Abstract
PTPN22 is a gene encoding the protein tyrosine phosphatase Lyp. A missense mutation changing residue 1858 from cytosine to thymidine (1858C/T) is associated with multiple autoimmune disorders. Studies have demonstrated that Lyp has an inhibitory effect on TCR signaling; however, the presence of autoantibodies in all of the diseases associated with the 1858T variant and recent evidence that Ca2+ flux is altered in B cells of 1858T carriers indicate a role for Lyp in B cell signaling. In this study we show that B cell signal transduction is impaired in individuals who express the variant. This defect in signaling is characterized by a deficit in proliferation, a decrease in phosphorylation of key signaling proteins, and is reversed by inhibition of Lyp. These findings suggest that the PTPN22 1858T variant alters BCR signaling and implicate B cells in the mechanism by which the PTPN22 1858T variant contributes to autoimmunity.
Identifying the functional significance of genetic polymorphisms associated with autoimmunity will allow us to develop insights into the pathogenesis of these diseases. One such variant is a single nucleotide polymorphism (SNP)4 in the coding region of the protein tyrosine phosphatase N22 (PTPN22). PTPN22 encodes the lymphoid tyrosine phosphatase Lyp. The disease-associated SNP is a missense mutation at position 1858 (C→T), resulting in a change from an Arg (R) at position 620 to Trp (W). The diseases associated with this SNP include type 1 diabetes, rheumatoid arthritis, systemic lupus erythematosus, Graves disease, and myasthenia gravis (1–4). Autoantibodies play a prominent role in each of these diseases, implicating B cells in the functional outcome related to this variant.
Lyp has been shown to be a negative regulator of T cell activation (5) via its dephosphorylation of the activating tyrosines of Lck, Fyn, and ZAP-70 as well as phosphorylation sites on TCRζ, CD3ε, Vav, and valosin-containing protein (6, 7). Lyp620W results in a dominant gain of inhibitory function in T cells leading to a blunted response to TCR stimulation in individuals both homozygous and heterozygous for the 1858T variant (8, 9). Lyp is expressed in B lymphocytes (10), but its role in BCR-mediated signaling has not been established. Recently, our group has shown that subjects who carry the 1858T variant have a decrease in memory B cells, which also exhibit impaired calcium flux upon BCR ligation, suggesting a B cell-intrinsic defect in individuals who express the Lyp620W variant (9). In this current study, we sought to determine whether the PTPN22 1858T allele alters BCR signal transduction.
Materials and Methods
Subjects
Samples for this study were obtained from participants in the Benaroya Research Institute Immune Mediated Disease Registry and Juvenile Diabetes Research Foundation Center for Translational Research (Seattle, WA). The control population was selected based on a lack of personal or family history of diabetes, autoimmunity, or asthma. Research protocols were approved by the Benaroya Research Institute Institutional Review Board.
FACS analysis
Purified B cells were surface stained with CD19-PerCP (eBioscience), IgD-PE, CD27-allophycocyanin, CD20-Alexa Fluor 488, IgM-FITC, and B220-PerCP (BD Pharmingen). PE-conjugated anti-phosphotyrosine and anti-phospholipase C (PLC)γ2 (pY759) Abs for intracellular phosphoprotein staining were obtained from BD Biosciences. Flow cytometry was conducted on a BD FACSCalibur with analysis by FlowJo software (Tree Star).
B cell purification, activation, and proliferation
Miltenyi microbeads were used to isolate B cells from PBMC. Total (CD19+) and naive (CD19+CD27-) B cells were isolated via negative selection, and memory (CD19+CD27+) B cells were isolated by positive selection. B cells were activated by F(ab′)2 goat anti-human IgG plus IgM (Jackson Immuno-Research Laboratories) at 20 μg/ml or PMA/ionomycin. Cells (106/well) were cultured in triplicate in 96-well round-bottom plates (Costar; Corning), [3H]thymidine was then added after 48 h (1 μCi/well), and cells were harvested after an additional 24 h. Thymidine incorporation was quantified using a Wallac Microbeta Plus liquid scintillation counter (PerkinElmer). Apoptosis was assessed by quantifying cleaved caspase-3 (Asp175; Cell Signaling Technology) by intracellular flow cytometry.
Western blotting
B cells were activated with anti-human IgM F(ab′)2 (20 μg/ml) (Jackson ImmunoResearch Laboratories) for the amount of time indicated, followed by cell lysis. Protein lysates were quantified using Bradford assays (BioRad), resolved by gradient SDS-PAGE, and transferred onto polyvinylidene difluoride membranes (Millipore). The following Abs were used: phospho-Syk (Tyr525/526); Syk, phospho-PLCγ2 (Tyr759); phospho-Akt (Ser473); Akt, and phosphop38 MAPK (Thr180/Tyr182) (Cell Signaling). Anti-Lyp (R&D Systems), anti-mouse β-actin (Abcam), or anti-Syk antisera were used to verify equal protein loading.
Inhibitor studies
The Lyp specific inhibitor I-C11 was synthesized as described previously (13). Purified total B cells were rested overnight in RPMI 1640 medium supplemented with 1% human serum (MP Biomedicals). Cells were treated for 1 h with 0.1–10 μM I-C11 or 0.2% DMSO vehicle followed by stimulation with or without 20 μg/ml anti-IgM or anti-kappa (Southern Biotechnology Associates) (F(ab′)2) for 5 min. Immediately thereafter, cells were washed with ice-cold PBS and lysed as above for Western blotting or fixed and processed for intracellular phosphoprotein staining with anti-phospho-PLCγ2 according to the manufacturerg's instructions (BD Biosciences).
Statistical analysis
Data were analyzed by unpaired t tests as indicated using GraphPad Prism version 4.02 for Windows. Values of p < 0.05 were considered significant.
Results and Discussion
Expression of the PTPN22 1858T variant results in impaired B cell activation
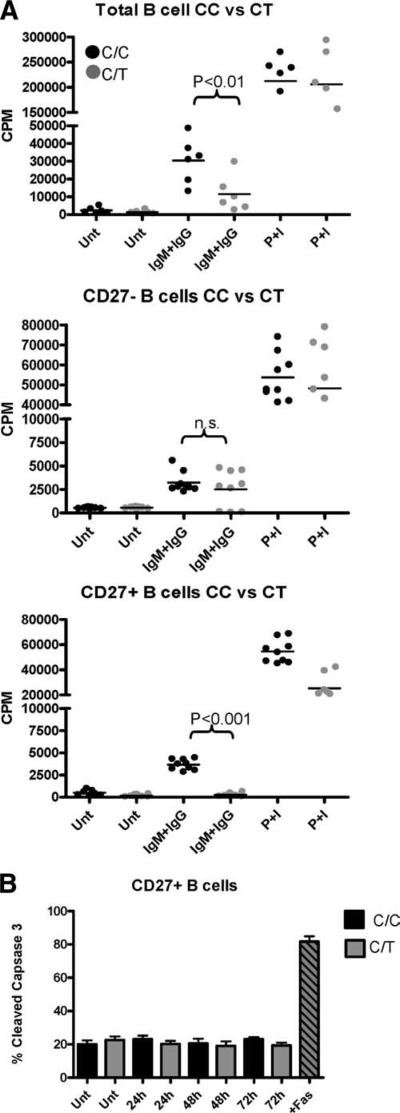
We have previously shown that healthy individuals heterozygous for the PTPN22 1858T variant allele exhibit reduced BCR-mediated calcium mobilization, consistent with the finding that this is a functionally dominant variant. To address the impact of the variant on the outcome of B cell activation, we compared Ag-mediated B cell proliferation in healthy PTPN22 C/C homozygote and PTPN22 C/T heterozygote subjects. Total (CD19+), naive (CD19+CD27-), and memory (CD19+CD27+) B cells were purified and then cultured with anti-IgM/IgG F(ab′)2 or PMA/ionomycin and proliferation was assessed at 72 h. The CD19+ B cells harboring the 1858T variant displayed reduced proliferation when stimulated via IgM/IgG (p < 0.01). When naive and memory B cells were examined separately, the impairment in proliferation was most pronounced in the memory B cell population (p < 0.001), whereas the proliferation of naive B cells was modestly diminished (Fig. 1A), consistent with the observation that the impairment of Ca2+ flux in the B cells of 1858T subjects is most profound in the memory B cell population (11).
FIGURE 1.
B cell proliferation is diminished in 1858C/T subjects. A, Total (CD19+; top), naive (CD19+CD27-; middle), and memory (CD19+CD27+; bottom) B cells were purified and activated with anti-IgG plus IgM F(ab′)2 (IgM+IgG) or PMA/ionomycin (P+I). Proliferation was measured at 72 h. Each dot represents a unique individual. B, B cells were activated as above and the CD27+ and CD27- populations were assessed by FACS for cleaved caspase-3 (n = 4 per group). Unt, Untreated.
Apoptosis is known to occur upon activation via the BCR. To address the possibility that a decrease in proliferation is due to enhanced apoptosis, naive and memory B cells were activated for 24, 48, and 72 h and the level of cleaved caspase-3 was assessed by FACS. Using this method, no increase in cleaved caspase-3 activity was seen among the naive or memory B cells of C/T heterozygote subjects (Fig. 1B and data not shown). These results indicate that the reduced proliferation observed in the memory B cell population of PTPN22 C/T subjects is not due to increased apoptosis of these cells. Additionally, cell surface expression of IgM or IgG was equivalent in both subject groups (data not shown). Together, these findings suggest that Lyp620W affects early events in BCR signaling, resulting in impaired proliferation.
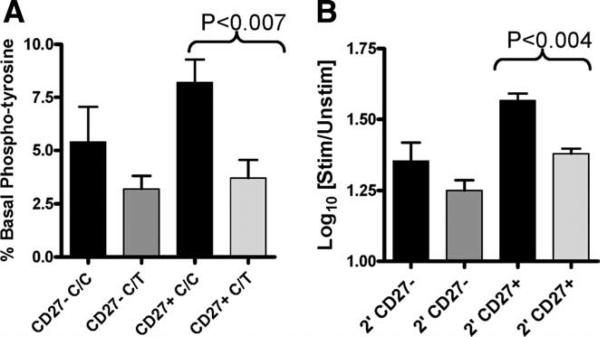
To address this possibility, we next assessed early activation events in response to BCR engagement. Intracellular total protein tyrosine phosphorylation was measured by FACS, allowing us to measure signaling events in both naive and memory B cell subsets. Using this approach we found that the basal tyrosine phosphorylation was decreased in memory B cells from subjects with the 1858C/T genotype (p < 0.007), with a similar trend seen in naive B cells (Fig. 2A). Similarly, activation-induced tyrosine phosphorylation was significantly reduced in the memory B cell population (p < 0.01) from individuals with the 1858C/T genotype, with a similar trend present in naive B cells (Fig. 2B). To exclude the possibility that differences in surface IgM expression contributed to our findings, we simultaneously verified that the expression of surface IgM was equivalent between samples (data not shown). These findings suggest that protein tyrosine phosphatase activity is increased in B cells harboring the 1858T variant both at baseline and upon BCR activation.
FIGURE 2.
Total phosphorylated tyrosine is significantly decreased in memory B cells of PTPN22 1858C/T subjects. Pan-tyrosine phosphorylation was assessed in B cells by intracellular flow cytometry. A, The percentage of unstimulated CD27+ (memory) and CD27- (naive) B cells with basal tyrosine phosphorylation is shown. B, B cells were activated for 2 min (2′) with anti-IgM/IgG. The log10-fold change in the mean fluorescence intensity of phosphorylation relative to the unstimulated control for CD27- naive and CD27+ memory B cells is shown. Black bars represent C/C samples (n = 6) and gray bars represent C/T samples (n = 6).
The PTPN22 1858T variant results in reduced phosphorylation of Syk and downstream effectors upon BCR stimulation
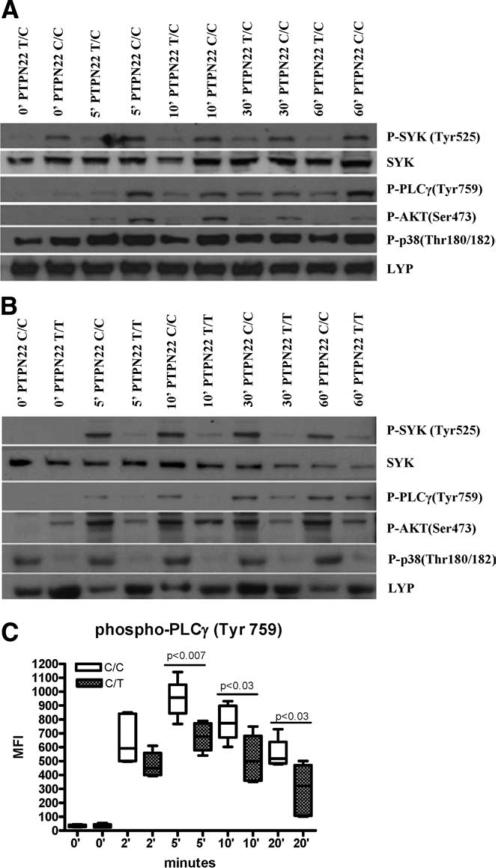
The role of Lyp in BCR signal transduction has not been explored. Thus, given the primary deficit in BCR-mediated phosphotyrosine activation, calcium mobilization, and B cell proliferation in subjects harboring the 1858T mutation, we further assessed early BCR-mediated signaling in these subjects. BCR cross-linking by Ag promotes Src family kinase activity and the subsequent recruitment and transphosphorylation of Syk and BTK family kinases. The concerted action of Syk and BTK lead to the activation of PI3K and PLCγ2, both of which are crucial for the proper dissemination of signal transduction. PI3K and PLCγ2 generate and bind lipid products that result in the release of intracellular calcium and the activation of protein kinase C, followed by activation of the MAPK family of kinases. To assess the competency of these signaling cascades in healthy subjects harboring the 1858T substitution, total B cells were isolated and activated using soluble anti-IgM. Following activation, cells were lysed and protein equivalents were resolved by SDS-PAGE followed by Western blotting analyses. We found reduced phosphorylated levels of Syk, PLCγ2, and Akt in subjects heterozygous for the 1858T allele (Fig. 3A). When similar studies were performed on samples obtained from a healthy subject homozygous for the variant 1858T allele, the level of phosphorylation was even more dramatically reduced. In addition, phospho-p38 MAPK levels were clearly reduced in this subject (Fig. 3B). These data demonstrate reduced activation of major BCR signaling pathways in subjects heterozygous for the 1858T variant, and more pronounced changes in a subject homozygous for the variant. The decrease in activation of Syk, PLCγ2, and Akt was not due to a decrease in total Syk, Akt, or Lyp. Western blots show that the levels of Syk and Lyp are similar among subjects, irrespective of PTPN22 genotype (Fig. 3A and data not shown). When the naive and memory populations were independently evaluated for intracellular phospho-PLCγ2 using flow cytometry, the decrease in phosphorylation was most pronounced in the memory B cell population, whereas a more modest decrease was seen in the naive B cell population (Fig. 3C and data not shown).
FIGURE 3.
B cells from subjects harboring the 1858 T variant display BCR-mediated signaling defects. B cells were isolated and then activated with anti-IgM for the indicated time in minutes (′), followed by cell lysis. Western blotting was performed with the following Abs: phospho (P)-Akt-Ser473, phospho-PLCγ2-Tyr759, Syk, phospho-Syk-Tyr525/526, phospho-p38 MAPK-Thr180/Tyr182, and Lyp. A, Two control subjects were compared, one homozygous for 1858C/C and the other heterozygous for 1858C/T. B, Two control subjects were compared, one homozygous for 1858C/C and the other homozygous for the1858T/T variant. Blots are representative of three independent experiments. C, B cells were stimulated with soluble anti-IgM plus IgG F(ab′)2 for the indicated times. Total phospho-PLCγ2 (Tyr759) was assessed by intracellular flow cytometry in the CD27+ memory population of 1858 C/C and 1858 C/T subjects (n = 6). Mean fluorescence intensity (MFI) values for phospho-PLCγ2 are shown.
Inhibition of Lyp results in reversal of the PTPN22 1858T-associated BCR signaling defect
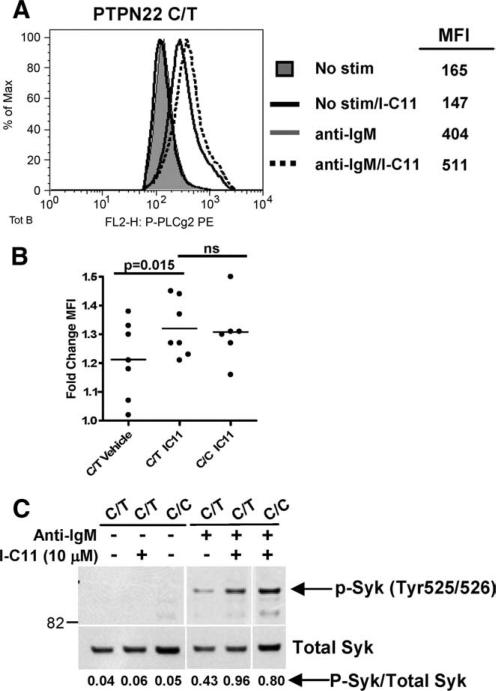
The mechanisms by which the single amino acid change at position 620 of Lyp may enhance phosphatase activity in B cells include increased expression or an altered function of Lyp. The variant allele is not differentially expressed in human lymphocytes, at rest or upon activation (12). This suggests that the amino acid change at position 620 results in enhanced function of Lyp, either by directly altering its phosphatase activity or by the interaction of Lyp with its target substrates. The alterations in BCR signal transduction and proliferation that we have observed in the B cells of subjects who express the Lyp620W variant support this possibility. To directly test whether a gain of phosphatase function by the Lyp620W variant is the source of altered B cell signaling in these subjects, we utilized the Lyp-specific inhibitor I-C11 (13). I-C11 is a salicylic acid-based Lyp inhibitor that binds to the catalytic site of Lyp in a specific manner and has been shown to enhance TCR signaling (13). When B cells isolated from 1858C/T subjects were treated with 0.1–10 μM I-C11 before BCR stimulation, an increase in phospho-PLCγ2 was readily apparent at 5 μM (Fig. 4A) and was significantly enhanced (p = 0.015) when compared with paired vehicle-treated B cells (Fig. 4B). Moreover, PLCγ2 phosphorylation in I-C11-treated 1858C/T B cells was no different from that of I-C11-treated 1858C/C B cells (Fig. 4B). We observed a similar rescue of Syk phosphorylation and total phosphotyrosine in Western blots following treatment of 1858C/T B cells with I-C11 and stimulation with anti-IgM (Fig. 4C and data not shown).
FIGURE 4.
Inhibition of Lyp by I-C11 enhances B cell signaling in PTPN22 C/T subjects. A, Freshly isolated B cells were treated with vehicle or 5 μM I-C11 followed by stimulation (stim) for 5 min with anti-IgM and intra-cellular staining for phospho-PLCγ2. B, Total B cells were treated as in A followed by stimulation with anti-kappa and intracellular staining for phospho-PLCγ2. Each dot represents the fold change in mean fluorescence intensity (MFI) relative to the unstimulated control for a unique individual. C, B cells were treated with vehicle or I-C11 (10 μM) and stimulated as in A. Whole cell lysates were immunoblotted using anti-phospho-Syk (p-Syk; Tyr525/526) and then reprobed with anti-Syk antisera (lower panel). Band intensities were quantified by densitometry (ImageQuant TL software). The ratio of phospho-Syk (P-Syk) to total Syk protein is shown below each lane. Adjacent panels depicted in C are from the same gel. Data shown are representative of three experiments.
Taken together, our results demonstrate a clear association between the PTPN22 1858T variant and altered B cell signal transduction. We show that the Lyp620W variant influences Ag receptor-driven proliferation and several key mediators of proximal BCR signaling, consistent with enhanced inhibitory activity when compared with Lyp620R, as in T cells. Importantly, we demonstrate a direct role of Lyp in BCR signaling by reversal of the Lyp620W phenotype using a Lyp-specific inhibitor. Of note, we did not observe a significant difference in BCR activation between vehicle- and I-C11-treated 1858C/C B cells (J. Buckner and T. Habib, unpublished observations), potentially reflecting a mechanism of BCR inhibition unique to the Lyp620W variant in B cells.
We previously reported that expression of the 1858T allele is associated with a decrease in the size of the memory B cell compartment and with reduced BCR activation (as measured by Ca2+ flux), which is most profound in memory B cells (11). In the current study, we further demonstrate a defect in the proliferation of memory B cells and diminished phosphorylation of key signaling proteins upon BCR stimulation. Studies of Jurkat cells have shown that the Lyp620W variant has enhanced phosphatase function in the context of TCR signal transduction. These findings have been confirmed in unmanipulated T cells (8, 9). Our studies reveal alterations in the BCR signaling cascade that are also consistent with enhanced phosphatase activity, characterized by diminished Syk and PLCγ phosphorylation. This is further supported by the dose effect observed in subjects homozygous for the 1858T allele. Although similar alterations in the BCR signaling pathway are present in both naive and memory B cells, the impact of this variant is most pronounced in the memory B cell population. It has been shown that the response to BCR stimulation differs at various stages of B cell development; one example of this is the delay in the initiation of proliferation and less rapid cell division in naive human B cells when compared with memory B cells (14). Our observation that the Lyp620W variant more profoundly affects memory B cells may be due in part to such differences.
The genetic association of the PTPN22 1858T allele with autoimmune disease is now well established, and the genetic evidence indicates that the missense mutation at PTPN22 1858 is the causative SNP (15). Yet, the mechanism by which this SNP contributes to the development of autoimmunity is still not known. Alterations in T cell activation in the presence of the Lyp620W variant is well established and could result in a loss of tolerance by reduced negative selection of autoreactive T cells, impaired T cell regulation, or death. However, a common feature of each of the autoimmune diseases for which PTPN22 1858 SNP has been associated is the presence of autoantibodies (16). Such autoantibodies could be the result of a loss of T cell tolerance and T cell help. Alternatively, the 1858T variant could directly affect B cell maturation, selection, and function by altering BCR signal transduction. Our findings support this latter possibility and indicate that the Lyp620W variant results in a B cell-intrinsic defect. Our data implicate alterations in BCR signal transduction in the pathogenic mechanisms involved in autoimmunity and a role for B cell-directed therapeutics in the treatment of these diseases.
Acknowledgments
We thank the Benaroya Research Institute Translational Research Program and Clinical Core for subject recruitment and sample processing and handling. We thank Heather Shilling and Shan Wei for genotyping. We also thank Dr. Gerald Nepom, Dr. David Rawlings, and Dr. Steven Ziegler for helpful discussions and critical reviews of this manuscript.
Footnotes
This work was supported by funds from the Juvenile Diabetes Research Foundation (to the Center for Translational Research at Benaroya Research Institute). Y.H., X.Z., and Z.-Y.Z. were supported by National Institutes of Health Grant CA69202.
Abbreviations used in this paper: SNP, single nucleotide polymorphism; PLC, phospholipase C.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Begovich AB, Carlton VE, Honigberg LA, Schrodi SJ, Chokkalingam AP, Alexander HC, Ardlie KG, Huang Q, Smith AM, Spoerke JM, et al. A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. Am. J. Hum. Genet. 2004;75:330–337. doi: 10.1086/422827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Velaga MR, Wilson V, Jennings CE, Owen CJ, Herington S, Donaldson PT, Ball SG, James RA, Quinton R, Perros P, Pearce SH. The codon 620 tryptophan allele of the lymphoid tyrosine phosphatase (LYP) gene is a major determinant of Graves’ disease. J. Clin. Endocrinol. Metab. 2004;89:5862–5865. doi: 10.1210/jc.2004-1108. [DOI] [PubMed] [Google Scholar]
- 3.Smyth D, Cooper JD, Collins JE, Heward JM, Franklyn JA, Howson JM, Vella A, Nutland S, Rance HE, Maier L, et al. Replication of an association between the lymphoid tyrosine phosphatase locus (LYP/PTPN22) with type 1 diabetes, and evidence for its role as a general autoimmunity locus. Diabetes. 2004;53:3020–3023. doi: 10.2337/diabetes.53.11.3020. [DOI] [PubMed] [Google Scholar]
- 4.Bottini N, Musumeci L, Alonso A, Rahmouni S, Nika K, Rostamkhani M, MacMurray J, Meloni GF, Lucarelli P, Pellecchia M, et al. A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat. Genet. 2004;36:337–338. doi: 10.1038/ng1323. [DOI] [PubMed] [Google Scholar]
- 5.Cloutier JF, Veillette A. Cooperative inhibition of T-cell antigen receptor signaling by a complex between a kinase and a phosphatase. J. Exp. Med. 1999;189:111–121. doi: 10.1084/jem.189.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu J, Katrekar A, Honigberg LA, Smith AM, Conn MT, Tang J, Jeffery D, Mortara K, Sampang J, Williams SR, et al. Identification of substrates of human protein-tyrosine phosphatase PTPN22. J. Biol. Chem. 2006;281:11002–11010. doi: 10.1074/jbc.M600498200. [DOI] [PubMed] [Google Scholar]
- 7.Gjorloff-Wingren A, Saxena M, Williams S, Hammi D, Mustelin T. Characterization of TCR-induced receptor-proximal signaling events negatively regulated by the protein tyrosine phosphatase PEP. Eur. J. Immunol. 1999;29:3845–3854. doi: 10.1002/(SICI)1521-4141(199912)29:12<3845::AID-IMMU3845>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 8.Vang T, Congia M, Macis MD, Musumeci L, Orru V, Zavattari P, Nika K, Tautz L, Tasken K, Cucca F, et al. Autoimmune-associated lymphoid tyrosine phosphatase is a gain-of-function variant. Nat. Genet. 2005;37:1317–1319. doi: 10.1038/ng1673. [DOI] [PubMed] [Google Scholar]
- 9.Rieck M, Arechiga A, Onengut-Gumuscu S, Greenbaum C, Concannon P, Buckner JH. Genetic variation in PTPN22 corresponds to altered function of T and B lymphocytes. J. Immunol. 2007;179:4704–4710. doi: 10.4049/jimmunol.179.7.4704. [DOI] [PubMed] [Google Scholar]
- 10.Hasegawa K, Yajima H, Katagiri T, Ogimoto M, Arimura Y, Mitomo K, Mashima K, Mizuno K, Yakura H. Requirement of PEST domain tyrosine phosphatase PEP in B cell antigen receptor-induced growth arrest and apoptosis. Eur. J. Immunol. 1999;29:887–896. doi: 10.1002/(SICI)1521-4141(199903)29:03<887::AID-IMMU887>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 11.Rieck M, Arechiga A, Onegut-Gumuscu S, Greenbaum C, Concannon P, Buckner JH. Genetic Variation in PTPN22 Corresponds to Altered Function of T and B Lymphocytes. J. Immunol. 2007;17:4704–4710. doi: 10.4049/jimmunol.179.7.4704. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen C, Barington T, Husby S, Lillevang ST. Expression of human PTPN22 alleles. Genes Immun. 2007;8:131–137. doi: 10.1038/sj.gene.6364369. [DOI] [PubMed] [Google Scholar]
- 13.Yu X, Sun JP, He Y, Guo X, Liu S, Zhou B, Hudmon A, Zhang ZY. Structure, inhibitor, and regulatory mechanism of Lyp, a lymphoid-specific tyrosine phosphatase implicated in autoimmune diseases. Proc. Natl. Acad. Sci. USA. 2007;104:19767–19772. doi: 10.1073/pnas.0706233104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tangye SG, Avery DT, Deenick EK, Hodgkin PD. Intrinsic differences in the proliferation of naive and memory human B cells as a mechanism for enhanced secondary immune responses. J. Immunol. 2003;170:686–694. doi: 10.4049/jimmunol.170.2.686. [DOI] [PubMed] [Google Scholar]
- 15.Onengut-Gumuscu S, Buckner JH, Concannon P. A haplotype-based analysis of the PTPN22 locus in type 1 diabetes. Diabetes. 2006;55:2883–2889. doi: 10.2337/db06-0225. [DOI] [PubMed] [Google Scholar]
- 16.Gregersen PK. Gaining insight into PTPN22 and autoimmunity. Nat. Genet. 2005;37:1300–1302. doi: 10.1038/ng1205-1300. [DOI] [PubMed] [Google Scholar]