Abstract
Plasma viremia decreases coincident with the appearance of virus-specific CD8+ T cells during acute HIV or SIV infection. This finding, along with demonstrations of viral mutational escape from CD8+ T-cell responses and transient increase in plasma viremia after depletion of CD8+ T cells in SIV-infected monkeys strongly suggest a role for CD8+ T cells in controlling HIV/SIV. However, direct quantitative or qualitative correlates between CD8+ T-cell activity and virus control have not been established. To directly assess the impact of large numbers of virus-specific CD8+ T cells present at time of SIV infection, we transferred in vitro expanded autologous central and effector memory-derived Gag CM9-, Nef YY9- and Vif WY8-specific CD8+ T-cell clones to acutely infected rhesus macaques. The cells persisted in PBMC between 4 and 9 days but were not detected in gut-associated lymphoid tissue or lymph nodes. Interestingly, a high frequency of the infused cells localized to the lungs where they persisted at high frequency for more than 6 weeks. While persisting cells in the lungs were antigen reactive, there was no measurable effect on virus load. Sequencing of virus from the animal receiving Nef YY9-specific CD8+ T cells demonstrated an escape mutation in this epitope less than 3 weeks post infection, consistent with immune selection pressure by the infused cells. These studies establish methods for adoptive transfer of autologous SIV-specific CD8+ T cells for evaluating immune control during acute infection, and demonstrate that infused cells retain function and persist for at least 2 months in specific tissues.
Introduction
Despite more than 2 decades of intensive research, an efficacious vaccine against HIV and AIDS is lacking. Passive immunization using HIV neutralizing antibodies can provide sterilizing immunity against pathogenic HIV/SIV hybrid viruses (SHIV) in non-human primate (NHP) models (1-3). However, vaccine induction of broadly cross reactive neutralizing antibody responses that will likely be necessary for protection remains elusive, leading to an intense complementary research effort focusing on vaccines that can maximize the potential of adaptive cellular immunity (4-7).
Multiple indirect lines of evidence suggest that HIV and SIV virus replication can be partially controlled by CD8+ T-cell responses: i) the temporal correlation between CD8+ T cell expansion and reduced viremia during acute infection (8-10), ii) mutations leading to escape in defined viral CD8+ T-cell epitopes (11-14), iii) CD8+ cell depletion studies giving rise to increased viremia (15-17), as well as iv) partial viral control after vaccine induction of CD8+ T cell responses in the absence of neutralizing antibody, under certain experimental conditions (4-9, 18-21). However, no consistent cellular immune correlate with viral control has been demonstrated in HIV-infected patients or SIV-infected macaques asserting long-term control of viral replication, i.e. long-term non-progressors (LTNP, reviewed in (22)). Also, a recent clinical trial aimed at generating HIV-reactive T-cell responses failed to induce measurable protection against infection or reduce viral load after infection (23). The failure of current CD8+ T cell-based vaccines in HIV- and SIV-induced disease could be due to a quantitative defect, i.e. current vaccine protocols fail to generate a strong enough CD8+ T-cell response. Alternatively, the vaccines might not induce a qualitatively appropriate response with a phenotype suitable for viral control, or a combination of the two.
Adoptive transfer of antigen-specific T cells using syngeneic cells from inbred mice has helped to define effector mechanisms for immune-based tumor suppression (reviewed in (24)), as well as protection against some viral infections (25, 26). The achievements using murine models led to clinical protocols where patient CD8+ T cells specific for tumor-associated antigens or virus are generated and expanded in vitro to large numbers, and then adoptively transferred to the autologous host. Notwithstanding problems with in vivo persistence of infused T cells, positive clinical effects have been reported using T cells against different malignancies (27-30) and viral infections (31). Therefore, it has been suggested that adoptive transfer of SIV-specific CD8+ T cells could be an important tool for determining what phenotype is critical for effective SIV suppression in vivo (32, 33). One impediment to these types of studies is the difficulty in generation/isolation, long-term maintenance, and large scale expansion of virus-specific macaque T-cell clones in vitro. A study by Berger et al., in Macaca nemestrina using CMV as a model viral infection, made strides towards establishing such methods, with adoptive transfers of antigen-specific CD8+ T cells (34). Building on this protocol, we have developed methods to efficiently generate, maintain and expand SIV-specific CD8+ T-cell clones from SIV-infected rhesus macaques (35).
It has been suggested that current vaccines against SIV/HIV fail because by the time the memory CD8+ T-cell responses expand after the viral challenge, usually 2-3 weeks post infection (36), the virus has gained an upper hand during this crucial delay and the immune system can not catch up: a model described as the “too little too late” hypothesis (37). In this study, we investigated whether large numbers of SIV-specific CD8+ T cells can impact the acute course of infection. To this end, we generated and characterized central and effector memory-derived CD8+ T-cell clones from SIV DNA vaccinated rhesus macaques specific for epitopes within the SIV proteins Gag, Nef and Vif and performed autologous adoptive transfers after large scale expansion. The hosts were intravenously challenged with high dose SIVmac239 three days before the CD8+ T cell adoptive transfers. The impact of the transferred cells was monitored by tracking their distribution and persistence, measuring the viral load and assessing disease progression.
Materials and Methods
Animals and generation of SIV-specific CD8+ and autologous CD4+ T-cell clones
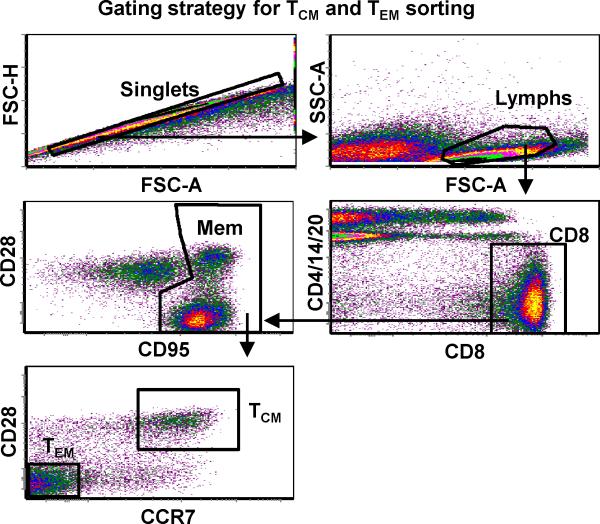
SIV-specific CD8+ T cells were generated from PBMC isolated from 2 uninfected Indian Rhesus macaques, Macaca mulatta (DBN2; Mamu A*01+/A*02+ and AZ15; Mamu A*02+ ) two months after a third immunization using electroporation and SIV DNA vaccine constructs. The DNA vaccine contructs consisted of full length SIVmac239 Gag, Pol, Vif, Tat and Nef sequences as previously described ((38), Patel et al., submitted for publication and Rosati et al., submitted for publication) and an optimized rhesus IL-12 expression plasmid as adjuvant (Jalah et al., in preparation). CD8+ T-cell clones against the SIV Gag181-189CM9 epitope (CM9) or pools of 15-mer overlapping peptides spanning SIV Gag and the accessory proteins Nef, Vif and regulatory protein Tat (Acc) were generated from PBMC from DBN2, and against the Gag and Acc peptide pools for AZ15, as described previously (39). Briefly, isolated PBMC were sorted into CD8+ central and effector memory T cells based on CD28, CD95 and CCR7 expression (central memory, TCM: CD28+CD95+CCR7+; effector memory, TEM: CD28−CD95+CCR7−; further details in next section) (Fig. 1). The sorted TCM and TEM cell fractions were stimulated for 1 week with irradiated autologous PBMC pulsed with CM9 peptide (1 μg/mL: SynPep Corp., Dublin, CA) (40), SIV Gag or Acc peptide pools (1 μg/mL: NIH AIDS Research and Reference Reagent Program, Germantown, MD) and the cells restimulated weekly with peptide-pulsed, irradiated autologous PBMC in the presence of recombinant human IL-2 (50 IU/mL: NIH AIDS Research and Reference Reagent Program). Following 2 rounds of peptide stimulation, the virus-specific CD8+ T cells were cloned by limiting dilution and maintained essentially as described in Riddell et al., (41) and Berger et al., (42) using bi-weekly stimulation with anti-CD3 monoclonal antibody (mAb) (30 ng/mL; clone SP34-2; BD Biosciences, San Jose, CA) and irradiated human PBMC and human Epstein-Barr virus transformed B-cell lines (TM B-LCL; kindly provided by Drs. S.R. Riddell and P. D. Greenberg, FHCRC, Seattle, WA) as feeder cells, but without anti-CD28 mAb stimulation. APC and feeder cells were irradiated in a Mark I Cs137 γ-irradiator (Shepherd & Associates, San Fernando, CA) at 6,000 and 12,500 rad for PBMC and TM B-LCL, respectively. Positive wells were tested for antigen specificity by flow cytometry using intracellular cytokine staining (ICS) for IFN-γ production and, in the case of SIV Gag CM9-specific CD8+ T cells, by staining with a CM9 peptide MHC class I/tetramer (Beckman Coulter, Miami, FL). SIV non-specific autologous CD4+ T-cell clones were generated from PBMC from DBN2 and AZ15 as described (39). Animal care was according to the guidelines of the Committee on the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council, and the Health and Human Services guidelines “Guide for the Care and Use of Laboratory Animals” (National Research Council, 1996, National Academy Press, Washington, D.C.), under an Institutional Animal Care and Use Committee approved protocol.
Figure 1.
Gating strategy and flow cytometry sorting analysis of central (TCM) and effector (TEM) memory CD8+ T-cell populations from a rhesus macaque (DBN2) immunized with a DNA vaccine construct containing full length SIVmac239 Gag, Pol, Vif, Tat, and Nef sequences. CD8+ lymphocytes were singlet-gated and defined as memory cells based on surface expression of CD95. TCM and TEM fractions were further defined and sorted based on CD28 and CCR7 expression.
Cell sorting and flow cytometry
Whole PBMC were stained with flourochrome conjugated mAbs (all mAbs from BD Biosciences, unless otherwise indicated) to CD4 (clone L200), CD8 (clone SK1), CD14 (clone MφP9), CD20 (clone 2H7), CD28 (clone CD28.2; Beckman Coulter), CD62L (clone SK11), CCR7 (clone 150503; R&D systems, Minneapolis, MN) and CD95 (clone DX2). The CD8+ T-cell fractions were then sorted into TCM (CD28+CD95+CCR7+) and TEM (CD28−CD95+CCR7−) by flow cytometry using a BD FACS Aria (BD Biosciences) prior to SIV peptide stimulation and cloning (Fig. 1). TEM clones were further sorted for the CD8+ T cell degranulation marker CD107a after SIV peptide stimulation using flourochrome conjugated CD107a mAb (clone H4A3).
Epitope mapping
CD8+ T cell epitopes were mapped by stimulation for 18-24 h with pools of 15-mer overlapping peptides spanning the entire SIV Gag and Acc peptide sequences in a peptide matrix IFN-γ ELISpot as described (43). Assays were repeated with each individual 15-mer or derivative 9-mer, for SIV Gag CM9 and Nef159-167YY9 (YY9) (44), or 8-mer, for SIV Vif97-104WY8 (WY8) (44, 45), peptide to confirm the epitope specificity.
In vitro functional and phenotypic characterization of CD8+ T-cell clones
In vitro reactivity of the virus-specific TCM- and TEM-derived CD8+ T-cell clones was assessed by measuring intracellular IFN-γ and surface CD107a expression following stimulation with autologous PBMC pulsed with SIV Gag CM9, Nef YY9, Vif WY8 peptides or the relevant Gag or Acc peptide pools and SIVmac239-infected autologous CD4+ T cells. Briefly, CD8+ T-cell clones were co-cultured with PBMC that were pre-pulsed for 1 h with the appropriate peptide or peptide pool (1 μg/mL), or with virus-infected autologous CD4+ T cells as described (35); a CD8+:CD4+ T cell ratio of 1:1 was used in 0.5 mL suspension of 1 × 106 cells total in 5 mL polypropylene tubes. Non-pulsed autologous PBMC or uninfected autologous CD4+ T cells were included as negative control APC. PE conjugated anti-human CD107a mAb was added to the cell suspensions before incubation. Monensin, 20 μL/test of a 1:20 dilution, (Golgi stop™; BD Biosciences) was added after 1 h of incubation and the cells incubated for additional 4 h. Cells were washed and surface and intracellular stained with PerCP-Cy5.5 conjugated anti-human CD8 mAb and FITC conjugated anti-human IFN-γ mAb (clone 4S.B3), respectively. Samples were acquired on a BD FACSCalibur flow cytometer (BD Biosciences) and data analyses performed using FCS Express Version 3 (De Novo Software, Los Angeles, CA). Dead cells were excluded from the analyses based on forward versus side scatter gating, and at least 100,000 live cell events collected for each sample.
Virus-specific CD8+ T-cell clones with confirmed in vitro reactivity to cognate peptide-pulsed or SIV-infected autologous CD4+ T cells were analyzed for surface expression of chemokine receptors/homing markers as well as PD-1 using the following mAbs (all mAbs were from BD Biosciences, unless otherwise indicated): CCR5 (clone 3A9), CCR7 (clone 150503), CCR8 (clone 191704, R&D Systems), CCR9 (clone 112509.111, R&D Systems), CD103 (clone 2G5), α4β7 (clone ACT1) and PD-1 (R&D Systems, catalog number BAF1086). An anti-human CD45 mAb (clone HI30) that does not cross-react with rhesus macaque cells was included to exclude human feeder cells. Sample acquisition and analyses was as described in Bolton et al. (submitted for publication).
Virus stocks
Virus stocks for infection of CD4+ T cells used as APC in in vitro assays were produced by transfection of HEK293T cells with SIVmac239 using TransIt®-293 reagent (Mirus Corporation, Madison, WI) as described (46). CD4+ T cells were infected by incubating with aliquots of virus stock for 2-3 h using the Viromag magnetofection™ reagents; a ratio of 7.5 μl of beads per mL of clarified transfection supernatant was used as recommended by the manufacturer (OzBiosciences, Marseille, France). Virus stocks with ~1 × 109 viral RNA copies Eq/mL in a volume of 250 μL were added per 1×106 CD4+ T cells. Virus exposed CD4+ T cells were cultured for 7 days, with IL-2 addition every 2-3 days at a final concentration of 50 IU/mL prior to use as APC to ensure optimal numbers of SIV-infected cells (35).
Infection of rhesus macaques
A stock of SIVmac239 with an in vivo titer of 3.2×105 AID50/mL was used for animal infections. This virus stock was a kind gift from Dr Ronald C. Desrosiers of the New England Regional Primate Research Center, Harvard Medical School, Southborough, MA (17). All three monkeys were infected 7 months after the third DNA vaccine administration intravenously (via the saphenous vein) and three days prior to CD8+ T cell infusion using a dose of 100 AID50.
In vitro CD8+ T cell expansion, adoptive transfer and monitoring of persistence in vivo
Two Gag CM9- and Vif WY8-specific CD8+ T-cell clones (one clone of each specificity TCM- and the other TEM-derived) from DBN2 as well as one Vif WY8-specific TCM-derived and two Nef YY9-specific TEM-derived CD8+ T-cell clones from AZ15 were selected for expansion and adoptive transfer. CD8+ T-cell clones were selected based on in vitro reactivity (IFN-γ, CD107a) to autologous peptide-pulsed PBMC and virus-infected CD4+ T cells. The CD8+ T-cell clones of interest were expanded in vitro for 6-8 weeks through repeated cycles of bi-weekly stimulation with anti-CD3 mAb (BD Biosciences) and irradiated human PBMC and human TM B-LCL as feeder cells to obtain billions of cells of each clone as described (35, 39). To track the tissue distribution and in vivo persistence of infused cells, half of each clonal population was stained with a fluorescent dye, TCM and TEM labeled with PKH26 (red dye; Sigma-Aldrich, St. Louis, MO) and CFSE (green dye; Invitrogen, Carlsbad, CA), respectively, and then pooled with their unlabeled counterparts.
TCM and TEM clones from each animal were combined, washed extensively, resuspended in 50 mL saline solution supplemented with 2% autologous serum, and infused (1.5 mL/min) intravenously to the autologous animal. AZ15 and DBN2 were infused with approximately 4 and 12 billion CD8+ T cells total, respectively. The animals were administered low dose (104 U/kg/day) IL-2 daily for 10 days to support infused T cell survival and proliferation (24), as was the control. Blood was collected from all three monkeys before and 30 min post infusion for the 2 monkeys that received cells and then from all three monkeys every other day during the first week post infusion and once a week thereafter. Bronchoalveolar lavage (BAL), gut-associated lymphoid tissue (GALT) and lymph node (LN) samples were also collected from all three animals 2 and 9 days post infusion (i.e. days 5 and 12 post challenge) and BAL once a week thereafter. Blood, BAL, GALT and LN biopsies were collected 3 weeks prior to virus challenge and 6 months after receiving the third DNA vaccine immunization to determine the baseline (memory vaccine-induced) CD8+ T-cell responses to SIV Gag and Acc peptide. CD4+ T-cell counts were monitored using BD Tru Count™ absolute cell counting tubes, according to the manufacturer's recommendations (BD Biosciences).
Viral DNA and RNA measurements
Cell-associated viral DNA was extracted from PBMC, BAL, LN and GALT samples using the Qiagen DNA Mini Kit as recommended by the manufacturer (Qiagen, Valencia, CA). The copy numbers of SIV DNA (gag-specific target) and of the macaque CCR5 gene, for cell equivalents, were co-determined in a duplex format QPCR. The assay was as described in Cline et al., (47) with omission of the reverse transcription step, and with the addition of primers and probe (100 nM final concentration each) specific for the macaque CCR5 sequence and use of the plasmid pR1-D, of the Macaca mulatta CCR5 gene promoter region (48) as a quantitation standard (Genbank Acc. No. AF252567; http://www.ncbi.nlm.nih.gov/nuccore/9488623?report=genbank; this was a kind donation of Dr. Sunil K. Ahuja of the University of Texas Health Science Center, San Antonio, Texas). The duplex QPCR assay was run on an MX3000P™ instrument (Stratagene, La Jolla, CA) and results are reported as nominal SIV gag DNA copy numbers per 100,000 cell equivalents determined by copy numbers of the CCR5 sequence and based on 2 nominal copies of CCR5 per rhesus macaque cell (M. Piatak, unpublished results).
Viral RNA was extracted from plasma, BAL, LN and GALT samples essentially as described previously (47). Viral replication was quantified using a FRET probe-based real time RT-PCR (TaqMan) assay described (17, 47). All RT-PCR reactions were run on ABI Prism 7700 Sequence Detection System and the fluorescent signal-based quantitation of viral RNA copy numbers in test samples were determined by ABI sequence detection software (Applied Biosystems, Foster City, CA).
Sequencing of SIVmac239 regions encoding relevant CD8+ T cell epitopes
SIVmac239 regions encoding Gag CM9, Nef YY9, Tat28-35SL8 (SL8), and Vif WY8 were sequenced as described (47). Briefly, cell-free plasma was obtained by centrifugation of EDTA anti-coagulated whole blood on a Ficoll density gradient and viral RNA extracted for use in RT-PCR as previously reported (47). RT was performed by using SuperScript first strand synthesis system for RT-PCR (Invitrogen). PCR reactions were carried out in 50 μL volumes; the PCR buffer contained 200 μM dNTP, 300 nM of each primer, 1.5 mM MgCl2 and 1.0 unit of platinum Taq polymerase. The PCR conditions were 94°C for 2 min followed by 45 cycles of 94°C for 30 sec, 52°C for 30 sec and 72°C for 30 sec. The primers for each epitope were; CM9 pair: 5’-ATGCCAAAACAAGTAGACCA-3’ and 5’-GATCCTGACGGCTCCCTAAG-3’, YY9 pair: 5’-GAGGCCAAAAGTTCCCCTAA-3’ and 5’-TCTTGCGGTTAGCCTTCTTC-3’, SL8 pair: 5’-AACCATGGGATGAATGGGTA-3’ and 5’-GCCTTAGCCTTTTTCGGAGT-3’ and WY8 pair: 5’-GTTTGCTATGTGCCCCATTT-3’ and 5’-TGTTTCCAGGTGGGATTCTC-3’. Both strands of each amplicon were directly sequenced and nucleotide sequences were aligned pair wise to the GenBank SIVmac239 sequence (Accession no. M33262.1; http://www.ncbi.nlm.nih.gov/nuccore/334647?report=Summary) (49). Nucleotide changes in and around the region encoding the epitope resulting in amino acid replacements as well as silent mutations were noted.
Results
Generation and characterization of SIV-specific TCM- and TEM-derived CD8+ T-cell clones
Attempts by our group to in vitro prime SIV-specific CD8+ T-cell clones from naïve rhesus macaques for use in autologous adoptive transfer studies using autologous peripheral blood monocyte-derived dendritic cells pulsed with viral peptides failed to generate clones of sufficient functional avidity to react to SIV-infected cells in vitro (data not shown). To facilitate efficient priming, we therefore chose to prime a cohort of rhesus macaques by DNA vaccination using electroporation of a mixture of optimized plasmids expressing the SIVmac239 proteins Gag, Pol, Nef, Tat and Vif, and an optimized rhesus IL-12 expression plasmid as adjuvant. All vaccinated monkeys mounted robust cellular and humoral responses to the relevant SIV antigens after three rounds of vaccination; the detailed analyses of the vaccine-induced responses are being reported elsewhere (Patel et al., submitted for publication). Virus-specific CD8+ T-cell clones were generated from two of the DNA vaccinated animals (DBN2, Mamu A*01+/A*02+; AZ15, Mamu A*02+) two months after the third DNA immunization. A third vaccinated monkey (DBK1, Mamu A*01+), was used as a control for vaccine-induced antiviral effect. Given a recent report by Berger et al., showing enhanced survival/persistence of TCM- compared to TEM-derived CMV-specific CD8+ T-cell clones in vivo (34), we sorted CD8+ T cells from PBMC from DBN2 and AZ15 into TCM and TEM based on CD28, CD95 and CCR7 expression (TCM: CD28+CD95+CCR7+ and TEM: CD28−CD95+CCR7−) (Fig. 1) prior to stimulation with SIV peptides or peptide pools and limiting dilution cloning (35, 39). We reasoned that targeting multiple epitopes from more than one SIV protein would provide a better chance for infused virus-specific CD8+ T cells to have an impact on virus replication in infected monkeys. We therefore isolated multiple CD8+ T-cell clones specific for the Mamu A*01-restricted SIV Gag CM9 epitope, and the Mamu A*02-restricted SIV Nef YY9, and Vif WY8 epitopes for their ability to produce IFN-γ and the CD8+ T cell degranulation marker CD107a upon peptide stimulation, as well as to proliferate robustly (data not shown). The clones were analyzed for intracellular IFN-γ and surface CD107a expression by flow cytometry following stimulation with SIV peptide-pulsed autologous PBMC and SIV-infected autologous CD4+ T-cell clones.
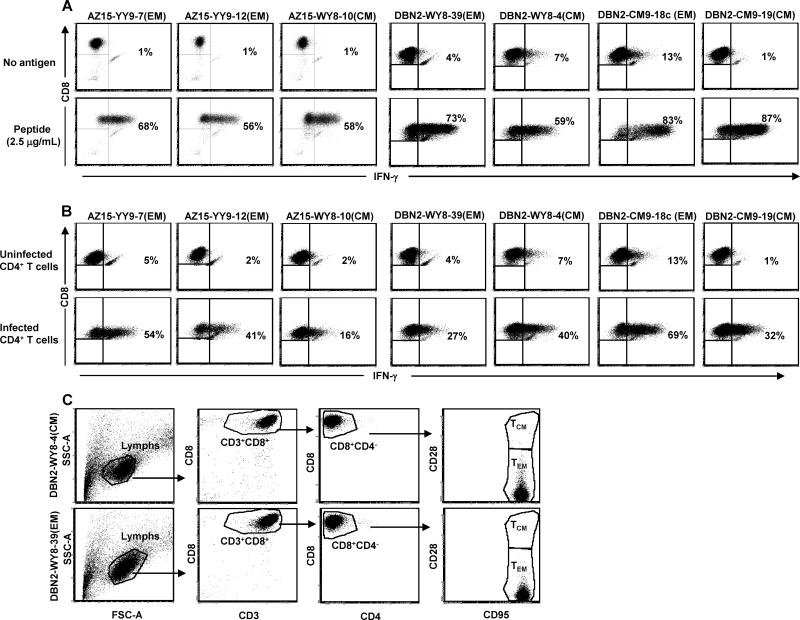
From these analyses, we selected a series of clones from the two macaques for infusion (Table 1). These TCM and TEM clones showed robust IFN-γ (Fig. 2A) and CD107a responses (data not shown) to peptide-pulsed APCs and to autologous SIV-infected CD4+ T cells (Fig. 2B), a more biologically relevant stimulus. Regardless of TCM or TEM origin, all clones had obtained an effector memory phenotype (CD8+CD28−CD95+CCR7−) after in vitro culture (Fig. 2C; data not shown), consistent with previous observations by Berger et al., for CMV-specific clones from M. nemestrina (34). Comprehensive analyses of the CD8+ T-cell clones selected for adoptive transfer for surface expression of chemokine receptors/homing markers and the marker of T-cell exhaustion, PD-1, showed clonal differences in the pattern and levels of expression of these markers after in vitro expansion not related to their in vivo origin i.e., TCM versus TEM. Overall, most of the clones were CCR5high, but showed low to negative expression of CCR9, CCR7 and CCR8 while expression of the gut homing markers, α4β7 and CD103, or the exhaustion marker, PD-1, varied between the clones (Supplemental Fig. 1; example for one TCM- and one TEM-derived clone).
Table I.
CD8+ T cell specificities, MHC class I restriction and number of cells infused
| Monkey ID (Mamu) | CD8+ T cell specificity (origin)a | Mamu restriction | bNumber of cells infused |
|---|---|---|---|
| AZ15 (A*02) | Nef-YY9-7 (TEM) | A*02 | 0.8×109 |
| Nef-YY9-12 (TEM) | A*02 | 1.4×109 | |
| Vif-WY8-10 (TCM) | A*02 | 1.6×109 | |
| DBN2 (A*01/*02) | Gag-CM9-18c (TEM) | A*01 | 5.8×109 |
| Gag-CM9-19 (TCM) | A*01 | 2.6×109 | |
| Vif-WY8-4 (TCM) | A*02 | 2.1×109 | |
| Vif-WY8-39 (TEM) | A*02 | 1.6×109 | |
| DBK1 (A*01) | No CD8+ T cells | N/A | N/A |
CD8+ T cells from AZ15 and DBN2 were sorted based on surface phenotype (central memory T cells, TCM: CD28+CD95+CCR7+; effector memory T cells, TEM: CD28− CD95+CCR7−) before generation of SIV-specific lines, cloning and characterization in vitro.
Total number of cells of each clone included in pool of cells infused intravenously; half of the cells from each clone were stained with either PKH26 (TCM) or CFSE (TEM). N/A, not applicable.
Figure 2.
TCM- and TEM-derived CD8+ T-cell clones exhibit similar functional reactivity and both display an effector memory surface phenotype after culture in vitro. TCM- and TEM-derived SIV-specific CD8+ T-cell clones from two rhesus macaques, AZ15 and DBN2, were stimulated with PBMC pulsed with the appropriate peptides (A) or autologous SIV-infected CD4+ T-cell clones (B). IFN-γ-expression by the TCM- and TEM-derived clones was measured by ICS and flow cytometry. The surface phenotype of TCM- and TEM-derived clones was determined using flourochrome conjugated mAbs to CD28 and CD95 followed by flow cytometric analyzes (C).
Challenge with SIVmac239 and adoptive transfer of autologous SIV-specific CD8+ T-cell clones
Data from a parallel study with chronically infected rhesus macaques showed limited persistence of infused autologous virus-specific CD8+ T cells in PBMC (Bolton et al., submitted for publication). Thus, adoptive transfer at time of infection could result in clearance of infused cells before appreciable virus replication starts. To avoid this scenario and to ensure that the maximum numbers of infused cells are present at time of a limited infection, the monkeys were infected intravenously with 100 AID50 of SIVmac239 virus three days before T cell adoptive transfer. Plasma viral loads were analyzed on day 3 post infection (day of infusion) by highly sensitive QPCR, and all three animals showed low but detectable viremia (AZ15 = 180 RNA copies/mL; DBN2 = 1200 copies/mL; DBK1 = 200 copies/mL; limit of detection > 30 RNA copies/mL).
SIV Gag CM9- and Vif WY8-specific clones (one TCM- and TEM-derived clone of each specificity) from DBN2 and two TEM-derived Nef YY9 and one TCM-derived Vif WY8-specific CD8+ T-cell clones from AZ15 were expanded in vitro to obtain 0.8-6×109 cells of each clone (Table 1). To track the different clones after transfer, half of the cells of each clonal population were stained with a fluorescent dye: TCM and TEM were labeled with PKH26 and CFSE, respectively. The cells were pooled and infused intravenously to the autologous animal. The animals received a low dose (104 U/kg/day) of IL-2 for 10 days post infusion to support survival and proliferation. Because Picker et al., have previously shown that increased CD4+ T cell activation following IL-15 treatment does not elevate plasma viremia in chronically infected rhesus macaques (50), we did not anticipate any effects of IL-2 on viral load. The treatment was well tolerated and no adverse effects were observed.
SIV-specific TCM- and TEM-derived CD8+ T cells show clonal differences in persistence in vivo independent of TCM versus TEM origin
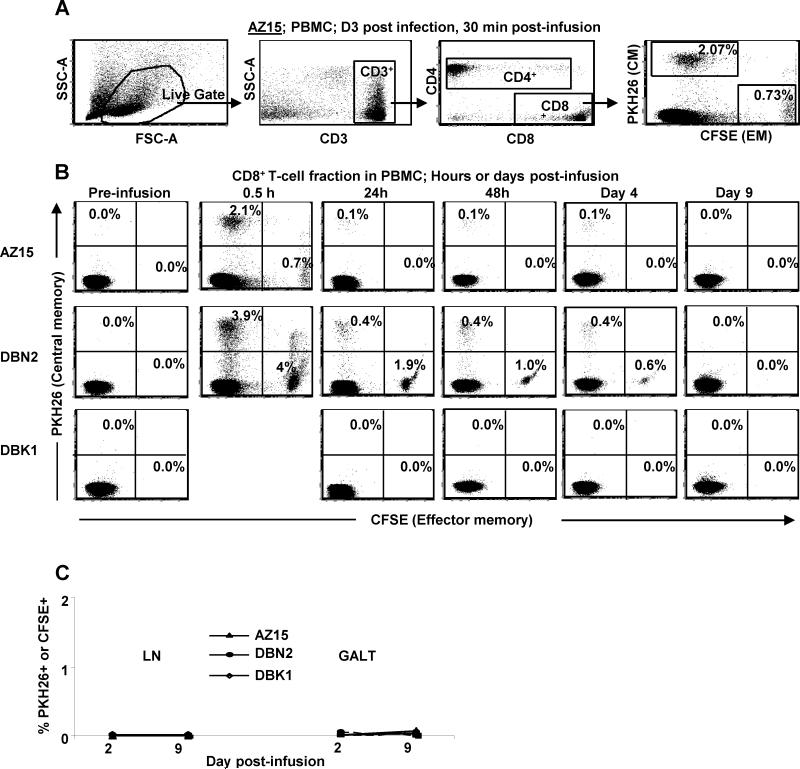
To assess the immediate engraftment of infused cells, we analyzed the CD3+CD8+ fraction of the lymphocyte gate in the 30 min post infusion blood samples for labeled cells (Fig. 3A). Taking into account that only half of the infused cells were labeled, approximately 5.6 and 16% of the CD8+ fraction of PBMC collected 30 min post infusion from AZ15 and DBN2, respectively, were infused cells (Fig. 3B). The TCM-derived infused cells were approximately 4 and 8% of the CD8+ T-cell fraction in PBMC from AZ15 and DBN2, respectively, and the corresponding numbers for TEM-derived cells were approximately 1.4 and 8%. The higher overall frequencies of the infused CD8+ T cells seen 30 min post infusion in PBMC from DBN2 correlated with the 3-fold higher number of cells infused in this monkey compared to AZ15 (Table 1). The frequency of labeled cells in PBMC declined with a similar kinetics in both animals and although present at day 4, no labeled cells could be detected by day 9 post infusion. The TCM-derived cells from AZ15 persisted better in PBMC than the TEM-derived clones, despite the close to 1.5-fold higher frequency of TEM- compared to TCM-derived cells in the initial pool of infused CD8+ T cells for this monkey. In contrast, the TEM-derived clones, which were about 1.5-fold more frequent than the TCM-derived cells in the initial pool of infused cells, persisted better in DBN2. To determine homing to lymphoid and mucosal tissue, biopsies from LN and GALT samples were analyzed on days 2 and 9 post infusion but no labeled cells could be detected in either tissue at any of these time points (Fig. 3C). Thus, the differences we observed in persistence of infused SIV-specific CD8+ T-cell clones in PBMC seemed to be clonal in nature, and not related to their TCM or TEM origin.
Figure 3.
Intravenously infused ex vivo expanded virus-specific TCM- and TEM-derived CD8+ T-cell clones show similar distribution and persistence in vivo. Half of the clonal population of TCM- and TEM-derived virus-specific CD8+ T-cell clones from two rhesus macaques, DBN2 and AZ15, were stained with PKH26 and CFSE, respectively, and the pool of stained and unstained cells adoptively transferred to the monkeys. The distribution and persistence of the infused cells was analyzed by flow cytometry by gating for the CD3+CD8+ T-cell fraction of the “live gate” of the forward and side scatter plot (A). PBMC (B), LN and GALT (C) samples were analyzed at indicated time points post infusion.
Transferred SIV-specific CD8+ T cells show no discernable impact on plasma virus load or CD4+ T cell preservation
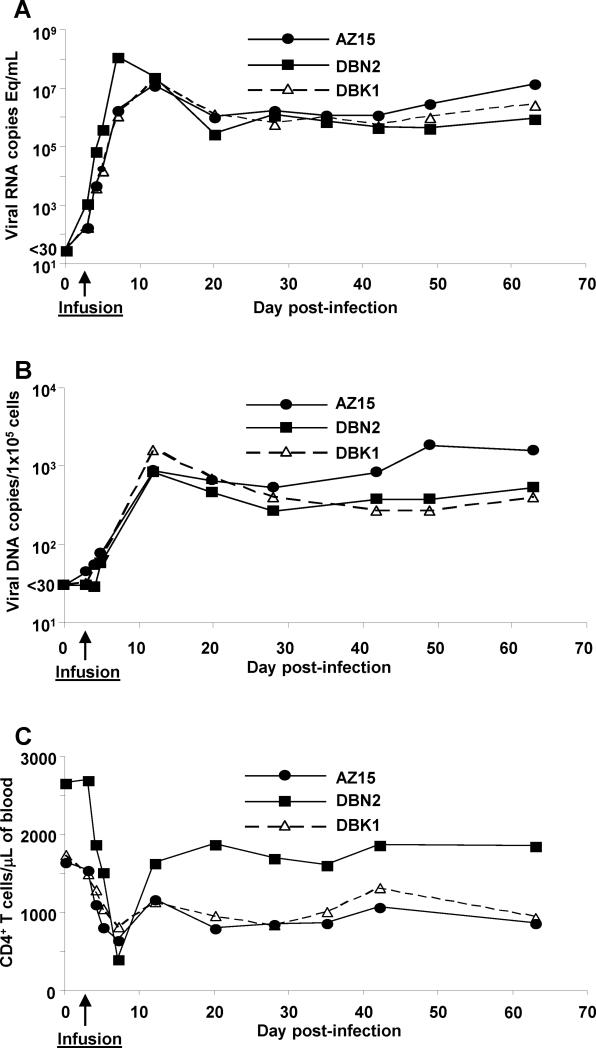
To assess the effect of the transferred virus-specific CD8+ T cells on virus replication in vivo, we measured viral RNA and DNA levels by QPCR in blood, LN and GALT samples at different time points post infection. While one infused monkey had a slightly faster kinetics and higher peak plasma RNA compared with the control and second infused animal, similar levels of plasma viral RNA (Fig. 4A) and cell-associated viral DNA and RNA per 100,000 PBMC (Fig. 4B) were observed for all three monkeys at ramp-up as well as set point. Similar to our findings in PBMC, comparable levels of cell-associated viral DNA and RNA were observed in LN and GALT biopsies (Table II). At certain time-points, viral load data from the control animal were elevated in either tissue compared with infused monkeys, but this was reversed at other time-points. Thus, the infused virus-specific CD8+ T cells had no measurable effect on either the amount of plasma virus or the number of infected cells in PBMC, LN or gut mucosa; the DNA vaccination, as used in the present study, did not appear to provide meaningful acute protective effect against high-dose intravenous challenge with SIVmac239.
Figure 4.
Adoptive transfer of ex vivo expanded virus-specific CD8+ T-cell clones show no measurable effect on the virus load or the number of circulating CD4+ T cells in peripheral blood. Virus load in two animals infused with virus-specific CD8+ T cells, DBN2 and AZ15, as well as a control animal, DBK1, was determined on day 0, 3 (day of infusion), 4, 5 and 7 post challenge and once a week thereafter, by analyzing cell-free plasma viral RNA (A) or cell-associated viral DNA (B) levels by QPCR. The frequency of circulating CD4+ T cells in PBMC was determined using the BD Tru Count™ kit (C).
Table II.
Cell-associated viral DNA and RNA in lymph node and GALT biopsies.
| Tissue | Parameter | DNAb | RNAc | ||||
|---|---|---|---|---|---|---|---|
| Day PIa | AZ15 | DBN2 | DBK1 | AZ15 | DBN2 | DBK1 | |
| GALT | 12 | 1.8×104 | 1.8×103 | 1×103 | 1.9×106 | 5.6×104 | 2.2×104 |
| Lymph node | 5 | 9.3×101 | 3.4×101 | 8×100 | 5.4×103 | 1.5×103 | 7×101 |
| 12 | 2.6×103 | 1.7×103 | 3.6×103 | 5.2×104 | 5.7×104 | 1.3×105 | |
PI, post infection
Cell-associated viral DNA copies per 100,000 cells
Cell-associated viral RNA copies per 100,000 cells. GALT; gut-associated lymphoid tissue i.e. jejunal biopsies collected by upper endoscopy.
We next investigated if virus-specific CD8+ T cell infusion during the acute phase of infection would lead to better preservation of CD4+ T cells. There was a steady loss of CD4+ T cells during the first week post challenge dipping to below 50% of pre-challenge baseline counts for AZ15 and DBK1, or as low as 20% for DBN2, before rebounding to a set point of about 60% of the frequency seen on the day of challenge (day 0) for all three monkeys (Fig. 4C). Thus, there was no measurable effect of the SIV-specific CD8+ T cell infusions on the CD4+ T cell counts.
Adoptively transferred SIV-specific CD8+ T cells persist for more than 6 weeks in lungs with no apparent impact on local viral load
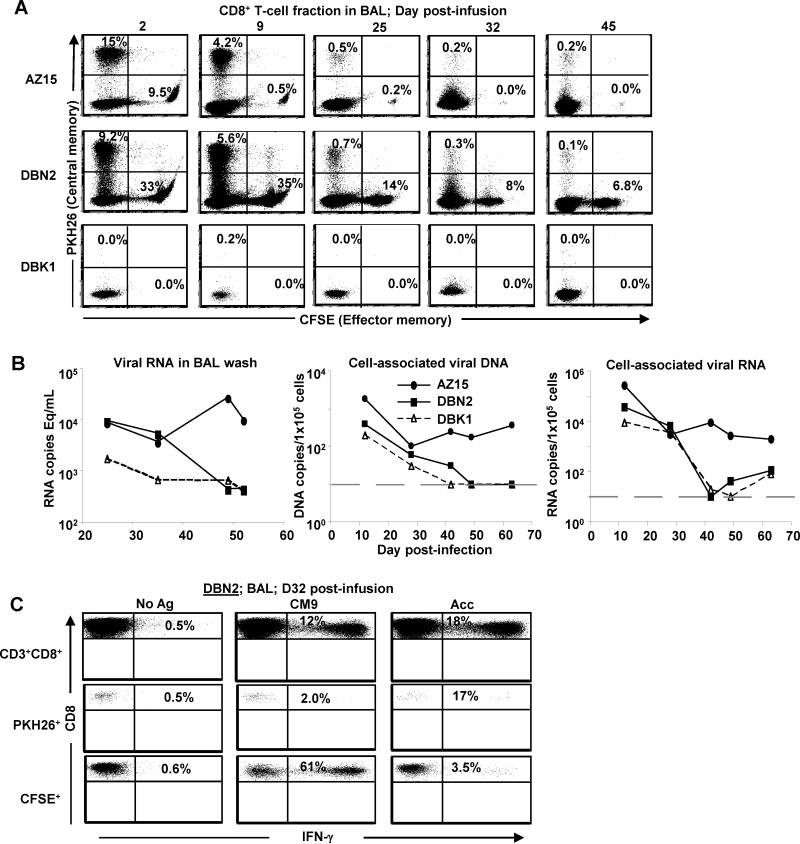
BAL represents a conveniently sampled compartment for assessment of immune responses at a mucosal site and it has been shown that lymphocytes present in the lungs share similarities with those present in the upper gastrointestinal tract (51). BAL samples were collected on day 2 post infusion and once a week thereafter and the frequency of CD3+CD8+PKH26+ (TCM) and CFSE+ (TEM) cells in the lungs of the monkeys was determined by flow cytometry.
After adjusting for labeling and assuming comparable survival and persistence of stained and unstained cells, the infused cells (PKH26+ and CFSE+) made up about 50 and 84% of CD8+ T cells in BAL samples from AZ15 and DBN2, respectively, 48 h post infusion (Fig. 5A). Strikingly, the infused cells persisted for a long period in the lungs with substantial frequencies observed more than 6 weeks post infusion (e.g ~14% of CD8+ T cells in BAL from DBN2 on day 45 post infusion were infused cells) (Fig. 5A). Similar to our observation in PBMC, the TCM-derived CD8+ T-cell clones persisted in the lungs longer than the TEM-derived cells in AZ15 while the TEM-derived cells in DBN2 showed better persistence than TCM-derived cells.
Figure 5.
T cell persistence, function and viral load in BAL samples. (A) Virus-specific TCM- and TEM-derived CD8+ T-cell clones from two rhesus macaques, DBN2 and AZ15, half of which were stained with PKH26 and CFSE, respectively, were infused 3 days after challenge with SIVmac239. Infused cells were tracked using flow cytometry at indicated time points (B). Cell-free viral RNA and cell-associated viral DNA and RNA were determined at indicated time-points by QPCR. DBN2 and AZ15 received virus-specific CD8+ T cells. Grey dashed lines show threshold of detection of cell-associated viral DNA and RNA (>10 copies/100,000 cells). (C) Cells from BAL from DBN2 were stimulated with SIV Acc peptide pool or the SIV Gag CM9 peptide as indicated. The frequency of IFN-γ-expressing cells was determined by ICS and flow cytometry.
Because the infused virus-specific CD8+ T cells persisted for long periods in the lungs, we investigated if virus could be detected in BAL. BAL supernatant or cell pellets from BAL were used to measure cell-free viral RNA or cell-associated viral DNA and RNA by QPCR. We detected cell-free and cell-associated viral RNA and DNA in BAL from all three animals (Fig. 5B). Initially the levels were similar, but from 4 weeks post infection the two Mamu A*01+ animals, DBN2 and DBK1, showed greater than 1-log lower cell-free and cell-associated viral load than the Mamu A*01 negative monkey, AZ15. Our data thus indicate that even though the infused virus-specific CD8+ T cells persisted in the lungs, they did not have any measurable effect on virus load in this mucosal compartment.
Adoptively transferred SIV-specific CD8+ T cells recovered from lungs one month post infusion retain antigen specific reactivity
Due to our finding of comparable virus load in the animals infused with virus-specific CD8+ T cells and the control monkey, we investigated whether the infused cells retained functional activities. Cells recovered from BAL samples collected five weeks after infection (day 32 post infusion) in DBN2 were stimulated with the SIV Gag CM9 peptide or Acc peptide pool and the frequency of IFN-γ-producing cells determined by flow cytometry. Approximately 12% of BAL-derived CD8+ T cells from DBN2 (Mamu*A01+) responded to SIV Gag CM9 peptide stimulation. When BAL cells from DBN2 were gated based on PKH26 and CFSE staining, 2% and 61% of the PKH26+ (TCM) and CFSE+ (TEM) cells, respectively, responded to SIV Gag CM9 peptide (Fig. 5C). The corresponding numbers for SIV Acc peptide pool reactive CD8+ T cells in BAL samples from DBN2 were approximately 18, 17 and 3.5%, respectively. As seen in Fig. 5A, few infused cells persisted in AZ15 at the time point for this analysis (day 32 post infusion). However, despite the low frequency, 11 and 40% of the persisting PKH26+ (TCM) and CFSE+ (TEM) cells, respectively, in BAL from this monkey showed IFN-γ responses following stimulation with SIV Acc peptide pool (data not shown). Thus, infused CD8+ T cells persisting in the lungs retained antigen specificity and reactivity 32 days post infusion.
Endogenous CD8+ T cell responses to SIV are moderately reduced in magnitude but the kinetics of anamnestic expansion are not affected by SIV-specific CD8+ T cell infusion
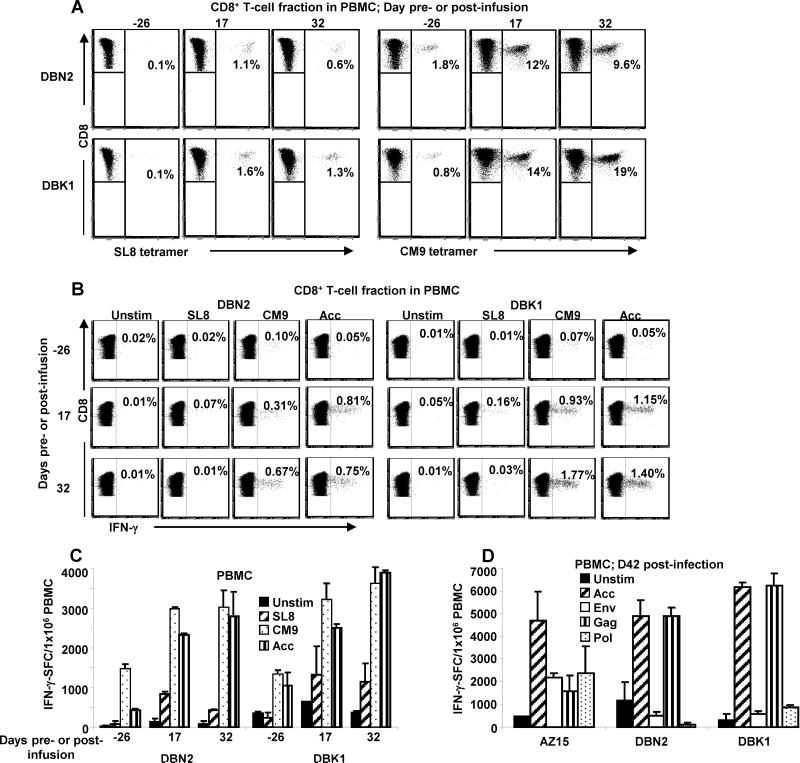
To investigate the potential impact of the infused CD8+ T cells on the magnitude and kinetics of endogenous responses, we next analyzed the development of virus-induced CD8+ T cell responses in the animals. The frequencies of SIV Gag CM9 and Tat SL8 responding CD8+ T cells in PBMC from the Mamu A*01+ animals, DBN2 (infused) and DBK1 (control), collected pre-infection and at two different time points post infection were determined by MHC class I/tetramer staining followed by flow cytometry (Fig. 6A). Overall, both animals had a similar expansion of endogenous CM9- and SL8-specific responses, with DBN2 having a somewhat lower response.
Figure 6.
Adoptive transfer of virus-specific CD8+ T cells does not significantly affect the endogenous SIV-specific T-cell response. (A) PBMC collected at the indicated time points from the two Mamu A*01 positive monkeys, DBN2 and DBK1 were stained with SIV Tat SL8 or Gag CM9 tetramers and CD8 mAb. (B) PBMC from DBN2 and DBK1 collected pre- and post- infusion were stimulated with SIV Tat SL8, Gag CM9 and Acc peptide pool and the percentage of CD8+ IFN-γ-producing T cells determined by flow cytometry, or (C) the number of IFN-γ-producing cells determined by ELISpot. (D) PBMC from AZ15, DBN2 and DBK1 collected on day 39 post infusion were stimulated with SIV Acc, Env, Gag and Pol peptide pools and the number of antigen-induced IFN-γ-producing T cells determined by ELISpot.
A similar pattern was observed when we evaluated virus-specific T-cell function by measuring the percentage of IFN-γ-producing CD8+ T cells in PBMC following stimulation with SIV Gag CM9, Tat SL8 and Acc peptide pool by flow cytometry (Fig. 6B) or the number of IFN-γ-producing cells in PBMC in response to these antigens by ELISpot (Fig. 6C). Thus, while the baseline levels of the endogenous vaccine-induced responses as well as the kinetics of development of virus-specific CD8+ T cell responses following challenge were similar for the infused animal DBN2 and the control animal DBK1, the CD8+ T-cell responses post challenge were somewhat higher in the control monkey. In line with the diminished endogenous responses in DBN2 compared with the control DBK1, we observed an escape mutation in the SIV Tat SL8 epitope between 3 and 8 weeks post infection in virus recovered from plasma from DBK1 but not from virus in DBN2 (data not shown). Because escape mutations are known to occur in this epitope very early during acute infection (36, 45), our finding may suggest a skewing of the early endogenous response, with a dampened Tat SL8 response in DBN2 following infusion of CD8+ T cells specific to other epitopes (Gag CM9 and Vif WY8) compared to the control animal DBK1. Our observation of slightly higher frequencies of SIV Tat SL8 MHC class I/tetramer positive (Fig. 6A) and SIV Tat SL8-reactive CD8+ T cells (Fig. 6B and C) in PBMC from the control monkey, DBK1, compared to the test monkey, DBN2, further point to a possible dampening of the endogenous responses in DBN2, although the differences did not reach statistical significance given our small sample size.
We next investigated if the diversity of the endogenous virus-induced CD8+ T cell responses was affected by the adoptive transfer. PBMC samples from all three monkeys were collected on day 42 post challenge (i.e. day 39 post infusion for AZ15 and DBN2) and stimulated with peptide pools spanning the entire Gag, Pol, Env and Acc SIV proteins and IFN-γ production measured by ELISpot. We could not detect consistent differences in SIV responses between infused animals AZ15 and DBN2, and the control DBK1 (Fig. 6D). The higher frequencies of SIV Gag peptide pool responding cells in PBMC from DBN2 and DBK1 compared to AZ15, seem to be largely due to the robust SIV Gag CM9-specific responses seen in these Mamu A*01+ monkeys (Fig. 6A). Overall, a similar spectrum of virus-induced endogenous CD8+ T cell responses was observed in PBMC from all three monkeys with a somewhat higher magnitude of SIV Acc- and Gag-induced endogenous responses seen in the control animal, DBK1.
Early selection for escape mutation in the Nef159-167YY9 epitope after adoptive transfer of virus-specific CD8+ T cells
Given the lack of a measurable effect of the infused CD8+ T cells on virus load in plasma or BAL, we investigated if amino acid changes had occurred in any of the targeted epitopes before the peak in endogenous virus-induced CD8+ T cell responses normally seen after 3 weeks post infection (36, 45). Regions spanning the CD8+ T cell epitopes, SIV Gag CM9, Nef YY9 and Vif WY8, were sequenced from virus extracted from plasma, and analyzed for amino acid changes 1, 2, 3, 5 and 9 weeks post infection.
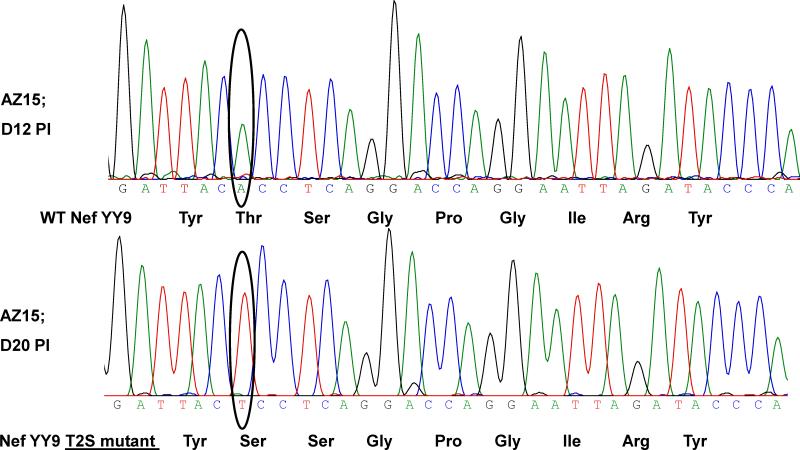
Sequencing of plasma virus from animal AZ15 revealed an A to T switch in the codon for the second amino acid (a.a) of the Mamu A*02-restricted SIV Nef YY9 epitope, leading to a Threonine to Serine (T2S) change between days 12 and 20 post infection (days 9 and 17 post infusion) (Table 3). This represented an amino acid replacement in the vast majority of the sequenced virus by this time point (<3 weeks post infection) as detected by the chromatogram from bulk sequencing (Fig. 7). There was an additional point mutation in the Nef YY9 epitope in virus from this monkey between days 38-63 post infection, with an A to T switch in the codon for the last a.a of the epitope leading to a tyrosine to phenylalanine switch (Y9F). The latter mutation was observed in circulating virus from the Mamu A*01/A*02 positive animal DBN2 by the same time point, but this animal did not receive T cells targeting this epitope. As expected, no mutation was seen in this epitope in virus from the control Mamu A*02 negative monkey DBK1 (data not shown). No amino acid changes where observed in the SIV Gag CM9 or Vif WY8 epitopes by week 9 post infection.
Table III.
Mutations in CD8+ T cell epitope, SIV Nef YY9, following adoptive transfer
| Monkey ID |
Peptide sequencec |
Sample dated |
% IFN-γ-producing cellse |
|---|---|---|---|
| AZ15 a(A*02) | Nef159-167YY9b YTSGPGIRY (wild type) | 74 | |
| YSSGPGIRY (T2S variant) | 9-17 (12-20) | 44 | |
| |
YSSGPGIRF (T2S; Y9F variant) |
35-60 (38-63) |
4 |
| DBN2 a(A*01/*02) | YTSGPGIRF (Y9F variant) | 35-60 (38-63) | 27 |
Monkey MHC class I (Mamu) type of interest.
Mamu A*02-restricted epitope.
Wild type epitope sequence and observed mutations in virus isolated from acutely infected rhesus macaques; amino acid replacements in variant peptides underlined in bold.
Time frame in days post infusion (infection) when mutation was observed i.e. mutation not seen at lower time point in range but seen at higher time point.
Frequency of cells positive for IFN-γ in clonal population of CD8+ T cells generated against WT peptide after stimulation with APC pulsed with 2 μg/mL of WT or variant peptide; analyses by ICS and flow cytometry.
Figure 7.
Escape mutation in the SIV Nef159-167YY9 epitope detected <3 weeks post infection. Virus isolated from plasma collected from the Mamu A*02-positive monkey AZ15 on days 12 and 20 after challenge with SIVmac239 (days 9 and 17 post T cell infusion) was analyzed by direct sequencing spanning the SIV Nef YY9 epitope. The nucleotide change is highlighted by black circles with the encoded amino acid indicated.
To test if any of the Nef YY9 mutations represented escape from CD8+ T cell recognition, IFN-γ responses by a Nef YY9-specific CD8+ T-cell clone was measured by flow cytometry following stimulation with autologous PBMC pulsed with synthetic WT and variant peptides. All three mutations (T2S alone, T2S and Y9F, and Y9F alone) resulted in diminished or complete loss of recognition of the variant peptide as demonstrated by markedly reduced IFN-γ responses (Table 3). Overall, our data showing a rapid escape in the Nef YY9 epitope in plasma virus from the animal AZ15 suggest a role of the infused CD8+ T cells on viral escape.
Discussion
A number of conditions must be achieved for autologous adoptive T-cell transfer to function as a tool for dissecting the role of CD8+ T cells during acute SIV/HIV infection. The first is reliable and consistent means of expansion of cells from a single cell to hundreds of millions, and this must be accomplished while maintaining the specificity and functional phenotype of the T cells. After adoptive transfer, the cells must home to and persist in tissues where viral replication takes place while maintaining their effector function. Targeting a diverse epitope repertoire and preferentially epitopes with a high fitness cost following mutations should help avoid viral escape.
Building on a method for cloning and expansion of human CD8+ T cells that was recently adapted for use in generation of CMV-specific CD8+ T-cell clones from M. nemestrina (34, 41), we developed a protocol for isolating and maintaining TCM- and TEM-derived SIV-specific T-cell clones from rhesus macaques (35, 39) immunized with a DNA vaccine construct. We were able to maintain CD8+ T-cell clones in culture for more than 5 months while characterizing them prior to adoptive transfer, thus overcoming one of the main problems hampering large scale autologous adoptive transfer of CD8+ T-cell clones in NHP models of HIV; the inability to keep rhesus macaque-derived SIV-specific CD8+ T-cell clones in culture for prolonged periods of time. Berger et al., showed that adoptively transferred TCM-derived CMV-specific T-cell clones persist better in PBMC compared with TEM-derived clones (34). We could not detect a consistent difference between TCM- and TEM-derived clones in our model, with a TCM-derived clone persisting better in one animal, and TEM-derived clones in the other. In a parallel set of experiments infusing autologous virus-specific CD8+ T cells intra-peritoneally (i.p.) compared to i.v. to rhesus macaques during chronic SIV infection, we found better persistence of i.p. compared to i.v. infused cells in PBMC (Bolton et al., submitted for publication). However, regardless of the route of infusion (i.p. versus i.v.), and similar to the infusions during acute infection, we observed clonal differences in persistence of the infused cells independent of their origin. This suggests that unknown clonal differences, independent of TCM/TEM origin, played an important role in determining survival in vivo. The difference between the two systems could be due to differences in the disease models, differences between M. nemestrina and M. mulatta, or subtle differences in the protocols used for generating the original clones. For example while we sorted TCM and TEM as CD28+CD95+CCR7+ and CD28−CD95+CCR7− CD8+ T-cell fractions, respectively, the TCM and TEM populations described in Berger et al., (34) were CD62L+CD28+Fashi and CD62L−CD28−Fashi CD8+ T cells, respectively. Furthermore, we did not try to maintain our TCM with IL-15 or IL-7 to improve in vivo survival.
The limited persistence of transferred antigen specific CD8+ T-cell clones in PBMC compared with bulk uncharacterized CD4+ (52) or CD8+ (53) lymphocytes is possibly related to the extensive in vitro culture of the antigen-specific cells before transfer. Although the infused cells were found to express α4β7 and CD103, markers commonly associated with homing to the gut, no infused cells could be detected in GALT and LN biopsies analyzed on day 2 post infusion. Similar results were obtained by Bolton et al., (submitted for publication) in a hemiallogeneic as well as autologous adoptive transfer model in the acute and chronic phase of infection, respectively. This suggests that the transferred T cells had either limited homing to these organs that are important for acute viral replication, or alternatively, that they did home there but were killed, possibly after contact with infected cells. Irrespective of the mechanism involved, the poor or lack of persistence of the infused cells in the GALT in this model, may partly explain the lack of an effect on viral replication in the ramp up phase as well as peak and set point viral load. Strikingly, whereas the infused cells could be detected only for a week in PBMC and not at all in LN and GALT, the cells persisted long-term in the lungs (BAL) and were antigen-reactive ex vivo. However, virologic analysis did not show any evidence of anti-viral effect in vivo. We detected cell free SIV RNA as well as cell-associated viral RNA and DNA in BAL in the infused monkeys and the control, at levels similar to those reported by others for SIV-infected rhesus macaques (54). Our route of infusion, via the femoral vein, entails initial passage through the pulmonary vasculature before gaining access to the systemic arterial circulation. It is possible that a substantial proportion of the infused cells were trapped in this tissue in a non-specific manner, and thus did not enter the arterial circulation and consequently failed to home to the LN or GALT. This together with the data on i.p. versus i.v. infusion by Bolton et al., (submitted for publication) suggest that different routes of infusion can affect homing and persistence of adoptively transferred cells. In addition, transfection of T cells to induce overexpression of receptors like α4β7, that promote homing to gut mucosa (reviewed in (55)), is currently being evaluated. To improve persistence in vivo, IL-15, which has been shown to provide a stronger survival and proliferation signal to antigen experienced cells than the low-dose IL-2 used in the current study (34, 50), as well as transduction with genes that enhance cell survival like telomerase (39, 56) could also be evaluated. However, it is important to underscore that the long-term persistence of transferred T cells in the lungs (BAL), together with their preserved capacity to respond to antigen stimulation ex vivo, point to an intrinsic capacity of the infused cells to survive and maintain function in vivo.
When we investigated the effect of the infused CD8+ T cells on the magnitude and kinetics of the endogenous virus-induced T-cell responses, a bias could be detected suggesting a dampening of the total SIV-induced response in animals that received transferred T cells with no detectable effect on the kinetics of the response. However, more infusions will be needed to address whether the observed slight decrease in the magnitude of the endogenous responses is statistically and biologically relevant. Measures of disease progression in the three animals (CD4 counts and viral load) have remained stable and similar between the test and control monkey out to day 200 post infection (data not shown).
A key impediment for CD8+ T-cell based vaccines and immunotherapy against HIV/SIV is the high rate of mutations in the virus leading to escape in T-cell epitopes (reviewed in (57)). We detected a very early (less than 3 weeks post infection) mutation in the Mamu A*02-restricted Nef YY9 epitope (T2S) in plasma virus from AZ15, before the peak in the endogenous virus-specific CD8+ T-cell response (36). This early mutation markedly reduced recognition by the cognate CD8+ T-cell clones, suggesting that the infused Nef YY9-specific CD8+ T-cell clones may have exerted a selective pressure on replicating virus. DBN2, the other Mamu A*02-positive monkey which did not receive CD8+ T cells specific for this epitope, did not develop mutations until 5 weeks post infection. Still, given the limited number of animals in the study, more experiments will be needed to determine the exact role of the transferred cells on the emergence of this escape mutation. Using a more diverse specificity of transferred clones as well as targeting epitopes less likely to mutate due to high fitness cost to the virus would presumably be of benefit.
The route of experimental SIV challenge that presumably reflects the most common mode of natural HIV infection is a limiting dose mucosal challenge, repeated until the animal is infected (58), typified by the involvement of only one or a few variants in establishment of the initial systemic infection (59). It is probable that a limiting dose mucosal challenge would give adoptively transferred SIV-specific T cells a better chance to impact the infection than the high dose i.v. challenge used in this study. High-dose i.v. challenge has recently been shown to result in infection by more than 20 (and possibly several-fold higher) founder viruses (60). It is possible that the transferred T cells were able to significantly reduce the number of founder viruses, but that this did not result in a biologically significant reduction in viral load. This is in agreement with the recent report by Hansen et al., suggesting that persistently activated CD8+ T cells can protect some animals from SIV challenge, but fail to impact the viral load in vaccinated animals that do get infected (61). Logistically, the repeated low-dose mucosal challenge approach is complex and labor intensive to apply in our model because the billions of expanded T cells can not be kept ready for infusion in a practical and manageable way. In addition, failing to lead to productive infection may prime cellular or humoral responses, further confounding the interpretation of the experiment. However, an optimized high-dose mucosal (as opposed to repeated low dose mucosal or high dose i.v) challenge model that better reflects the most common natural infection could be evaluated in future studies.
In conclusion, this first effort to prevent or significantly diminish the effects of acute SIV infection by adoptive transfer of large numbers of autologous SIV-specific CD8+ T-cell clones revealed a number of critical observations: i) SIV-specific CD8+ T-cell clones from rhesus macaques can be generated, characterized and expanded to numbers sufficient for adoptive transfer, ii) infusion of large numbers of T cells was safe with no adverse reactions detected, iii) the infused cells persisted and remained functional in lungs for an extended period of time (more than 2 months), but showed limited persistence and/or homing to gut mucosa and lymph-nodes, iv) viral sequence analysis suggested that infusion of SIV-specific CD8+ T-cell clones exerted immune pressure on the virus and finally, v) CD8+ T cell transfer did not impact the viral peak or set-point in a detectable way. Further experiments will address if the lack of impact on virus load is associated with the failure to demonstrate homing/persistence of the cells in gut mucosal tissues, and also investigate the impact of transferring clones with more diverse specificities. Alternatively, it is possible that CD8+ T cells exert a limited effect during acute SIV infection, and that the presented results reflect that reality. Finally, the described methods for adoptive transfer of macaque T-cell clones will also provide an important tool for immunological studies of different NHP infectious disease models.
Supplementary Material
Acknowledgements
The authors thank Fang Yuan, Gary Bowers, Kelli Oswald and Rebecca Shoemaker for help with the viral load (QPCR) analyses. Vicky Coalter, Adam Wiles and Rodney Wiles are thanked for help with sample processing. The authors are grateful to Dr Ronald C. Desrosiers for providing the SIVmac239 virus stock and Dr Brandon Keele for helpful discussions. The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: IL-2 from Hoffman-La Roche Inc., NJ; SL8 peptide/MHC tetramer from the NIH Tetramer Facility, Emory University, Atlanta, GA; SIVmac p27 hybridoma (55-2f12) from Dr Niels Pedersen.
Footnotes
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract numbers N01-C0-12400 and HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government.
References
- 1.Hofmann-Lehmann R, Vlasak J, Rasmussen RA, Smith BA, Baba TW, Liska V, Ferrantelli F, Montefiori DC, McClure HM, Anderson DC, Bernacky BJ, Rizvi TA, Schmidt R, Hill LR, Keeling ME, Katinger H, Stiegler G, Cavacini LA, Posner MR, Chou TC, Andersen J, Ruprecht RM. Postnatal passive immunization of neonatal macaques with a triple combination of human monoclonal antibodies against oral simian-human immunodeficiency virus challenge. J. Virol. 2001;75:7470–7480. doi: 10.1128/JVI.75.16.7470-7480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mascola JR, Lewis MG, Stiegler G, Harris D, VanCott TC, Hayes D, Louder MK, Brown CR, Sapan CV, Frankel SS, Lu Y, Robb ML, Katinger H, Birx DL. Protection of Macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 1999;73:4009–4018. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veazey RS, Shattock RJ, Pope M, Kirijan JC, Jones J, Hu Q, Ketas T, Marx PA, Klasse PJ, Burton DR, Moore JP. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat. Med. 2003;9:343–346. doi: 10.1038/nm833. [DOI] [PubMed] [Google Scholar]
- 4.Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, Evans RK, Zhang ZQ, Simon AJ, Trigona WL, Dubey SA, Huang L, Harris VA, Long RS, Liang X, Handt L, Schleif WA, Zhu L, Freed DC, Persaud NV, Guan L, Punt KS, Tang A, Chen M, Wilson KA, Collins KB, Heidecker GJ, Fernandez VR, Perry HC, Joyce JG, Grimm KM, Cook JC, Keller PM, Kresock DS, Mach H, Troutman RD, Isopi LA, Williams DM, Xu Z, Bohannon KE, Volkin DB, Montefiori DC, Miura A, Krivulka GR, Lifton MA, Kuroda MJ, Schmitz JE, Letvin NL, Caulfield MJ, Bett AJ, Youil R, Kaslow DC, Emini EA. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415:331–335. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- 5.Rose NF, Marx PA, Luckay A, Nixon DF, Moretto WJ, Donahoe SM, Montefiori D, Roberts A, Buonocore L, Rose JK. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell. 2001;106:539–549. doi: 10.1016/s0092-8674(01)00482-2. [DOI] [PubMed] [Google Scholar]
- 6.Belyakov IM, Hel Z, Kelsall B, Kuznetsov VA, Ahlers JD, Nacsa J, Watkins DI, Allen TM, Sette A, Altman J, Woodward R, Markham PD, Clements JD, Franchini G, Strober W, Berzofsky JA. Mucosal AIDS vaccine reduces disease and viral load in gut reservoir and blood after mucosal infection of macaques. Nat. Med. 2001;7:1320–1326. doi: 10.1038/nm1201-1320. [DOI] [PubMed] [Google Scholar]
- 7.Barouch DH, Santra S, Schmitz JE, Kuroda MJ, Fu TM, Wagner W, Bilska M, Craiu A, Zheng XX, Krivulka GR, Beaudry K, Lifton MA, Nickerson CE, Trigona WL, Punt K, Freed DC, Guan L, Dubey S, Casimiro D, Simon A, Davies ME, Chastain M, Strom TB, Gelman RS, Montefiori DC, Lewis MG, Emini EA, Shiver JW, Letvin NL. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science. 2000;290:486–492. doi: 10.1126/science.290.5491.486. [DOI] [PubMed] [Google Scholar]
- 8.Hel Z, Nacsa J, Tryniszewska E, Tsai WP, Parks RW, Montefiori DC, Felber BK, Tartaglia J, Pavlakis GN, Franchini G. Containment of simian immunodeficiency virus infection in vaccinated macaques: correlation with the magnitude of virus-specific pre- and postchallenge CD4+ and CD8+ T cell responses. J. Immunol. 2002;169:4778–4787. doi: 10.4049/jimmunol.169.9.4778. [DOI] [PubMed] [Google Scholar]
- 9.Hazuda DJ, Young SD, Guare JP, Anthony NJ, Gomez RP, Wai JS, Vacca JP, Handt L, Motzel SL, Klein HJ, Dornadula G, Danovich RM, Witmer MV, Wilson KA, Tussey L, Schleif WA, Gabryelski LS, Jin L, Miller MD, Casimiro DR, Emini EA, Shiver JW. Integrase inhibitors and cellular immunity suppress retroviral replication in rhesus macaques. Science. 2004;305:528–532. doi: 10.1126/science.1098632. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez CS, Smith MZ, Batten CJ, De Rose R, Reece JC, Rollman E, Venturi V, Davenport MP, Kent SJ. Vaccine-induced T cells control reversion of AIDS virus immune escape mutants. J. Virol. 2007;81:4137–4144. doi: 10.1128/JVI.02193-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barouch DH, Kunstman J, Kuroda MJ, Schmitz JE, Santra S, Peyerl FW, Krivulka GR, Beaudry K, Lifton MA, Gorgone DA, Montefiori DC, Lewis MG, Wolinsky SM, Letvin NL. Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature. 2002;415:335–339. doi: 10.1038/415335a. [DOI] [PubMed] [Google Scholar]
- 12.Ueno T, Idegami Y, Motozono C, Oka S, Takiguchi M. Altering Effects of Antigenic Variations in HIV-1 on Antiviral Effectiveness of HIV-Specific CTLs. J. Immunol. 2007;178:5513–5523. doi: 10.4049/jimmunol.178.9.5513. [DOI] [PubMed] [Google Scholar]
- 13.Loffredo JT, Burwitz BJ, Rakasz EG, Spencer SP, Stephany JJ, Vela JP, Martin SR, Reed J, Piaskowski SM, Furlott J, Weisgrau KL, Rodrigues DS, Soma T, Napoe G, Friedrich TC, Wilson NA, Kallas EG, Watkins DI. The antiviral efficacy of simian immunodeficiency virus-specific CD8+ T cells is unrelated to epitope specificity and is abrogated by viral escape. J. Virol. 2007;81:2624–2634. doi: 10.1128/JVI.01912-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loffredo JT, Sidney J, Wojewoda C, Dodds E, Reynolds MR, Napoe G, Mothe BR, O'Connor DH, Wilson NA, Watkins DI, Sette A. Identification of seventeen new simian immunodeficiency virus-derived CD8+ T cell epitopes restricted by the high frequency molecule, Mamu-A*02, and potential escape from CTL recognition. J. Immunol. 2004;173:5064–5076. doi: 10.4049/jimmunol.173.8.5064. [DOI] [PubMed] [Google Scholar]
- 15.Jin X, Bauer DE, Tuttleton SE, Lewin S, Gettie A, Blanchard J, Irwin CE, Safrit JT, Mittler J, Weinberger L, Kostrikis LG, Zhang L, Perelson AS, Ho DD. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Metzner KJ, Jin X, Lee FV, Gettie A, Bauer DE, Di Mascio M, Perelson AS, Marx PA, Ho DD, Kostrikis LG, Connor RI. Effects of in vivo CD8(+) T cell depletion on virus replication in rhesus macaques immunized with a live, attenuated simian immunodeficiency virus vaccine. J. Exp. Med. 2000;191:1921–1931. doi: 10.1084/jem.191.11.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lifson JD, Rossio JL, Piatak M, Parks T, Li L, Kiser R, Coalter V, Fisher B, Flynn BM, Czajak S, Hirsch VM, Reimann KA, Schmitz JE, Ghrayeb J, Bischofberger N, Nowak MA, Desrosiers RC, Wodarz D. Role of CD8(+) lymphocytes in control of simian immunodeficiency virus infection and resistance to rechallenge after transient early antiretroviral treatment. J. Virol. 2001;75:10187–10199. doi: 10.1128/JVI.75.21.10187-10199.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ellenberger D, Otten RA, Li B, Aidoo M, Rodriguez IV, Sariol CA, Martinez M, Monsour M, Wyatt L, Hudgens MG, Kraiselburd E, Moss B, Robinson H, Folks T, Butera S. HIV-1 DNA/MVA vaccination reduces the per exposure probability of infection during repeated mucosal SHIV challenges. Virology. 2006;352:216–225. doi: 10.1016/j.virol.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Barouch DH, Santra S, Kuroda MJ, Schmitz JE, Plishka R, Buckler-White A, Gaitan AE, Zin R, Nam JH, Wyatt LS, Lifton MA, Nickerson CE, Moss B, Montefiori DC, Hirsch VM, Letvin NL. Reduction of simian-human immunodeficiency virus 89.6P viremia in rhesus monkeys by recombinant modified vaccinia virus Ankara vaccination. J. Virol. 2001;75:5151–5158. doi: 10.1128/JVI.75.11.5151-5158.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amara RR, Villinger F, Altman JD, Lydy SL, O'Neil SP, Staprans SI, Montefiori DC, Xu Y, Herndon JG, Wyatt LS, Candido MA, Kozyr NL, Earl PL, Smith JM, Ma HL, Grimm BD, Hulsey ML, Miller J, McClure HM, McNicholl JM, Moss B, Robinson HL. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science. 2001;292:69–74. doi: 10.1126/science.1058915. [DOI] [PubMed] [Google Scholar]
- 21.Cafaro A, Caputo A, Fracasso C, Maggiorella MT, Goletti D, Baroncelli S, Pace M, Sernicola L, Koanga-Mogtomo ML, Betti M, Borsetti A, Belli R, Akerblom L, Corrias F, Butto S, Heeney J, Verani P, Titti F, Ensoli B. Control of SHIV-89.6P-infection of cynomolgus monkeys by HIV-1 Tat protein vaccine. Nat. Med. 1999;5:643–650. doi: 10.1038/9488. [DOI] [PubMed] [Google Scholar]
- 22.Pantaleo G, Koup RA. Correlates of immune protection in HIV-1 infection: what we know, what we don't know, what we should know. Nat. Med. 2004;10:806–810. doi: 10.1038/nm0804-806. [DOI] [PubMed] [Google Scholar]
- 23.Sekaly RP. The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development? J. Exp. Med. 2008;205:7–12. doi: 10.1084/jem.20072681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenberg PD. Adoptive T cell therapy of tumors: mechanisms operative in the recognition and elimination of tumor cells. Adv. Immunol. 1991;49:281–355. doi: 10.1016/s0065-2776(08)60778-6. [DOI] [PubMed] [Google Scholar]
- 25.Pahl-Seibert MF, Juelch M, Podlech J, Thomas D, Deegen P, Reddehase MJ, Holtappels R. Highly protective in vivo function of cytomegalovirus IE1 epitope-specific memory CD8 T cells purified by T-cell receptor-based cell sorting. J. Virol. 2005;79:5400–5413. doi: 10.1128/JVI.79.9.5400-5413.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasenkrug KJ, Dittmer U. Immune control and prevention of chronic Friend retrovirus infection. Front Biosci. 2007;12:1544–1551. doi: 10.2741/2167. [DOI] [PubMed] [Google Scholar]
- 27.Rooney CM, Smith CA, Ng CY, Loftin SK, Sixbey JW, Gan Y, Srivastava DK, Bowman LC, Krance RA, Brenner MK, Heslop HE. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92:1549–1555. [PubMed] [Google Scholar]
- 28.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bollard CM, Aguilar L, Straathof KC, Gahn B, Huls MH, Rousseau A, Sixbey J, Gresik MV, Carrum G, Hudson M, Dilloo D, Gee A, Brenner MK, Rooney CM, Heslop HE. Cytotoxic T lymphocyte therapy for Epstein-Barr virus+ Hodgkin's disease. J. Exp. Med. 2004;200:1623–1633. doi: 10.1084/jem.20040890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, Rogers LJ, Gracia GJ, Jones SA, Mangiameli DP, Pelletier MM, Gea-Banacloche J, Robinson MR, Berman DM, Filie AC, Abati A, Rosenberg SA. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J. Clin. Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walter EA, Greenberg PD, Gilbert MJ, Finch RJ, Watanabe KS, Thomas ED, Riddell SR. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N. Engl. J. Med. 1995;333:1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 32.Koff WC, Johnson PR, Watkins DI, Burton DR, Lifson JD, Hasenkrug KJ, McDermott AB, Schultz A, Zamb TJ, Boyle R, Desrosiers RC. HIV vaccine design: insights from live attenuated SIV vaccines. Nat. Immunol. 2006;7:19–23. doi: 10.1038/ni1296. [DOI] [PubMed] [Google Scholar]
- 33.Johnson RP. Mechanisms of protection against simian immunodeficiency virus infection. Vaccine. 2002;20:1985–1987. doi: 10.1016/s0264-410x(02)00083-x. [DOI] [PubMed] [Google Scholar]
- 34.Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J. Clin. Invest. 2008;118:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Minang JT, Barsov EV, Yuan F, Trivett MT, Piatak M, Jr., Lifson JD, Ott DE, Ohlen C. Efficient inhibition of SIV replication in rhesus CD4+ T-cell clones by autologous immortalized SIV-specific CD8+ T-cell clones. Virology. 2008;372:430–441. doi: 10.1016/j.virol.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 36.Allen TM, O'Connor DH, Jing P, Dzuris JL, Mothe BR, Vogel TU, Dunphy E, Liebl ME, Emerson C, Wilson N, Kunstman KJ, Wang X, Allison DB, Hughes AL, Desrosiers RC, Altman JD, Wolinsky SM, Sette A, Watkins DI. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature. 2000;407:386–390. doi: 10.1038/35030124. [DOI] [PubMed] [Google Scholar]
- 37.Reynolds MR, Rakasz E, Skinner PJ, White C, Abel K, Ma ZM, Compton L, Napoe G, Wilson N, Miller CJ, Haase A, Watkins DI. CD8+ T-lymphocyte response to major immunodominant epitopes after vaginal exposure to simian immunodeficiency virus: too late and too little. J. Virol. 2005;79:9228–9235. doi: 10.1128/JVI.79.14.9228-9235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosati M, Valentin A, Jalah R, Patel V, von Gegerfelt A, Bergamaschi C, Alicea C, Weiss D, Treece J, Pal R, Markham PD, Marques ET, August JT, Khan A, Draghia-Akli R, Felber BK, Pavlakis GN. Increased immune responses in rhesus macaques by DNA vaccination combined with electroporation. Vaccine. 2008;26:5223–5229. doi: 10.1016/j.vaccine.2008.03.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andersen H, Barsov EV, Trivett MT, Trubey CM, Giavedoni LD, Lifson JD, Ott DE, Ohlen C. Transduction with human telomerase reverse transcriptase immortalizes a rhesus macaque CD8(+) T cell clone with maintenance of surface marker phenotype and function. AIDS Res. Hum. Retroviruses. 2007;23:456–465. doi: 10.1089/aid.2006.0194. [DOI] [PubMed] [Google Scholar]
- 40.O'Connor DH, Mothe BR, Weinfurter JT, Fuenger S, Rehrauer WM, Jing P, Rudersdorf RR, Liebl ME, Krebs K, Vasquez J, Dodds E, Loffredo J, Martin S, McDermott AB, Allen TM, Wang C, Doxiadis GG, Montefiori DC, Hughes A, Burton DR, Allison DB, Wolinsky SM, Bontrop R, Picker LJ, Watkins DI. Major histocompatibility complex class I alleles associated with slow simian immunodeficiency virus disease progression bind epitopes recognized by dominant acute-phase cytotoxic-T-lymphocyte responses. J. Virol. 2003;77:9029–9040. doi: 10.1128/JVI.77.16.9029-9040.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riddell SR, Greenberg PD. The use of anti-CD3 and anti-CD28 monoclonal antibodies to clone and expand human antigen-specific T cells. J. Immunol. Methods. 1990;128:189–201. doi: 10.1016/0022-1759(90)90210-m. [DOI] [PubMed] [Google Scholar]
- 42.Berger C, Huang ML, Gough M, Greenberg PD, Riddell SR, Kiem HP. Nonmyeloablative immunosuppressive regimen prolongs In vivo persistence of gene-modified autologous T cells in a nonhuman primate model. J. Virol. 2001;75:799–808. doi: 10.1128/JVI.75.2.799-808.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frahm N, Korber BT, Adams CM, Szinger JJ, Draenert R, Addo MM, Feeney ME, Yusim K, Sango K, Brown NV, SenGupta D, Piechocka-Trocha A, Simonis T, Marincola FM, Wurcel AG, Stone DR, Russell CJ, Adolf P, Cohen D, Roach T, StJohn A, Khatri A, Davis K, Mullins J, Goulder PJ, Walker BD, Brander C. Consistent cytotoxic-T-lymphocyte targeting of immunodominant regions in human immunodeficiency virus across multiple ethnicities. J. Virol. 2004;78:2187–2200. doi: 10.1128/JVI.78.5.2187-2200.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robinson S, Charini WA, Newberg MH, Kuroda MJ, Lord CI, Letvin NL. A commonly recognized simian immunodeficiency virus Nef epitope presented to cytotoxic T lymphocytes of Indian-origin rhesus monkeys by the prevalent major histocompatibility complex class I allele Mamu-A*02. J. Virol. 2001;75:10179–10186. doi: 10.1128/JVI.75.21.10179-10186.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Connor DH, Allen TM, Vogel TU, Jing P, DeSouza IP, Dodds E, Dunphy EJ, Melsaether C, Mothe B, Yamamoto H, Horton H, Wilson N, Hughes AL, Watkins DI. Acute phase cytotoxic T lymphocyte escape is a hallmark of simian immunodeficiency virus infection. Nat. Med. 2002;8:493–499. doi: 10.1038/nm0502-493. [DOI] [PubMed] [Google Scholar]
- 46.Minang JT, Trivett MT, Coren LV, Barsov EV, Piatak M, Jr., Chertov O, Chertova E, Ott DE, Ohlen C. The Mamu B 17-restricted SIV Nef IW9 to TW9 mutation abrogates correct epitope processing and presentation without loss of replicative fitness. Virology. 2008;375:307–314. doi: 10.1016/j.virol.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cline AN, Bess JW, Piatak M, Jr., Lifson JD. Highly sensitive SIV plasma viral load assay: practical considerations, realistic performance expectations, and application to reverse engineering of vaccines for AIDS. J. Med. Primatol. 2005;34:303–312. doi: 10.1111/j.1600-0684.2005.00128.x. [DOI] [PubMed] [Google Scholar]
- 48.Mummidi S, Bamshad M, Ahuja SS, Gonzalez E, Feuillet PM, Begum K, Galvis MC, Kostecki V, Valente AJ, Murthy KK, Haro L, Dolan MJ, Allan JS, Ahuja SK. Evolution of human and non-human primate CC chemokine receptor 5 gene and mRNA. Potential roles for haplotype and mRNA diversity, differential haplotype-specific transcriptional activity, and altered transcription factor binding to polymorphic nucleotides in the pathogenesis of HIV-1 and simian immunodeficiency virus. J. Biol. Chem. 2000;275:18946–18961. doi: 10.1074/jbc.M000169200. [DOI] [PubMed] [Google Scholar]
- 49.Kestler H, Kodama T, Ringler D, Marthas M, Pedersen N, Lackner A, Regier D, Sehgal P, Daniel M, King N, et al. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science. 1990;248:1109–1112. doi: 10.1126/science.2160735. [DOI] [PubMed] [Google Scholar]
- 50.Picker LJ, Reed-Inderbitzin EF, Hagen SI, Edgar JB, Hansen SG, Legasse A, Planer S, Piatak M, Jr., Lifson JD, Maino VC, Axthelm MK, Villinger F. IL-15 induces CD4 effector memory T cell production and tissue emigration in nonhuman primates. J. Clin. Invest. 2006;116:1514–1524. doi: 10.1172/JCI27564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brenchley JM, Paiardini M, Knox KS, Asher AI, Cervasi B, Asher TE, Scheinberg P, Price DA, Hage CA, Kholi LM, Khoruts A, Frank I, Else J, Schacker T, Silvestri G, Douek DC. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112:2826–2835. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Villinger F, Brice GT, Mayne AE, Bostik P, Mori K, June CH, Ansari AA. Adoptive transfer of simian immunodeficiency virus (SIV) naive autologous CD4(+) cells to macaques chronically infected with SIV is sufficient to induce long-term nonprogressor status. Blood. 2002;99:590–599. doi: 10.1182/blood.v99.2.590. [DOI] [PubMed] [Google Scholar]
- 53.Greene JM, Burwitz BJ, Blasky AJ, Mattila TL, Hong JJ, Rakasz EG, Wiseman RW, Hasenkrug KJ, Skinner PJ, O'Connor SL, O'Connor DH. Allogeneic lymphocytes persist and traffic in feral MHC-matched mauritian cynomolgus macaques. PLoS One. 2008;3:e2384. doi: 10.1371/journal.pone.0002384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barber SA, Gama L, Li M, Voelker T, Anderson JE, Zink MC, Tarwater PM, Carruth LM, Clements JE. Longitudinal analysis of simian immunodeficiency virus (SIV) replication in the lungs: compartmentalized regulation of SIV. J. Infect. Dis. 2006;194:931–938. doi: 10.1086/507429. [DOI] [PubMed] [Google Scholar]
- 55.Agace WW. Tissue-tropic effector T cells: generation and targeting opportunities. Nat. Rev. Immunol. 2006;6:682–692. doi: 10.1038/nri1869. [DOI] [PubMed] [Google Scholar]
- 56.Zhou J, Dudley ME, Rosenberg SA, Robbins PF. Selective growth, in vitro and in vivo, of individual T cell clones from tumor-infiltrating lymphocytes obtained from patients with melanoma. J. Immunol. 2004;173:7622–7629. doi: 10.4049/jimmunol.173.12.7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walker BD, Burton DR. Toward an AIDS vaccine. Science. 2008;320:760–764. doi: 10.1126/science.1152622. [DOI] [PubMed] [Google Scholar]
- 58.Wilson NA, Reed J, Napoe GS, Piaskowski S, Szymanski A, Furlott J, Gonzalez EJ, Yant LJ, Maness NJ, May GE, Soma T, Reynolds MR, Rakasz E, Rudersdorf R, McDermott AB, O'Connor D H, Friedrich TC, Allison DB, Patki A, Picker LJ, Burton DR, Lin J, Huang L, Patel D, Heindecker G, Fan J, Citron M, Horton M, Wang F, Liang X, Shiver JW, Casimiro DR, Watkins DI. Vaccine-Induced Cellular Immune Responses Reduce Plasma Viral Concentrations after Repeated Low-Dose Challenge with Pathogenic Simian Immunodeficiency Virus SIVmac239. J. Virol. 2006;80:5875–5885. doi: 10.1128/JVI.00171-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, Sun C, Grayson T, Wang S, Li H, Wei X, Jiang C, Kirchherr JL, Gao F, Anderson JA, Ping LH, Swanstrom R, Tomaras GD, Blattner WA, Goepfert PA, Kilby JM, Saag MS, Delwart EL, Busch MP, Cohen MS, Montefiori DC, Haynes BF, Gaschen B, Athreya GS, Lee HY, Wood N, Seoighe C, Perelson AS, Bhattacharya T, Korber BT, Hahn BH, Shaw GM. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl. Acad. Sci. U S A. 2008;105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keele BF, Li H, Learn GH, Hraber P, Giorgi EE, Grayson T, Sun C, Chen Y, Yeh WW, Letvin NL, Mascola JR, Nabel GJ, Haynes BF, Bhattacharya T, Perelson AS, Korber BT, Hahn BH, Shaw GM. Low-dose rectal inoculation of rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. J. Ex Med. 2009;206:1117–1134. doi: 10.1084/jem.20082831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, Axthelm MK, Oswald K, Trubey CM, Piatak M, Jr., Lifson JD, Nelson JA, Jarvis MA, Picker LJ. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat. Med. 2009;15:293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.