Abstract
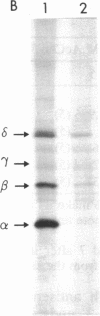
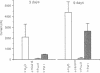
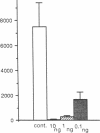
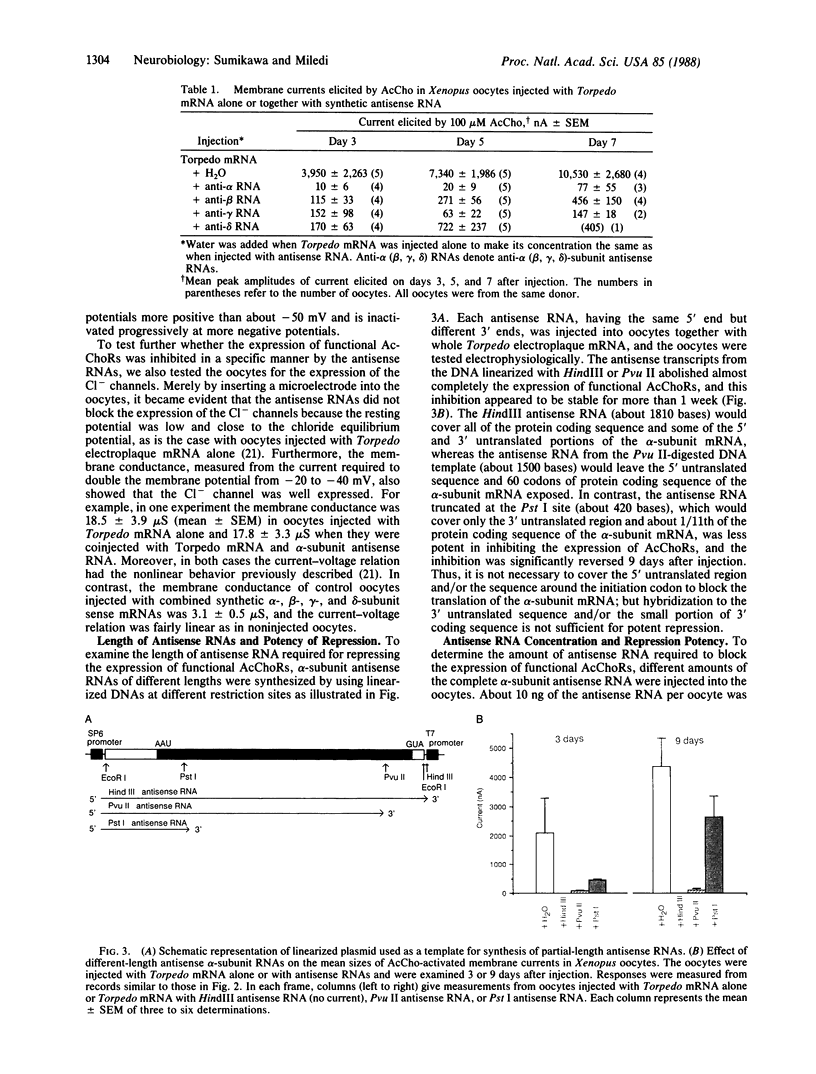
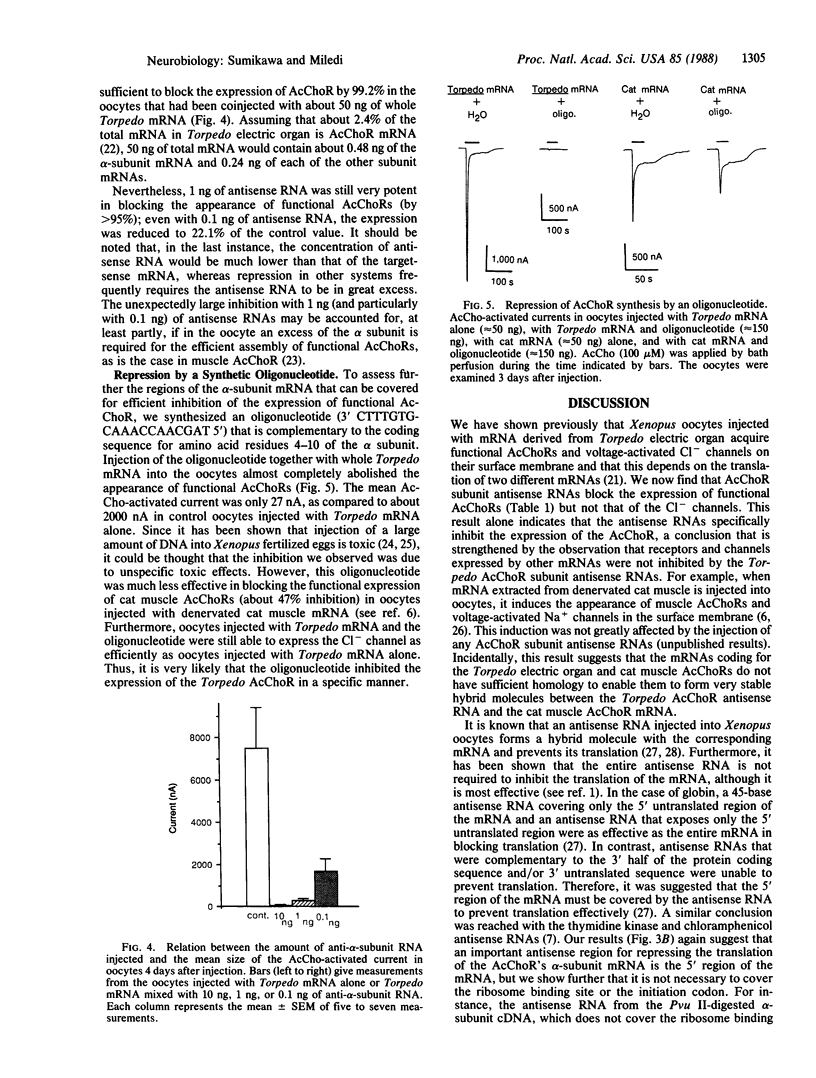
Four antisense RNAs, synthesized from cDNA clones coding for the four subunits of the acetylcholine receptor of Torpedo electroplaques, were used to study their effect on the expression of functional Torpedo acetylcholine receptors in Xenopus oocytes. All antisense RNAs inhibited the appearance of functional receptors in the oocyte's surface membrane for at least 1 week. This inhibition was specific because the antisense RNAs did not block the expression of the Cl- channels, also encoded by Torpedo electroplaque mRNA. Experiments with incomplete antisense RNAs and a synthetic oligonucleotide indicate that covering the ribosome binding site or the initiation codon in the mRNA is not a necessary requirement for efficient blocking. Thus, the use of antisense RNAs combined with the Xenopus oocyte system provides a novel approach to screen cDNA libraries for the genes coding for multisubunit neurotransmitter receptors.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. J., Blobel G. In vitro synthesis, glycosylation, and membrane insertion of the four subunits of Torpedo acetylcholine receptor. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5598–5602. doi: 10.1073/pnas.78.9.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard E. A., Miledi R., Sumikawa K. Translation of exogenous messenger RNA coding for nicotinic acetylcholine receptors produces functional receptors in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1982 May 22;215(1199):241–246. doi: 10.1098/rspb.1982.0040. [DOI] [PubMed] [Google Scholar]
- Barrantes F. J. Recent developments in the structure and function of the acetylcholine receptor. Int Rev Neurobiol. 1983;24:259–341. doi: 10.1016/s0074-7742(08)60224-x. [DOI] [PubMed] [Google Scholar]
- Claudio T., Ballivet M., Patrick J., Heinemann S. Nucleotide and deduced amino acid sequences of Torpedo californica acetylcholine receptor gamma subunit. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1111–1115. doi: 10.1073/pnas.80.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claudio T. Stable expression of transfected Torpedo acetylcholine receptor alpha subunits in mouse fibroblast L cells. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5967–5971. doi: 10.1073/pnas.84.16.5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti-Tronconi B. M., Raftery M. A. The nicotinic cholinergic receptor: correlation of molecular structure with functional properties. Annu Rev Biochem. 1982;51:491–530. doi: 10.1146/annurev.bi.51.070182.002423. [DOI] [PubMed] [Google Scholar]
- Green M. R., Maniatis T., Melton D. A. Human beta-globin pre-mRNA synthesized in vitro is accurately spliced in Xenopus oocyte nuclei. Cell. 1983 Mar;32(3):681–694. doi: 10.1016/0092-8674(83)90054-5. [DOI] [PubMed] [Google Scholar]
- Green P. J., Pines O., Inouye M. The role of antisense RNA in gene regulation. Annu Rev Biochem. 1986;55:569–597. doi: 10.1146/annurev.bi.55.070186.003033. [DOI] [PubMed] [Google Scholar]
- Gundersen C. B., Miledi R., Parker I. Voltage-operated channels induced by foreign messenger RNA in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1983 Nov 22;220(1218):131–140. doi: 10.1098/rspb.1983.0092. [DOI] [PubMed] [Google Scholar]
- Harland R., Weintraub H. Translation of mRNA injected into Xenopus oocytes is specifically inhibited by antisense RNA. J Cell Biol. 1985 Sep;101(3):1094–1099. doi: 10.1083/jcb.101.3.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE DEVELOPMENT OF ACETYLCHOLINE SENSITIVITY IN NERVE-FREE SEGMENTS OF SKELETAL MUSCLE. J Physiol. 1964 Mar;170:389–396. doi: 10.1113/jphysiol.1964.sp007339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosaki T., Fukuda K., Konno T., Mori Y., Tanaka K., Mishina M., Numa S. Functional properties of nicotinic acetylcholine receptor subunits expressed in various combinations. FEBS Lett. 1987 Apr 20;214(2):253–258. doi: 10.1016/0014-5793(87)80065-0. [DOI] [PubMed] [Google Scholar]
- Kusano K., Miledi R., Stinnakre J. Cholinergic and catecholaminergic receptors in the Xenopus oocyte membrane. J Physiol. 1982 Jul;328:143–170. doi: 10.1113/jphysiol.1982.sp014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom J., Gullick W., Conti-Tronconi B., Ellisman M. Proteolytic nicking of the acetylcholine receptor. Biochemistry. 1980 Oct 14;19(21):4791–4795. doi: 10.1021/bi00562a012. [DOI] [PubMed] [Google Scholar]
- MILEDI R. FORMATION OF EXTRA NERVE-MUSCLE JUNCTIONS IN INNERVATED MUSCLE. Nature. 1963 Sep 21;199:1191–1192. doi: 10.1038/1991191a0. [DOI] [PubMed] [Google Scholar]
- Melton D. A. Injected anti-sense RNAs specifically block messenger RNA translation in vivo. Proc Natl Acad Sci U S A. 1985 Jan;82(1):144–148. doi: 10.1073/pnas.82.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez B., Valenzuela P., Martial J. A., Baxter J. D. Cell-free synthesis of acetylcholine receptor polypeptides. Science. 1980 Aug 8;209(4457):695–697. doi: 10.1126/science.7394526. [DOI] [PubMed] [Google Scholar]
- Merlie J. P., Lindstrom J. Assembly in vivo of mouse muscle acetylcholine receptor: identification of an alpha subunit species that may be an assembly intermediate. Cell. 1983 Oct;34(3):747–757. doi: 10.1016/0092-8674(83)90531-7. [DOI] [PubMed] [Google Scholar]
- Merlie J. P., Sebbane R., Tzartos S., Lindstrom J. Inhibition of glycosylation with tunicamycin blocks assembly of newly synthesized acetylcholine receptor subunits in muscle cells. J Biol Chem. 1982 Mar 10;257(5):2694–2701. [PubMed] [Google Scholar]
- Popot J. L., Changeux J. P. Nicotinic receptor of acetylcholine: structure of an oligomeric integral membrane protein. Physiol Rev. 1984 Oct;64(4):1162–1239. doi: 10.1152/physrev.1984.64.4.1162. [DOI] [PubMed] [Google Scholar]
- Rusconi S., Schaffner W. Transformation of frog embryos with a rabbit beta-globin gene. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5051–5055. doi: 10.1073/pnas.78.8.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumikawa K., Houghton M., Emtage J. S., Richards B. M., Barnard E. A. Active multi-subunit ACh receptor assembled by translation of heterologous mRNA in Xenopus oocytes. Nature. 1981 Aug 27;292(5826):862–864. doi: 10.1038/292862a0. [DOI] [PubMed] [Google Scholar]
- Sumikawa K., Parker I., Amano T., Miledi R. Separate fractions of mRNA from Torpedo electric organ induce chloride channels and acetylcholine receptors in Xenopus oocytes. EMBO J. 1984 Oct;3(10):2291–2294. doi: 10.1002/j.1460-2075.1984.tb02128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumikawa K., Parker I., Miledi R. Partial purification and functional expression of brain mRNAs coding for neurotransmitter receptors and voltage-operated channels. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7994–7998. doi: 10.1073/pnas.81.24.7994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. M., Mayne K. M., Lester H. A., Davidson N. Mouse-Torpedo hybrid acetylcholine receptors: functional homology does not equal sequence homology. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4852–4856. doi: 10.1073/pnas.82.14.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormington W. M. Stable repression of ribosomal protein L1 synthesis in Xenopus oocytes by microinjection of antisense RNA. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8639–8643. doi: 10.1073/pnas.83.22.8639. [DOI] [PMC free article] [PubMed] [Google Scholar]