Abstract
The power of fMRI in assessing neural activities is hampered by inter‐subject variations in basal physiologic parameters, which may not be related to neural activation but has a modulatory effect on fMRI signals. Therefore, normalization of fMRI signals with these parameters is useful in reducing variations and improving sensitivity of this important technique. Recently, we have shown that basal venous oxygenation is a significant modulator of fMRI signals and individuals with higher venous oxygenation tend to have lower fMRI signals. In this study, we aim to test the utility of venous oxygenation normalization in distinguishing subject groups. A “model” condition was used in which two visual stimuli with different flashing frequencies were used to stimulate two subject groups, respectively, thereby simulating the situation of control and patient groups. It was found that visual‐evoked BOLD signal is significantly correlated with baseline venous T2 (P = 0.0003) and inclusion of physiologic modulator in the regression analysis can substantially reduce P values of group‐level statistical tests. When applied to voxel‐wise analysis, the normalization process can allow the detection of more significant voxels. The utility of other basal parameters, including blood pressure, heart rate, arterial oxygenation, and end‐tidal CO2, in BOLD normalization was also assessed and it was found that the improvement was less significant. Time‐to‐peak of the BOLD responses was also studied and it was found that subjects with higher basal venous oxygenation tend to slower BOLD responses. Hum Brain Mapp, 2010. © 2009 Wiley‐Liss, Inc.
Keywords: fMRI, normalization, venous oxygenation, TRUST MRI, visual
INTRODUCTION
Functional magnetic resonance imaging (fMRI) studies often require the use of multiple subjects, in which fMRI data from a group of participants are processed together to yield a population activation result [Friston et al., 1996]. This is particularly the case for neurological and psychiatric studies that aim for better understanding of disease mechanism. A major challenge in finding differences in fMRI signals between patients and controls is that the disease effect is often “buried” inside the large noise attributed to inter‐subject variations. Therefore, it is of great interest to understand the mechanisms of inter‐subject variations in fMRI signals and to develop an approach to normalize the signals before the group comparison [Handwerker et al., 2007; Kannurpatti and Biswal, 2008; Lu et al., 2008; Thomason et al., 2007].
Recently, we have developed a T2‐Relaxation‐Under‐Spin‐Tagging (TRUST) MRI technique to quantitatively estimate cerebral venous oxygenation (Y v) in units of percentage [Lu and Ge, 2008], and have shown that task‐evoked fMRI signal is inversely correlated with Y v,baseline across subjects [Lu et al., 2008]. Specifically, individuals with higher baseline oxygenation tend to have a smaller fMRI signal and vice versa, when using identical stimuli. This provides an opportunity to use TRUST MRI as a normalization factor to reduce inter‐subject variations, and to improve the sensitivity of fMRI in detecting group differences. This improvement will be beneficial for the study of psychiatric disorders and comparison of pre/postdrug treatment, thereby substantially extending the potential clinical utility of fMRI.
In the present study, we aim to test the utility of TRUST MRI in distinguishing subject groups in a model situation, in which the neural activity differences between two subject groups are known and we ask the question that whether or not fMRI can detect these regional differences with and without the use of TRUST MRI. The extent of brain activation by flashing checkerboards is known to be dependent on flashing frequency, with 8 Hz stimulus inducing the greatest activation which decays at higher or lower frequencies [Fox and Raichle, 1984; Lin et al., 2008; Parkes et al., 2004; Singh et al., 2003]. Thus, we presented checkerboards flashing at 8 Hz and 4 Hz to two healthy control groups, respectively, to simulate the cases of “controls” and “patients.” Comparison between the groups with and without using Y v‐normalization was conducted. Alternative normalization parameters, including blood pressure, heart rate, arterial oxygenation, end‐tidal CO2, and breathing rate, were also assessed. Several normalization strategies were presented, and recommendations for ROI‐based and voxel‐based analysis were provided.
MATERIALS AND METHODS
MRI Experiment
All MR imaging experiments were conducted on a 3‐Tesla MR system (Philips Medical System, Best, The Netherlands). A total of 20 healthy subjects (29.3 ± 6.6 years old, 11 men and nine women) were scanned for this study. Each subject gave an informed written consent before participating in the study. The study protocol was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center.
The subjects were divided into two groups, one group receiving 8 Hz visual stimuli (n = 10, six males and four females) and the other group receiving 4 Hz stimuli (n = 10, five males and five females). The vision of the subjects was corrected using MR‐compatible corrective lenses, whenever necessary. This is important to minimize the confounding effect of visual acuity on the fMRI signals. Vital physiologic signs, including blood pressure, heart rate, arterial oxygenation (MEDRAD, Pittsburgh, PA), and end‐tidal CO2 (Capnogard, Model 1265, Novametrix Medical Systems, CT), were measured before they entered the magnet room.
FMRI of visual stimulation was conducted with flashing blue‐yellow checkerboard (visual angle = 25°). The functional paradigm consisted of 10‐s stimulation followed by 50 s of fixation on a cross sign in the center of the screen, and was repeated four times. An additional fixation period of 50 s was used at the beginning of the experiment. BOLD fMRI used the following parameters: TR = 500 ms, flip angle 37° (to allow for T1 relaxation and to reduce inflow effect), TE = 30 ms, SENSE factor 2, matrix 64 × 64, nine slices, voxel size 3.44 × 3.44 × 5 mm3, 1 mm gap. The relatively high temporal resolution is used so that we can also study the time‐to‐peak (TTP) of the BOLD responses. The slices were positioned axially covering the occipital lobes.
TRUST MRI was used to assess baseline venous oxygenation in the sagittal sinus [Lu and Ge, 2008]. The subjects were instructed to rest quietly, but not to fall asleep, as sleeping can change the blood oxygenation [Bangash et al., 2008]. The scan parameters were: TR = 8,000 ms, TE = 7 ms, labeling slab thickness 80 mm, gap between labeling slab and imaging slice 20 mm, single shot EPI, voxel size 3.44 × 3.44 × 5 mm3, four different T2‐weightings with TE of 0, 40, 80, and 160 ms, corresponding to 0, 4, 8, and 16 refocusing pulses in the T2‐preparation (τCPMG = 10 ms). For each TE, four pairs of tag and control images were acquired to improve signal‐to‐noise ratio (SNR). The total scan time of TRUST MRI was 4 min and 16 s.
Data Processing
Preliminary definition of brain activation was based on cross‐correlation (cc > 0.15) between the signal time‐course and the expected BOLD response. Standard response curve is available in most fMRI data processing softwares and is often based on a gamma variate impulse response function [Boynton et al., 1996]. However, BOLD signal is known to have nonlinear components [Gu et al., 2005] and, for long duration stimuli (e.g., 10 s), it would be more accurate to use an experimentally determined template response. We have therefore used a template BOLD response determined in one of our previous studies using identical stimuli (10‐s flashing checkerboard in visual cortex) [Lu et al., 2006]. A threshold on the cluster size (three voxels) was also used. The uncorrected P value was 0.0001, and the familywise error was P < 0.06. We note that this is a preliminary activation mask. The final mask used for region‐of‐interest (ROI) analysis was based on a subset of voxels defined later. Thus, the cutoff P value was different for different subjects. The largest activation cluster was determined and this always corresponds to the occipital lobe regions (visually verified). To ensure the numbers of voxels averaged are the same for all subjects, only the 500 voxels that have the highest cc values were included in the ROI analysis. This number was chosen because all subjects had at least 500 activated voxels in the visual cortex (voxel number 1,854 ± 816, mean ± SD, n = 20).
The BOLD time course was obtained by spatially averaging the MRI signals of the final mask and normalizing against the signals during the fixation period. Two parameters, TTP and percentage change, were then quantified from the time course. The TTP was determined by shifting the standard BOLD template at 500 ms steps and, for each step, calculating the cc with the experiment BOLD time course. The shift at which maximum cc value is achieved is used as the TTP for the individual time course. The BOLD percentage change is then calculated by linear regression of the signal time course to the optimally shifted BOLD template.
TRUST MRI data were used to estimate venous blood T2, which was in turn converted to venous oxygenation using a calibration plot [Lu and Ge, 2008]. This value in the sagittal sinus was used as the baseline venous oxygenation, which is thought to be homogenous across the brain at resting state [An and Lin, 2000; Fox and Raichle, 1986]. The baseline oxygenation is recently shown to be an important modulator of the BOLD fMRI signal [Lu et al., 2008].
Statistical Analysis Comparing BOLD Responses Between 8 Hz and 4 Hz Groups
For standard comparison of 8 Hz and 4 Hz responses, a two‐sample t‐test was used to compare the percentage signal changes between these two frequencies.
Next, we tested to see whether the additional measurements of basal physiologic parameters can help in distinguishing these two groups. Both ROI based and voxel‐based analyses were performed. For the ROI analysis, we used multiple regression method. The advantage of the regression method is that it does not depend on the mechanism or biophysical model of the BOLD signal [Buxton et al., 1998; Ogawa et al., 1993; van Zijl et al., 1998]. Instead, additional regressors are added to see if they are significant factors in explaining the variations in the BOLD data. Thus, the BOLD signal can be written as:
| (1) |
where X i is the ith regressor (independent variable), a i is the coefficient associated with the regressor, a ij is the coefficient associated with the interaction between X i and X j, a 0 is a constant. Note that the two‐sample t‐test described above is essentially a regression with flashing frequency being the only regressor. In our regression analysis, two regressors, X 1 = flashing frequency, X 2 = baseline venous T2, were used and the statistical significance of the coefficients, a 1, a 2, and a 12, was tested. Similar analysis was performed using the other measured physiologic parameters (blood pressure, heart rate, arterial oxygenation, and end‐tidal CO2) as X 2. Furthermore, to assess the benefits of including multiple signal modulators in the regression (i.e., adding more than one variable from the list of venous T2, blood pressure, heart rate, arterial oxygenation, and end‐tidal CO2), a stepwise regression method (with matlab routine stepwise.m) was used in which new regressors are added to the model in a step‐by‐step manner, from the highest ranked regressor to lowest ranked regressor. The Analysis of Variance (ANOVA) was used to access the statistical significance of the variance explained by the new regressor.
For the voxel‐based analysis, multiple regression analysis was performed on a voxel‐by‐voxel basis with flashing frequency and venous T2 as the regressors (using SPM2's “Multiple regression with constant” functionality). For each voxel, if the coefficient a 1 was determined to be significantly (P < 0.05, cluster size 800 mm3) different from zero, this voxel will be showed in color in the group comparison map.
In addition, a secondary analysis based on a BOLD biophysical model was also applied in the voxel‐wise data. According to the biophysical model used by Davis et al. [1998] and others [Chiarelli et al., 2007a, b; Hoge et al., 1999; Uludag et al., 2004], the BOLD signal is affected by baseline venous oxygenation as: S BOLD ∝ (1−Y v)β. The index β is associated with vascular geometry and magnetic susceptibility effect of deoxyhemoglobin [Boxerman et al., 1995]. The value of β is typically 1.5 at 1.5 T [Davis et al., 1998]. At 3 T, due to the increased contribution of extravascular BOLD effect, a value of 1 has been used in several previous studies [Kannurpatti and Biswal, 2008; Thomason et al., 2007; Uludag et al., 2004). Thus, the normalized BOLD signal in the voxel‐based analysis was calculated by:
| (2) |
This normalization was performed for all voxels inside the brain, yielding a map of S BOLD,n. Voxel‐by‐voxel comparison between the 8 Hz and 4 Hz groups was performed using two‐sample t‐test to detect brain regions that show significant differences (P < 0.05, cluster size 800 mm3) in BOLD responses, for both S BOLD and S BOLD,n. The reason for testing the model‐based normalization approach is that, due to the lower sensitivity in a single voxel compared with an ROI, regression analysis may not be feasible (that is, a 2 in Eq. (1) cannot be estimated accurately on a voxel‐by‐voxel basis). We note that the model‐based approach has its own limitations in that additional assumptions are made: (i) the relationship between venous T2 and venous oxygenation [Lu and Ge, 2008]; (ii) the analytical relationship between baseline venous oxygenation and the BOLD signal [Davis et al., 1998; Hoge et al., 1999; Uludag et al., 2004].
RESULTS
Robust fMRI activations were detected in all subjects. Examples of the 8 Hz and 4 Hz activation maps are shown in Figures 1a,b. Figure 1c shows the averaged BOLD time courses for both groups. The vital sign parameters are listed in the Table I. TRUST MRI data yielded baseline venous blood T2 of 55.8 ± 11.2 ms (n = 10, mean ± D) and 52.4 ± 14.5 ms (n = 10) for the 8 Hz and 4 Hz, respectively. Using an in vitro calibration plot [Lu and Ge, 2008], these T2 values correspond to venous oxygenation of 61.1 ± 7.8% and 58.5 ± 9.9%, respectively. No significant differences were found between the groups in any of these basal physiologic parameters (P > 0.4 for all parameters).
Figure 1.
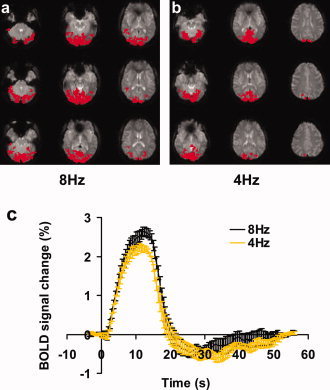
Single subject visual activation results using 8 Hz and 4 Hz flashing checkerboard. (a, b) Activation maps in two healthy subjects using an 8 Hz (a) and a 4 Hz (b) flashing stimulus, respectively. The activated voxels in the occipital lobe are shown in red. (c) BOLD signal time‐courses averaged over all subjects (n = 10 for each group). Error bars indicate the standard error across subjects. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Table I.
Summary of physiologic parameters for the two groups of subjects and P values of statistical comparisons
| Parameter | Mean blood pressure (mm Hg) | Heart rate (beats per min) | Arterial blood oxygenation (%) | End‐tidal CO2 (mm Hg) | Breathing rate (times per min) |
|---|---|---|---|---|---|
| 8Hz (n = 10) | 89.9 ± 7.4 | 76.3 ± 10.4 | 97.9 ± 0.9 | 37.3 ± 4.5 | 15.8 ± 5.4 |
| 4Hz (n = 10) | 91.1 ± 10.5 | 75.1 ± 10.9 | 98.0 ± 1.3 | 36.0 ± 2.8 | 15.3 ± 3.4 |
| P value | 0.764 | 0.804 | 0.845 | 0.446 | 0.806 |
The BOLD percentage changes, S BOLD, in the ROIs were 2.64 ± 0.33% and 2.27 ± 0.27% for the 8 Hz and 4 Hz groups, respectively. Regression with flashing frequency as the sole regressor (essentially a two‐sample t‐test) showed a marginal difference between the two frequency groups with a P value of 0.0126. Addition of the venous T2 into the regression significantly (P = 0.0003, using F‐test to compare two models) improved the model. The significance level of the frequency dependence becomes P = 0.0003. The coefficient associated with the flashing frequency, a 1, and its 95% confidence interval (CI) were 0.093 and (0.026, 0.160), respectively, without consideration of venous T2 and these values were 0.108 and (0.061, 0.155), respectively, after venous T2 was added to the regression model. The CI of the coefficient estimation became narrower after accounting for venous T2, suggesting a more accurate estimation of the frequency effect. Further addition of an interaction term between frequency‐dependence and venous T2‐dependence did not improve the model (P = 0.3334). We therefore only used the linear terms in further analysis. For conceptual understanding of the mechanism, it is sometimes helpful to calculate the “normalized” BOLD signal, in which the venous T2‐dependence of the BOLD signal is first quantified and then its effect is subtracted from the original BOLD signal, i.e., S BOLD,n = S BOLD − a · (T 2,v − T 2,v), where T 2,v is the group average of venous T2. The normalized BOLD signal, S BOLD,n, were 2.67 ± 0.23% and 2.24 ± 0.18% for the 8 Hz and 4 Hz groups, respectively, and the P value from the two‐sample t‐test was 0.0002, which is comparable with the multiple regression results. It can also be seen that the intragroup standard deviation of the normalized BOLD signals decreased compared with the original BOLD signals, and showed a 30 and 33% reduction for the 8 Hz and 4 Hz groups, respectively. Reduction of intragroup variations explains why BOLD normalization is helpful in detecting group differences.
Figure 2 shows the scatter plot between baseline venous T2 and BOLD percentage change for all subjects. It can be seen that both groups show a negative slope, suggesting that subjects with higher baseline venous T2 tend to have lower BOLD signal.
Figure 2.
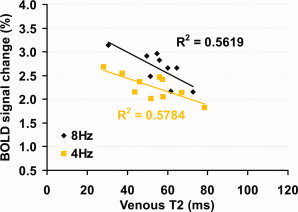
Scatter plot between baseline venous T2 and BOLD signal changes. Each symbol in the plot represents data from one subject. The solid curves show the linear fitting of the experimental data. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Regression analysis was also performed using each of the other physiologic parameters and it was found that heart rate (P = 0.0099) was a significant regressor. However, the benefits in group differentiation only improved slightly (P = 0.0063) compared to simple two‐sample t‐test (P = 0.0126). To assess whether the inclusion of multiple physiologic regressors are more advantageous than a single regressor, stepwise regression was performed in which regressors were added to the model from the most significant to the least significant ones until no more significant regressors are available. The order of the significant regressors and their respective significant levels were: venous T2 (P = 0.0003) and mean blood pressure (P = 0.0350). After each step of the regression, the significance level of the flashing frequency dependence was: P = 0.0126 (no physiologic regressor), 0.0003 (with venous T2), 0.0001 (with venous T2 and mean blood pressure).
Voxel‐wise analysis was also performed to test the utility of baseline physiologic state in improving fMRI power. Group‐level one‐sample t test between 8 Hz flashing and fixation (n = 10) showed robust activation (Fig. 3a) in the thalamus and occipital cortex, which are the main visual areas in the brain. Figures 3b,c show the activation patterns after normalization with two different approaches, regression‐based and model‐based normalization. Increased number of activated voxels can be seen in the thalamus regions. Group‐level two‐sample t‐test between 8 Hz (n = 10) and 4 Hz (n = 10) fMRI signals without normalization showed a few clusters with significant differences across groups (Fig. 3d). Figure 3e,f show such maps after regression and model‐based normalization, respectively. It can be seen that more voxels are detected after the signal normalization, especially with the model‐based approach (Fig. 3f). As a quantitative demonstration of the improvement, histograms of the t scores in the visual‐activated voxels (red voxels in Fig. 3c) are plotted in Figure 4. It is clear that the histogram shifted to the right after the normalization, again with the model‐based approach showing greater improvement. The histograms were significantly different for all three analyses (P < 0.0001).
Figure 3.
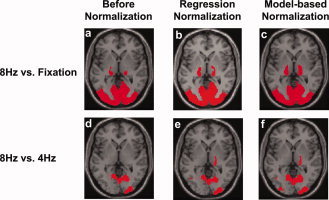
Group‐level activation maps with un‐normalized and normalized fMRI signals. (a–c) Activation maps comparing 8 Hz flashing checkerboard and fixation (n = 10). Threshold: P value < 0.005, cluster > 800 mm3. (d–f) Difference maps comparing two subject groups receiving 8 Hz (n = 10) and 4 Hz (n = 10) stimulus, respectively. Threshold: P value < 0.05, cluster > 800 mm3. Results from two normalization approaches, a regression based and a model‐based, are presented. Only activations in thalamus and occipital lobes are shown. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Figure 4.
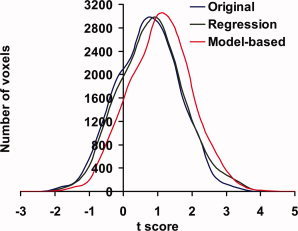
Histograms of t scores of the frequency effect in the visual‐activated voxels. The voxels were selected from the functional activation maps in Figure 3c. The t scores of the un‐normalized signals were obtained from the group‐level two‐sample t‐tests between 8 Hz and 4 Hz subjects performed on a voxel‐by‐voxel basis. The regression‐normalized t scores were obtained from a multiple regression with frequency and venous T2 as the regessors and testing whether the coefficient associated with frequency is different from zero. The model‐based t‐scores were obtained from the two‐sample t‐tests on the signals calculated according to Eq. (2). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
In addition to the BOLD signal amplitude, we have studied the TTP of the BOLD responses. No significant differences in TTP were found between the 8 Hz and 4 Hz groups (P = 0.68). This is still the case after including the basal physiologic parameters as regressors. We therefore grouped the 8 Hz and 4 Hz subjects together, and studied the correlation between BOLD TTP and each of the basal parameters. A significant correlation was found between the basal venous T2 and the BOLD TTP (P = 0.0123, Fig. 5). Subjects with greater venous T2 tend to have a longer TTP in their BOLD responses. This intersubject correlation is in agreement with previous findings that, within a subject, when the basal blood flow is elevated [Cohen et al., 2002] the BOLD TTP becomes longer. Similar, when the basal blood flow is reduced [Liu et al., 2004], the BOLD TTP is shorter.
Figure 5.
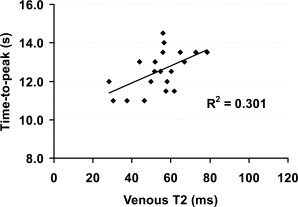
Scatter plot between baseline venous T2 and time‐to‐peak (TTP) of the BOLD responses. Each symbol in the plot represents data from one subject. Since there are no differences in TTP between 8 Hz and 4 Hz groups, the data are shown in one group (n = 20). The solid curve shows the linear fitting of the experimental data. The significant correlation (P = 0.0123) suggests that subjects with higher baseline oxygenation tend to have a slower BOLD response and those with lower baseline oxygenation tend to have a more rapid response.
DISCUSSION
In this study, we used basal venous T2 to normalize fMRI signals across subjects, and demonstrated in a “model” situation that the normalization process can substantially reduce P values of statistical tests and can allow the detection of more significant voxels. The utility of other basal parameters, including blood pressure, heart rate, arterial oxygenation, and end‐tidal CO2, in BOLD normalization was also assessed and it was found that the improvement was less significant. The results also suggest that there is a significant correlation between basal physiologic state and time‐to‐peak of the BOLD response.
It is well known that physiologic noise is the largest source of noise in MRI data [Hu and Kim, 1994; Kruger and Glover, 2001]. In the analysis of group fMRI data, intersubject variations in basal physiology present an important, if not the largest, source of noise, because many of these physiologic parameters are modulators of the BOLD fMRI signal [Cohen et al., 2002; Corfield et al., 2001; Liu et al., 2004; Lu et al., 2008; Mulderink et al., 2002]. Therefore, when using fMRI to assess neural activities, measurement of these basal parameters is not only important for correct interpretation of fMRI results (e.g., whether the fMRI signal difference before/after drug treatment is due to changes in neural activities or to changes in basal parameters), but is also beneficial in improving the detection power by reducing intersubject variations. Thus, while the typical protocol for an fMRI session currently consists of localizer, anatomic scans, and functional scans, we propose to also include a scan to measure basal physiologic parameters.
To determine exactly which physiologic parameter to measure for best normalization results, a survey of the BOLD biophysical model [Davis et al., 1998; Hoge et al., 1999; Kim and Ugurbil, 1997] suggests that the following parameters are possible candidates and are also accessible with MRI techniques: basal CBV, basal CBF, and basal venous oxygenation. The present study and one of our earlier studies [Lu et al., 2008] have demonstrated the utility of basal venous oxygenation. We have also assessed the use of basal CBF previously [Lu et al., 2008]. It was found that the correlation between basal CBF as assessed by ASL MRI and BOLD signal is less significant than that of venous oxygenation, although a trend can be clearly seen [Lu et al., 2008] and is recently confirmed by a study from Liau et al. [2008]. Basal CBV may be another modulator of the fMRI signals as more blood in the voxel would result in greater BOLD signal with activation. This has not been sufficiently assessed because the measurement of absolute CBV typically requires the injection of Gd‐DTPA contrast agent [Kuppusamy et al., 1996; Lu et al., 2005; Moseley et al., 1992; Schwarzbauer et al., 1993] and, in many cases, the estimation of arterial input function [Ostergaard et al., 1996]. Another basal physiologic parameter in the BOLD model, Cerebral Metabolic Rate of Oxygen or CMRO2, cannot yet be accurately determined in humans with existing MRI techniques [Hyder et al., 1996; Zhu et al., 2005].
Intersubject variations in BOLD signals can also be attributed to differences in vessel elasticity across subjects. This factor may be particularly important in conditions where the vessel elasticity is known to change, such as in aging [D'Esposito et al., 2003]. Recently, Handwerker et al. [2007] and Thomason et al. [2007] have used breathhold (increasing arterial CO2 content) to assess vascular reactivity and used this information to normalize fMRI signals. These methods are complementary to the basal physiology normalization methods, and if used together, may provide the best results. One confounding factor in breathhold experiment is that the extent and efficacy of modulating arterial CO2 is highly dependent on subject cooperation and may not be feasible in all elderly subjects. Furthermore, some evidences have suggested that hypercapnia may cause a reduction in neural activity [Zappe et al., 2008] and CMRO2, [Xu et al., 2009] and thus is not a purely vascular maneuver. More recently, it was suggested that spontaneous fluctuation in breathing patterns can cause BOLD signal changes [Birn et al., 2006], which can be used to estimate the vessel elasticity [Kannurpatti and Biswal, 2008]. Such techniques will be very useful in controlling for intersubject variations if fully developed.
It should be noted that the normalization method used in the present study is based on a global measurement of venous T2 in the sagittal sinus. Although a number of studies have suggested that the oxygen extraction fraction, i.e., venous oxygenation, is homogenous across brain regions in healthy controls [Fox and Raichle, 1986; He and Yablonskiy, 2007], the baseline venous oxygenation may be region‐dependent in patient populations. Therefore, the effect of regional oxygenation variation on BOLD fMRI signal is not accounted for in this study. Robust mapping of venous oxygenation in the brain is still challenging. The TRUST method can be potentially applied to small veins or venules to estimate local Y v. However, one would need to develop a method to label the tissue and wait for the labeled spin to enter the venules, where its T2 is determined. In this regard, tissue‐based techniques, such as the ones used by An and Lin [2003], He and Yablonskiy [2007], and He et al. [2008], may have an advantage if the biophysical model used can be shown to be applicable for all vessel sizes and orientations. Another utility of regional measurement of basal physiologic parameters is that one can account for both interregional and intersubject variability [Liau et al., 2008], which may further improve the power of fMRI.
The results in the present study should be interpreted in the context of a few limitations. First, the discussion of BOLD variations across subjects has primarily focused on vascular physiology. The implicit assumption is that identical visual stimulus (e.g., 8 Hz flashing checkerboard) will evoke identical neural response in all subjects, which may not be exactly correct. Thus, the intersubject variations in neural response will not be able to be corrected by the normalization method proposed in this study or those in previous studies. A more realistic goal of the proposed normalization method is then to reduce the intersubject variations (the part that is related to vascular physiology) and to improve the statistical power to some extent. Indeed, with venous oxygenation normalization, the intersubject variations in our data only reduced by ∼30%, suggesting that many other sources of variations are not yet understood and have not be accounted for. Second, in the present study, each subject only received visual stimulation with one frequency (8 Hz or 4 Hz). Therefore, we were only able to make frequency‐dependent comparison across groups, but not within‐subject in which the 8 Hz/4 Hz signal difference would not be affected by physiologic differences across subjects. As a result, we were not able to compare the pre/post normalization data with that of a paired comparison. Finally, our study has used a “model” situation in a group of healthy volunteers. The application of the normalization method in patient populations using a cognitive paradigm will need to be further tested.
CONCLUSIONS
Basal venous oxygenation can be used to normalize fMRI signals across subjects. The utility of this method was demonstrated in a “model” situation in which two visual stimuli with different flashing frequencies were used to stimulate two subject groups, respectively, thereby simulating the situation of control and patient groups. It was found that the normalization process can substantially reduce P values of statistical tests and can allow the detection of more significant voxels. Thus, the measurement of basal physiologic parameters may be a useful addition to the typical protocol of an fMRI session.
Acknowledgements
The authors are grateful to Kelly Lewis‐Amezcua for assistance with subject recruitment.
REFERENCES
- An H, Lin W ( 2000): Quantitative measurements of cerebral blood oxygen saturation using magnetic resonance imaging. J Cereb Blood Flow Metab 20: 1225–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An H, Lin W ( 2003): Impact of intravascular signal on quantitative measures of cerebral oxygen extraction and blood volume under normo‐ and hypercapnic conditions using an asymmetric spin echo approach. Magn Reson Med 50: 708–716. [DOI] [PubMed] [Google Scholar]
- Bangash MF, Xie A, Skatrud JB, Reichmuth KJ, Barczi SR, Morgan BJ ( 2008): Cerebrovascular response to arousal from NREM and REM sleep. Sleep 31: 321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Diamond JB, Smith MA, Bandettini PA ( 2006): Separating respiratory‐variation‐related fluctuations from neuronal‐activity‐related fluctuations in fMRI. Neuroimage 31: 1536–1548. [DOI] [PubMed] [Google Scholar]
- Boxerman JL, Hamberg LM, Rosen BR, Weisskoff RM ( 1995): MR contrast due to intravascular magnetic susceptibility perturbations. Magn Reson Med 34: 555–566. [DOI] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ ( 1996): Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci 16: 4207–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton RB, Wong EC, Frank LR ( 1998): Dynamics of blood flow and oxygenation changes during brain activation: The balloon model. Magn Reson Med 39: 855–864. [DOI] [PubMed] [Google Scholar]
- Chiarelli PA, Bulte DP, Piechnik S, Jezzard P ( 2007a) Sources of systematic bias in hypercapnia‐calibrated functional MRI estimation of oxygen metabolism. Neuroimage 34: 35–43. [DOI] [PubMed] [Google Scholar]
- Chiarelli PA, Bulte DP, Wise R, Gallichan D, Jezzard P ( 2007b) A calibration method for quantitative BOLD fMRI based on hyperoxia. Neuroimage 37: 808–820. [DOI] [PubMed] [Google Scholar]
- Cohen ER, Ugurbil K, Kim SG ( 2002): Effect of basal conditions on the magnitude and dynamics of the blood oxygenation level‐dependent fMRI response. J Cereb Blood Flow Metab 22: 1042–1053. [DOI] [PubMed] [Google Scholar]
- Corfield DR, Murphy K, Josephs O, Adams L, Turner R ( 2001): Does hypercapnia‐induced cerebral vasodilation modulate the hemodynamic response to neural activation? Neuroimage 13( 6 Part 1): 1207–1211. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Deouell LY, Gazzaley A ( 2003): Alterations in the BOLD fMRI signal with ageing and disease: A challenge for neuroimaging. Nat Rev Neurosci 4: 863–872. [DOI] [PubMed] [Google Scholar]
- Davis TL, Kwong KK, Weisskoff RM, Rosen BR ( 1998): Calibrated functional MRI: Mapping the dynamics of oxidative metabolism. Proc Natl Acad Sci USA 95: 1834–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Raichle ME ( 1984): Stimulus rate dependence of regional cerebral blood flow in human striate cortex, demonstrated by positron emission tomography. J Neurophysiol 51: 1109–1120. [DOI] [PubMed] [Google Scholar]
- Fox PT, Raichle ME ( 1986): Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci USA 83: 1140–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes A, Poline JB, Price CJ, Frith CD ( 1996): Detecting activations in PET and fMRI: Levels of inference and power. Neuroimage 4( 3 Part 1): 223–235. [DOI] [PubMed] [Google Scholar]
- Gu H, Stein EA, Yang Y ( 2005): Nonlinear responses of cerebral blood volume, blood flow and blood oxygenation signals during visual stimulation. Magn Reson Imaging 23: 921–928. [DOI] [PubMed] [Google Scholar]
- Handwerker DA, Gazzaley A, Inglis BA, D'Esposito M ( 2007): Reducing vascular variability of fMRI data across aging populations using a breathholding task. Hum Brain Mapp 28: 846–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Yablonskiy DA ( 2007): Quantitative BOLD: Mapping of human cerebral deoxygenated blood volume and oxygen extraction fraction: Default state. Magn Reson Med 57: 115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Zhu M, Yablonskiy DA ( 2008): Validation of oxygen extraction fraction measurement by qBOLD technique. Magn Reson Med 60: 882–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB ( 1999): Linear coupling between cerebral blood flow and oxygen consumption in activated human cortex. Proc Natl Acad Sci USA 96: 9403–9408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Kim SG ( 1994): Reduction of signal fluctuation in functional MRI using navigator echoes. Magn Reson Med 31: 495–503. [DOI] [PubMed] [Google Scholar]
- Hyder F, Chase JR, Behar KL, Mason GF, Siddeek M, Rothman DL, Shulman RG ( 1996): Increased tricarboxylic acid cycle flux in rat brain during forepaw stimulation detected with 1H[13C]NMR. Proc Natl Acad Sci USA 93: 7612–7617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannurpatti SS, Biswal BB ( 2008): Detection and scaling of task‐induced fMRI‐BOLD response using resting state fluctuations. Neuroimage 40: 1567–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SG, Ugurbil K ( 1997): Comparison of blood oxygenation and cerebral blood flow effects in fMRI: Estimation of relative oxygen consumption change. Magn Reson Med 38: 59–65. [DOI] [PubMed] [Google Scholar]
- Kruger G, Glover GH ( 2001): Physiological noise in oxygenation‐sensitive magnetic resonance imaging. Magn Reson Med 46: 631–637. [DOI] [PubMed] [Google Scholar]
- Kuppusamy K, Lin W, Cizek GR, Haacke EM ( 1996): In vivo regional cerebral blood volume: Quantitative assessment with 3D T1‐weighted pre‐ and postcontrast MR imaging. Radiology 201: 106–112. [DOI] [PubMed] [Google Scholar]
- Liau J, Perthen JE, Liu TT. The relation between BOLD amplitude and baseline cerebral blood flow depends on the analysis scale; 2008; Proceedings of the 16th Annual Meeting of ISMRM, Toronto, p 854.
- Lin AL, Fox PT, Yang Y, Lu H, Tan LH, Gao JH ( 2008): Evaluation of MRI models in the measurement of CMRO2 and its relationship with CBF. Magn Reson Med 60: 380–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TT, Behzadi Y, Restom K, Uludag K, Lu K, Buracas GT, Dubowitz DJ, Buxton RB ( 2004): Caffeine alters the temporal dynamics of the visual BOLD response. Neuroimage 23: 1402–1413. [DOI] [PubMed] [Google Scholar]
- Lu H, Donahue MJ, van Zijl PC ( 2006): Detrimental effects of BOLD signal in arterial spin labeling fMRI at high field strength. Magn Reson Med 56: 546–552. [DOI] [PubMed] [Google Scholar]
- Lu H, Ge Y ( 2008): Quantitative evaluation of oxygenation in venous vessels using T2‐relaxation‐under‐spin‐tagging MRI. Magn Reson Med 60: 357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Law M, Johnson G, Ge Y, van Zijl PC, Helpern JA ( 2005): Novel approach to the measurement of absolute cerebral blood volume using vascular‐space‐occupancy magnetic resonance imaging. Magn Reson Med 54: 1403–1411. [DOI] [PubMed] [Google Scholar]
- Lu H, Zhao C, Ge Y, Lewis‐Amezcua K ( 2008): Baseline blood oxygenation modulates response amplitude: Physiologic basis for intersubject variations in functional MRI signals. Magn Reson Med 60: 364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley ME, Chew WM, White DL, Kucharczyk J, Litt L, Derugin N, Dupon J, Brasch RC, Norman D ( 1992): Hypercarbia‐induced changes in cerebral blood volume in the cat: A 1H MRI and intravascular contrast agent study. Magn Reson Med 23: 21–30. [DOI] [PubMed] [Google Scholar]
- Mulderink TA, Gitelman DR, Mesulam MM, Parrish TB ( 2002): On the use of caffeine as a contrast booster for BOLD fMRI studies. Neuroimage 15: 37–44. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Menon RS, Tank DW, Kim SG, Merkle H, Ellermann JM, Ugurbil K ( 1993): Functional brain mapping by blood oxygenation level‐dependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model. Biophys J 64: 803–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostergaard L, Sorensen AG, Kwong KK, Weisskoff RM, Gyldensted C, Rosen BR ( 1996): High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. II. Experimental comparison and preliminary results. Magn Reson Med 36: 726–736. [DOI] [PubMed] [Google Scholar]
- Parkes LM, Fries P, Kerskens CM, Norris DG ( 2004): Reduced BOLD response to periodic visual stimulation. Neuroimage 21: 236–243. [DOI] [PubMed] [Google Scholar]
- Schwarzbauer C, Syha J, Haase A ( 1993): Quantification of regional blood volumes by rapid T1 mapping. Magn Reson Med 29: 709–712. [DOI] [PubMed] [Google Scholar]
- Singh M, Kim S, Kim TS ( 2003): Correlation between BOLD‐fMRI and EEG signal changes in response to visual stimulus frequency in humans. Magn Reson Med 49: 108–114. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Foland LC, Glover GH ( 2007): Calibration of BOLD fMRI using breath holding reduces group variance during a cognitive task. Hum Brain Mapp 28: 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uludag K, Dubowitz DJ, Yoder EJ, Restom K, Liu TT, Buxton RB ( 2004): Coupling of cerebral blood flow and oxygen consumption during physiological activation and deactivation measured with fMRI. Neuroimage 23: 148–155. [DOI] [PubMed] [Google Scholar]
- van Zijl PC, Eleff SM, Ulatowski JA, Oja JM, Ulug AM, Traystman RJ, Kauppinen RA ( 1998): Quantitative assessment of blood flow, blood volume and blood oxygenation effects in functional magnetic resonance imaging. Nat Med 4: 159–167. [DOI] [PubMed] [Google Scholar]
- Xu F, Yezhuvath U, Brier MR, Hart J, Kraut MA, Moore C, Lu H ( 2009): CO2 breathing suppresses cerebral metabolic rate of oxygen. In Proceedings of the 17th Annual Meeting of ISMRM, Honolulu, Hawaii. p 215.
- Zappe AC, Uludag K, Oeltermann A, Ugurbil K, Logothetis NK ( 2008): The influence of moderate hypercapnia on neural activity in the anesthetized nonhuman primate. Cereb Cortex 18: 2666–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XH, Zhang N, Zhang Y, Zhang X, Ugurbil K, Chen W ( 2005): In vivo 17O NMR approaches for brain study at high field. NMR Biomed 18: 83–103. [DOI] [PubMed] [Google Scholar]


