). Symbols represent different dosing conditions for the same monkeys. Asterisk represents a significant difference from the vehicle condition for all time points (*P < .05).
Table 1.
Behavioral Responses of Intrathecal Administration of N/OFQ Over a Wide Range of Ultra-Low Doses as Compared to a Single Dose of DAMGO and Substance P.
| Warm Water Tail-Withdrawal Latency (sec)* |
|||
|---|---|---|---|
| Itch/Scratching† | |||
| Compound/Dose | 46°C | 50°C | Number/15 min |
| N/OFQ | |||
| 0 (vehicle) | 20 ± 0‡ | 1.6 ± 0.1 | 50.0 ± 12.9 |
| 1 fmol | 20 ± 0 | 1.7 ± 0.1 | 33.8 ± 9.9 |
| 10 fmol | 20 ± 0 | 1.6 ± 0.2 | 49.3 ± 15.7 |
| 100 fmol | 20 ± 0 | 1.4 ± 0.2 | 44.3 ± 14.0 |
| 1 pmol | 20 ± 0 | 1.7 ± 0.1 | 57.2 ± 15.3 |
| 10 pmol | 20 ± 0 | 1.8 ± 0.1 | 57.5 ± 10.2 |
| 100 pmol | 20 ± 0 | 1.9 ± 0.2 | 41.0 ± 15.1 |
| 1 nmol | 20 ± 0 | 1.6 ± 0.2 | 35.2 ± 7.1 |
| Substance P | |||
| 100 nmol | 4.9 ± 1.4§ | 1.2 ± 0.1 | 48.5 ± 8.6 |
| DAMGO | |||
| 10 nmol | 20 ± 0 | 16.8 ± 2.1§ | 910.5 ± 103.9§ |
The latency was measured at 15 min after intrathecal administration of test compound.
The scratching number was scored between 15th and 30th min after intrathecal administration of test compound.
Each value represents mean ± S.E.M. (n = 6).
The asterisk represents a significant difference from the vehicle condition (P < 0.05).
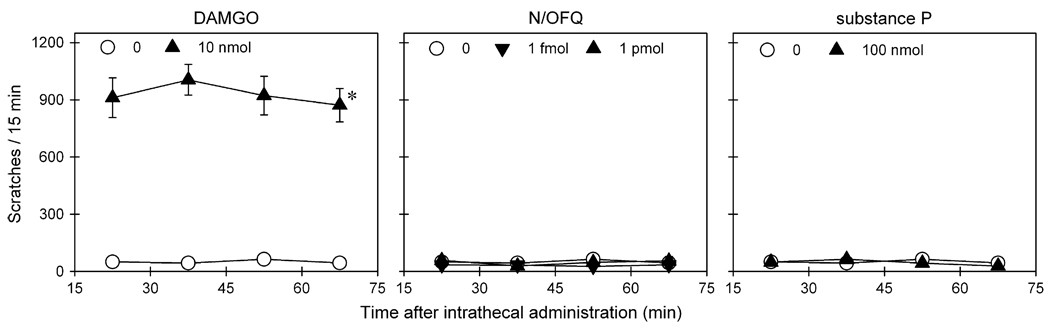
Fig 2 compares distinct behavioral responses of monkeys after intrathecal administration of N/OFQ, DAMGO, and substance P. Intrathecal N/OFQ over a wide dose range of ultra-low doses did not elicit scratching responses (Table 1). Although intrathecal substance P 100 nmol significantly produced hyperalgesic effects, this dose of substance P did not elicit scratching responses. In contrast, intrathecal DAMGO 10 nmol significantly evoked scratching responses [F(1,5) = 124.3; P < .05] in addition to its antinociceptive effects. Scratching evoked by intrathecal DAMGO peaked at the first observation period (ie, 15 minutes after intrathecal administration) and continued throughout the 1 hour observation period (Fig 2 and Table 1). It is worth noting that intrathecal administration of N/OFQ, DAMGO, and substance P at these doses did not cause any observable side effects including sedation and muscle relaxation.
Figure 2.
Comparison of itch/scratching responses of intrathecally administered N/OFQ, DAMGO, and substance P. Behavioral responses were scored for each 15-minute session after intrathecal administration of test compound, using a single dosing procedure. Each value represents mean ± SEM (n = 6). Symbols represent different dosing conditions for the same monkeys. Asterisk represents a significant difference from the vehicle condition for all time periods (*P < .05).
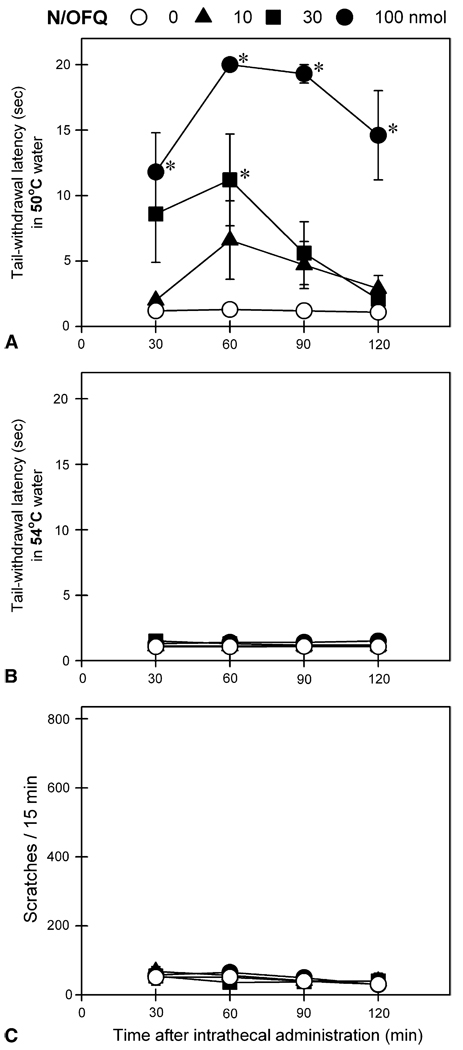
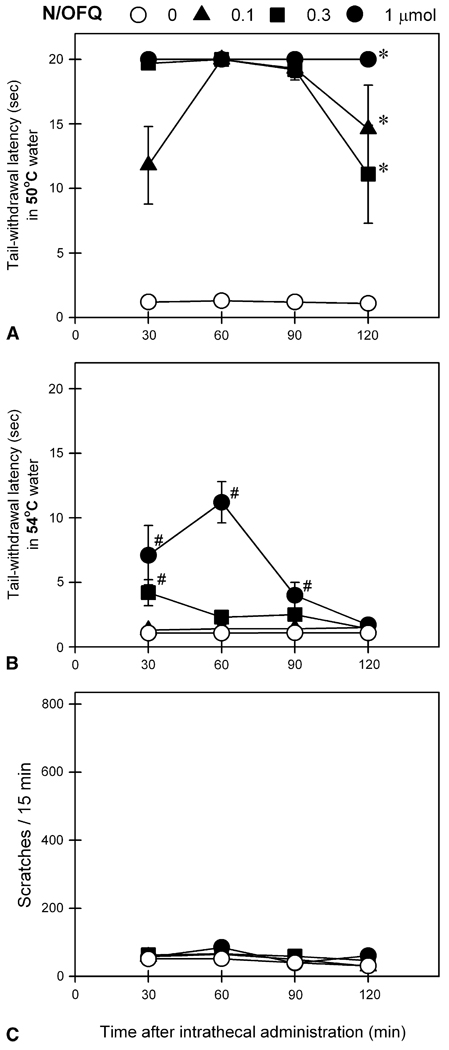
Fig 3 shows behavioral responses of intrathecal N/OFQ at doses between 10 and 100 nmol. Intrathecal N/OFQ dose-dependently produced antinociceptive effects against a nociceptive stimulus, 50°C water [F(3,15) = 28.1; P < .05]. However, N/OFQ at these doses did not produce significant antinociception against a higher intensity of nociceptive stimulus, 54°C water and it did not elicit scratching responses under these conditions. For comparison, Fig 4 shows behavioral responses of intrathecal N/OFQ at higher doses from 0.1 to 1 µmol. All 3 doses of intrathecal N/OFQ produced significant antinociception against 50°C water [F(3,15) = 198.4; P < .05]. In addition, N/OFQ dose-dependently produced antinociceptive effects against 54°C water [F(3,15) = 15.1, P < .05] without evoking scratching responses. It is worth noting that intrathecal administration of N/OFQ at these doses did not cause any observable side effects including sedation and motor impairment.
Figure 3.
Behavioral responses of intrathecally administered N/OFQ at doses between 10 and 100 nmol. A and B, tail-withdrawal latency in 50° and 54°C water, respectively. C, itch/scratching responses for each 15-minute session crossing the time points, 30, 60, 90, or 120 minutes after intrathecal N/OFQ (ie, scratching number between 23rd and 38th minutes for the time point, 30 minutes). Each value represents mean ± SEM (n = 6). Symbols represent different experimental conditions for the same monkeys. Asterisk represents a significant difference from the vehicle condition at corresponding time point (*P < .05).
Figure 4.
Behavioral responses of intrathecally administered N/OFQ at doses between 0.1 and 1 µmol. A and B, tail-withdrawal latency in 50° and 54°C water, respectively. C, itch/scratching responses for each 15-minute session crossing the time points, 30, 60, 90, or 120 minutes after intrathecal N/OFQ. Each value represents mean ± SEM (n = 6). Symbols represent different experimental conditions for the same monkeys. Asterisk represents a significant difference from the vehicle condition for all time points (*P < .05). #Significant difference from the vehicle condition at corresponding time point (P < .05). See Fig 3 for other details.
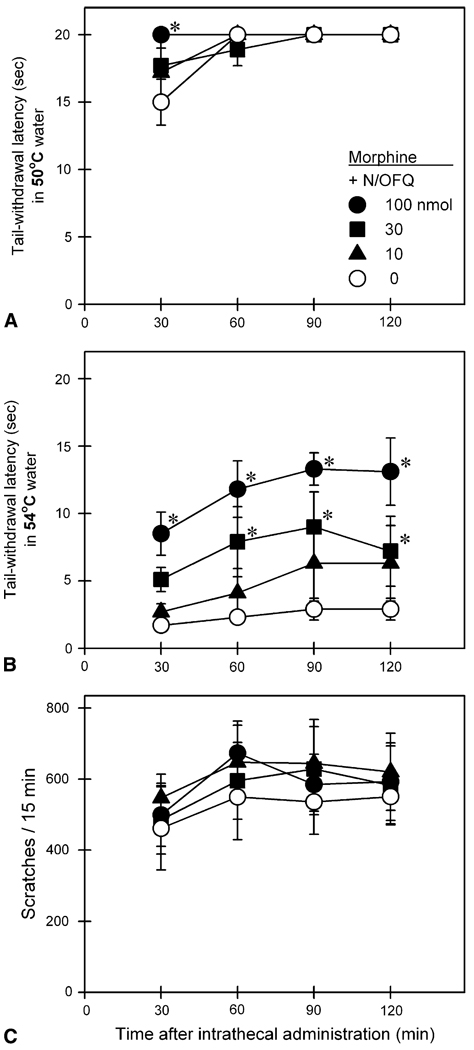
Fig 5 illustrates behavioral responses of intrathecal N/OFQ in combination with morphine. A single dose of intrathecal morphine 50 nmol produced antinociceptive effects against 50°C, not 54°C water (top 2 panels). This antinociceptive effect of intrathecal morphine was accompanied by profound scratching responses (bottom panel). When N/OFQ was combined with intrathecal morphine, N/OFQ dose-dependently increased the mixture’s antinociceptive effects against 54°C water [F(3,15) = 14.2; P < .05]. Under these conditions, increasing doses of N/OFQ did not attenuate intrathecal morphine-induced scratching responses.
Figure 5.
Behavioral responses of intrathecally administered N/OFQ in combination with morphine. Open circles represent the effects of intrathecal morphine 50 nmol alone. Other symbols represent effects of the same dose of morphine in combination with different doses of N/OFQ in the same monkeys. Each value represents mean ± SEM (n = 6). Asterisk represents a significant difference from the control condition (ie, intrathecal morphine alone) at corresponding time point (*P < .05). See Fig 3 for other details.
Discussion
The present study showed that intrathecal administration of N/OFQ over a wide dose range (ie, from 1 fmol to 1 µmol) produced thermal antinociception in the absence of hyperalgesia, scratching, sedation, and muscle relaxation. There were no sequelae to intrathecal N/OFQ, administered over several occasions consecutively in the same primates. For comparison, intrathecal administration of substance P 100 nmol significantly produced pronociceptive/hyperalgesic effects, manifested as reduced tail-withdrawal latencies in 46°C water. These results agree with rodent studies, indicating that intrathecal substance P causes hyperalgesic effects.27,30 Intrathecal administration of substance P and N/OFQ both produced a similar degree of hyperalgesic effects, as shown by decreased response latency approximately for 2 to 3 seconds in rodents.30,39 It has been suggested that intrathecal N/OFQ-induced hyperalgesia may be mediated by tachykinin NK1 receptors in the mouse spinal cord.39,40 Although intrathecal N/OFQ did not produce hyperalgesic effects like intrathecal substance P in monkeys, more studies are warranted to elucidate the relationship of intrathecal substance P with other neurotransmitter systems in the modulation of nociceptive processing of the primate spinal cord.
In contrast, intrathecal administration of DAMGO 10 nmol significantly produced antinociceptive effects, manifested as elevated tail-withdrawal latencies in 50°C water. These effects are consistent with rodent studies, indicating that intrathecal DAMGO is a potent µ-opioid antinociceptive agent.29,41 By testing intrathecal N/OFQ, substance P, and DAMGO in the same animals, they displayed distinct effects on modulating the nociceptive threshold. Such findings may suggest that intrathecal N/OFQ over a wide dose range does not produce pronociceptive/hyperalgesic responses in monkeys under this context.
Intrathecal administration of either N/OFQ or substance P did not significantly elicit scratching responses, but only intrathecal DAMGO elicited profound scratching responses (Fig 2 and Table 1). Behavioral responses of intrathecal DAMGO are expected because previous studies have demonstrated that antinociceptive doses of µ-opioid receptor agonists elicited scratching responses in monkeys.18,20,26 It is well known that intrathecal morphine produces pain relief accompanied by simultaneous itch sensation in humans.1,34 These findings strongly support the notion that increased scratching responses in monkeys may represent a behavioral end point selective for itch sensation18,21 and may suggest that intrathecal N/OFQ and substance P do not elicit itch sensation in primates.
It is interesting to know that intrathecal administration of substance P and N/OFQ both elicited scratching responses in rodents.3,13,15,40 Nevertheless, rodents’ scratching behavior may be neither necessary nor sufficient to be indicative of pain or itch sensation. For example, early studies showed that intrathecal substance P–induced scratching was not attenuated by pretreatment with analgesics, indicating that scratching is not pain-related.3,13 In contrast, increased scratching is considered as a sign of chronic pain in arthritic rats.11 Perhaps a series of behavioral responses including scratching, biting, and licking15,40 after intrathecal substance P or N/OFQ represents a general behavioral spectrum in rodents under the state of pain or/and agitation, especially when additional measurements such as decreased response latency to a noxious stimulus were provided.30,39 On the other hand, increased scratching is also considered as a behavioral response to itch sensation in rodents receiving pruritogenic agents.16,23,25 Whether scratching behavior is pain-related or itch-related depends on the context. Several factors such as administration routes and species differences may also contribute to different results or interpretations in the behavioral pharmacology of itch. Therefore, it is very important to conduct more psychophysical studies in humans and functional studies in animals9,17,42 to further integrate and elucidate the physiological role of each neurotransmitter in the modulation of itch and pain sensation.
Intrathecal administration of N/OFQ at the dose range from 10 nmol to 1 µmol dose-dependently produced antinociception against a noxious stimulus in 2 intensities (Fig 3 and Fig 4). The magnitude of N/OFQ’s antinociceptive effects in this assay is potentially similar to that of clinically available µ-opioid analgesics, such as nalbuphine, morphine, and fentanyl.5,18,43 Importantly, these antinociceptive doses of intrathecal N/OFQ did not elicit scratching responses. As previously demonstrated, intrathecal N/OFQ-induced antinociception was blocked by pretreatment with a selective NOP receptor antagonist, J-11339733 but not by a classic opioid receptor antagonist, naltrexone.22 These findings together suggest that intrathecal N/OFQ or other NOP receptor agonists may have the therapeutic potential as spinal analgesics without side effects derived from µ-opioid receptor agonists. The degree of antinociception produced by an experimental compound depends on its intrinsic efficacy and the nociceptive stimulus intensity.12,35,38 Future studies are needed to further investigate whether intrathecal N/OFQ or other NOP receptor agonists produce the same degree of antinociception as µ-opioid receptor agonists in monkeys under different pain modalities. In particular, long-lasting NOP receptor agonists32,37 such as UFP-112 have been identified, and it would be important to study such agonists in the context of spinal delivery in primates.
When N/OFQ was combined with a single dose of intrathecal morphine, this addition potentiated intrathecal morphine-induced antinociception, manifested as elevated tail-withdrawal latencies in 54°C water, by increasing the dose of N/OFQ (Fig 5). Interestingly, addition of intrathecal N/OFQ did not attenuate intrathecal morphine-elicited scratching responses. These results may indicate that intrathecal N/OFQ potentiates morphine-induced antinociception without producing motor-related side effects because monkeys still display profound scratching responses. Furthermore, in contrast to antimorphine actions of supraspinal N/OFQ,7,45 intrathecal N/OFQ did not produce anti-morphine actions, indicating that N/OFQ has different actions on spinal versus supraspinal sites.45 It would be reasonable to expect that intrathecal administration of a mixture of morphine with NOP receptor agonists produces antinociceptive effectiveness with fewer side effects. It also would be interesting to investigate the development of tolerance to antinociceptive effects of spinally administered morphine or/and NOP receptor agonists in future studies.
In summary, this study reveals a unique functional profile of intrathecal N/OFQ in primates. Unlike dual actions (ie, both pronociceptive and antinociceptive effects) of intrathecal N/OFQ observed in rodents, intrathecal N/OFQ over a wide dose range only produced antinociception. More importantly, intrathecal N/OFQ did not produce anti-morphine actions when it was combined with intrathecal morphine. The therapeutic potential of N/OFQ and the NOP receptors has been emphasized for its broad medical applications.24,45 Given that intrathecal N/OFQ produces antinociception without eliciting itch/scratching responses in monkeys, N/OFQ or other NOP receptor agonists represent a viable target as spinal analgesics for future clinical trials.
Acknowledgments
We thank John Pascoe, Kathryn Craig, Katie Hamelink, and Karthik Murali for technical assistance. All authors declare that they have no conflicts of interest.
Supported by United States Department of Defense, Peer Reviewed Medical Research Program, Grant No. W81XWH-07-1-0162.
References
- 1.Bailey PL, Rhondeau S, Schafer PG, Lu JK, Timmins BS, Foster W, Pace NL, Stanley TH. Dose-response pharmacology of intrathecal morphine in human volunteers. Anesthesiology. 1993;79:49–59. doi: 10.1097/00000542-199307000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Berthele A, Platzer S, Dworzak D, Schadrack J, Mahal B, Butter A, Aβmus HP, Wurster K, Zieglgansberger W, Conrad B, Tolle TR. [3H]-Nociceptin ligand-binding and nociception opioid receptor mRNA expression in the human brain. Neuroscience. 2003;121:629–640. doi: 10.1016/s0306-4522(03)00484-6. [DOI] [PubMed] [Google Scholar]
- 3.Bossut D, Frenk H, Mayer DJ. Is substance P a primary afferent neurotransmitter for nociceptive input? II: Spinalization does not reduce and intrathecal morphine potentiates behavioral responses to substance P. Brain Res. 1988;455:232–239. doi: 10.1016/0006-8993(88)90081-9. [DOI] [PubMed] [Google Scholar]
- 4.Bridge KE, Wainwright A, Reilly K, Oliver KR. Autoradio-graphic localization of 125I[Tyr14]nociception/orphanin FQ binding sites in macaque primate CNS. Neuroscience. 2003;118:513–523. doi: 10.1016/s0306-4522(02)00927-2. [DOI] [PubMed] [Google Scholar]
- 5.Butelman ER, Lewis JW, Woods JH. Methoclocinnamox: Agonist and antagonist effects of a novel long-lasting opioid in rhesus monkeys. J Pharmacol Exp Ther. 1996;279:934–938. [PubMed] [Google Scholar]
- 6.Butelman ER, Harris TJ, Kreek MJ. Effects of E-2078, a stable dynorphin A(1–8) analog, on sedation and serum prolactin levels in rhesus monkeys. Psychopharmacology. 1999;147:73–80. doi: 10.1007/s002130051144. [DOI] [PubMed] [Google Scholar]
- 7.Calò G, Rizzi A, Marzola G, Guerrini R, Salvadori S, Beani L, Regoli D, Bianchi C. Pharmacological characterization of the nociceptin receptor mediating hyperalgesia in the mouse tail withdrawal assay. Br J Pharmacol. 1998;125:373–378. doi: 10.1038/sj.bjp.0702087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cousins MJ, Mather LE. Intrathecal and epidural administration of opioids. Anesthesiology. 1984;61:276–310. [PubMed] [Google Scholar]
- 9.Davidson S, Zhang X, Yoon CH, Khasabov SG, Simone DA, Giesler G., Jr The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci. 2007;27:10007–10014. doi: 10.1523/JNEUROSCI.2862-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeBalli P, Breen TW. Intrathecal opioids for combined spinal-epidural analgesia during labour. CNS Drugs. 2003;17:889–904. doi: 10.2165/00023210-200317120-00003. [DOI] [PubMed] [Google Scholar]
- 11.De Castro-Costa M, Gybels J, Kupers R, Van Hees J. Scratching behavior in arthritic rats: A sign of chronic pain or itch? Pain. 1987;29:123–131. doi: 10.1016/0304-3959(87)90186-2. [DOI] [PubMed] [Google Scholar]
- 12.Dirig DM, Yaksh TL. Differential right shifts in the dose-response curve for intrathecal morphine and sufentanil as a function of stimulus intensity. Pain. 1995;62:321–328. doi: 10.1016/0304-3959(95)00006-E. [DOI] [PubMed] [Google Scholar]
- 13.Frenk H, Bossut D, Urca G, Mayer DJ. Is substance P a primary afferent neurotransmitter for nociceptive input? I: Analysis of pain-related behaviors resulting from intrathecal administration of substance P and 6 excitatory compounds. Brain Res. 1988;455:223–231. doi: 10.1016/0006-8993(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 14.Ganesh A, Maxwell LG. Pathophysiology and management of opioid-induced pruritus. Drugs. 2007;67:2323–2333. doi: 10.2165/00003495-200767160-00003. [DOI] [PubMed] [Google Scholar]
- 15.Inoue M, Shimohira I, Yoshida A, Zimmer A, Takeshima H, Sakurada T, Ueda H. Dose-related opposite modulation by nociceptin/orphanin FQ of substance P nociception in the nociceptors and spinal cord. J Pharmacol Exp Ther. 1999;291:308–313. [PubMed] [Google Scholar]
- 16.Jinks SL, Carstens E. Responses of superficial dorsal horn neurons to intradermal serotonin and other irritants: Comparison with scratching behavior. J Neurophysiol. 2002;87:1280–1289. doi: 10.1152/jn.00431.2001. [DOI] [PubMed] [Google Scholar]
- 17.Johanek LM, Meyer RA, Hartke T, Hobelmann JG, Maine DN, LaMotte RH, Ringkamp M. Psychophysical and physiological evidence parallel afferent pathways mediating the sensation of itch. J Neurosci. 2007;27:7490–7497. doi: 10.1523/JNEUROSCI.1249-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ko MCH, Naughton NN. An experimental itch model in monkeys: Characterization of intrathecal morphine-induced scratching and antinociception. Anesthesiology. 2000;92:795–805. doi: 10.1097/00000542-200003000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ko MCH, Naughton NN, Traynor JR, Song MS, Woods JH, Rice KC, McKnight AT. Orphanin FQ inhibits capsaicin-induced thermal nociception in monkeys by activation of peripheral ORL1 receptors. Br J Pharmacol. 2002;135:943–950. doi: 10.1038/sj.bjp.0704535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ko MCH, Lee H, Song MS, Sobczyk-Kojiro K, Mosberg HI, Kishioka S, Woods JH, Naughton NN. Activation of κ-opioid receptors inhibits pruritus evoked by subcutaneous or intrathecal administration of morphine in monkeys. J Pharmacol Exp Ther. 2003;305:173–179. doi: 10.1124/jpet.102.044909. [DOI] [PubMed] [Google Scholar]
- 21.Ko MCH, Song MS, Edwards T, Lee H, Naughton NN. The role of central mu opioid receptors in opioid-induced itch in primates. J Pharmacol Exp Ther. 2004;310:169–176. doi: 10.1124/jpet.103.061101. [DOI] [PubMed] [Google Scholar]
- 22.Ko MCH, Wei H, Woods JH, Kennedy RT. Effects of intrathecally administered nociceptin/orphanin FQ in monkeys: Behavioral and mass spectrometric studies. J Pharmacol Exp Ther. 2006;318:1257–1264. doi: 10.1124/jpet.106.106120. [DOI] [PubMed] [Google Scholar]
- 23.Kuraishi Y, Nagasawa T, Hayashi K, Satoh M. Scratching behavior induced by pruritogenic but not algesiogenic agents in mice. Eur J Pharmacol. 1995;275:229–233. doi: 10.1016/0014-2999(94)00780-b. [DOI] [PubMed] [Google Scholar]
- 24.Lambert DG. The nociceptin/orphanin FQ receptor: A target with broad therapeutic potential. Nat Rev Drug Discov. 2008;7:694–710. doi: 10.1038/nrd2572. [DOI] [PubMed] [Google Scholar]
- 25.Lee H, Naughton NN, Woods JH, Ko MCH. Characterization of scratching responses in rats following centrally administered morphine or bombesin. Behav Pharmacol. 2003;14:501–508. doi: 10.1097/01.fbp.0000095082.80017.0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee H, Naughton NN, Woods JH, Ko MC. Effects of butorphanol on morphine-induced itch and analgesia in primates. Anesthesiology. 2007;107:478–485. doi: 10.1097/01.anes.0000278876.20263.a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsumura H, Sakurada T, Hara A, Sakurada S, Kisara K. Characterization of the hyperalgesic effect induced by intrathecal injection of substance P. Neuropharmacology. 1985;24:421–426. doi: 10.1016/0028-3908(85)90027-9. [DOI] [PubMed] [Google Scholar]
- 28.Meunier J-C, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour J-L, Guillemot J-C, Ferrara P, Monsarrat B, Mazargull H, Vassart G, Parmentier M, Costentin J. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- 29.Mjanger E, Yaksh TL. Characteristics of dose-dependent antagonism by β-funaltrexamine of the antinociceptive effects of intrathecal mu agonists. J Pharmacol Exp Ther. 1991;258:544–550. [PubMed] [Google Scholar]
- 30.Moochhala SM, Sawynok J. Hyperalgesia produced by intrathecal substance P and related peptides: Desensitization and cross desensitization. Br J Pharmacol. 1984;82:381–388. doi: 10.1111/j.1476-5381.1984.tb10773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neal CR, Jr, Akil H, Watson SJ. Expression of orphanin FQ and the opioid receptor-like (ORL1) receptor in the developing human and rat brain. J Chem Neuroanat. 2001;22:219–249. doi: 10.1016/s0891-0618(01)00135-1. [DOI] [PubMed] [Google Scholar]
- 32.Okada K, Sujaku T, Chuman Y, Nakashima R, Nose T, Costa T, Yamada Y, Yokoyama M, Nagahisa A, Shimohigashi Y. Highly potent nociception analog containing the Arg-Lys triple repeat. Biochem Biophys Res Commun. 278:493–498. doi: 10.1006/bbrc.2000.3822. [DOI] [PubMed] [Google Scholar]
- 33.Ozaki S, Kawamoto H, Itoh Y, Miyaji M, Azuma T, Ichikawa D, Nambu H, Iguchi T, Iwasawa Y, Ohta H. In vitro and in vivo pharmacological characterization of J-113397, a potent and selective non-peptidyl ORL1 receptor antagonist. Eur J Pharmacol. 2000;402:45–53. doi: 10.1016/s0014-2999(00)00520-3. [DOI] [PubMed] [Google Scholar]
- 34.Palmer CM, Emerson S, Volgoropolous D, Alves D. Dose-response relationship of intrathecal morphine for postcesarean analgesia. Anesthesiology. 1999;90:437–444. doi: 10.1097/00000542-199902000-00018. [DOI] [PubMed] [Google Scholar]
- 35.Parsons CG, Headley PM. Spinal antinociceptive actions of mu- and kappa-opioids: The importance of stimulus intensity in determining “selectivity” between reflexes to different modalities of noxious stimulus. Br J Pharmacol. 1998;98:523–532. doi: 10.1111/j.1476-5381.1989.tb12626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reinscheid RK, Nothacker H-P, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Jr, Civelli O, Orphanin FQ. A neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- 37.Rizzi A, Spagnolo B, Wainford RD, Fischette C, Guerrini R, Marzola G, Baldisserotto A, Salvadori S, Regoli D, Kapusta DR, Calo G. In vitro and in vivo studies on UFP-112, a novel potent and long lasting agonist selective for the nociception/orphanin FQ receptor. Peptides. 2007;28:1240–1251. doi: 10.1016/j.peptides.2007.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saeki S, Yaksh TL. Suppression of nociceptive responses by spinal mu opioid agonists: Effects of stimulus intensity and agonist efficacy. Anesth Analg. 1993;77:265–274. doi: 10.1213/00000539-199308000-00010. [DOI] [PubMed] [Google Scholar]
- 39.Sakurada C, Sakurada S, Katsuyama S, Sasaki J, Tan-No K, Sakurada T. Involvement of tachykinin NK1 receptors in nociceptin-induced hyperalgesia in mice. Brain Res. 1999;841:85–92. doi: 10.1016/s0006-8993(99)01800-4. [DOI] [PubMed] [Google Scholar]
- 40.Sakurada T, Katsuyama S, Sakurada S, Inoue M, Tan-No K, Kisara K, Sakurada C, Ueda H, Sasaki J. Nociception-induced scratching, biting, and licking in mice: Involvement of spinal NK1 receptors. Br J Pharmacol. 1999;127:1712–1718. doi: 10.1038/sj.bjp.0702698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xie H, Woods JH, Traynor JR, Ko MC. The spinal antinociceptive effects of endomorphins in rats: Behavioral and G protein functional studies. Anesth Analg. 2008;106:1873–1881. doi: 10.1213/ane.0b013e31817300be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yosipovitch G, Carstens E. Report from the 4th international workshop for the study of itch. J Invest Dermatol. 2008;128:256–257. doi: 10.1038/sj.jid.5701218. [DOI] [PubMed] [Google Scholar]
- 43.Walker EA, Butelman ER, DeCosta BR, Woods JH. Opioid thermal antinociception in rhesus monkeys: Receptor mechanisms and temperature dependency. J Pharmacol Exp Ther. 1993;267:280–286. [PubMed] [Google Scholar]
- 44.Witta J, Palkovits M, Rosenberger J, Cox BM. Distribution of nociception/orphanin FQ in adult human brain. Brain Res. 2004;997:24–29. doi: 10.1016/j.brainres.2003.08.066. [DOI] [PubMed] [Google Scholar]
- 45.Zeilhofer HU, Calo G. Nociceptin/orphanin FQ and its receptor–potential targets for pain therapy? J Pharmacol Exp Ther. 2003;306:423–429. doi: 10.1124/jpet.102.046979. [DOI] [PubMed] [Google Scholar]