Abstract
Hypersensitivity to metallic implants remains relatively unpredictable and poorly understood. We initially hypothesized that metal-induced lymphocyte proliferation responses to soluble metal challenge (ions) are mediated exclusively by early T-cell activation (not B-cells), typical of a Delayed-Type-Hypersensitivity response. We tested this by comparing proliferation (6-days) of primary lymphocytes with early T-cell and B-cell activation (48-hours) in three groups of subjects likely to demonstrate elevated metal-reactivity: Group 1(n=12) history of metal-sensitivity with no implant; Group 2a(n=6) well performing metal-on-metal THRs, and Group 2b(n=20) subjects with poorly performing metal-on-polymer total joint arthroplasties (TJA). Group 1 showed 100%(12/12) metal reactivity (Stimulation Index>2) to Ni. Group 2a&2b were 83%(5/6) and 75%(15/22) metal reactive (to Co, Cr or Ni) respectively. Of the n=32 metal reactive subjects to Co, Cr or Ni (SI>2), n=22/32 demonstrated >2-fold elevations in % of T-cell or B-cell activation (CD25+,CD69+) to metal challenge compared to untreated control. 18/22 metal-activated subjects demonstrated an exclusively T-cell or B-cell activation response to metal challenge, where 6/18 demonstrated exclusively B-cell activation and 12/18 demonstrated a T-cell only response, as measured by surface activation markers CD25+ and CD69+. However, there was no direct correlation (R2<0.1) between lymphocyte proliferation and % T-cell or B-cell activation (CD25+:CD69+). Proliferation assays (LTT) showed greater ability to detect metal reactivity than did subject-dependent results of flow-cytometry analysis of T-cell or B-cell activation. The high incidence of lymphocyte reactivity and activation, indicate that more complex than initially hypothesized immune responses may contribute to the etiology of debris induced osteolysis in metal-sensitive individuals.
Keywords: T-cell, B-cell, Metals, Hypersensitivity, Bioreactivity, Lymphocyte, Lymphocyte Transformation Test, Arthroplasty, Cobalt, Chromium, Nickel
Introduction
While hypersensitivity to metallic implants is well documented in case reports1, 5, 30, 40, 54, 62, 68, 74 and group studies,12, 17, 18, 20, 22-25, 29, 31, 34, 43, 53, 56 it remains a relatively unpredictable and poorly understood phenomenon.6, 22, 27 All metals in contact with biological systems corrode11, 46 and the released ions, while not sensitizers on their own, can activate the immune system by forming complexes with native proteins.55, 75, 76 These metal-protein complexes are considered to be candidate antigens (or more loosely termed, allergens) for eliciting metal hypersensitivity responses. Although little is known about the short-and long-term pharmacodynamics and bioavailability of circulating metal degradation products in vivo,10, 44, 45, 55 it is known that the degradation products of metallic biomaterials include particulate wear debris, colloidal organometallic complexes and metallic ions (both specifically and non-specifically bound to proteins), inorganic metal salts/oxides, and precipitated organometallic storage forms.
Immunologic responses (e.g. cell-mediated hypersensitivity) associated with metal components may be partly responsible for this differential reactivity. The most common metals which are allergens/sensitizers include nickel,29, 42, 47, 51 cobalt,51 and chromium,51 while occasional responses have been reported to tantalum,4 titanium50, 60 and vanadium4. Nickel is the most common metal sensitizer in humans followed by cobalt and chromium6, 29, 42, 47 and cross reactivity between nickel and cobalt is common.6 While the prevalence of metal sensitivity among the general population is approximately 10% as noted above, the prevalence of metal sensitivity among patients with well functioning and poorly functioning implants has been reported to be approximately 25% and 60%, respectively, as measured by dermal patch testing.34 However, the specific metals that mediate implant related metal hypersensitivity reactions, the true clinical prevalence of metal hypersensitivity, and the clinical impact of metal hypersensitivity on TJR patient populations are unknown.
In this study of metal induced responses of peripheral blood mononuclear cell populations, the term “metal hypersensitivity” will be used to describe elevated responses by T-cells and B-cells to metal challenge. However, generally, metal hypersensitivity has been characterized in past clinical and in vitro studies as mediated by T-cells in classical delayed type hypersensitivity type responses, DTH type IV.51, 67 The effector phase of a DTH response is initiated by contact of sensitized T cells with an antigen presented in class II MHC by antigen presenting cells (APCs). In this phase T cells, which are antigen-activated, are characterized as THelper cells and, in conjunction with APC's, can secrete a variety of cytokines that recruit and activate macrophages, monocytes, neutrophils and other inflammatory cells. These released cytokines include: IL-3 and GM-CSF which promote hematopoesis of granulocytes; monocyte chemotactic activating factor (MCAF) which promotes chemotaxis of monocytes toward areas of DTH activation; IFN-γ and TNF-β which produce a number of effects on local endothelial cells facilitating infiltration; and migration inhibitory factor (MIF) which inhibits the migration of macrophages away from the site of a DTH reaction. Activated macrophages, because of their increased ability to present class II MHC complexes and IL-1, can trigger the activation of more THelper cells, which in turn activates more macrophages, resulting in a vicious cycle. This DTH self-perpetuation response can create extensive tissue damage.
The specific lymphocyte subpopulations associated with metal hypersensitivity are among the many facets of metal “allergy” that remain uncharacterized. To what degree do T-cells or B-cells mediate lymphocyte proliferation in response to specific metals when tested in vitro? We hypothesized that metal-induced proliferation reactivity (peripheral blood mononuclear cell, PBMC) responses to soluble metal challenge are mediated exclusively by early T-cell activation (not B-cells). We tested this hypothesis by comparing metal induced proliferation of primary lymphocytes with early T-cell and B-cell activation (48 hours) in subjects with and without joint replacement implants.
Materials and Methods
T-cell vs. B-cell reactivity was characterized using metal reactive subjects (recruited from populations previously determined to have a high incidence of metal reactivity36, 37), which fall into two general categories; those with implant and those without. Those subjects with an implant were further partitioned into kind of implant (metal-on-metal or metal-on-polymer articulating bearing). Group 1 metal-reactive control subjects had no implants but had a clinical history of metal allergy manifested as a cutaneous reaction to “cheap” nickel or chromium containing jewelry (Group 1, no implant and history of metal sensitivity and osteoarthritis, n=12, with 11 females and 1 male, average age = 61.5yrs). Group 2 was subdivided into Group 2a (subjects with well performing pain free metal-on-metal bearing THA surface replacements, n=6, one of the six had a history of surface effusions associated with the implant) and Group2b (subjects with poorly performing metal-on-polymer bearing TJAs, n=20 pre-revision surgery, 18 TKA and 2 THA; all 2b subjects had painful joints with osteolysis in excess of 0.5cm2 8). Group 2 had either a total knee arthroplasty (TKA) or a total hip arthroplasty (THA) implant (n=26, with 17 females and 9 males, average age = 57.6). Subjects within both groups were recruited on an all-comer basis and subject groups were selected to maximize the incidence and recruitment of subjects with in vitro hypersensitivity to metals. Our previous studies have shown that people with no implants and no clinical history of metal hypersensitivity do not demonstrate in vitro activation of metal-challenged PBMCs.14, 15, 36, 37 Thus metal induced hypersensitivity responses were compared to unchallenged PBMCs from the same individuals for contrast with negative controls. Similarly, people with well performing joint arthplasties have a low incidence of metal hypersensitivity (estimated at approx 1-3%),34 and thus subjects in this study were limited to individuals most likely to demonstrate in vitro metal hypersensitivity.
Serum and lymphocytes were obtained from all subjects by peripheral venipuncture after obtaining Rush University Medical Center Institutional Review Board approval and subject informed consent. Human primary lymphocytes were isolated using Ficoll gradient separation of mononuclear cells from approximately 30 mls of blood (15-30 × 106 cells per subject) and incubated with DMEM and 10% autologous serum with either no metal (plain medium) as a negative control, 0.01 mg/ml phytohemagglutinin (PHA) as a positive control, 0.1 mM CrCl3, 0.1 mM NiCl2, 0.1 mM CoCl2 (Sigma, St. Louis, MO). These concentrations of metal challenge have been extensively investigated previously for toxicity, apoptosis and DNA damage,13, 35, 37, 38 where these concentrations have been well documented as non-toxic, within the limited context of in vitro LTT testing of PBMCs. The amount of lymphocyte proliferation, activation and cytokine production was normalized to that of the negative control (no treatment), providing the stimulation index (SI), activation index and normalized cytokine ratio.
In vivo circulating levels of metal from implant degradation are generally an order of magnitude less than that used to challenge lymphocytes, see Table 1.13, 35, 38 In addition, the autologous serum (with metal from an individual with an implant) was used in cell culture at a concentration of only 10%, which results in more than two orders of magnitude difference between in vitro metal challenge levels and that in the autologous serum used to supplemented culture medium. However, the metal challenge concentrations used in this study are clinically significant given that local concentrations of metal found in peri-implant tissue (Table 1) are highly elevated, which demonstrates that local peri-implant concentrations are similar to those used to challenge lymphocytes in the current study.
TABLE 1.
Results of metal reactivity (Metal reactive criteria: SI>2, p<0.05, t-test) and activation are shown for all study subjects where degree of metal reactivity is graded from low, medium and high by shading (see legend). “% Increase Activation” indicates the percent increase over untreated controls paired for each individual.
| Group | Subject # | Cr Stimulation Index | Cr T-cell %Increase Activation | Cr B-cell %Increase Activation | Co Stimulation Index | Co T-cell %Increase Activation | Co B-cell %Increase Activation | Ni Stimulation Index | Ni T-cell %Increase Activation | Ni B-cell %Increase Activation |
|---|---|---|---|---|---|---|---|---|---|---|
| Group1 OA-Hist of Metal Sens | 1 |
**
|
*
|
*
|
87 |
45 |
57 |
8 |
9 |
3 |
| 2 |
4 |
*
|
2 |
33 |
*
|
7 |
93 |
5 |
9 |
|
| 3 |
**
|
*
|
*
|
**
|
*
|
*
|
3 |
1 |
*
|
|
| 4 |
**
|
*
|
*
|
**
|
*
|
*
|
9 |
*
|
*
|
|
| 5 |
7 |
2 |
*
|
67 |
6 |
*
|
200 |
6 |
5 |
|
| 6 |
**
|
*
|
*
|
**
|
*
|
*
|
27 |
5 |
1 |
|
| 7 |
**
|
*
|
*
|
**
|
*
|
*
|
6 |
6 |
*
|
|
| 8 |
2 |
1 |
*
|
3 |
*
|
*
|
3 |
*
|
*
|
|
| 9 |
29 |
*
|
*
|
49 |
1 |
3 |
55 |
3 |
1 |
|
| 10 |
**
|
*
|
*
|
**
|
*
|
*
|
3 |
*
|
*
|
|
| 11 |
**
|
*
|
*
|
**
|
*
|
*
|
2 |
5 |
*
|
|
| 12 | ** | * | * | ** | * | * | 2 | 3 | * | |
|
| ||||||||||
| Group2a TJA-M-on-M | 13 |
**
|
*
|
*
|
**
|
*
|
*
|
11 |
1 |
5 |
| 14 |
**
|
*
|
*
|
**
|
*
|
*
|
**
|
*
|
*
|
|
| 15 |
**
|
*
|
*
|
**
|
*
|
*
|
6 |
3 |
10 |
|
| 16 |
**
|
*
|
*
|
35 |
17 |
5 |
**
|
*
|
*
|
|
| 17 |
**
|
*
|
*
|
2 |
3 |
1 |
3 |
4 |
*
|
|
| 18 | 2 | 1 | * | ** | * | * | 2 | 1 | 2 | |
|
| ||||||||||
| Group2b TJA-Poly (pre-revision) | 19 |
**
|
*
|
*
|
**
|
*
|
*
|
2 |
5 |
*
|
| 20 |
**
|
*
|
*
|
3 |
2 |
*
|
6 |
3 |
4 |
|
| 21 |
**
|
*
|
*
|
**
|
*
|
*
|
**
|
*
|
*
|
|
| 22 |
**
|
*
|
*
|
27 |
*
|
3 |
3 |
1 |
*
|
|
| 23 |
**
|
*
|
*
|
2 |
*
|
2 |
3 |
*
|
*
|
|
| 24 |
3 |
3 |
16 |
**
|
*
|
*
|
7 |
3 |
10 |
|
| 25 |
2 |
1 |
*
|
**
|
*
|
*
|
12 |
3 |
*
|
|
| 26 |
3 |
5 |
*
|
**
|
*
|
*
|
10 |
*
|
1 |
|
| 27 |
3 |
3 |
1 |
**
|
*
|
*
|
4 |
6 |
*
|
|
| 28 |
**
|
*
|
*
|
**
|
*
|
*
|
**
|
*
|
*
|
|
| 29 |
**
|
1 |
9 |
**
|
*
|
*
|
**
|
*
|
*
|
|
| 30 |
**
|
*
|
*
|
**
|
*
|
*
|
2 |
*
|
20 |
|
| 31 |
**
|
1 |
|
**
|
*
|
*
|
**
|
2 |
|
|
| 32 |
**
|
*
|
*
|
29 |
*
|
2 |
**
|
*
|
*
|
|
| 33 |
**
|
*
|
*
|
**
|
*
|
*
|
3 |
2 |
*
|
|
| 34 |
4 |
1 |
1 |
**
|
*
|
*
|
6 |
2 |
1 |
|
| 35 |
**
|
*
|
*
|
33 |
4 |
4 |
3 |
1 |
3 |
|
| 36 |
**
|
*
|
*
|
**
|
*
|
*
|
**
|
*
|
*
|
|
| 37 |
**
|
*
|
*
|
**
|
*
|
*
|
5 |
6 |
*
|
|
| 38 | 3 | * | * | ** | * | * | 5 | 1 | * | |
 : SI > 8 = high reactivity
: SI > 8 = high reactivity
 : 8> SI > 4 = moderate reactivity
: 8> SI > 4 = moderate reactivity
 : 4 > SI > 2 = mild reactivity
: 4 > SI > 2 = mild reactivity
≤ 1% increase in actiavation over paired untreated controls
Stimulation Index (SI) < 2
Proliferation assays
Proliferation of isolated lymphocytes, also known as Lymphocyte Transformation Testing (LTT), was conducted using [3H]-thymidine (Amersham International, Arlington Heights, IL) incorporation into DNA. Other techniques such as CFSE (Carboxyfluorescein succinimidyl ester) dye using multi-color flow cytometry analysis to co-localized proliferation to cell type was not possible because of 1) the time difference of flow marker analysis (48 hours) and proliferation analysis typical of Delayed Type Hypersensitivity LTT testing (6 days), and 2) the practical limitation of cells available for testing from 48-60mLs (6× 10mL vacationers) of heparinized peripheral blood per subject. LTT proliferation assays were performed using lymphocytes cultured in 96-well cell-culture plates (Sigma) with metal treatments, at a density of approximately 0.2×106 cells/well with [3H]-thymidine (1 μCi [3H]-thymidine/well) for 48 hours in 150μL of DMEM/well, 10% FBS at 37° C and 0.5% CO2. Each metal concentration was tested in quadruplicate (4 wells/metal concentration) yielding a total of 384 samples. [3H]-thymidine was added during the last 12 hours of a 6-day incubation and was measured using liquid scintillation Beta plate analysis (Wollac Gatesburg, MD). The amount of [3H]-thymidine incorporation for each metal treatment was normalized to that of the non-treated control producing a ratio, referred to here as the stimulation index, SI (also known as the “proliferation factor”, “proliferation index”, or “proliferation ratio”). The stimulation index was calculated using measured radiation counts per minute (cpm) as follows: Stimulation Index = (mean cpm with treatment) / (mean cpm without treatment). Proliferation tests were conducted in quadruplicate. The term “metal hypersensitivity” will be used to refer to in vitro elevated proliferation and surface marker expression of activation.
Flow Cytometry
Flow cytometry was used to characterize subpopulations and activation of lymphocytes. The fluorescent markers used to characterize lymphocytes included CD4 (T-helper cells), CD19 (B-cells), CD25 (T-cell activation) and CD69 (B-cell activation). Lymphocytes (6×106 from each individual) were cultured in 48-well plates at a density of 1×106 cells/well and 1 metal challenge agent per well for 48 hours. Cells collected from each of the 6 wells/subject were stained with CD markers (following manufacturers protocol, BD Pharmingen, San Diego Ca) which were used in 4 paired combinations to determine: 1) the ratio of activated T-helper cells (CD4/CD25), and 2) the ratio of activated B-cells (CD19/CD25). A FACScan flow cytometer (BD Biosciences, San Diego, CA) was used to measure cell florescence where a total of 10,000 cells were counted per each CD marker pair. The function of surface CD marker is as follows:
CD25 is a surface receptor on T-cells also known as IL-2 R alpha, and is a precursor type I membrane protein with a signal peptide. CD25 binds IL-2 with low affinity, when by itself. However, when CD25 associates with IL-2 R beta and gamma a high affinity heterotrimeric receptor complex that transduces IL-2 signals is formed. T-regulatory cells express constitutively high amounts of CD25, but represent a small proportion of circulating T-cells (<2% in humans) and act as powerful suppressor cells that, if activated by metals, would result in decreasing overall metal induced T-cell proliferation. The role of T-regulatory cells in metal activation was beyond the scope of this investigation.
Confocal Microscopy
Confocal Microscopy (Nikon TE200 Confocal fitted, Melville, NY) was used to visualize patterns of CD3 and CD4 receptor distribution on metal-treated lymphocytes from five Ni reactive individuals selected from Groups 1 and 2. Anti-CD4/CD4 fluorescent antibodies were used to stain isolated and treated lymphocytes (BD Biosciences, Franklin Lakes, NJ) following the manufacturer's protocol. Antibodies were used to determine the pattern(s) of CD4 receptor distribution that takes place upon lymphocyte and T-cell activation to visually help identify possible mechanisms of metal induced activation (e.g. superantigen receptor clustering vs. antigen-presenting-cell dependent interactions).
Statistical Analysis
Proliferation and activation marker data were subjected to statistical analysis using Student's t-tests. Student's t-tests for independent samples with unequal or equal variances were used to test equality of the mean values at a minimum 95% confidence interval (p<0.05) allowed. A power analysis at 80% confidence was used to determine the goal for group sizes which, because of the hypothesis of this investigation was assumed to have at least a 50% difference at p<0.05, means assuming a mean value of 4 and a standard deviation of 0.5 where the group size was required to be n=12.
Results
Metal-Induced Proliferation Assays
All groups demonstrated high levels of in vitro metal hypersensitivity where 100% of Group 1 and 76% of Group 2 subjects showed hypersensitivity to Ni, Co or Cr (Table 2). Nickel induced the greatest incidence of hypersensitivity where 100% of the subjects in Group 1 and 69% of the subjects in Group 2 showed Ni hypersensitivity (SI>2). Stimulation Indices calculated from cpm's (counts per minute) were used to normalize, average and compare individual responses. Figure 1a shows the proliferation responses of all subjects tested (n=38). All metals induced an increase in proliferation where the average of all subjects was significantly above controls (p<0.05, paired t-test), where Ni proliferation was significantly greater than Cr (p<0.05, paired t-test). The high (SI>8) or low reactors in the bimodal distribution observed in subject proliferation to Co (Fig 1a) were not specific to any of the groups tested. The average lymphocyte proliferation responses of groups 1, 2a and 2b are shown in Fig 1b, where all three groups demonstrated statistically elevated proliferation (stimulation index >2) to at least 2 of the 3 metals challenge agents (Cr, Co or Ni). The highest levels of proliferation to any of the metals was found in the average responses of Group 1 subjects to Ni which are significantly greater than Group 2a and 2b (p<0.05). Intergroup comparison of lymphocyte proliferation to the positive control, PHA (a lymphocyte mitogen), revealed no statistically significant differences between any of the groups. Group 2 differed in its pattern of metal proliferation from Group 1 in that it demonstrated a higher proliferation to Co (>6 Stimulation Index) than Ni but this increase was not significantly above that of Ni or Cr. Both Groups 1 and 2 showed statistically significant increased proliferation to Ni and Co (Figure 1b), where the incidence of SI>2 metal proliferation to Ni, Co and/or Cr was 100% in Group 1 and 70% in Group 2. The averaged responses of Group 1 showed the most elevated proliferation to Ni. In contrast, Groups 2a and 2b demonstrated the highest average proliferation responses to Co (Figure 1b). Group proliferation to Ni, Co or Cr is shown in Table 2. Ni induced the greatest incidence of proliferation (Table 2), where 30 of all 32 metal reactive subjects were reactive to Nickel (SI>2).
Table 2.
Results of metal reactivity (Metal reactive criteria: SI>2, p<0.05, t-test) and activation are shown for all study subjects where degree of metal reactivity is graded from low, medium and high by shading (see legend). “% Increase Activation” indicates the percent increase over untreated controls paired for each individual. Implanted TJA materials included cobalt-alloy (Co-alloy), titanium-alloy (Ti-alloy), Bone cement (PMMA) and ultrahigh molecular weight polyethylene (PE).
| Group | Subject | IMPLANT TYPE |
Sex | IN SITU months |
Age | Implant Material |
Dermal Allergy |
Cr Stim Index |
Cr T-cell Activation |
Cr B-cell Activation |
Co Stim Index |
Co T-cell Activation |
Co B-cell Activation |
Ni Stim Index |
Ni T-cell Activation |
Ni B-cell Activation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Group1 OA-Hist of Metal Sens |
1 | F | N/A | 43 | Jewelry |
**
|
*
|
*
|
87 |
45 |
57 |
8 |
9 |
3 |
||
| 2 | F | N/A | 71 | Silver |
4 |
*
|
2 |
33 |
*
|
7 |
93 |
5 |
9 |
|||
| 3 | F | N/A | 74 | Jewelry |
**
|
*
|
*
|
**
|
*
|
*
|
3 |
1 |
*
|
|||
| 4 | F | N/A | 46 | Jewelry |
**
|
*
|
*
|
**
|
*
|
*
|
9 |
*
|
*
|
|||
| 5 | F | N/A | 62 | Ni |
7 |
2 |
*
|
67 |
6 |
*
|
200 |
6 |
5 |
|||
| 6 | F | N/A | 55 | Ni - Jewerly |
**
|
*
|
*
|
**
|
*
|
*
|
27 |
5 |
1 |
|||
| 7 | F | N/A | 58 | ZippersJewelry |
**
|
*
|
*
|
**
|
*
|
*
|
6 |
6 |
*
|
|||
| 8 | F | N/A | 69 | Jewelry |
2 |
1 |
*
|
3 |
*
|
*
|
3 |
*
|
*
|
|||
| 9 | F | N/A | 66 | Ag and Au |
29 |
*
|
*
|
49 |
1 |
3 |
55 |
3 |
1 |
|||
| 10 | F | N/A | 74 | Ni |
**
|
*
|
*
|
**
|
*
|
*
|
3 |
*
|
*
|
|||
| 11 | M | N/A | 66 | Ni |
**
|
*
|
*
|
**
|
*
|
*
|
2 |
5 |
*
|
|||
| 12 | F | N/A | 54 | Ni | ** | * | * | ** | * | * | 2 | 3 | * | |||
|
| ||||||||||||||||
|
Group2a TJA-M-on-M |
13 | THA-MMSR | M | 83 | 47 | Co alloy, PMMA |
**
|
*
|
*
|
**
|
*
|
*
|
2 |
5 |
*
|
|
| 14 | THA-bilat MM | F | N/I | NA | Co alloy, PMMA | N/I |
**
|
*
|
*
|
**
|
*
|
*
|
**
|
*
|
*
|
|
| 15 | THA-MM | M | 10 | 67 | Co alloy, PMMA |
**
|
*
|
*
|
**
|
*
|
*
|
6 |
3 |
10 |
||
| 16 | THA-MMSR | M | * | 73 | Co alloy, PMMA | effusion problems |
**
|
*
|
*
|
35 |
17 |
5 |
**
|
*
|
*
|
|
| 17 | THA-MMSR | M | 34 | 58 | Co alloy, PMMA |
**
|
*
|
*
|
2 |
3 |
1 |
3 |
4 |
*
|
||
| 18 | THA-MMSR | M | 23 | 33 | Co alloy, PMMA | 2 | 1 | * | ** | * | * | 2 | 1 | 2 | ||
|
| ||||||||||||||||
|
Group2b TJA-Poly (pre-revision) |
19 | THA | F | 14 | 70 | N/I |
**
|
*
|
*
|
**
|
*
|
*
|
11 |
1 |
5 |
|
| 20 | TKA | F | 30 | 68 | UHMWPE, PMMA |
**
|
*
|
*
|
3 |
2 |
*
|
6 |
3 |
4 |
||
| 21 | THA | F | 17 | 58 | UHMWPE, PMMA | Jewelry |
**
|
*
|
*
|
**
|
*
|
*
|
**
|
*
|
*
|
|
| 22 | TKA | F | N/I | 50 | Co and Ti alloys, PE |
**
|
*
|
*
|
27 |
*
|
3 |
3 |
1 |
*
|
||
| 23 | TKA | F | N/I | 35 | Co and Ti alloys, PE |
**
|
*
|
*
|
2 |
*
|
2 |
3 |
*
|
*
|
||
| 24 | TKA | F | 7 | 64 | UHMWPE, PMMA |
3 |
3 |
16 |
**
|
*
|
*
|
7 |
3 |
10 |
||
| 25 | TKA | F | 10 | 77 | UHMWPE, PMMA |
2 |
1 |
*
|
**
|
*
|
*
|
12 |
3 |
*
|
||
| 26 | TKA | F | 18 | 53 | Co and Ti alloys, PE |
3 |
5 |
*
|
**
|
*
|
*
|
10 |
*
|
1 |
||
| 27 | TKA | F | 25 | 72 | N/I |
3 |
3 |
1 |
**
|
*
|
*
|
4 |
6 |
*
|
||
| 28 | TKA | F | 10 | 64 | Co and Ti alloys, PE |
**
|
*
|
*
|
**
|
*
|
*
|
**
|
*
|
*
|
||
| 29 | TKA | M | 9 | 66 | Co and Ti alloys, PE |
**
|
1 |
9 |
**
|
*
|
*
|
**
|
*
|
*
|
||
| 30 | TKA | M | 10 | 66 | Co and Ti aloys, PE |
**
|
*
|
*
|
**
|
*
|
*
|
2 |
*
|
20 |
||
| 31 | TKA | M | 52 | 55 | UHMWPE, PMMA |
**
|
1 |
|
**
|
*
|
*
|
**
|
2 |
|
||
| 32 | TKA | F | 12 | 49 | UHMWPE, PMMA |
**
|
*
|
*
|
29 |
*
|
2 |
**
|
*
|
*
|
||
| 33 | TKA | F | 18 | 40 | UHMWPE, PMMA | Jewelry |
**
|
*
|
*
|
**
|
*
|
*
|
3 |
2 |
*
|
|
| 34 | TKA | F | 60 | 50 | UHMWPE, PMMA | Ni +2 Patch Test |
4 |
1 |
1 |
**
|
*
|
*
|
6 |
2 |
1 |
|
| 35 | TKA | F | 19 | 60 | N/I | Jewelry, Glasses |
**
|
*
|
*
|
33 |
4 |
4 |
3 |
1 |
3 |
|
| 36 | TKA | F | 6 | 67 | UHMWPE, PMMA | Jewelry |
**
|
*
|
*
|
**
|
*
|
*
|
**
|
*
|
*
|
|
| 37 | TKA | F | 20 | 40 | UHMWPE, PMMA | Ni, Jewelry |
**
|
*
|
*
|
**
|
*
|
*
|
5 |
6 |
*
|
|
| 38 | TKA | F | N/I | N/I | N/I | N/I | 3 | * | * | ** | * | * | 5 | 1 | * | |
 : SI > 8 = high reactivity
: SI > 8 = high reactivity
 : 8> SI > 4 = moderate reactivity
: 8> SI > 4 = moderate reactivity
 : 4 > SI > 2 = mild reactivity
: 4 > SI > 2 = mild reactivity
Surface Marker Activaiton = ≤ 1% increase in surface marker expresstion of actiavation over paired untreated controls
Proliferation = Stimulation Index (SI) < 2
NA=Not Applicable : none=No Information Available
MMSR=Metal on metal surface replacement, THA=total hip arthroplasty, TKA=total knee arthroplasty, Bilat-MM=Bilateral metal on metal total hip arthplast
Figure 1.
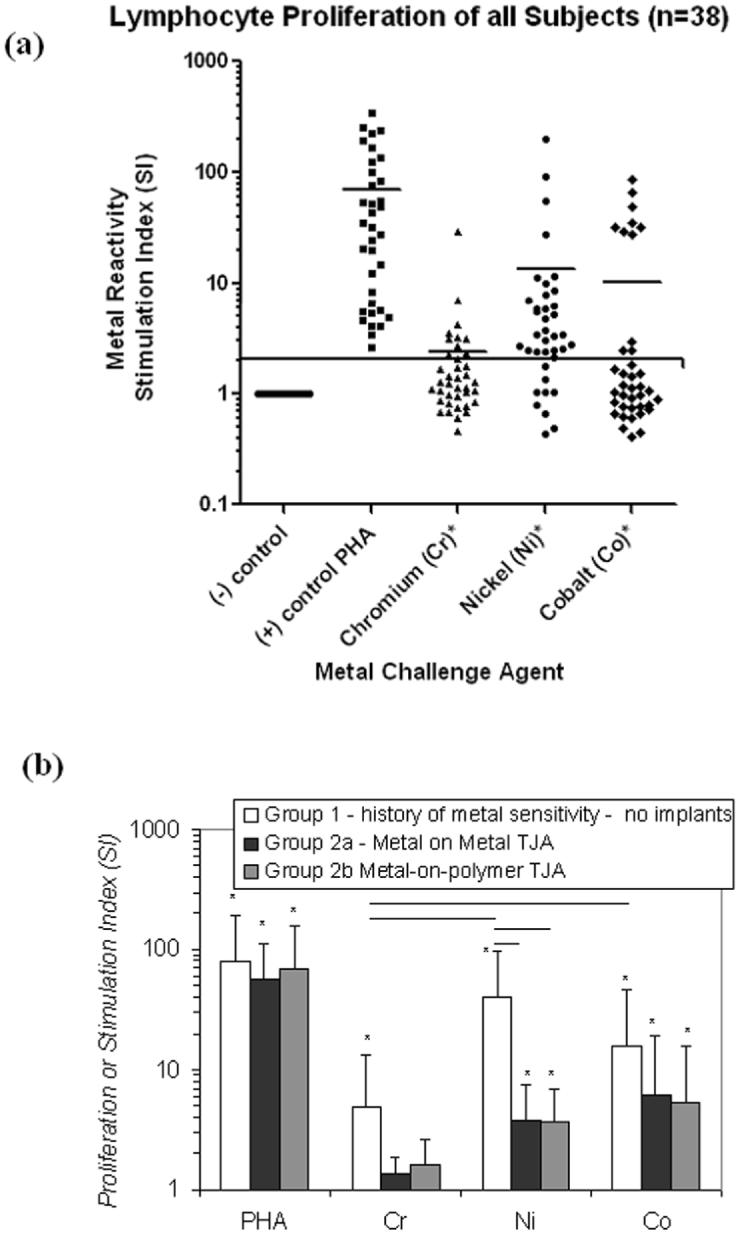
(1a) All subjects proliferation values in responses to Cr, Ni and Co are shown, where an SI >2 was considered positive for metal reactivity. Reactivity to Co is demonstrably bimodal with high or low reactors that are not subject of any one group tested but are comprised of Groups 1 and 2. Negative controls are shown as a line at SI=1 to underscore that the stimulation index represents normalized data that facilitates compilation, group comparison and comparison to threshold values of high proliferation (e.g. SI>8 = high reactivity) of different individuals within a group. However, statistical comparison was based on paired t-testing of raw cpm proliferation data. (1b) The averaged proliferation responses of metal challenged subjects in Groups 1 and 2 are shown. There were no intergroup differences in reactivity to PHA (a lymphocyte mitogen). Group 1 subjects with THA demonstrated elevated reactivity to Cr (p<0.05) with respect to Group 2a and 2b. Group 1 subjects demonstrated significantly elevated responses to Ni (p<0.02) with respect to Group 2a and 2b. Note: An * = p<0.05, t-test compared to untreated controls.
Lymphocyte Activation- Flow Cytometry
The expression of surface markers CD25 and CD69 were used to measure the amount of T-cell and B-cell activation, respectively. The activation of T-cells and B-cells to each of the metal challenge agents (Cr, Co, Ni and PHA) was measured using flow cytometry for each subject at the relatively early-activation time point of 48 hours where the surface marker expression of CD25 or CD69 in 10,000 cells was measured to calculate the % of activated T-cells or B-cells. Flow cytometry testing of individuals was conducted on separate days, thus calibration of the instrument varied from day to day such that gating to positive controls of CD4+ FITC and PE yielded different gate settings on different days. This resulted in surface marker activation of untreated cells (negative controls) remaining in the range of approximately 2-3%. Activation by metal challenge agents were measured using the magnitude of the increase above negative control activation for that same individual, facilitating comparison between individuals with different negative control levels of activation. These results showed a person dependent pattern of T-cell and/or B-cell activation (Figures 2, 3 and 4). Three individual subjects are used to highlight the different types of T-cell and B-cell activation found to be associated with proliferation. Figure 2 shows both the proliferation and flow cytometry results of the most reactive subject tested (i.e. with an over 80 fold proliferation response to Co) who demonstrated both T-cell (47%) and B-cell (59%) activation in response to cobalt challenge. Subject B (Figure 3) demonstrated a T-cell dominated response (19% activation T-cell to 5% B-cell activation, SI>40). This is in contrast to the results in Figure 4 of Subject C where Ni and Co proliferation responses are associated with B-cell dominated expression of cell surface activation markers. The correlation of activation of all subjects CD4+ T-cells vs. CD19+ B-cells with the level of metal induced proliferation is shown in Figure 5. This scatter graph shows the order of magnitude increase in the PHA induced activation of T-cells and B-cells when compared to metal induced activation (CD25+ and CD69+). The % activations of metal-challenged T-cells and B-cells of all subjects are shown in Figure 5, where the average of all metal treated T-cells and B-cells are above that of negative (untreated) controls and where 2-3 individuals showed activation levels as high the positive control (PHA), a powerful lymphocyte mitogen. T-cell activation induced by Co, Cr and Ni was high enough and consistent enough to be significantly (p<0.05, t-test) increased over controls. B-cell activation induced by Ni alone was significantly elevated over controls (p<0.05, t-test). Nickel induced the greatest level of activation and was significantly elevated over Cr activation (p<0.01, t-test). The average activation of untreated controls was 3% for T-cells and B-cells (Fig 5); thus a 2 fold increase in metal-induced activation required >6% T-cell or B-cell activation. This >2 fold activation index was used as a conservative measure to calculate the incidence of positive activation within Groups 1 and 2 (threshold conservatively based on guidelines previously established for proliferation testing).16, 26, 36, 37, 39 There was no significant difference in metal-specific T-cell or B-cell surface marker expression of activation between Groups 1 and 2 or Groups 1, 2a and 2b.
Figure 2.
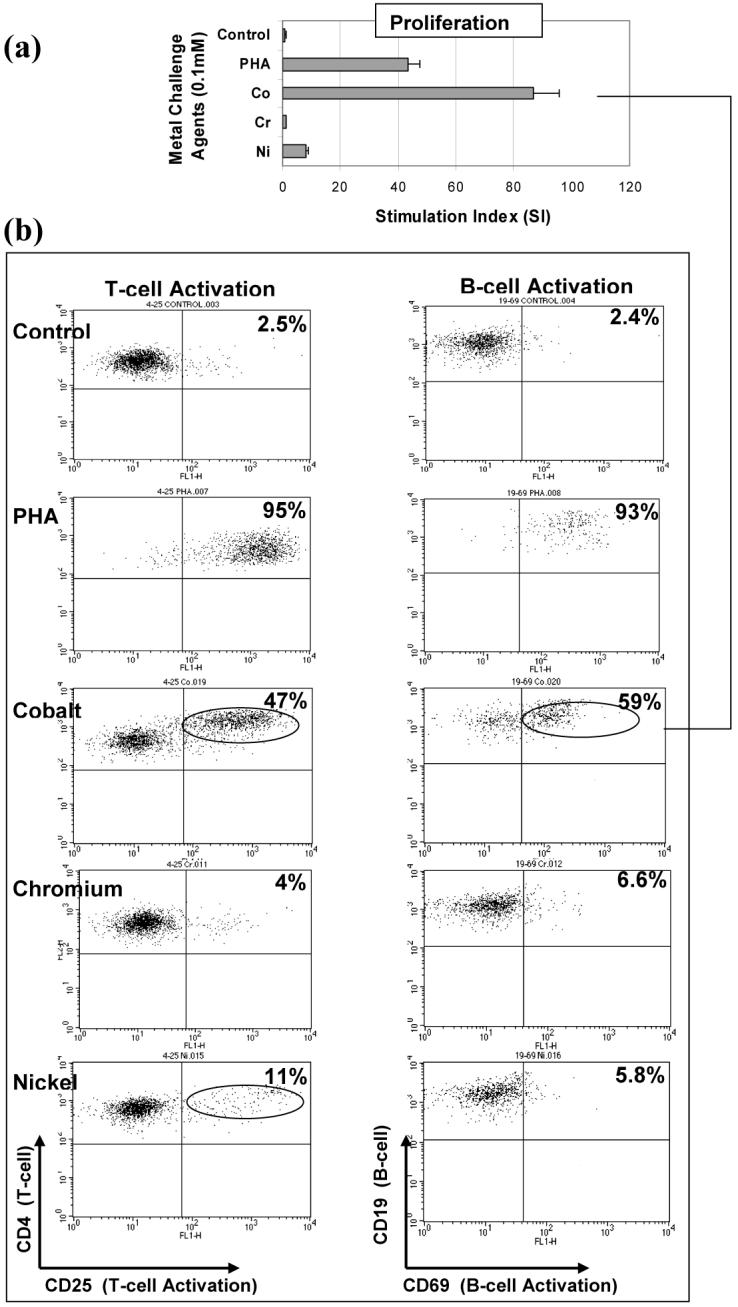
T-cell and B-cell activation: Flow cytometric analysis of a highly Co reactive subject (SI>40) is shown to contain both T-cell (47% CD4+CD25+) and B-cell (59% CD19+CD69+) activation at 48 hours. Control activation for non-challenged T-cells and B-cells from this subject was below 2.5%. An increase in T-cell and B-cell activation is also demonstrated with Ni where a 11% and 6% increase in activation, respectively, is associated with a SI>5. Subject A: Group 1, 43 year old female with a history of dermal sensitivity to jewelry.
Figure 3.
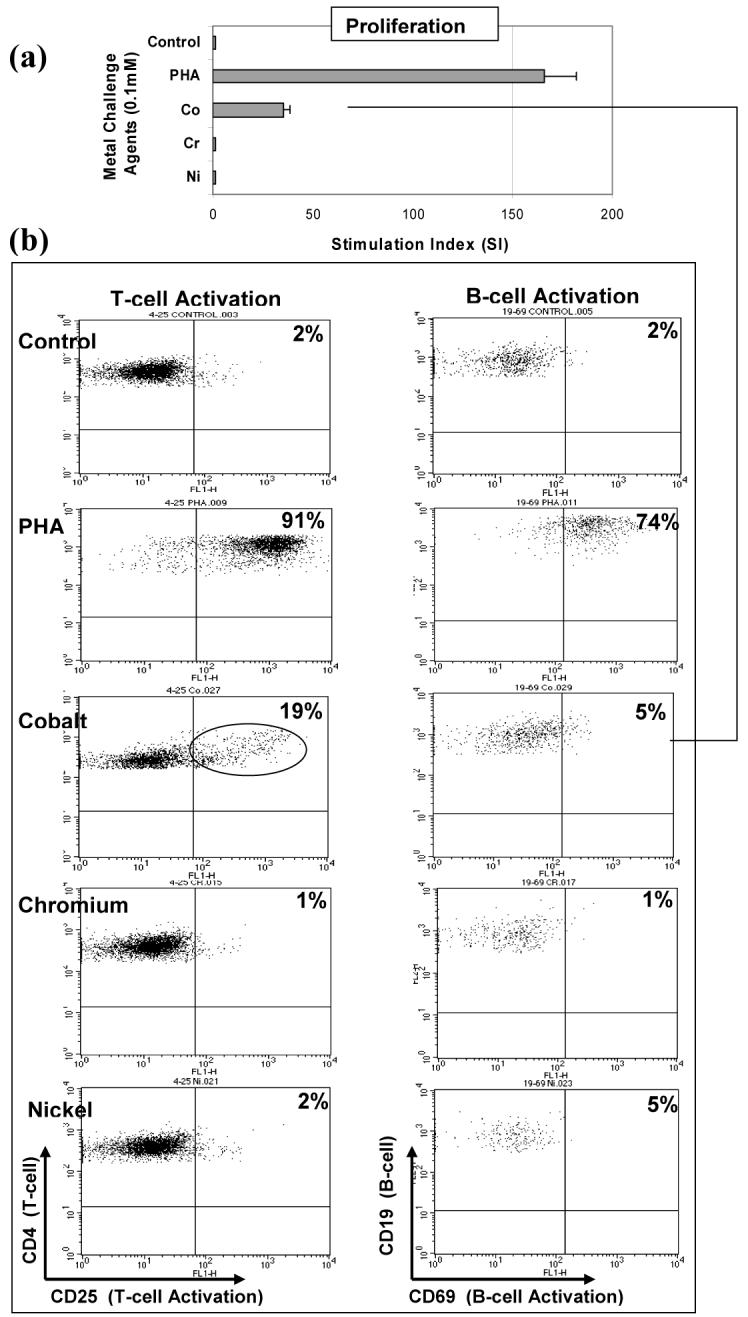
T-cell activation: Flow cytometric analysis of a highly Co reactive subject (SI>30) subject is shown to contain high T-cell (19% CD4+CD25+) activation and low B-cell (5% CD19+CD69+) activation at 48 hours. Control activation of for non-challenged T-cells and B-cells from this subject was below 2%. Subject B: Group 2b 73 year male with a metal-on-metal total hip arthroplasty surface replacement well performing, but had a history of effusions problems associated with the implant.
Figure 4.
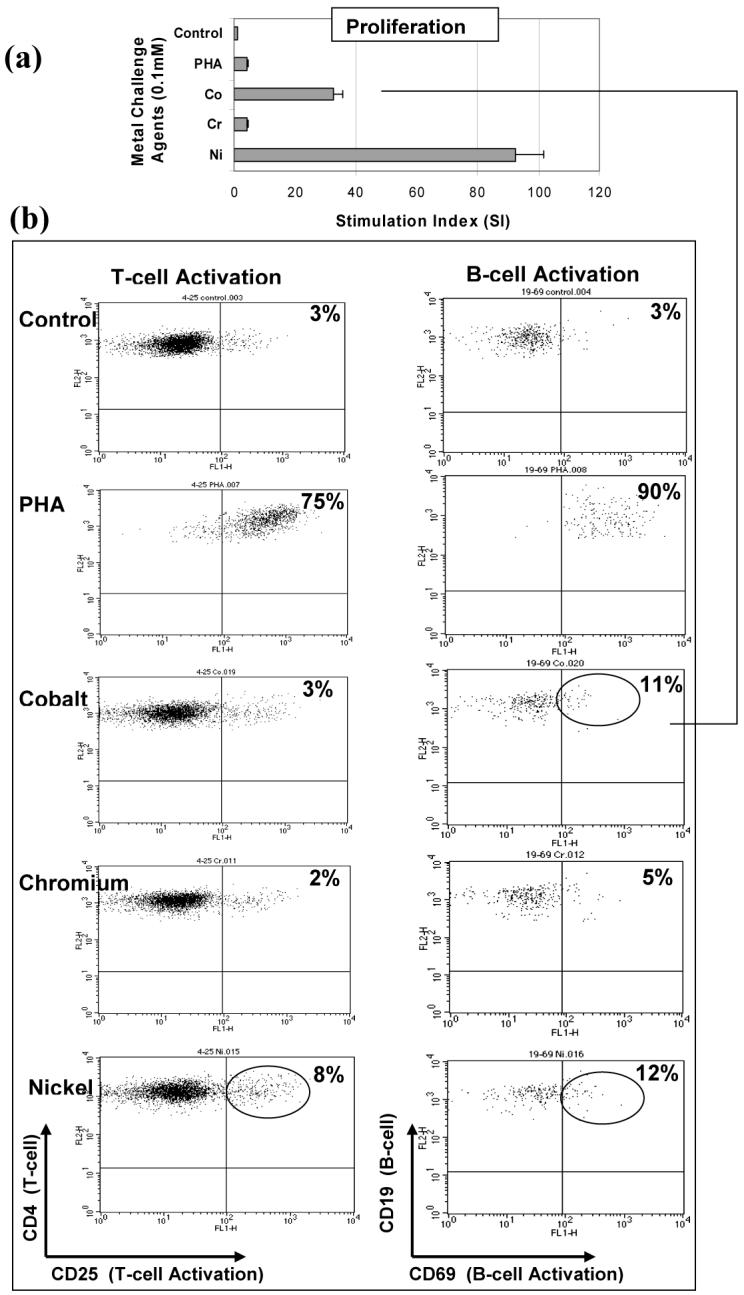
B-cell activation: Flow cytometric analysis of a highly Co reactive subject (SI>30) is shown to contain high B-cell (11% CD4+CD25+) activation and no increase in T-cell (3% CD19+CD69+) activation at 48 hours. Control activation for non-challenged T-cells and B-cells from this subject was below 3%. An increase in T-cell and B-cell activation is also demonstrated with Ni where a 8% and 12% increase in activation, respectively, is associated with a SI>90. Subject C: Group 1, 71 year old female with a history of dermal hypersensitivity to nickel and silver.
Figure 5.
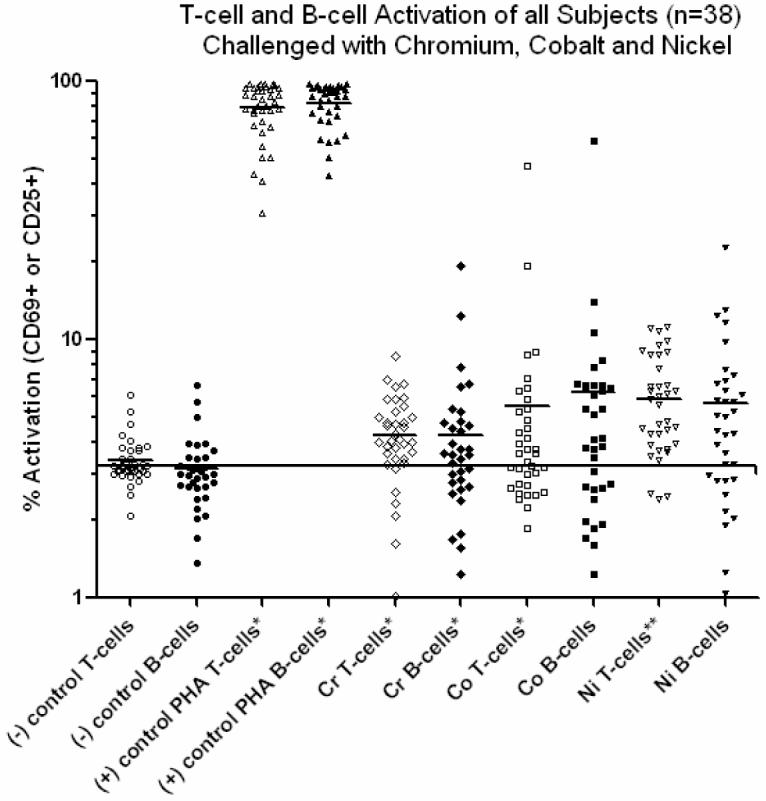
All subjects activation values in responses to Cr, Ni and Co are shown, where an activation level >6% represented a >2 fold over unchallenged controls. The horizontal line represents the average of the control values, approx 3%. Reactivity to Cr, Co and Ni demonstrated levels of activation that were significantly above controls for both T-cells and B-cells. There were no significant differences in the elevated levels of reactivity to Cr, Co and Ni. Additionally, there were no intergroup (Group 1 vs. Group 2) differences in reactivity to the metal challenge agents (a lymphocyte mitogen). Note: An * = p<0.005, paired t-test compared to untreated controls. An ** = p<0.06, paired t-test compared to untreated controls.
Among the n=32 metal reactive subjects (SI>2) in Groups 1 and 2, there were 22 subjects who demonstrated >2 fold activation to metal challenge above that of controls (i.e. >6% increase above unchallenged self-controls expression of CD25 or CD69 per 10,000 cells). However, only 18 subjects of these 22 demonstrated an exclusively T-cell or B-cell activation response to Co, Cr or Ni challenge, where 6 of these 18 subjects demonstrated a B-cell only activation response (>fold) and 12 of 18 demonstrated a T-cell only >2 fold activation response to Co, Cr or Ni. 9 of 22 subjects with >2 fold activation showed a T-cell and B-cell >2 fold activation to challenge with Co, Cr or Ni (Table 2).
Ni and Co induced the greatest incidence of T-cell and/or B-cell activation: where 18 of 29 “Ni reactive” (SI>2) subjects and 7 of 11 “Co reactive” subjects had >2 fold increase in T-cell and/or B-cell activation over subject matched negative control activation. Only 3 of 11 “Cr reactive” subjects demonstrated a >2 fold increase in activation of T-cells and/or B-cells. Of the 29 Ni-reactive subjects, 9 demonstrated only T-cell activation, 3 demonstrated only B-cell activation, 6 demonstrated T-cell and B-cell activation, and 9 demonstrated neither T-cell nor B-cell activation at 48 hours after challenge with Ni (Table 2). Of the 11 Co reactive subjects, 3 demonstrated only T-cell activation, 3 demonstrated only B-cell activation, 3 demonstrated T-cell and B-cell activation, and 3 demonstrated neither T-cell nor B-cell activation at 48 hours after challenge with Co (Table 2). Of the 11 Cr reactive subjects, 1 demonstrated only T-cell activation, 0 demonstrated only B-cell activation, 1 demonstrated T-cell and B-cell activation, and 9 demonstrated neither T-cell nor B-cell activation at 48 hours after challenge with Cr (Table 2).
Confocal Microscopy
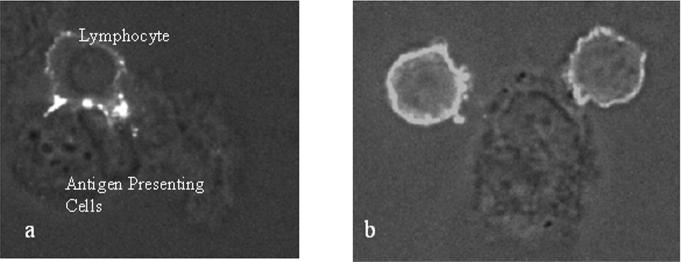
Metal treated lymphocytes from five selected Ni-reactive subjects demonstrated two mechanisms of activation, using the distribution of anti-CD3 and anti-CD4 fluorescently labeled T-cell receptors. Examples of T-cell and antigen presenting cell contact under control (non-activated) and metal-activated conditions are shown in Figures 6, 7 and 8. In these figures antigen presenting cells (APCs) are characteristic of PBMC derived monocytes which have differentiated into monocyte-macrophages during the course of the 48 hour in vitro testing. B-cells (CD3+ or CD19+) were not observed as APC in contact with T-cells. Figure 8 demonstrates the less common occurrence of individual T-cell receptor clustering into discrete node-like patterns or clustering onto a single side of the lymphocyte (so called capping) on the T-cell surface, independent of an APC. T-cell/antigen presenting cell coupled T-cell activation was the dominant mechanism detected visually and was associated with T-cell activation, only <10% of 100 visually identified activated T-cells were involved in APC independent clustering per subject.
Figure 6.

3-D reconstruction and midsection confocal micrographs of CD4 antibody-labeled lymphocyte/macrophage interactions under conditions of (a, b) 0.1 mM nickel chloride, 48 hour Ni challenge and (c, d) control untreated conditions. Images (a and c) are three-dimensional reconstructions of 50 fluorescent confocal microscope serial image slices through the lymphocyte-macrophage complex. Mid-section image slices (of a and c) are depicted in b and d. Note CD4+ clustering at the immunologic synapse and up-regulated expression of T cell receptor complexes (CD4) in the Ni challenged lymphocyte. The CD4 containing tendrils of the CD4+ T cells cover the surface of the antigen presenting cell (monocyte-macrophage) during metal activation, facilitating visualization of the entire antigen presenting cell surface. This “tendril coverage” is not observed with the unchallenged control lymphocyte-antigen presenting cell complex (c and d).
Figure 7.
Antigen presenting cell/lymphocyte mediated metal activation: Images of CD3 antibody labeled lymphocyte/macrophage interactions under conditions of (a) Ni challenge and (b) control untreated conditions. Images (a) and (b) are combined phase contrast bright-field and fluorescent confocal microscope images to visualize the CD3 labeled lymphocytes interacting with the macrophages. (a) Primary human lymphocyte/macrophage interactions under Ni challenge (0.1mM) demonstrate clustering and up-regulated expression of T cell receptor complexes at the macrophage/lymphocyte border. (b) Unchallenged control lymphocytes in contact with a macrophage do not demonstrate redistribution of the CD3 T-cell receptors to the macrophage-lymphocyte synapse.
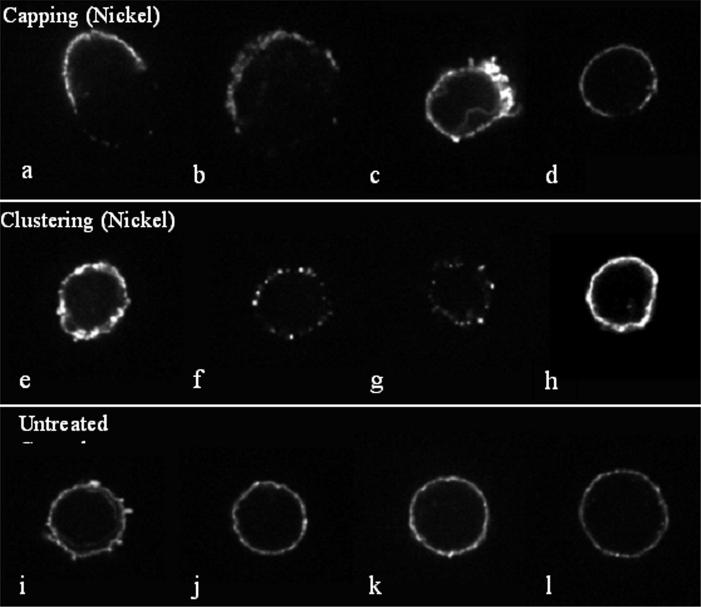
Figure 8.
Metal induced effects on T-cell receptor clustering of lymphocytes show a variety of patterns. Confocal microscopy of Ni challenged (0.1 mM, 48 hour exposure) and control untreated lymphocytes stained with anti-CD4 are shown in cluster pattern (indicative of activation) independent of antigen presenting cells. Four examples of each pattern are shown. (a-d) Metal treated lymphocytes (0.1 mM Ni) are shown with antigen-induced receptor redistribution during activation termed “capping”. (e-h) Metal challenged lymphocytes (0.1 mM Ni, 48 hour exposure) show clustering of lymphocyte CD4-containing receptors into node-like segments, characteristic of superantigen-like cross-linking of surface receptors. (i-l) Untreated control lymphocytes are shown with their characteristic smooth distribution of CD4 containing receptors.
Discussion
Lymphocyte analysis of primary human peripheral blood mononuclear cells did not demonstrate a correlation between in vitro lymphocyte proliferation to metal challenge and T-cell activation (CD69+ or CD25+). Thus, our results did not support our original hypothesis that early lymphocyte expression of activation is exclusively T-cell mediated. Only 12 of 32 metal-reactive subjects (Stimulation Index >2) demonstrated T-cell activation exclusively. Four conditions were all shown to be associated with metal induced PBMC proliferation to one (or more) of the metals tested: 1) exclusive T-cell surface expression of activation markers, 2) exclusive B-cell activation, 3) both T-cell and B-cell surface expression of activation markers, and 4) neither T-cell or B-cell surface expression of activation markers. This heterogeneity of expression of surface activation marker responses indicates that T-cells and/or B-cells can be associated with in vitro metal hypersensitivity. The activation of B-cells without commiserate T-cell activation suggests a potential role for B-cells in eliciting metal reactivity in vitro and thus may further complicate mitigating and diagnosing metal hypersensitivity responses in some individuals.
The subjects tested did not have unexpectedly high incidence of in vitro metal hypersensitivity given that the subject groups were selected based on their high likelihood for predisposition for metal hypersensitivity, based on past studies.34,36, 37 Group 1 individuals with a history of dermal metal sensitivity were found to be 100% (12 of 12) metal reactive to Ni. Group 2a individuals with well functioning metal-on-metal implants were found to be 83% (5 of 6) metal reactive (to Ni, Co or Cr) and Group 2b subjects with a poorly functioning metal-on-poly TJA (pre-revision surgery) were found to be 75% (15 of 22) metal reactive (to Co, Cr or Ni). These incidences of in vitro assayed metal hypersensitivity are slightly higher than incidence levels previously reported for similar groups.34, 36, 37 The general prevalence of dermal metal sensitivity among patients with well functioning and poorly functioning total joint replacements implants has been reported to be approximately 25% and 60%, respectively.34 Past investigations of patients with metal-on-metal implants using patch testing have found similarly high incidences of sensitivity from 10% to 74% with an average of approximately 46% incidence (subject-weighted average of 6 previous investigations with n=157 subjects cumulatively),7, 23-25, 34, 36, 37, 58 whereas the prevalence of dermal metal sensitivity among the general population is approximately 10%.34
Group 1 (subjects with a history of metal sensitivity and no implant) demonstrated the highest proliferative hypersensitivity to Ni (p<0.05), whereas Group 2 individuals with TJAs were most proliferative to Co (p<0.05), the major metal within their TJA implants. This indicates that priming or sensitization may occur in vivo, however, the expression profile of surface markers of activation of T-cells or B-cells was not significantly different from Group 1 individuals without implants (Figure 1b).
The practical limitations necessitating a limited time-point study of B-cell and T-cell metal-induced activation (at 48 hours) in metal-reactive individuals reflects only an early cross-section of many cumulative events, which includes late and early competing and cooperative immune cell dynamics (e.g. mechanisms of tolerance, synergy, APC activation, individual dependent B-cell to T-cell ratio, etc.). Practical limitations associated with the amount of blood draw (6 × 10mL vacutainers) did not facilitate the analysis of multi-time point activation analysis. Thus 48 hours was chosen based on prior previous reports.28, 63, 64, 71 However, the goal of this investigation was to link more causal “early” T-cell or B-cell activation, with “late” proliferation responses. Thus an important finding of this study was that metal-hypersensitivity as detected by proliferation assay is not well correlated with the T-cell and/or B-cell surface expression of activation markers using a single 24 hour time point.60 This indicates that there may be limits on the utility of flow cytometry analysis in the diagnosis of metal hypersensitivity given the variability in relatively upstream activation of surface marker expression when compared to proliferation.
Our results of both T-cell and B-cell activation associated with hypersensitivity are novel in that they show a mixed picture of early activation, i.e. no ubiquitous exclusive early T-cell activation and some cases of exclusive B-cell activation (5 of 32 metal-reactive subjects). These findings are consistent with past studies that have shown abnormalities in circulating white blood cell populations associated with loose implants49 and metal-on-metal implants41. Hart et al41 reported that in patients with metal-on-metal implant (surface replacements) a threshold level of circulating cobalt and chromium ions was significantly correlated with reduced CD8+ T-cell counts.41 However, other studies of metal sensitive individuals without implants or heterogeneous populations of orthopedic patients3, 9 have not shown such altered circulating immune cell function to exposure correlations. This demonstrates that alterations in circulating immune cell (e.g. T-cell or B-cell) number or activity is likely a subtle phenomenon that requires either more specific subject groups and analysis41 and/or requires amplification of any altered immune reactivity using in vitro challenge and assay, such as the current study.
The amount of metal induced T-cell proliferation did not generally correlate (R2<0.5, p<0.05) with the amount of T-cell or B-cell surface marker expression of activation levels. This lack of correlations suggests a de-coupled more generalized mechanism of “polyclonal-like” metal induced adaptive immune reactivity. This more generalized respones was observed with confocal microscopy (Figs 6, 7 and 8), i.e. both classical antigen presentation by antigen-presenting cells and superantigen-like T-cell receptor clustering. This is surprising given that it has been well established that T-cell activation will result in proliferation and non-antigen dependent activation of B-cells.60, 65 This underscores the utility of downstream assays such as proliferation testing (LTT) for diagnosis and the inability of shorter-term flow analysis of activation using flow cytometry to indicate in vitro metal reactivity. This is important, as proliferation testing has been reported to show similar incidence of metal reactivity as that of dermal patch testing.19, 26, 61, 66, 69, 70
Metal induced reactivity associated with APC independence is consistent with past reports by Gamerdinger et al 28, 72 that show metals such as Ni can act in a superantigen-like manner. In this case normal antigen processing is not required to activate lymphocytes. This superantigen-like enhancement of T cell receptor-protein can occur when metals act as facilitating agents in the crosslinking of receptors (e.g. VB17 of CDR1 T-cell receptor and others CD3, CD4 and CD45).32, 33, 48, 57, 59, 72, 73 Despite these reports of APC independent antigen processing for activating T-cells, there is a general consensus implicating the T cell receptor (MHCII and TCR) in metal induced activation.32, 33, 48
While the majority of early activation responses involved elevated T-cell activation (CD25+), the activation of B-cells (CD69+) without commiserate T-cell activation suggests a potential role for B-cells in eliciting and mediating metal reactivity in some individuals. End point proliferation assay results (LTT) showed greater utility for the diagnosis of metal reactivity than did flow cytometry analysis of T-cell or B-cell activation at early time points.
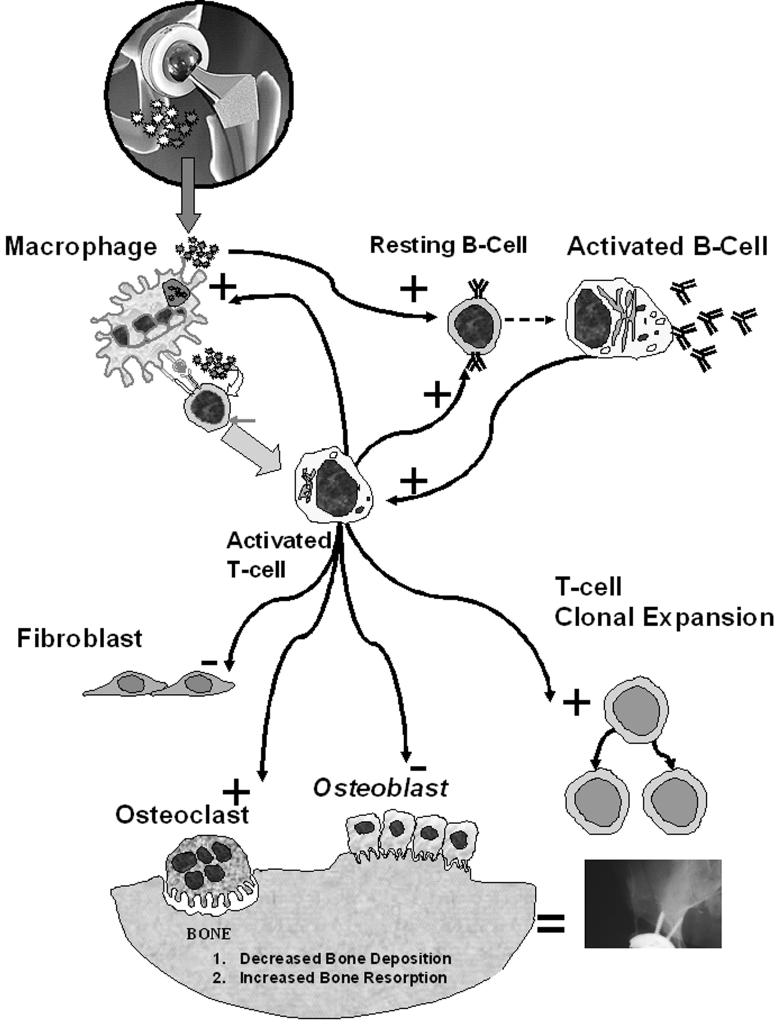
Because lymphocytes reside within the periprosthetic space it is likely that metal-induced lymphocyte proliferation may contribute to the cascade of events leading to osteoclastogenesis2, 21, 52 osteolysis and aseptic loosening (Figure 9). The high incidence of reactivity within the groups of subjects with implants provides evidence that adaptive cell mediated immunity responses likely play a role in the etiology of osteolysis in susceptible individuals. However, without the linkage between adaptive hypersensitivity reactions and bone loss (e.g. osteoclastogenesis) this remains incomplete. Prospective, longitudinal clinical studies combined with in vitro mechanistic studies will provide the basis for assessing the clinical relevance of this phenomenon and will provide the requisite knowledge for potential prophylactic and therapeutic interventions for implant related metal hypersensitivity responses.
Figure 9.
A variety of cell types in the periprosthetic space can be affected by T-cell and B-cell activation and/or reactivity resulting from a response to metal implant debris (where stimulation and inhibition of function are shown by “+“ and “-”, respectively). All cell types within the periprosthetic space participate in the cascade of events that result in osteolysis and aseptic loosening.
Acknowledgments
We would like to thank the NIH/NIAMS, the Crown Family Chair of Orthopedics, and the Rush Arthritis and Orthopaedics Institute for support in conducting this research.
Reference List
- 1.Abdallah HI, Balsara RK, O'Riordan AC. Pacemaker contact sensitivity: Clinical recognition and management. Annals of Thoracic Surgery. 1994;57:1017–1018. doi: 10.1016/0003-4975(94)90228-3. [DOI] [PubMed] [Google Scholar]
- 2.Abu-Amer Y, Abbas S, Hirayama T. TNF receptor type 1 regulates RANK ligand expression by stromal cells and modulates osteoclastogenesis. J Cell Biochem. 2004;93:980–989. doi: 10.1002/jcb.20197. [DOI] [PubMed] [Google Scholar]
- 3.Al-Tawil NG, Marcusson JA, Moller E. T and B lymphocytes in patients with nickel sensitivity. Scand J Immunol. 1985;22:495–502. doi: 10.1111/j.1365-3083.1985.tb01908.x. [DOI] [PubMed] [Google Scholar]
- 4.Angle C. Organ-specific Therapeutic Intervention. In: Goyer RA, Klaassen CD, Waalkes MP, editors. Metal Toxicology. Academic Press; New York: 1995. pp. 71–110. [Google Scholar]
- 5.Barranco VP, Solloman H. Eczematous dermatitis from nickel. Journal of the American Medical Association. 1972;220:1244. [PubMed] [Google Scholar]
- 6.Basketter DA, Briatico-Vangosa G, Kaestner W, Lally C, Bontinck WJ. Nickel, cobalt and chromium in consumer products: a role in allergic contact dermatitis? Contact Dermatitis. 1993;28:15–25. doi: 10.1111/j.1600-0536.1993.tb03318.x. [DOI] [PubMed] [Google Scholar]
- 7.Benson MK, Goodwin PG, Brostoff J. Metal sensitivity in patients with joint replacement arthroplasties. British Medical Journal. 1975;4:374–375. doi: 10.1136/bmj.4.5993.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berger RA, Quigley LR, Smink D, Sheinkop M, Jacobs JJ, Rosenberg AG, Galante JO. Factors contributing to osteolysis and failure in primary cementless THA. Orthop Trans. 1999;22:129. [Google Scholar]
- 9.Bjurholm A, al-Tawil NA, Marcusson JA, Netz P. The lymphocyte response to nickel salt in patients with orthopedic implants. Acta Orthop Scand. 1990;61:248–250. doi: 10.3109/17453679008993510. [DOI] [PubMed] [Google Scholar]
- 10.Black J. Orthopaedic Biomaterials in Research and Practice. Churchill Livingstone; New York: 1988. [Google Scholar]
- 11.Black J. Systemic effects of biomaterials. Biomater. 1984;5:12–17. doi: 10.1016/0142-9612(84)90061-9. [DOI] [PubMed] [Google Scholar]
- 12.Brown GC, Lockshin MD, Salvati EA, Bullough PG. Sensitivity to metal as a possible cause of sterile loosening after cobalt-chromium total hip-replacement arthroplasty. J Bone Joint Surg Am. 1977;59-A:164–168. [PubMed] [Google Scholar]
- 13.Caicedo M, Jacobs JJ, Reddy A, Hallab NJ. Analysis of metal ion-induced DNA damage, apoptosis, and necrosis in human (Jurkat) T-cells demonstrates Ni(2+) and V(3+) are more toxic than other metals: Al(3+), Be(2+), Co(2+), Cr(3+), Cu(2+), Fe(3+), Mo(5+), Nb(5+), Zr(2+) J Biomed Mater Res A. 2007 doi: 10.1002/jbm.a.31789. [DOI] [PubMed] [Google Scholar]
- 14.Caicedo M, Reddy A, Jacobs JJ, Hallab NJ. Characterizing lymphocyte reactivity to molybdenum. Trans 53rd Orthopedic Research Society. 2007;53:328. [Google Scholar]
- 15.Caicedo M, Reddy A, Samee I, Jacobs JJ, Hallab N. Cobalt Ions and Co-Cr-Mo alloy particles induce human monocyte co-stimulatory molecules CD-86, ICAM-1 and the cytokine IL-8: Implications for innate activation of adaptive immune responses. 6th Combined Meeting of the Orthopaedic Research Societies.2007. p. 535. [Google Scholar]
- 16.Carando S, Cannas M, Rossi P, Portigliatti-Barbos M. The lymphocytic transformation test (L.T.T.) in the evaluation of intolerance in prosthetic implants. Ital J Orthop Traumatol. 1985;11:475–481. [PubMed] [Google Scholar]
- 17.Carlsson AS, Macnusson B, Moller H. Metal sensitivity in patients with metal-to-plastic total hip arthroplasties. Acta Orthop Scand. 1980;51:57–62. doi: 10.3109/17453678008990769. [DOI] [PubMed] [Google Scholar]
- 18.Carpenter RR. In vitro studies of cellular hypersensitivity. J Immun. 1963;91:803–818. [PubMed] [Google Scholar]
- 19.Cederbrant K, Hultman P, Marcusson JA, Tibbling L. In vitro lymphocyte proliferation as compared to patch test using gold, palladium and nickel. Int Arch Allergy Immunol. 1997;112:212–217. doi: 10.1159/000237456. [DOI] [PubMed] [Google Scholar]
- 20.Christiansen K, Holmes K, Zilko PJ. Metal sensitivity causing loosened joint prostheses. Ann Rheum Dis. 1980;39:476–480. doi: 10.1136/ard.39.5.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clohisy JC, Yamanaka Y, Faccio R, Abu-Amer Y. Inhibition of IKK activation, through sequestering NEMO, blocks PMMA-induced osteoclastogenesis and calvarial inflammatory osteolysis. J Orthop Res. 2006;24:1358–1365. doi: 10.1002/jor.20184. [DOI] [PubMed] [Google Scholar]
- 22.Cramers M, Lucht U. Metal sensitivity in patients treated for tibial fractures with plates of stainless steel. Acta Orthopedica Scandinavia. 1977;48:245–249. doi: 10.3109/17453677708988763. [DOI] [PubMed] [Google Scholar]
- 23.Deutman R, Mulder TH, Brian R, Nater JP. Metal sensitivity before and after total hip arthroplasty. J Bone Joint Surg [Am] 1977;59-A:862–865. [PubMed] [Google Scholar]
- 24.Elves MW, Wilson JN, Scales JT, Kemp HB. Incidence of metal sensitivity in patients with total joint replacements. British Medical Journal. 1975;4:376–378. doi: 10.1136/bmj.4.5993.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans EM, Freeman MA, Miller AJ, Vernon-Roberts B. Metal sensitivity as a cause of bone necrosis and loosening of the prosthesis in total joint replacement. The Journal of Bone and Joint Surgery. 1974;56-B:626–642. doi: 10.1302/0301-620X.56B4.626. [DOI] [PubMed] [Google Scholar]
- 26.Everness KM, Gawkrodger DJ, Botham PA, Hunter JA. The discrimination between nickel-sensitive and non-nickel-sensitive subjects by an in vitro lymphocyte transformation test. Br J Dermatol. 1990;122:293–298. doi: 10.1111/j.1365-2133.1990.tb08276.x. [DOI] [PubMed] [Google Scholar]
- 27.Fisher AA. Allergic dermatitis presumably due to metallic foreign bodies containing nickel or cobalt. Current Contact News. 1977;19:285–295. [PubMed] [Google Scholar]
- 28.Gamerdinger K, Moulon C, Karp DR, Van BJ, Koning F, Wild D, Pflugfelder U, Weltzien HU. A new type of metal recognition by human T cells: contact residues for peptide-independent bridging of T cell receptor and major histocompatibility complex by nickel. J Exp Med. 2003;197:1345–1353. doi: 10.1084/jem.20030121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gawkrodger DJ. Nickel sensitivity and the implantation of orthopaedic prostheses. Contact Dermatitis. 1993;28:257–259. doi: 10.1111/j.1600-0536.1993.tb03427.x. [DOI] [PubMed] [Google Scholar]
- 30.Gillespie WJ, Frampton CM, Henderson RJ, Ryan PM. The incidence of cancer following total hip replacement. J Bone Joint Surg [Br] 1988;70:539–542. doi: 10.1302/0301-620X.70B4.3403594. [DOI] [PubMed] [Google Scholar]
- 31.Granchi D, Ciapetti G, Stea S, Cavedagna D, Bettini N, Bianco T, Fontanesi G, Pizzoferrato A. Evaluation of several immunological parameters in patients with aseptic loosening of hip arthroplasty. Chir Organi Mov. 1995;80:399–408. [PubMed] [Google Scholar]
- 32.Griem P, Gleichmann E. Metal ion induced autoimmunity. Curr Opin Immunol. 1995;7:831–838. doi: 10.1016/0952-7915(95)80056-5. [DOI] [PubMed] [Google Scholar]
- 33.Griem P, von Vultee C, Panthel K, Best SL, Sadler PJ, Shaw CF. T cell cross-reactivity to heavy metals: identical cryptic peptides may be presented from protein exposed to different metals. Eur J Immunol. 1998;28:1941–1947. doi: 10.1002/(SICI)1521-4141(199806)28:06<1941::AID-IMMU1941>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 34.Hallab N, Merritt K, Jacobs JJ. Metal sensitivity in patients with orthopaedic implants. J Bone Joint Surg Am. 2001;83-A:428–436. doi: 10.2106/00004623-200103000-00017. [DOI] [PubMed] [Google Scholar]
- 35.Hallab NJ, Anderson S, Caicedo M, Brasher A, Mikecz K, Jacobs JJ. Effects of soluble metals on human peri-implant cells. J Biomed Mater Res A. 2005;74:124–140. doi: 10.1002/jbm.a.30345. [DOI] [PubMed] [Google Scholar]
- 36.Hallab NJ, Anderson S, Caicedo M, Skipor A, Campbell P, Jacobs JJ. Immune responses correlate with serum-metal in metal-on-metal hip arthroplasty. J Arthroplasty. 2004;19:88–93. doi: 10.1016/j.arth.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 37.Hallab NJ, Anderson S, Stafford T, Glant T, Jacobs JJ. Lymphocyte responses in patients with total hip arthroplasty. J Orthop Res. 2005;23:384–391. doi: 10.1016/j.orthres.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Hallab NJ, Caicedo M, Finnegan A, Jacobs JJ. Th1 type lymphocyte reactivity to metals in patients with total hip arthroplasty. J Orthop Surg. 2008;3:6. doi: 10.1186/1749-799X-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hallab NJ, Mikecz K, Vermes C, Skipor A, Jacobs JJ. Differential lymphocyte reactivity to serum-derived metal-protein complexes produced from cobalt-based and titanium-based implant alloy degradation. J Biomed Mater Res. 2001;56:427–436. doi: 10.1002/1097-4636(20010905)56:3<427::aid-jbm1112>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 40.Halpin DS. An unusual reaction in muscle in association with a vitallium plate: A report of possible metal hypersensitivity. The Journal of Bone and Joint Surgery. 1975;57-B:451–453. [PubMed] [Google Scholar]
- 41.Hart AJ, Hester T, Sinclair K, Powell JJ, Goodship AE, Pele L, Fersht NL, Skinner J. The association between metal ions from hip resurfacing and reduced T-cell counts. J Bone Joint Surg Br. 2006;88:449–454. doi: 10.1302/0301-620X.88B4.17216. [DOI] [PubMed] [Google Scholar]
- 42.Haudrechy P, Foussereau J, Mantout B, Baroux B. Nickel release from nickel-plated metals and stainless steels. Contact Dermatitis. 1994;31:249–255. doi: 10.1111/j.1600-0536.1994.tb01997.x. [DOI] [PubMed] [Google Scholar]
- 43.Hensten-Pettersen A. Allergy and hypersensitivity. In: Morrey BF, editor. Biological, Material, and Mechanical Considerations of Joint Replacements. Raven Press; New York: 1993. pp. 353–360. [Google Scholar]
- 44.Jacobs JJ, Gilbert JL, Urban RM. Corrosion of metallic implants. In: Stauffer RN, editor. Advances in Orthopaedic Surgery. Vol 2. Mosby; St. Louis: 1994. pp. 279–319. [Google Scholar]
- 45.Jacobs JJ, Rosenbaum DH, Hay RM, Gitelis S, Black J. Early sarcomatous degeneration near a cementless hip replacement. A case report and review. J Bone Joint Surg Br. 1992;74:740–744. doi: 10.1302/0301-620X.74B5.1326562. [DOI] [PubMed] [Google Scholar]
- 46.Jacobs JJ, Shanbhag A, Glant TT, Black J, Galante JO. Wear Debris in Total Joint Replacements. J Am Acad Orthop Surg. 1994;2:212–220. doi: 10.5435/00124635-199407000-00004. [DOI] [PubMed] [Google Scholar]
- 47.Kanerva L, Sipilainen-Malm T, Estlander T, Zitting A, Jolanki R, Tarvainen K. Nickel release from metals, and a case of allergic contact dermatitis from stainless steel. Contact Dermatitis. 1994;31:299–303. doi: 10.1111/j.1600-0536.1994.tb02023.x. [DOI] [PubMed] [Google Scholar]
- 48.Kubicka-Muranyi M, Griem P, Lubben B, Rottmann N, Luhrmann R, Gleichmann E. Mercuric-chloride-induced autoimmunity in mice involves up-regulated presentation by spleen cells of altered and unaltered nucleolar self antigen. Int Arch Allergy Immunol. 1995;108:1–10. doi: 10.1159/000237110. [DOI] [PubMed] [Google Scholar]
- 49.Ladon D, Doherty A, Newson R, Turner J, Bhamra M, Case CP. Changes in metal levels and chromosome aberrations in the peripheral blood of patients after metal-on-metal hip arthroplasty. J Arthroplasty. 2004;19:78–83. doi: 10.1016/j.arth.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 50.Lalor PA, Revell PA, Gray AB, Wright S, Railton GT, Freeman MA. Sensitivity to titanium. A cause of implant failure. The Journal of Bone and Joint Surgery. 1991;73-B:25–28. doi: 10.1302/0301-620X.73B1.1991768. [DOI] [PubMed] [Google Scholar]
- 51.Liden C, Maibach HI, Howard I, Wahlberg JE. In: Skin. Goyer RA, Klaasen CD, Waalkes M, editors. Academic Press; New York: 1995. pp. 447–464. [Google Scholar]
- 52.Merkel KD, Erdmann JM, McHugh KP, Abu-Amer Y, Ross FP, Teitelbaum SL. Tumor necrosis factor-alpha mediates orthopedic implant osteolysis. Am J Pathol. 1999;154:203–210. doi: 10.1016/s0002-9440(10)65266-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Merritt K, Brown S. Metal sensitivity reactions to orthopedic implants. International Journal of Dermatology. 1981;20:89–94. doi: 10.1111/j.1365-4362.1981.tb00408.x. [DOI] [PubMed] [Google Scholar]
- 54.Merritt K, Brown SA. Biological effects of corrosion products from metal. American Society for Testing and Materials; Philadelphia: 1985. [Google Scholar]
- 55.Merritt K, Rodrigo JJ. Immune response to synthetic materials. Sensitization of patients receiving orthopaedic implants. Clin Orthop Rel Res. 1996;326:71–79. [PubMed] [Google Scholar]
- 56.Milavec-Puretic V, Orlic D, Marusic A. Sensitivity to metals in 40 patients with failed hip endoprosthesis. Arch Orthop Trauma Surg. 1998;117:383–386. doi: 10.1007/s004020050272. [DOI] [PubMed] [Google Scholar]
- 57.Moulon C, Vollmer J, Weltzien HU. Characterization of processing requirements and metal cross-reactivities in T cell clones from patients with allergic contact dermatitis to nickel. Eur J Immunol. 1995;25:3308–3315. doi: 10.1002/eji.1830251216. [DOI] [PubMed] [Google Scholar]
- 58.Munro-Ashman D, Miller AJ. Rejection of metal to metal prosthesis and skin sensitivity to cobalt. Contact Dermatitis. 1976;2:65. doi: 10.1111/j.1600-0536.1976.tb02986.x. [DOI] [PubMed] [Google Scholar]
- 59.Nakashima Y, Sun D-H, Trindade MCD, Maloney WJ, Goodman SB, Schurman DJ, Smith RL. Signaling pathways for tumor necrosis factor-alpha and interleukin-6 expression in human macrophages exposed to titanium alloy particulate debris in vitro. J Bone Joint Surg. 1999;81A doi: 10.2106/00004623-199905000-00002. [DOI] [PubMed] [Google Scholar]
- 60.Parker DC. T cell-dependent B cell activation. Annu Rev Immunol. 1993;11:331–360. doi: 10.1146/annurev.iy.11.040193.001555. [DOI] [PubMed] [Google Scholar]
- 61.Pichler WJ, Tilch J. The lymphocyte transformation test in the diagnosis of drug hypersensitivity. Allergy. 2004;59:809–820. doi: 10.1111/j.1398-9995.2004.00547.x. [DOI] [PubMed] [Google Scholar]
- 62.Poss R, Thornhill TS, Ewald FC, Thomas WH, Batte NJ, Sledge CB. Factors influencing the incidence and outcome of infection following total joint arthoplasty. Clin Orthop. 1984;182 [PubMed] [Google Scholar]
- 63.Silvennoinen-Kassinen S, Ikaheimo I, Karvonen J, Kauppinen M, Kallioinen M. Mononuclear cell subsets in the nickel-allergic reaction in vitro and in vivo. J Allergy Clin Immunol. 1992;89:794–800. doi: 10.1016/0091-6749(92)90433-3. [DOI] [PubMed] [Google Scholar]
- 64.Silvennoinen-Kassinen S, Poikonen K, Ikaheimo I. Characterization of nickel-specific T cell clones. Scand J Immunol. 1991;33:429–434. doi: 10.1111/j.1365-3083.1991.tb01791.x. [DOI] [PubMed] [Google Scholar]
- 65.Stout RD, Suttles J. T-cell signalling of macrphage activation: cell contact-dependent and cytokine signals. R.G. Landes Company; Austin: 1995. [Google Scholar]
- 66.Svejgaard E, Morling N, Svejgaard A, Veien NK. Lymphocyte transformation induced by nickel sulphate: an in vitro study of subjects with and without a positive nickel patch test. Acta Derm Venereol. 1978;58:245–250. [PubMed] [Google Scholar]
- 67.Thierse HJ, Moulon C, Allespach Y, Zimmermann B, Doetze A, Kuppig S, Wild D, Herberg F, Weltzien HU. Metal-protein complex-mediated transport and delivery of Ni2+ to TCR/MHC contact sites in nickel-specific human T cell activation. J Immunol. 2004;172:1926–1934. doi: 10.4049/jimmunol.172.3.1926. [DOI] [PubMed] [Google Scholar]
- 68.Thomas RH, Rademaker M, Goddard NJ, Munro DD. Severe eczema of the hands due to an orthopaedic plate made of Vitallium. British Medical Journal. 1987;294:106–107. doi: 10.1136/bmj.294.6564.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Veien NK, Svejgaard E. Lymphocyte transformation in patients with cobalt dermatitis. Br J Dermatol. 1978;99:191–196. doi: 10.1111/j.1365-2133.1978.tb01981.x. [DOI] [PubMed] [Google Scholar]
- 70.Veien NK, Svejgaard E, Menne T. In vitro lymphocyte transformation to nickel: a study of nickel-sensitive patients before and after epicutaneous and oral challenge with nickel. Acta Derm Venereol. 1979;59:447–451. [PubMed] [Google Scholar]
- 71.Vollmer J, Fritz M, Dormoy A, Weltzien HU, Moulon C. Dominance of the BV17 element in nickel-specific human T cell receptors relates to severity of contact sensitivity. Eur J Immunol. 1997;27:1865–1874. doi: 10.1002/eji.1830270808. [DOI] [PubMed] [Google Scholar]
- 72.Vollmer J, Weltzien HU, Gamerdinger K, Lang S, Choleva Y, Moulon C. Antigen contacts by Ni-reactive TCR: typical alphass chain cooperation versus alpha chain-dominated specificity. Int Immunol. 2000;12:1723–1731. doi: 10.1093/intimm/12.12.1723. [DOI] [PubMed] [Google Scholar]
- 73.Vollmer J, Weltzien HU, Moulon C. TCR reactivity in human nickel allergy indicates contacts with complementarity-determining region 3 but excludes superantigen-like recognition. J Immunology. 1999;163:2723–2731. [PubMed] [Google Scholar]
- 74.Wang JY, Wicklund BH, Gustilo RB, Tsukayama DT. Prosthetic metals interfere with the functions of human osteoblast cells in vitro. Clin Orthop. 1997:216–226. doi: 10.1097/00003086-199706000-00030. [DOI] [PubMed] [Google Scholar]
- 75.Yang J, Black J. Competitive binding of chromium cobalt and nickel to serum proteins. Biomater. 1994;15:262–268. doi: 10.1016/0142-9612(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 76.Yang J, Merritt K. Production of monoclonal antibodies to study corrosion of Co-Cr biomaterials. J Biomed Mater Res. 1996;31:71–80. doi: 10.1002/(SICI)1097-4636(199605)31:1<71::AID-JBM9>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]