Abstract
A significant body of evidence demonstrates that diets rich in fruit and vegetables promote health, and attenuate, or delay, the onset of various diseases, including cardiovascular disease (CVD), diabetes, certain cancers, and several other age-related degenerative disorders. The concept that moderate chocolate consumption could be part of a healthy diet has gained acceptance in the last years based on the health benefits ascribed to selected cocoa components. Specifically, cocoa as a plant and chocolate as food contain a series of chemicals that can interact with cell and tissue components providing protection against the development and amelioration of pathological conditions. The most relevant effects of cocoa and chocolate have been related to CVD. The mechanisms behind these effects are still under investigation. However the maintenance or restoration of vascular NO production and bioavailability and the antioxidant effects are the mechanisms most consistently supported by experimental data. This review will summarize the most recent research on the cardiovascular effects of cocoa flavanoles and related compounds.
Keywords: flavonoids, flavanols, hypertension, oxygen radicals, antioxidant, cardiovascular health
CARDIOVASCULAR DISEASE AND DIET
CVD, including stroke, is the leading cause of death and disability in developed countries. Atherosclerosis, vascular dysfunction, platelet aggregation, and other inflammation-associated conditions are central to CVD. Diet is a major factor contributing to the onset and development of CVD, by primarily affecting all the above mentioned conditions. Nevertheless, high intake of calories and certain fats increase the risk for CVD1, diet also provides micronutrients that appear fundamental in controlling CVD2. Defining specific modifications of dietary habits in a population can have a major impact on CVD, especially during the long period in which the disease is silent3. To set new goals to improve human diets, it is important to understand how macro and micronutrients can interact with biological systems to enhance health.
CARDIOVASCULAR DISEASE AND COCOA
Robust epidemiological evidence demonstrates that diets rich in fruits and vegetables promote health, and attenuate, or delay the onset of CVD3–6 (and references therein). The questions that remain open are: i) are all fruits and vegetables equivalent? if not, can we identify those with best health benefits?; and ii) how can we recognize the compounds responsible for such effects? A pioneer population study showed an inverse association between flavonoid consumption and the risk of coronary heart disease7. Under this paradigm cocoa and chocolate have been intensively studied in the last years mostly driven by the large content of flavanols and related compounds in cocoa beans that are generally conserved in the commercially available chocolate.
High cocoa and chocolate consumption have been associated with a decreased risk for CVD in a few population studies. A sub-study of a population from the Zutphen Elderly Study showed that cocoa consumption was associated with a decrease in blood pressure and overall cardiovascular mortality8. A case-control study done in Italy showed that the risk for myocardial infarction was inversely associated to chocolate consumption, reaching a 77% decrease in risk when comparing the population that ate more than three portions of chocolate per day with the population that consumed less than one9. Several dietary intervention studies in humans and animals demonstrated that cocoa10–18 and other flavanol-rich foods/beverages19–21 may exert protective vascular effects.
Although data for the above mentioned population and clinical studies show a similar trend suggesting flavanols as cardioprotective agents, the biochemical mechanisms behind that cardioprotection are not conclusively identified. Based on a number of in vitro studies, it has been speculated that biochemical mechanisms contributing to the health effects of flavanols include: antioxidant effects22; modulation of cell signaling and gene expression23–24; and alterations of certain cell membrane properties and receptor functions25–26. In addition to the above mentioned mechanisms, flavanols can inhibit several enzyme activities27–30. These mechanisms are not necessary independent, and then could occur concurrently or synergistically, as will be discussed in the following sections.
CHEMICAL STRUCTURE OF COCOA FLAVANOLS AND PROCYANIDINS
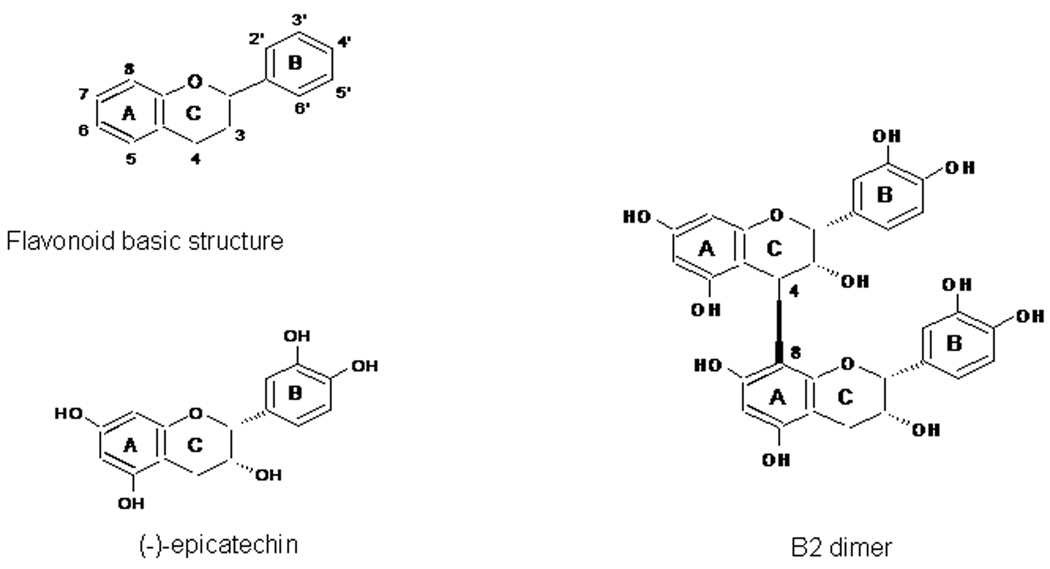
Polyphenols occur as plant secondary metabolites. Their ubiquitous occurrence in plants and plant foods, favors animal consumption and indeed human and animal tissue presence. Flavonoids are a chemically defined family of polyphenols that includes several thousand compounds. The flavonoids have a basic structure (Fig. 1), and several subclasses of flavonoids are characterized by a substitution pattern in the B- and C-rings. The main subclasses include flavanols, flavanones, flavones, isoflavones, flavonols, and anthocyanidins31. Flavanols are compounds present in high concentrations not only in cocoa, but also in grapes, apples, pomegranates, tea among other widely consumed fruits and vegetables. In cocoa and cocoa products, flavanols are present as: i) monomers, i. e. (−)-epicatechin (EC) and (+)-catechin (CT); and ii) oligomers of EC (procyanidins)32. EC (and CT) oligomers are denominated procyanidins, condensed tannins, or proanthocyanidins. Different plants present a particular pattern of monomers and oligomerization derivatives, e.g. cocoa procyanidins are mostly of the denominated B-type, e.g. B2-dimer (Fig. 1); while in tea (Camellia sinensis) predominate the gallolyated catechins.
Fig. 1.
Chemical structure of flavanols and procyanidins. B2-dimer is a characteristic cocoa procyanidin formed by two (−)-epicatechin units linked by 4→8 bonds.
BIOAVAILABILITY AND METABOLISM OF COCOA FLAVANOLS AND PROCYANIDINS
When discussing the biological activity of flavonoids in general, and flavanols in particular, four are the major factors to be considered: i) bioavailability from foods; ii) absorption and metabolism at the gastrointestinal tract; iii) tissue and cellular distribution after absorption; and iv) which are the chemical form(s) biologically available to the cell/tissue and their potential metabolism at cellular level. As indicated above, flavanols are present as monomers or forming procyanidins. Although it was initially thought that the procyanidins could not pass the acidic conditions of the stomach33, data from human subjects show that flavanols and procyanidins are stable during gastric transit34–35. Once in the mesenteric circulation, flavanols predominately exist in a conjugated form, both as methylated and glucuronidated flavanols36–38. In the liver, further glucuronidation and methylation can take place, as well as sulfation36–38. Metabolic studies have confirmed the presence of these conjugates in the plasma and urine of rodents and humans38–41, as well as in the bile38 and brain of rats42. It has been reported that colonic microflora can break flavonoids flavan structure to form simple phenolics and ring-fission metabolites that may be physiologically relevant43–44. In summary, non-metabolized flavanols or metabolites of flavanols can exert biological effects depending essentially on flavanol metabolism and presence in the target tissue28–30.
In humans, plasma concentrations of EC plus EC-metabolites can be found in the micromolar range as soon as 1 h after cocoa consumption12, 39, 45, with the major metabolite being 4’-o-methyl-epicatechin-7-β-D-glucuronide12. There is some evidence that certain flavanols are better absorbed than others. After human subjects consumed a cocoa beverage containing EC and CT in a 1:1 ratio, peak plasma CT concentrations were typically less than 10% of EC (0.16 vs. 5.92 µM)45. Part of these differences in plasma flavanol concentrations, could be due to procyanidin degradation; for example, dimers has been shown to form EC and methylated EC under certain conditions41, 46–47, although the physiological relevance of such degradation remains to be confirmed.
While several research groups have examined the bioavailability of the monomeric flavanols, there is limited information on the bioavailability and metabolism of procyanidins. B2-dimer has been detected in the plasma of humans and rats41, 45, 49; B5-dimer (EC-(4β-6)-EC) has been detected in very minute quantities in simulated gastric and intestinal juice47; and no other type of dimer has been detected in rats50. It is important to note that the dimers that have been detected in the plasma are those made up of EC, not of CT subunits. A chemical explanation for these discrepancies is the different hydroxyl group orientation in the C3 position of the flavonoid B ring, which affects the interaction between the 3-OH on the C ring and the B ring resulting in dissimilar biological actions51–52.
COCOA FLAVANOLS AS ANTIOXIDANTS: FREE RADICAL SCAVENGERS AND METAL CHELATORS
Plant polyphenols has been considered for long as physiologically relevant antioxidants based on the facts that: i) polyphenols have chemical structures favoring antioxidant actions, i.e. free radical scavenging and chelation of redox-active metals; ii) many polyphenols retain key features of their structure after ingestion and metabolism by mammals; and iii) certain polyphenols can provide physiological benefits in pathological situations associated with high free radical production, e. g. hypertension. Flavanols shared these properties, as confirmed by the extensive literature demonstrating that flavanols have free radical scavenging activity in a myriad of biochemical and ex vivo systems23, 25, 26, 53–61, and also in animal models and in humans62–64. In theory, these antioxidant actions can result in a reduction of the steady state concentration of free radicals and other oxidants, diminishing the subsequent oxidation of target molecules such as lipids, proteins and nucleic acids. However, one important limitation for the “antioxidant action” hypothesis resides in the relatively low flavanol and procyanidin plasma concentrations observed even after the consumption of foods rich in these compounds45, 65. The actual concentrations that can be reached in plasma of humans subjected to realistic polyphenol consumption are in the nanomolar range and are transient in nature (peaking at 2–4 h)12, 45. This low bioavailability leads to a kinetically unfavourable condition with respect to other compounds with similar free radical scavenger capabilities, that are present in blood in significantly higher micromolar concentrations i.e. tocopherols and ascorbate. Thus, a function of flavanols as direct free radical scavengers is unlikely to be relevant, and could be limited to the blood and other tissues directly exposed after consumption, i.e. gastrointestinal tract. It has been suggested that other mechanisms, compatible with the physiological levels reached by flavanols, may explain the observed changes in cell or tissue oxidation levels after flavanol consumption. These mechanisms are beyond the ability of flavanols and other flavonoids to directly prevent free radical-mediated tissue damage65–66.
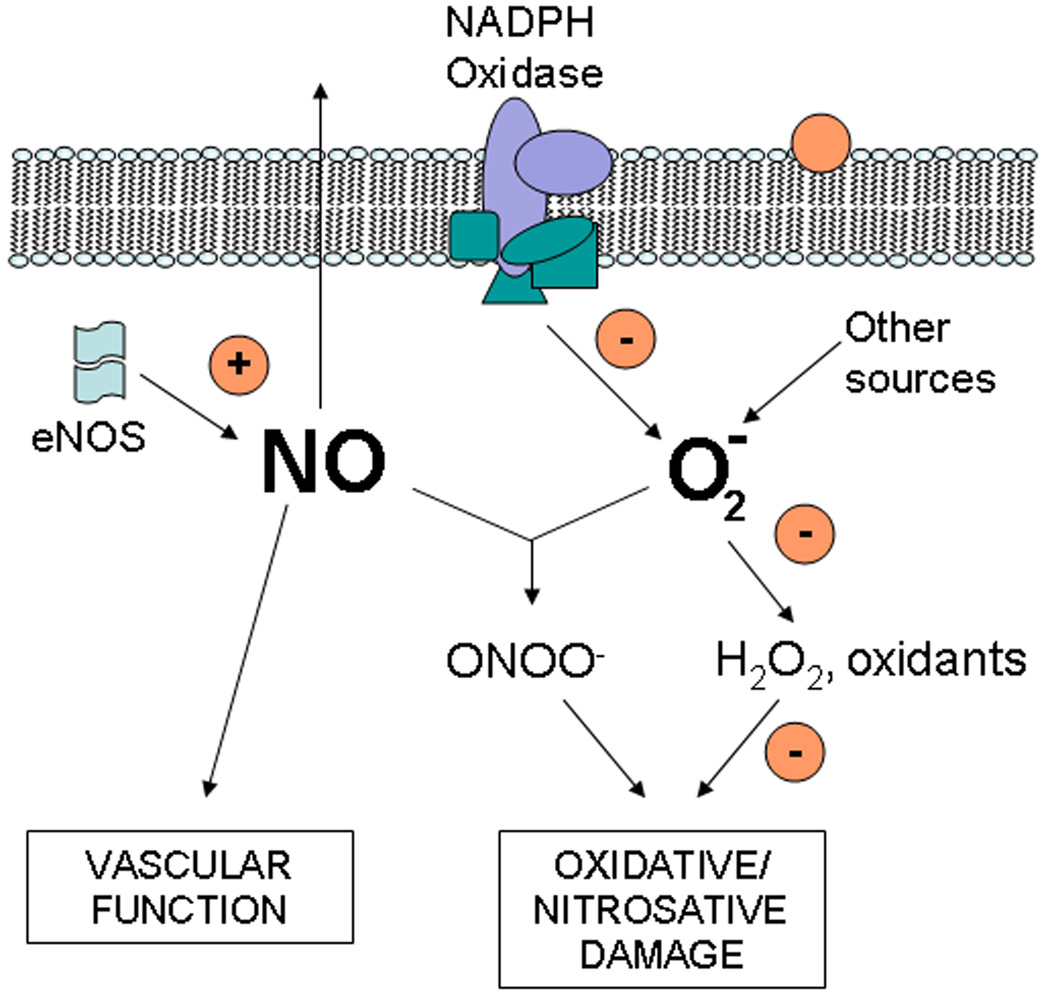
Another relevant detrimental effect of oxidants is the reaction of nitric oxide (NO) with superoxide to form peroxynitrite67. This reaction, that occur in a near diffusion controlled rate, and is important at the vascular level, leads to two undesirable conditions: i) reduction of NO availability necessary for proper smooth muscle cell function in vessel relaxation; and ii) increased formation of peroxynitrite that will promote oxidative and nitrosative damage (Fig. 2). NO production in mammalian cells is catalyzed by the enzyme nitric oxide synthase, which activity is by four isoforms, i.e. endothelial (eNOS) expressed mostly in endothelial cells; neuronal (nNOS) present mainly in neurons; inducible (iNOS) expressed in response a variety of proinflammatory stimuli; and mitochondrial (mtNOS) present in the inner membrane of the mitochondrion68–69. In the vascular environment, superoxide is not only originated by the mitochondria respiratory chain70–71, xanthine oxidase, and cytochrome P45072–73, but by other two enzymes: a non phagocytic NADPH oxidase74 and an uncoupled endothelial NOS75. Then, flavanols and procyanidins present in circulation or in the vasculature can improve NO vascular concentration by interfering with the reaction between NO and superoxide by: i) inhibiting NADPH oxidase-dependent superoxide production 30; ii) optimizing NO generation by NOS76; and/or iii) scavenging superoxide, H2O2, and other oxidants that mediate damage to cell components with superoxide and other oxidants77; and iv) modifying membrane-related events leading to changes in NO and superoxide production.
Fig. 2.
Scheme relating flavanols (circles) with NO and superoxide (O2 −) metabolism in endothelial cells. Flavanols could act by: i) inhibiting NADPH oxidase-dependent superoxide production, ii) activating eNOS; iii) scavenging superoxide, H2O2, and other oxidants that mediate damage to cell components; and iv) modifying membrane-related events leading to changes in NO and superoxide production. NO generated in endothelial cells will diffuse outside the cell and reach smooth muscle cells where it will induce vascular relaxation.
COCOA FLAVANOLS AS ANTIOXIDANTS: PROTEIN AND LIPID INTERACTIONS
Flavanols and procyanidins have multiple phenolic hydroxyl groups (Fig. 1) that favour their interaction with biological membranes which can occur via the formation of hydrogen bonds. Furthermore, the presence of both, hydrophobic and hydrophilic residues within the flavanol molecule, allows these compounds to interact with phospholipid head groups and be adsorbed onto the surface of membranes. These interactions can result in changes in a number of membrane properties leading to alterations in the regulation of membrane associated molecules and events, including, enzymes, receptors, and other functional proteins receptors78–80.
Some enzymes affected by cocoa consumptions are directly associated with CVD and oxidant m e tabolism, such as 5-lipoxygenase81–82, cyclooxygenase-283–84, and metalloproteinases85. The interactions of flavanols and proteins can also lead to changes in the modulation of gene expression. A direct interaction between nucleic acids and flavanols is thermodynamically feasible86, but the possibility that these compounds reach the DNA and achieve mechanistically-relevant concentrations is rather low. The modulation of signaling pathways by flavanols has been extensively studied87–89. More specifically, the effects of flavanols and procyanidins on the oxidant-regulated NF-kB activation pathway have received special attention. In Jurkat T cells, we demonstrated that EC and CT, and B2-dimer inhibited phorbol mirystate acetate (PMA)-induced IL-2 production, and interfere with several steps of the NF-κB activation cascade23. Essentially, since monomers and dimers (and their metabolites) can be transported into the cells they can act by: i) attenuating intracellular oxidants associated with select stimuli, and the subsequent activation of NF-κB (antioxidant effect); and/or ii) interacting with specific proteins, resulting in the inhibition of the phosphorylation and/or degradation of the inhibitory protein IκBα, the transport of active NF-κB from the cytosol into the nucleus, and/or the binding of NF-κB to κB DNA23, 90. Large procyanidins (with 3 or more units), that are mostly affecting cells from outside, modulate NF-κB activation by modulating the binding of the ligand (stimuli) to its receptor, as we observed in Caco-2 cells exposed to tumor necrosis factor alpha91. Another example of flavanols interaction with membranes is the finding that EC, B2-dimer and C1-trimer modulate intracellular calcium in Jurkat T cells92.
COCOA, LIPID METABOLISM, AND ATHEROSCLEROSIS
Alterations in plasma cholesterol concentration, especially increased levels of LDL-cholesterol, and decreased levels of HDL-cholesterol are associated to the development of atherosclerosis93–94 and CVD1. Reductions in LDL-cholesterol plasma levels have been reported after treatments with polyphenols from different sources95–98. Regarding the action of cocoa, mild hypercholesterolemic subjects lowered significantly (~5%) their LDL cholesterol level after 4 weeks of a dietary intervention with cocoa powder (81–163 mg/day of EC+CT)99. Even in normocholesterolemic young subjects a 15% reduction was observed in LDL-cholesterol level after 14 days of a daily consumption of 105 g of flavanol-containing milk chocolate (168 mg of flavanols)100. Patients with essential hypertension showed 11% of decrease in LDL cholesterol after 15 days receiving 100 g/day dark chocolate (88 mg of flavonols)10. Under an equivalent protocol a similar result was observed in glucose-intolerant hypertensive patients (−7.5%)101. Although the above studies did not investigate potential mechanisms, other works have proposed that LDL-cholesterol decrease associated to flavanoids consumption from different sources include: i) inhibition of cholesterol absorption in the digestive tract96; ii) inhibition of LDL biosynthesis in the liver98; iii) suppression of the hepatic secretion of apolipoprotein B10095; and/or iv) increased expression of LDL receptors in the liver97. All these mechanisms should be the result of interactions between flavanols and membranes, as whole structures, or with particular lipids or proteins. An increase in HDL-cholesterol has been demonstrated in normo and mildly hypercholesterolemic subjects after dark chocolate or cocoa powder supplementation99, 102–103. The mechanisms responsible for these effects on HDL concentrations remain unclear.
It is accepted that oxidized LDL has a role in the development of atherosclerosis104. Numerous studies in animals and humans showed that isolated LDLs are more resistant to in vitro oxidation after the consumption of cocoa products57, 102, 105–109. One study showed a decrease in plasma levels of oxidized LDL plasma levels after dietary cocoa powder supplementation99. All these studies suggest a role for cocoa components in the in vivo protection of LDL. These effects have been mostly ascribed to the scavenging of oxidants formed in the surface of the LDL, and to the chelation of metals catalyst of free radical formation; but they could also be the result of changes in the LDL surface rendering LDL less susceptible to oxidation.
COCOA, ENDOTHELIAL FUNCTION AND HYPERTENSION
The regulation of the vascular tone is the result of a complex network of molecules that includes catecholamines, vasoactive peptides (angiotensin-II or vasopressin), prostaglandins, and importantly, NO. Consumption of a high flavanol cocoa drink (providing 176–185 mg) by patients with cardiovascular risk factors, increased the bioavailability of NO, and an augmented flow-mediated vasodilation, effects that were reversed by the infusion of a NO synthesis inhibitor13–15. Studies with isolated flavonoids showed that EC was able to reproduce the vascular effects observed with cocoa products, suggesting that this flavanol should be responsible for the vascular effects12.
Observational and epidemiological studies indicate that diets rich in polyphenols decrease blood pressure and prevent the increase in blood pressure associated to several pathologies. For years, red wine was considered to have beneficial effects on cardiovascular health, a relationship supported by the French Paradox110. Cocoa and cocoa derived products have gained attention because their potential antihypertensive effects. A sub-study of the Zutphen population showed that cocoa consumption was associated with a decrease in blood pressure8. In addition, an association between the intake of cocoa and a low incidence of hypertension was observed in an indigenous population living on the Kuna Islands111. The Kuna Indians of Panama have a very low incidence of hypertension and cardiovascular disease, but when members of this tribe moved to urban places, their blood pressure was increased. The relocation led to cultural changes and to a decrease in the consumption of cocoa, suggesting that this dietary change was responsible of the observed changes in blood pressure. These results were complemented by other studies that showed an enhancement of endothelial function including acute positive effects on flow mediated dilation by cocoa consumption in humans12–13, 15–17.
Consistent with the association between cocoa consumption and low incidence of hypertension are the results from several short term clinical studies showing that the intake of certain chocolates can decrease blood pressure in humans10–11, 100, 112–113. Grassi et al. studied 15 healthy young adults with typical Italian diets isocalorically supplemented daily with daily 100 g dark chocolate or 90 g white chocolate (assuming 500 and 0 mg of polyphenols, respectively). They observed that the dark chocolate supplement was associated with decreased systolic blood pressure, whereas the white chocolate had no effect112. Results were extended to essential hypertensive patients10 and more recently to glucose-intolerant hypertensive patients101. We studied the effects of the regular consumption of a flavanol-containing milk chocolate on blood pressure and on oxidative stress parameters in healthy young soccer-players100. The consumption of the flavanol-containing milk chocolate was significantly associated with a decrease in blood pressure, and an amelioration of oxidative stress. Taubert et al. studied the effects of low doses of polyphenol-rich dark chocolate in humans during 18 weeks11. Dark chocolate intake reduced mean, systolic, and diastolic blood pressure and oxidative stress. Blood pressure decrease was accompanied by a sustained increase of S-nitrosoglutathione, suggesting an improve formation of NO. The above studies provide support for the involvement of oxidative stress in the vascular tone regulation by NO availability. A meta-analysis of 5 studies relating cocoa consumption with decreases in blood pressure confirmed the individual results114. Significantly, the reductions in systolic (4.7 mm Hg) and diastolic (2.8 mm Hg) blood pressure associated to cocoa and chocolate consumption were similar to those obtained with antihypertensive drugs114. In another study, a dark chocolate and a sugar-free cocoa drink were effective in improving endothelial function and decreasing blood pressure in overweight adults115. In accordance with all these results, several studies have shown significant decreases in blood pressure following the consumption of other flavanol-containing beverages such as wine19, 110, 116 and tea117–120. On the other hand, one study showed that the administration of 900 mg of flavanoles per day during two weeks enhanced insulin-mediated vasodilation but did not modify blood pressure in patients with essential hypertension. These authors concluded that the treatment was not long enough121.
COCOA, PLATELET ACTIVATION AND THROMBOSIS
Platelet activation is a central event in coagulation, but it is also related to the acute development of thrombosis and to the long term CVD pathogenesis. A thrombi release from an unstable atherosclerotic plaque is very often the first clinical manifestation of a myocardial ischemia or infarction or stroke122–123. Regarding the effects of cocoa on platelet function, Rein et al. administered a cocoa beverage (~897 mg of total EC and oligomeric procyanidins) or placebo to healthy subjects. Blood obtained 2 h after was stimulated with epinephrine and ADP. Platelets present in those samples were studied by the detection of activated conformation of the fibrinogen-binding receptor GPIIb-IIIa and the expression of CD62P (associated with platelet activation). Both parameters were significantly reduced in cocoa-treated individuals. Cocoa also inhibited coagulation, by reducing the formation of hemostatically active platelet microparticles, and increasing platelet-related hemostasis time124. In another study, platelet function was evaluated in smokers 2 h after receiving 40 g of dark chocolate (~47 mg of EC+CT)125. Platelet adherence as a result of a shear stress that mimics severely stenotic or disrupted plaques was significantly reduced (−5%) in association with dark chocolate supplementation. Using a similar assay in a protocol including heart transplant recipients, it was also found a reduction in platelet adherence126. Both studies propose that the antioxidant properties of cocoa flavanols can lead to an increase in NO bioavailability, which would be associated to the decrease in platelet reactivity, given the potent action of NO as platelet inhibitor127. In isolated platelets it was shown that a mixture of CT and quercetin can cause a similar effect increasing NO levels through the inhibition of protein kinase C-dependent NADPH oxidase128. Other mechanisms for the effects of flavonoids on platelet reactivity that may be associated with a NO availability because the inhibition of oxidant production include: i) a the reduction in phospholipase C activity associated to hydrogen peroxide production129; ii) the modulation of eicosanoide metabolism130; iii) the blockade of platelets TxA2 receptors131; and iv) the inhibition of platelet lipooxygenase132–133.
COCOA AND INFLAMMATION
There is increasing evidence that inflammation is pivotal in the induction and perpetuation of CVD. A participation of dietary flavonoids in the modulation of inflammation would contribute to reduce cardiovascular risk. An inverse association was observed between dietary flavonoid intake and serum C-reactive protein (CRP), that is both a biomarker for chronic inflammation and a sensitive risk factor for CVD134. Moreover, data from the NHANES 1999–2002 has shown an inverse association between particular flavonols, i.e. quercetin and kaempferol and C-reactive protein. A significant association between inflammation and moderate consumption of cocoa products was found in a study comparing subjects that ate chocolate regularly in the form of dark chocolate (n = 824) with subjects that did not eat chocolate for at least one year (n = 1,317). Serum C-reactive protein concentration in the subgroup having up to one serving (20 g of cocoa) every 3 days was significantly lower than in both, non consumers and subjects having higher consumption135. The possible mechanisms involved in the anti-inflammatory effects of cocoa products have been lately revised136–137. Of interest, are cell experiments showing the inhibition of MAPK kinase activities by cocoa procyanidins138–139, and cocoa extracts140.
CONCLUDING REMARKS
A full range of health benefits can today be associated to the actions of flavanols and procyanidins on vascular function. These benefits are mainly ascribed to diets rich in flavanols and procyanidins, and chocolate and cocoa derivatives are among the most valuable components of such a diet. Considering the fact that CVD is associated with a series of conditions that can trigger oxidant production and oxidant-regulated cell signaling, it would be logical to relate the free radical scavenging and metal chelating properties of cocoa flavanols to CVD protective effects. However, other biochemical mechanisms related to specific flavanol-lipid and flavanol-protein interactions can partially explain the observed in vitro and in vivo antioxidant effects. These mechanisms are more consistent with the in vivo flavanol and procyanidins levels observed in most human and animal tissues.
Acknowledgments
Supported by NIH AT2966, CHNR-State of California Vitamin Price Fixing Consumer Settlement Fund; UBACyT B801-B802, Argentina.
Abbreviations
- CT
(+)-catechin
- EC
(−)-epicatechin
- CVD
cardiovascular disease
REFERENCES
- 1.Lichtenstein AH, Appel LJ, Brands M, et al. Summary of American Heart Association Diet and Lifestyle Recommendations revision 2006. Arterioscler Thromb Vasc Biol. 2006;26:2186–2191. doi: 10.1161/01.ATV.0000238352.25222.5e. [DOI] [PubMed] [Google Scholar]
- 2.Knoops KT, de Groot LC, Kromhout D, et al. Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women: the HALE project. JAMA. 2004;292:1433–1439. doi: 10.1001/jama.292.12.1433. [DOI] [PubMed] [Google Scholar]
- 3.Appel LJ, Champagne CM, Harsha DW, et al. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. J Am Med Assoc. 2003;289:2083–2093. doi: 10.1001/jama.289.16.2083. [DOI] [PubMed] [Google Scholar]
- 4.Appel LJ, Moore TJ, Obarzanek E, et al. DASH Collaborative Research Group. A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 5.Bazzano LA, He J, Ogden LG, et al. Fruit and vegetable intake and risk of cardiovascular disease in US adults: the first National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Am J Clin Nutr. 2002;76:93–99. doi: 10.1093/ajcn/76.1.93. [DOI] [PubMed] [Google Scholar]
- 6.Hung HC, Joshipura KJ, Jiang R, et al. Fruit and vegetable intake and risk of major chronic disease. J Natl Cancer Inst. 2004;96:1577–1584. doi: 10.1093/jnci/djh296. [DOI] [PubMed] [Google Scholar]
- 7.Hertog MG, Feskens EJ, Hollman PC, et al. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- 8.Buijsse B, Feskens EJ, Kok FJ, et al. Cocoa intake, blood pressure, and cardiovascular mortality: the Zutphen Elderly Study. Arch Intern Med. 2006;166:411–417. doi: 10.1001/archinte.166.4.411. [DOI] [PubMed] [Google Scholar]
- 9.Gallus S, Tavani A, La Vecchia C. Response to Chocolate, well-being and health among elderly men: chocolate and acute myocardial infarction in a case-control study from Italy. Eur J Clin Nutr. 2009;63:588–589. doi: 10.1038/sj.ejcn.1602945. [DOI] [PubMed] [Google Scholar]
- 10.Grassi D, Necozione S, Lippi C, et al. Cocoa reduces blood pressure and insulin resistance and improves endothelium-dependent vasodilation in hypertensives. Hypertension. 2005;46:398–405. doi: 10.1161/01.HYP.0000174990.46027.70. [DOI] [PubMed] [Google Scholar]
- 11.Taubert D, Roesen R, Lehmann C, et al. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: a randomized controlled trial. J Am Med Assoc. 2007;298:49–60. doi: 10.1001/jama.298.1.49. [DOI] [PubMed] [Google Scholar]
- 12.Schroeter H, Heiss C, Balzer J, et al. (−)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci U S A. 2006;103:1024–1029. doi: 10.1073/pnas.0510168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heiss C, Dejam A, Kleinbongard P, et al. Vascular effects of cocoa rich in flavan-3-ols. J Am Med Assoc. 2003;290:1030–1031. doi: 10.1001/jama.290.8.1030. [DOI] [PubMed] [Google Scholar]
- 14.Heiss C, Finis D, Kleinbongard P, et al. Sustained increase in flow-mediated dilation after daily intake of high-flavanol cocoa drink over 1 week. J Cardiovasc Pharmacol. 2007;49:74–80. doi: 10.1097/FJC.0b013e31802d0001. [DOI] [PubMed] [Google Scholar]
- 15.Heiss C, Kleinbongard P, Dejam A, et al. Acute consumption of flavanol-rich cocoa and the reversal of endothelial dysfunction in smokers. J Am Coll Cardiol. 2005;46:1276–1283. doi: 10.1016/j.jacc.2005.06.055. [DOI] [PubMed] [Google Scholar]
- 16.Heiss C, Schroeter H, Balzer J, et al. Endothelial function, nitric oxide, and cocoa flavanols. J Cardiovasc Pharmacol. 2006;47 Suppl 2:S128–S135. doi: 10.1097/00005344-200606001-00007. discussion S172-S126. [DOI] [PubMed] [Google Scholar]
- 17.Fisher ND, Hughes M, Gerhard-Herman M, et al. Flavanol-rich cocoa induces nitric-oxide-dependent vasodilation in healthy humans. J Hypertens. 2003;21:2281–2286. doi: 10.1097/00004872-200312000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Murphy KJ, Chronopoulos AK, Singh I, et al. Dietary flavanols and procyanidin oligomers from cocoa (Theobroma cacao) inhibit platelet function. Am J Clin Nutr. 2003;77:1466–1473. doi: 10.1093/ajcn/77.6.1466. [DOI] [PubMed] [Google Scholar]
- 19.Diebolt M, Bucher B, Andriantsitohaina R. Wine polyphenols decrease blood pressure, improve NO vasodilatation, and induce gene expression. Hypertension. 2001;38:159–165. doi: 10.1161/01.hyp.38.2.159. [DOI] [PubMed] [Google Scholar]
- 20.Duffy SJ, Keaney JF, Jr, Holbrook M, et al. Short- and long-term black tea consumption reverses endothelial dysfunction in patients with coronary artery disease. Circulation. 2001;104:151–156. doi: 10.1161/01.cir.104.2.151. [DOI] [PubMed] [Google Scholar]
- 21.Freedman JE, Parker C, 3rd, Li L, et al. Select flavonoids and whole juice from purple grapes inhibit platelet function and enhance nitric oxide release. Circulation. 2001;103:2792–2798. doi: 10.1161/01.cir.103.23.2792. [DOI] [PubMed] [Google Scholar]
- 22.Rice-Evans C. Flavonoid antioxidants. Curr Med Chem. 2001;8:797–807. doi: 10.2174/0929867013373011. [DOI] [PubMed] [Google Scholar]
- 23.Mackenzie GG, Carrasquedo F, Delfino JM, et al. Epicatechin, catechin, and dimeric procyanidins inhibit PMA-induced NF-kappaB activation at multiple steps in Jurkat T cells. Faseb J. 2004;18:167–169. doi: 10.1096/fj.03-0402fje. [DOI] [PubMed] [Google Scholar]
- 24.Saliou C, Rihn B, Cillard J, et al. Selective inhibition of NF-kappaB activation by the flavonoid hepatoprotector silymarin in HepG2. Evidence for different activating pathways. FEBS Lett. 1998;440:8–12. doi: 10.1016/s0014-5793(98)01409-4. [DOI] [PubMed] [Google Scholar]
- 25.Erlejman AG, Verstraeten SV, Fraga CG, et al. The interaction of flavonoids with membranes: potential determinant of flavonoid antioxidant effects. Free Radic Res. 2004;38:1311–1320. doi: 10.1080/10715760400016105. [DOI] [PubMed] [Google Scholar]
- 26.Verstraeten SV, Keen CL, Schmitz HH, et al. Flavan-3-ols and procyanidins protect liposomes against lipid oxidation and disruption of the bilayer structure. Free Radic Biol Med. 2003;34:84–92. doi: 10.1016/s0891-5849(02)01185-1. [DOI] [PubMed] [Google Scholar]
- 27.Middleton E, Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- 28.Steffen Y, Gruber C, Schewe T, et al. Mono-O-methylated flavanols and other flavonoids as inhibitors of endothelial NADPH oxidase. Arch Biochem Biophys. 2008;469:209–219. doi: 10.1016/j.abb.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 29.Steffen Y, Jung T, Klotz LO, et al. Protein modification elicited by oxidized low-density lipoprotein (LDL) in endothelial cells: protection by (−)-epicatechin. Free Radic Biol Med. 2007;42:955–970. doi: 10.1016/j.freeradbiomed.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 30.Steffen Y, Schewe T, Sies H. (−)-Epicatechin elevates nitric oxide in endothelial cells via inhibition of NADPH oxidase. Biochem Biophys Res Commun. 2007;359:828–833. doi: 10.1016/j.bbrc.2007.05.200. [DOI] [PubMed] [Google Scholar]
- 31.Crozier A, Jaganath IB, Clifford MN. Phenols, polyphenols and tannins: an overview. In: MN, Clifford CA, Ashihara H, editors. Plant Secondary Metabolites-Ocurrence, Structure and Role in the Human Diet. Oxford, UK: Blackwell Publishing Ltd.; 2006. pp. 1–24. [Google Scholar]
- 32.Adamson GE, Lazarus SA, Mitchell AE, et al. HPLC method for the quantification of procyanidins in cocoa and chocolate samples and correlation to total antioxidant capacity. J Agric Food Chem. 1999;47:4184–4188. doi: 10.1021/jf990317m. [DOI] [PubMed] [Google Scholar]
- 33.Spencer JP, Chaudry F, Pannala AS, et al. Decomposition of cocoa procyanidins in the gastric milieu. Biochem Biophys Res Commun. 2000;272:236–241. doi: 10.1006/bbrc.2000.2749. [DOI] [PubMed] [Google Scholar]
- 34.Mullen W, Borges G, Donovan JL, et al. Milk decreases urinary excretion but not plasma pharmacokinetics of cocoa flavan-3-ol metabolites in humans. Am J Clin Nutr. 2009;89:1784–1791. doi: 10.3945/ajcn.2008.27339. [DOI] [PubMed] [Google Scholar]
- 35.Rios LY, Bennett RN, Lazarus SA, et al. Cocoa procyanidins are stable during gastric transit in humans. Am J Clin Nutr. 2002;76:1106–1110. doi: 10.1093/ajcn/76.5.1106. [DOI] [PubMed] [Google Scholar]
- 36.Spencer JP. Metabolism of tea flavonoids in the gastrointestinal tract. J Nutr. 2003;133:3255S–3261S. doi: 10.1093/jn/133.10.3255S. [DOI] [PubMed] [Google Scholar]
- 37.Piskula MK, Terao J. Accumulation of (−)-epicatechin metabolites in rat plasma after oral administration and distribution of conjugation enzymes in rat tissues. J Nutr. 1998;128:1172–1178. doi: 10.1093/jn/128.7.1172. [DOI] [PubMed] [Google Scholar]
- 38.Donovan JL, Crespy V, Manach C, et al. Catechin is metabolized by both the small intestine and liver of rats. J Nutr. 2001;131:1753–1757. doi: 10.1093/jn/131.6.1753. [DOI] [PubMed] [Google Scholar]
- 39.Baba S, Osakabe N, Yasuda A, et al. Bioavailability of (−)-epicatechin upon intake of chocolate and cocoa in human volunteers. Free Radic Res. 2000;33:635–641. doi: 10.1080/10715760000301151. [DOI] [PubMed] [Google Scholar]
- 40.Donovan JL, Bell JR, Kasim-Karakas S, et al. Catechin is present as metabolites in human plasma after consumption of red wine. J Nutr. 1999;129:1662–1668. doi: 10.1093/jn/129.9.1662. [DOI] [PubMed] [Google Scholar]
- 41.Baba S, Osakabe N, Natsume M, et al. Absortion and urinary excretion of procyanidin B2 [Epicatechin-(4β-8)-epicatechin] in rats. Free Radic. Biol. Med. 2002;33:142–148. doi: 10.1016/s0891-5849(02)00871-7. [DOI] [PubMed] [Google Scholar]
- 42.Abd El Mohsen MM, Kuhnle G, Rechner AR, et al. Uptake and metabolism of epicatechin and its access to the brain after oral ingestion. Free Radic Biol Med. 2002;33:1693–1702. doi: 10.1016/s0891-5849(02)01137-1. [DOI] [PubMed] [Google Scholar]
- 43.Rechner AR, Kuhnle G, Bremner P, et al. The metabolic fate of dietary polyphenols in humans. Free Radic Biol Med. 2002;33:220–235. doi: 10.1016/s0891-5849(02)00877-8. [DOI] [PubMed] [Google Scholar]
- 44.Unno T, Tamemoto K, Yayabe F, et al. Urinary excretion of 5-(3',4'-dihydroxyphenyl)-gamma-valerolactone, a ring-fission metabolite of (−)-epicatechin, in rats and its in vitro antioxidant activity. J Agric Food Chem. 2003;51:6893–6898. doi: 10.1021/jf034578e. [DOI] [PubMed] [Google Scholar]
- 45.Holt RR, Lazarus SA, Sullards MC, et al. Procyanidin dimer B2 [epicatechin-(4beta-8)-epicatechin] in human plasma after the consumption of a flavanol-rich cocoa. Am J Clin Nutr. 2002;76:798–804. doi: 10.1093/ajcn/76.4.798. [DOI] [PubMed] [Google Scholar]
- 46.Spencer JP, Schroeter H, Shenoy B, et al. Epicatechin is the primary bioavailable form of the procyanidin dimers B2 and B5 after transfer across the small intestine. Biochem Biophys Res Commun. 2001;285:588–593. doi: 10.1006/bbrc.2001.5211. [DOI] [PubMed] [Google Scholar]
- 47.Zhu QY, Holt RR, Lazarus SA, et al. Stability of the flavan-3-ols epicatechin and catechin and related dimeric procyanidins derived from cocoa. J Agric Food Chem. 2002;50:1700–1705. doi: 10.1021/jf011228o. [DOI] [PubMed] [Google Scholar]
- 48.Steinberg FM, Holt RR, Schmitz HH, et al. Cocoa procyanidin chain length does not determine ability to protect LDL from oxidation when monomer units are controlled. J Nutr Biochem. 2002;13:645–652. doi: 10.1016/s0955-2863(02)00215-2. [DOI] [PubMed] [Google Scholar]
- 49.Urpi-Sarda M, Monagas M, Khan N, et al. Epicatechin, procyanidins, and phenolic microbial metabolites after cocoa intake in humans and rats. Anal Bioanal Chem. 2009 doi: 10.1007/s00216-009-2676-1. [DOI] [PubMed] [Google Scholar]
- 50.Gonthier MP, Donovan JL, Texier O, et al. Metabolism of dietary procyanidins in rats. Free Radic Biol Med. 2003;35:837–844. doi: 10.1016/s0891-5849(03)00394-0. [DOI] [PubMed] [Google Scholar]
- 51.Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 52.Schroeder P, Klotz LO, Sies H. Amphiphilic properties of (−)-epicatechin and their significance for protection of cells against peroxynitrite. Biochem Biophys Res Commun. 2003;307:69–73. doi: 10.1016/s0006-291x(03)01132-x. [DOI] [PubMed] [Google Scholar]
- 53.Actis-Goretta L, Mackenzie GG, Oteiza PI, et al. Comparative study on the antioxidant capacity of wines and other plant-derived beverages. Ann N Y Acad Sci. 2002;957:279–283. doi: 10.1111/j.1749-6632.2002.tb02925.x. [DOI] [PubMed] [Google Scholar]
- 54.Erlejman AG, Fraga CG, Oteiza PI. Procyanidins protect Caco-2 cells from bile acid- and oxidant-induced damage. Free Radic Biol Med. 2006;41:1247–1256. doi: 10.1016/j.freeradbiomed.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 55.Fraga CG, Martino VS, Ferraro GE, et al. Flavonoids as antioxidants evaluated by in vitro and in situ liver chemiluminescence. Biochem Pharmacol. 1987;36:717–720. doi: 10.1016/0006-2952(87)90724-6. [DOI] [PubMed] [Google Scholar]
- 56.Guo Q, Zhao B, Li M, et al. Studies on protective mechanisms of four components of green tea polyphenols against lipid peroxidation in synaptosomes. Biochim Biophys Acta. 1996;1304:210–222. doi: 10.1016/s0005-2760(96)00122-1. [DOI] [PubMed] [Google Scholar]
- 57.Kurosawa T, Itoh F, Nozaki A, et al. Suppressive effect of cocoa powder on atherosclerosis in Kurosawa and Kusanagi-hypercholesterolemic rabbits. J Atheroscler Thromb. 2005;12:20–28. doi: 10.5551/jat.12.20. [DOI] [PubMed] [Google Scholar]
- 58.Lotito SB, Actis-Goretta L, Renart ML, et al. Influence of oligomer chain length on the antioxidant activity of procyanidins. Biochem Biophys Res Commun. 2000;276:945–951. doi: 10.1006/bbrc.2000.3571. [DOI] [PubMed] [Google Scholar]
- 59.Lotito SB, Fraga CG. (+)-Catechin prevents human plasma oxidation. Free Radic Biol Med. 1998;24:435–441. doi: 10.1016/s0891-5849(97)00276-1. [DOI] [PubMed] [Google Scholar]
- 60.Lotito SB, Fraga CG. Catechins delay lipid oxidation and alpha-tocopherol and beta-carotene depletion following ascorbate depletion in human plasma. Proc Soc Exp Biol Med. 2000;225:32–38. doi: 10.1046/j.1525-1373.2000.22504.x. [DOI] [PubMed] [Google Scholar]
- 61.Verstraeten SV, Hammerstone JF, Keen CL, et al. Antioxidant and membrane effects of procyanidin dimers and trimers isolated from peanut and cocoa. J Agric Food Chem. 2005;53:5041–5048. doi: 10.1021/jf058018m. [DOI] [PubMed] [Google Scholar]
- 62.Orozco TJ, Wang JF, Keen CL. Chronic consumption of a flavanol- and procyanindin-rich diet is associated with reduced levels of 8-hydroxy-2'-deoxyguanosine in rat testes. J Nutr Biochem. 2003;14:104–110. doi: 10.1016/s0955-2863(02)00273-5. [DOI] [PubMed] [Google Scholar]
- 63.Osakabe N, Natsume M, Adachi T, et al. Effects of cacao liquor polyphenols on the susceptibility of low-density lipoprotein to oxidation in hypercholesterolemic rabbits. J Atheroscler Thromb. 2000;7:164–168. doi: 10.5551/jat1994.7.164. [DOI] [PubMed] [Google Scholar]
- 64.Videla LA, Fraga CG, Koch OR, et al. Chemiluminescence of the in situ rat liver after acute ethanol intoxication--effect of (+)-cyanidanol-3. Biochem Pharmacol. 1983;32:2822–2825. doi: 10.1016/0006-2952(83)90099-0. [DOI] [PubMed] [Google Scholar]
- 65.Fraga CG. Plant polyphenols: how to translate their in vitro antioxidant actions to in vivo conditions. IUBMB Life. 2007;59:308–315. doi: 10.1080/15216540701230529. [DOI] [PubMed] [Google Scholar]
- 66.Fraga CG. Cocoa, diabetes, and hypertension: should we eat more chocolate? Am J Clin Nutr. 2005;81:541–542. doi: 10.1093/ajcn/81.3.541. [DOI] [PubMed] [Google Scholar]
- 67.Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci U S A. 2004;101:4003–4008. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ghafourifar P, Richter C. Nitric oxide synthase activity in mitochondria. FEBS Lett. 1997;418:291–296. doi: 10.1016/s0014-5793(97)01397-5. [DOI] [PubMed] [Google Scholar]
- 69.Giulivi C, Poderoso JJ, Boveris A. Production of nitric oxide by mitochondria. J Biol Chem. 1998;273:11038–11043. doi: 10.1074/jbc.273.18.11038. [DOI] [PubMed] [Google Scholar]
- 70.Boveris A, Cadenas E. Mitochondrial production of superoxide anions and its relationship to the antimycin insensitive respiration. FEBS Lett. 1975;54:311–314. doi: 10.1016/0014-5793(75)80928-8. [DOI] [PubMed] [Google Scholar]
- 71.Turrens JF, Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem J. 1980;191:421–427. doi: 10.1042/bj1910421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sarkis A, Roman RJ. Role of cytochrome P450 metabolites of arachidonic acid in hypertension. Curr Drug Metab. 2004;5:245–256. doi: 10.2174/1389200043335603. [DOI] [PubMed] [Google Scholar]
- 73.Ward R, Cirkovic-Vellichovia T, Ledeque F, et al. Neuroprotection by taurine and taurine analogues. Adv Exp Med Biol. 2006;583:299–306. doi: 10.1007/978-0-387-33504-9_33. [DOI] [PubMed] [Google Scholar]
- 74.Jones SA, O'Donnell VB, Wood JD, et al. Expression of phagocyte NADPH oxidase components in human endothelial cells. Am J Physiol. 1996;271:H1626–H1634. doi: 10.1152/ajpheart.1996.271.4.H1626. [DOI] [PubMed] [Google Scholar]
- 75.Touyz RM. Recent advances in intracellular signalling in hypertension. Curr Opin Nephrol Hypertens. 2003;12:165–174. doi: 10.1097/00041552-200303000-00007. [DOI] [PubMed] [Google Scholar]
- 76.Park YC, Rimbach G, Saliou C, et al. Activity of monomeric, dimeric, and trimeric flavonoids on NO production, TNF-alpha secretion, and NF-kappaB-dependent gene expression in RAW 264.7 macrophages. FEBS Lett. 2000;465:93–97. doi: 10.1016/s0014-5793(99)01735-4. [DOI] [PubMed] [Google Scholar]
- 77.Jovanovic SD, Steenken S, Tosic M, et al. Flavonoids as antioxidants. J Am Chem Soc. 1994;116:4846–4851. [Google Scholar]
- 78.Oteiza PI, Erlejman AG, Verstraeten SV, et al. Flavonoid-membrane interactions: a protective role of flavonoids at the membrane surface? Clin Dev Immunol. 2005;12:19–25. doi: 10.1080/10446670410001722168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rosenkranz S, Knirel D, Dietrich H, et al. Inhibition of the PDGF receptor by red wine flavonoids provides a molecular explanation for the "French paradox". FASEB J. 2002;16:1958–1960. doi: 10.1096/fj.02-0207fje. [DOI] [PubMed] [Google Scholar]
- 80.Tachibana H, Koga K, Fujimura Y, et al. A receptor for green tea polyphenol EGCG. Nat Struct Mol Biol. 2004;11:380–381. doi: 10.1038/nsmb743. [DOI] [PubMed] [Google Scholar]
- 81.Schewe T, Kuhn H, Sies H. Flavonoids of cocoa inhibit recombinant human 5-lipoxygenase. J Nutr. 2002;132:1825–1829. doi: 10.1093/jn/132.7.1825. [DOI] [PubMed] [Google Scholar]
- 82.Schewe T, Sadik C, Klotz LO, et al. Polyphenols of cocoa: inhibition of mammalian 15-lipoxygenase. Biol Chem. 2001;382:1687–1696. doi: 10.1515/BC.2001.204. [DOI] [PubMed] [Google Scholar]
- 83.Lee KW, Kundu JK, Kim SO, et al. Cocoa polyphenols inhibit phorbol ester-induced superoxide anion formation in cultured HL-60 cells and expression of cyclooxygenase-2 and activation of NF-kappaB and MAPKs in mouse skin in vivo. J Nutr. 2006;136:1150–1155. doi: 10.1093/jn/136.5.1150. [DOI] [PubMed] [Google Scholar]
- 84.Zhang WY, Liu HQ, Xie KQ, et al. Procyanidin dimer B2 [epicatechin-(4beta-8)-epicatechin] suppresses the expression of cyclooxygenase-2 in endotoxin-treated monocytic cells. Biochem Biophys Res Commun. 2006;345:508–515. doi: 10.1016/j.bbrc.2006.04.085. [DOI] [PubMed] [Google Scholar]
- 85.Oak MH, El Bedoui J, Anglard P, et al. Red wine polyphenolic compounds strongly inhibit pro-matrix metalloproteinase-2 expression and its activation in response to thrombin via direct inhibition of membrane type 1-matrix metalloproteinase in vascular smooth muscle cells. Circulation. 2004;110:1861–1867. doi: 10.1161/01.CIR.0000142617.52881.F4. [DOI] [PubMed] [Google Scholar]
- 86.Ottaviani JI, Carrasquedo F, Keen CL, et al. Influence of flavan-3-ols and procyanidins on UVC-mediated formation of 8-oxo-7,8-dihydro-2'-deoxyguanosine in isolated DNA. Arch Biochem Biophys. 2002;406:203–208. doi: 10.1016/s0003-9861(02)00455-1. [DOI] [PubMed] [Google Scholar]
- 87.Fraga CG, Oteiza PI. Flavanols and NF-κB activation: relevance for inflammation and associated diseases. In: Surh YJ, Dong Z, Cadenas E, Packer L, editors. Dietary Modulation of Cell Signaling Pathways. Boca Raton, FL: CRC Press; 2008. pp. 137–151. [Google Scholar]
- 88.Khan N, Afaq F, Saleem M, et al. Targeting multiple signaling pathways by green tea polyphenol (−)-epigallocatechin-3-gallate. Cancer Res. 2006;66:2500–2505. doi: 10.1158/0008-5472.CAN-05-3636. [DOI] [PubMed] [Google Scholar]
- 89.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 90.Mackenzie GG, Oteiza PI. Modulation of transcription factor NF-kappaB in Hodgkin's lymphoma cell lines: effect of (−)-epicatechin. Free Radic Res. 2006;40:1086–1094. doi: 10.1080/10715760600788396. [DOI] [PubMed] [Google Scholar]
- 91.Erlejman AG, Jaggers G, Fraga CG, et al. TNFalpha-induced NF-kappaB activation and cell oxidant production are modulated by hexameric procyanidins in Caco-2 cells. Arch Biochem Biophys. 2008;476:186–195. doi: 10.1016/j.abb.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 92.Verstraeten SV, Mackenzie GG, Oteiza PI, et al. (−)-Epicatechin and related procyanidins modulate intracellular calcium and prevent oxidation in Jurkat T cells. Free Radic Res. 2008;42:864–872. doi: 10.1080/10715760802471452. [DOI] [PubMed] [Google Scholar]
- 93.Shepherd J, Cobbe SM, Ford I, et al. West of Scotland Coronary Prevention Study Group. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med. 1995;333:1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 94.Stamler J, Wentworth D, Neaton JD. Is relationship between serum cholesterol and risk of premature death from coronary heart disease continuous and graded? Findings in 356,222 primary screenees of the Multiple Risk Factor Intervention Trial (MRFIT) J Am Med Assoc. 1986;256:2823–2828. [PubMed] [Google Scholar]
- 95.Borradaile NM, de Dreu LE, Wilcox LJ, et al. Soya phytoestrogens, genistein and daidzein, decrease apolipoprotein B secretion from HepG2 cells through multiple mechanisms. Biochem J. 2002;366:531–539. doi: 10.1042/BJ20020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ikeda I, Imasato Y, Sasaki E, et al. Tea catechins decrease micellar solubility and intestinal absorption of cholesterol in rats. Biochim Biophys Acta. 1992;1127:141–146. doi: 10.1016/0005-2760(92)90269-2. [DOI] [PubMed] [Google Scholar]
- 97.Pal S, Ho N, Santos C, et al. Red wine polyphenolics increase LDL receptor expression and activity and suppress the secretion of ApoB100 from human HepG2 cells. J Nutr. 2003;133:700–706. doi: 10.1093/jn/133.3.700. [DOI] [PubMed] [Google Scholar]
- 98.Wilcox LJ, Borradaile NM, de Dreu LE, et al. Secretion of hepatocyte apoB is inhibited by the flavonoids, naringenin and hesperetin, via reduced activity and expression of ACAT2 and MTP. J Lipid Res. 2001;42:725–734. [PubMed] [Google Scholar]
- 99.Baba S, Natsume M, Yasuda A, et al. Plasma LDL and HDL cholesterol and oxidized LDL concentrations are altered in normo- and hypercholesterolemic humans after intake of different levels of cocoa powder. J Nutr. 2007;137:1436–1441. doi: 10.1093/jn/137.6.1436. [DOI] [PubMed] [Google Scholar]
- 100.Fraga CG, Actis-Goretta L, Ottaviani JI, et al. Regular consumption of a flavanol-rich chocolate can improve oxidant stress in young soccer players. Clin Dev Immunol. 2005;12:11–17. doi: 10.1080/10446670410001722159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Grassi D, Desideri G, Necozione S, et al. Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate. J Nutr. 2008;138:1671–1676. doi: 10.1093/jn/138.9.1671. [DOI] [PubMed] [Google Scholar]
- 102.Baba S, Osakabe N, Kato Y, et al. Continuous intake of polyphenolic compounds containing cocoa powder reduces LDL oxidative susceptibility and has beneficial effects on plasma HDL-cholesterol concentrations in humans. Am J Clin Nutr. 2007;85:709–717. doi: 10.1093/ajcn/85.3.709. [DOI] [PubMed] [Google Scholar]
- 103.Mursu J, Voutilainen S, Nurmi T, et al. Dark chocolate consumption increases HDL cholesterol concentration and chocolate fatty acids may inhibit lipid peroxidation in healthy humans. Free Radic Biol Med. 2004;37:1351–1359. doi: 10.1016/j.freeradbiomed.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 104.Steinberg D, Parthasarathy S, Carew TE, et al. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 105.Kondo K, Hirano R, Matsumoto A, et al. Inhibition of LDL oxidation by cocoa. Lancet. 1996;348:1514. doi: 10.1016/s0140-6736(05)65927-2. [DOI] [PubMed] [Google Scholar]
- 106.Mathur S, Devaraj S, Grundy SM, et al. Cocoa products decrease low density lipoprotein oxidative susceptibility but do not affect biomarkers of inflammation in humans. J Nutr. 2002;132:3663–3667. doi: 10.1093/jn/132.12.3663. [DOI] [PubMed] [Google Scholar]
- 107.Osakabe N, Baba S, Yasuda A, et al. Daily cocoa intake reduces the susceptibility of low-density lipoprotein to oxidation as demonstrated in healthy human volunteers. Free Radic Res. 2001;34:93–99. doi: 10.1080/10715760100300091. [DOI] [PubMed] [Google Scholar]
- 108.Rein D, Lotito S, Holt RR, et al. Epicatechin in human plasma: in vivo determination and effect of chocolate consumption on plasma oxidation status. J Nutr. 2000;130:2109S–2114S. doi: 10.1093/jn/130.8.2109S. [DOI] [PubMed] [Google Scholar]
- 109.Wan Y, Vinson JA, Etherton TD, et al. Effects of cocoa powder and dark chocolate on LDL oxidative susceptibility and prostaglandin concentrations in humans. Am J Clin Nutr. 2001;74:596–602. doi: 10.1093/ajcn/74.5.596. [DOI] [PubMed] [Google Scholar]
- 110.Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339:1523–1526. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- 111.McCullough ML, Chevaux K, Jackson L, et al. Hypertension, the Kuna, and the epidemiology of flavanols. J Cardiovasc Pharmacol. 2006;47:S103–S109. doi: 10.1097/00005344-200606001-00003. [DOI] [PubMed] [Google Scholar]
- 112.Grassi D, Lippi C, Necozione S, et al. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am J Clin Nutr. 2005;81:611–614. doi: 10.1093/ajcn/81.3.611. [DOI] [PubMed] [Google Scholar]
- 113.Taubert D, Berkels R, Roesen R, et al. Chocolate and blood pressure in elderly individuals with isolated systolic hypertension. J Am Med Assoc. 2003;290:1029–1030. doi: 10.1001/jama.290.8.1029. [DOI] [PubMed] [Google Scholar]
- 114.Taubert D, Roesen R, Schomig E. Effect of cocoa and tea intake on blood pressure: a meta-analysis. Arch Intern Med. 2007;167:626–634. doi: 10.1001/archinte.167.7.626. [DOI] [PubMed] [Google Scholar]
- 115.Faridi Z, Njike VY, Dutta S, et al. Acute dark chocolate and cocoa ingestion and endothelial function: a randomized controled crossover trial. Am J Clin Nutr. 2008;88:58–63. doi: 10.1093/ajcn/88.1.58. [DOI] [PubMed] [Google Scholar]
- 116.Ralay Ranaivo H, Diebolt M, Andriantsitohaina R. Wine polyphenols induce hypotension, and decrease cardiac reactivity and infarct size in rats: involvement of nitric oxide. Br J Pharmacol. 2004;142:671–678. doi: 10.1038/sj.bjp.0705833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bingham SA, Vorster H, Jerling JC, et al. Effect of black tea drinking on blood lipids, blood pressure and aspects of bowel habit. Br J Nutr. 1997;78:41–55. doi: 10.1079/bjn19970117. [DOI] [PubMed] [Google Scholar]
- 118.Negishi H, Xu JW, Ikeda K, et al. Black and green tea polyphenols attenuate blood pressure increases in stroke-prone spontaneously hypertensive rats. J Nutr. 2004;134:38–42. doi: 10.1093/jn/134.1.38. [DOI] [PubMed] [Google Scholar]
- 119.Stensvold I, Tverdal A, Solvoll K, et al. Tea consumption. Relationship to cholesterol, blood pressure, and coronary and total mortality. Prev Med. 1992;21:546–553. doi: 10.1016/0091-7435(92)90062-m. [DOI] [PubMed] [Google Scholar]
- 120.Yang YC, Lu FH, Wu JS, et al. The protective effect of habitual tea consumption on hypertension. Arch Intern Med. 2004;164:1534–1540. doi: 10.1001/archinte.164.14.1534. [DOI] [PubMed] [Google Scholar]
- 121.Muniyappa R, Hall G, Kolodziej TL, et al. Cocoa consumption for 2 wk enhances insulin-mediated vasodilatation without improving blood pressure or insulin resistance in essential hypertension. Am J Clin Nutr. 2008;88:1685–1696. doi: 10.3945/ajcn.2008.26457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Osterud B. A global view on the role of monocytes and platelets in atherogenesis. Thromb Res. 1997;85:1–22. doi: 10.1016/s0049-3848(96)00205-8. [DOI] [PubMed] [Google Scholar]
- 123.Pearson DA, Holt RR, Rein D, et al. Flavanols and platelet reactivity. Clin Dev Immunol. 2005;12:1–9. doi: 10.1080/10446670410001722140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rein D, Paglieroni TG, Wun T, et al. Cocoa inhibits platelet activation and function. Am J Clin Nutr. 2000;72:30–35. doi: 10.1093/ajcn/72.1.30. [DOI] [PubMed] [Google Scholar]
- 125.Hermann F, Spieker LE, Ruschitzka F, et al. Dark chocolate improves endothelial and platelet function. Heart. 2006;92:119–120. doi: 10.1136/hrt.2005.063362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Flammer AJ, Hermann F, Sudano I, et al. Dark chocolate improves coronary vasomotion and reduces platelet reactivity. Circulation. 2007;116:2376–2382. doi: 10.1161/CIRCULATIONAHA.107.713867. [DOI] [PubMed] [Google Scholar]
- 127.de Graaf JC, Banga JD, Moncada S, et al. Nitric oxide functions as an inhibitor of platelet adhesion under flow conditions. Circulation. 1992;85:2284–2290. doi: 10.1161/01.cir.85.6.2284. [DOI] [PubMed] [Google Scholar]
- 128.Pignatelli P, Di Santo S, Buchetti B, et al. Polyphenols enhance platelet nitric oxide by inhibiting protein kinase C-dependent NADPH oxidase activation: effect on platelet recruitment. FASEB J. 2006;20:1082–1089. doi: 10.1096/fj.05-5269com. [DOI] [PubMed] [Google Scholar]
- 129.Pignatelli P, Pulcinelli FM, Celestini A, et al. The flavonoids quercetin and catechin synergistically inhibit platelet function by antagonizing the intracellular production of hydrogen peroxide. Am J Clin Nutr. 2000;72:1150–1155. doi: 10.1093/ajcn/72.5.1150. [DOI] [PubMed] [Google Scholar]
- 130.Schramm DD, Wang JF, Holt RR, et al. Chocolate procyanidins decrease the leukotriene-prostacyclin ratio in humans and human aortic endothelial cells. Am J Clin Nutr. 2001;73:36–40. doi: 10.1093/ajcn/73.1.36. [DOI] [PubMed] [Google Scholar]
- 131.Navarro-Nunez L, Castillo J, Lozano ML, et al. Thromboxane A2 receptor antagonism by flavonoids: structure-activity relationships. J Agric Food Chem. 2009;57:1589–1594. doi: 10.1021/jf803041k. [DOI] [PubMed] [Google Scholar]
- 132.Sadik CD, Sies H, Schewe T. Inhibition of 15-lipoxygenases by flavonoids: structureactivity relations and mode of action. Biochem Pharmacol. 2003;65:773–781. doi: 10.1016/s0006-2952(02)01621-0. [DOI] [PubMed] [Google Scholar]
- 133.Vasquez-Martinez Y, Ohri RV, Kenyon V, et al. Structure-activity relationship studies of flavonoids as potent inhibitors of human platelet 12-hLO, reticulocyte 15-hLO-1, and prostate epithelial 15-hLO-2. Bioorg Med Chem. 2007;15:7408–7425. doi: 10.1016/j.bmc.2007.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chun OK, Chung SJ, Claycombe KJ, et al. Serum C-reactive protein concentrations are inversely associated with dietary flavonoid intake in U.S. adults. J Nutr. 2008;138:753–760. doi: 10.1093/jn/138.4.753. [DOI] [PubMed] [Google Scholar]
- 135.di Giuseppe R, Di Castelnuovo A, Centritto F, et al. Regular consumption of dark chocolate is associated with low serum concentrations of C-reactive protein in a healthy Italian population. J Nutr. 2008;138:1939–1945. doi: 10.1093/jn/138.10.1939. [DOI] [PubMed] [Google Scholar]
- 136.Ramiro-Puig E, Castell M. Cocoa: antioxidant and immunomodulator. Br J Nutr. 2009;101:931–940. doi: 10.1017/S0007114508169896. [DOI] [PubMed] [Google Scholar]
- 137.Selmi C, Cocchi CA, Lanfredini M, et al. Chocolate at heart: the anti-inflammatory impact of cocoa flavanols. Mol Nutr Food Res. 2008;52:1340–1348. doi: 10.1002/mnfr.200700435. [DOI] [PubMed] [Google Scholar]
- 138.Cho ES, Jang YJ, Kang NJ, et al. Cocoa procyanidins attenuate 4-hydroxynonenal-induced apoptosis of PC12 cells by directly inhibiting mitogen-activated protein kinase kinase 4 activity. Free Radic Biol Med. 2009;46:1319–1327. doi: 10.1016/j.freeradbiomed.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 139.Kang NJ, Lee KW, Lee DE, et al. Cocoa procyanidins suppress transformation by inhibiting mitogen-activated protein kinase kinase. J Biol Chem. 2008;283:20664–20673. doi: 10.1074/jbc.M800263200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jenny M, Santer E, Klein A, et al. Cacao extracts suppress tryptophan degradation of mitogen-stimulated peripheral blood mononuclear cells. J Ethnopharmacol. 2009;122:261–267. doi: 10.1016/j.jep.2009.01.011. [DOI] [PubMed] [Google Scholar]