Abstract
Over decades, anesthesiologists have used intravenous adenosine as mainstay therapy for diagnosing or treating supraventricular tachycardia in the perioperative setting. More recently, specific adenosine receptor therapeutics or gene-targeted mice deficient in extracellular adenosine production or individual adenosine receptors became available. These models enabled physicians and scientists to learn more about the biological functions of extracellular nucleotide metabolism and adenosine signaling. Such functions include specific signaling effects through adenosine receptors expressed by many mammalian tissues, for example vascular endothelia, myocytes, heptocytes, intestinal epithelia or immune cells. At present, pharmacological approaches to modulate extracellular adenosine signaling are evaluated for their potential use in perioperative medicine, including attenuation of acute lung injury, renal, intestinal, hepatic and myocardial ischemia, or vascular leakage. If these laboratory studies can be translated into clinical practice, adenosine receptor based therapeutics may become an integral pharmacological component of daily anesthesiology practice.
Introduction
The observation that intravenous adenosine causes a temporary heart block dates back many years. In 1927, Drury and Szent-Gyorgyi from the University of Cambridge, United Kingdom, performed an experiment where they injected extracts from cardiac tissues intravenously into a whole animal. They were surprised to notice a transient disturbance of the cardiac rhythm and slowing of the heart rate.1 Following several purification steps, the authors were able to identify the biologically active compound of the extract as an “adenine compound”.1 Adenine is a purine-based nucleobase (similar to guanine) involved in many biological functions, including cellular respiration, or protein biosynthesis (as component of DNA and RNA). Looking back from today’s perspective, it seems likely that the induced slowing of the heart-rate was caused by the pharmacological activity of adenosine.1 Adenosine belongs to the molecular group of nucleosides, composed of an adenine-group attached to a ribose sugar (fig. 1). It took almost 50 years from these early discoveries of the heart-rate-slowing effects of “adenine compounds”1 to the clinical use of adenosine in treating patients with supraventricular tachycardia.2 However, intravenous adenosine has remained a mainstay form of clinical therapy for diagnosing or treating patients with supraventricular arrhythmias since the 1980ies.3,4 In fact, intravenous adenosine is among the most frequently used anti-arrhythmic medications in the clinical practice of anesthesiology,4 including treatment of supraventricular tachycardia in many perioperative settings, such as cardiothoracic anesthesia,5 critical care medicine,6 or obstetric anesthesia.7 In addition, adenosine-induced induction of a transient cardiac arrest is frequently used for assisting accurate deployment of vascular stent grafts in the major blood vessels.8,9
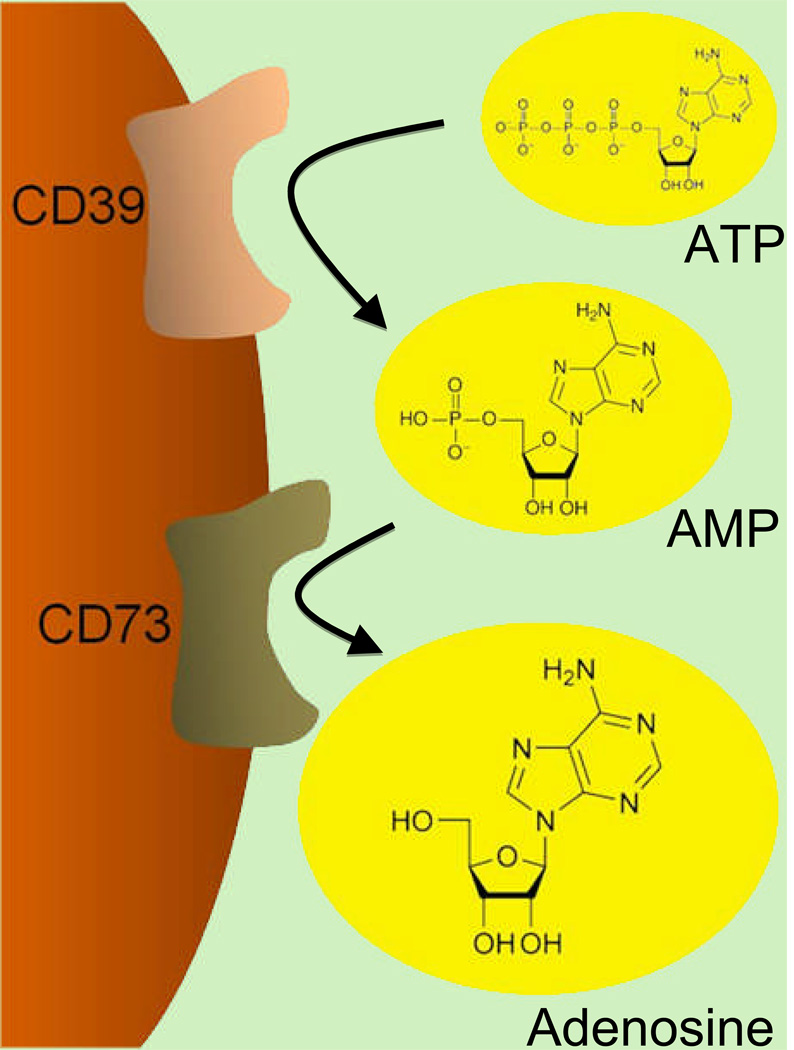
Figure 1. Extracellular Adenosine Generation.
Adenosine is an extracellular signaling molecule that is generated from its precursor molecules 5’-adenosine triphosphate (ATP) and 5’-adenosine monophosphate (AMP). This process consists of a two-step enzymatic reaction. Extracellular ATP released by multiple cell types (e.g. platelets, endothelia, epithelia or inflammatory cells) is rapidly converted to AMP by the ecto-apyrase (CD39). As second step in extracellular adenosine generation, AMP is converted by the 5’-ecto-nucleotdase (CD73) to adenosine. Thus, extracellular adenosine is available on the cell surface to activate its receptors.
In addition to its clinical role as anti-arrhythmic agent, adenosine has been implicated in diverse areas of medicine. An important clinical application for extracellular adenosine signaling is its potent effect as arterial vasodilator. For example, the adenosine-uptake inhibitor dipyridamole is used during pharmacologically-induced stress-echocardiography to enhance vascular adenosine levels, causing coronary vasodilatation, and unmasking a clinically relevant coronary artery obstruction.10 In addition, adenosine functions as platelet aggregation inhibitor.11 For example, a recent study investigated different platelet inhibitors in the prevention of recurrent stroke, and found that extended release dipyridamole in combination with aspirin is equally effective as the 5’-adenosine triphosphate (ATP)-receptor antagonist clopidogrel.12 Moreover, the nonspecific adenosine receptor antagonist caffeine has been suggested for the prevention or treatment of postdural puncture headache.13 While this indication has been challenged, 13 caffeine remains an important therapeutic agent in the treatment or prevention of caffeine withdrawal headache in perioperative patients.14,15 Similarly, the non-specific adenosine receptor antagonist theophylline has been used in the past for treating obstructive airway disease, but has been replaced by inhaled long-acting beta-agonist bronchodilators due to less drug-drug interactions and toxicity from drug overdosing.16
In addition to these well established clinical applications of adenosine, basic research has implicated extracellular adenosine as an endogenous distress molecule17 with profound impact on immune response,17,18 and adaptation to limited oxygen availability (hypoxia).19–23 In fact only recently, the research field of extracellular adenine nucleotide metabolism and adenosine signaling rapidly expanded to become an extremely dynamic and exciting field of investigation, as genetic models and specific pharmacological agents became available. For example, mice with genetic defects in the enzymes responsible for extracellular adenosine generation (cd39−/− or cd73−/− mice, see “Extracellular Adenosine Generation”), or for individual adenosine receptors allowed scientists to specifically target the contribution of extracellular adenosine generation or signaling through individual receptors in a wide range of disease models.
Biology of Extracellular Adenosine
Extracellular Adenosine Generation
Adenosine is implicated in a wide variety of basic biological functions, including nucleotide biosynthesis or cellular energy metabolism. On the outside of the cell, adenosine mainly serves as a signaling molecule and its biological functions occur through the activation of adenosine receptors (ARs) localized on the extracellular surface of the cell membranes. Particularly during conditions of cellular distress (inflammation, hypoxia, acute injury), extracellular adenosine stems from poshpo-ester hydrolysis of its precursor molecules, ATP, 5’-adenosine diphosphate (ADP) or 5’-adenosine monophosphate (AMP).24 These molecules (so called nucleotides) consist of the nucleoside adenosine, bound to a varying number of phospho-esters attached to the 5’-designated atom of its ribose sugar ring (fig. 1). In the absence of catalytic enzymes capable of hydrolyzing nucleotides, extracellular ATP, ADP and AMP would be relatively stable. However, most cell types express enzymes on their cell surface that catalyze nucleotide-phospho-hydrolyses. Typically, this process occurs in a three-step reaction. As first step, multiple cell types release intracellular stored nucleotides, particularly in the form of ATP and ADP.24–26 It is important to point out that intracellular ATP levels are very high (5–8 mmolar). Therefore, nucleotide release from intracellular sources can occur during cellular damage or death (lysis, necrosis, apoptosis, etc.), or through specific gradient driven channels.27,28 Activated platelets that release ADP from stored intracellular vesicles via granular release provide an additional source of extracellular nucleotides.29
As second step (Figure 1), extracellular ATP and ADP are rapidly converted to AMP by the ecto-apyrase (CD39). CD39 is a widely expressed surface-bound enzymes that is expressed on multiple cell types30 and serves in a dual role. On the one hand, CD39 is responsible for extracellular adenosine production by generation of AMP. As such, pharmacological inhibition or genetic deletion of CD39 is associated with attenuated extracellular adenosine levels and defects in extracellular adenosine signaling.31,32 At the same time, CD39 catalyzes the key step for extracellular breakdown of ATP and ADP, which are both important signaling molecules, particularly during vascular thrombosis or inflammation.33,34 Thus, pharmacological inhibition or genetic deletion of CD39 is also associated with elevated ATP or ADP signaling effects.35 Therefore, studies in cd39−/− mice have to address the question if an observed phenotype is related to enhanced nucleotide (ATP/ADP), or attenuated adenosine signaling.31,32,36 While gene-targeted mice for CD39 are viable and do not exhibit obvious immunologic defects,33 experimental approaches have identified several phenotypes, including a disordered homeostasis and thromboregulation,33 and increased susceptibility to renal,31,37 myocardial,32,38,39 or central nervous34,40 system ischemia or acute lung injury.36,41 In addition, CD39 plays an important role in organ transplantation38 or hepatic regeneration following partial hepatectomy 42.
The third and final step in extracellular adenosine generation (fig. 1) is catalyzed by the 5’-ecto-nucleotidase (CD73), a membrane bound glycoprotein that rapidly converts extracellular AMP to adenosine. CD39 and CD73 belong to a family of ecto-nucleotidases that rapidly hydrolyze ATP/ADP to AMP (CD39), or AMP to adenosine (CD73), respectively.17,43 Genetic deletion or pharmacological inhibition of CD73 is associated with elevated AMP and attenuated adenosine levels within the extracellular fluids.44–48 Similar to gene-target mice for CD39, cd73−/− mice appear to have a normal immune system and are healthy when housed in a specific pathogen-free animal facility.49 Comparative measurements of CD73 activity in wild-type or cd73−/− mice revealed that CD73 levels are particularly high in the intestine, brain, kidneys and lungs.49 Studies of cd73−/− mice indicate that these animals experience profound vascular leakage and pulmonary edema when exposed to ambient hypoxia (8% oxygen over 4 h) compared to littermate controls.49 Consistent with the high enzymatic levels of CD73 in the lungs, kidneys and the intestine,49 other studies also unveiled a critical role of CD73 in pulmonary41 or intestinal barrier function during ambient hypoxia,48 colitis,50 renal45 or intestinal ischemia.46,51 Taken together, extracellular adenosine mainly stems from phospho-hydrolysis of precursor nucleotides, a metabolic pathway that is highly regulated by transcriptionally controlled enzymes (CD39 and CD73, fig. 1).
Extracellular Adenosine Signaling
Extracellular adenosine mainly serves signaling molecule that can activate any of four ARs.17 At present, four different receptors have been described (A1AR, A2AAR, A2BAR, and A3AR; fig. 2). Due to the fact that individual ARs result in different biologic functions, it is important to point out that extracellular adenosine signaling effects strongly depend on the relative expression pattern of ARs on the extracellular surface of an individual cell types or a specific tissue.18,24,41,44,51–55 ARs contain seven trans-membrane spanning domains and are coupled to intracellular GTP-binding proteins, utilizing intracellular cyclic AMP (cAMP) as second messenger.56 Adenosine activates A1, A2A or A3ARS with EC50 values between 10 nM to 1 µM.56 In contrast, activation of the A2BAR generally requires adenosine levels that exceed 10 µM (EC50 of 24 µM).56,57 Under physiological conditions, typical adenosine concentrations remain lower than 1 µM.56 Therefore, activation of A1, A2A or A3ARs can occur during physiological conditions. While it remains difficult to estimate the adenosine concentrations locally present during an intimate cell-cell contact, it appears that the higher adenosine concentrations required for activation of the A2BAR are mainly achieved during pathophysiological conditions (hypoxia, inflammation, ischemia). 17,39,44,46,47,51,53–55,58. In addition, the A2BAR is dually coupled to cAMP and calcium signaling pathways, with activation of the A2BAR leading to a rise in intracellular calcium.59 As biological functions elicited by adenosine signaling depend on the adenosine concentrations at the cell surface, several other factors including receptor density and the functionality of the intracellular signalling pathways coupled to adenosine receptors, are important determinants of signaling effects.56 Moreover, specific transcriptional changes in the pattern of AR expression during pathophysiological conditions such as hypoxia, ischemia or inflammation have the potential to significantly alter AR signaling events.24,51,53,60,61
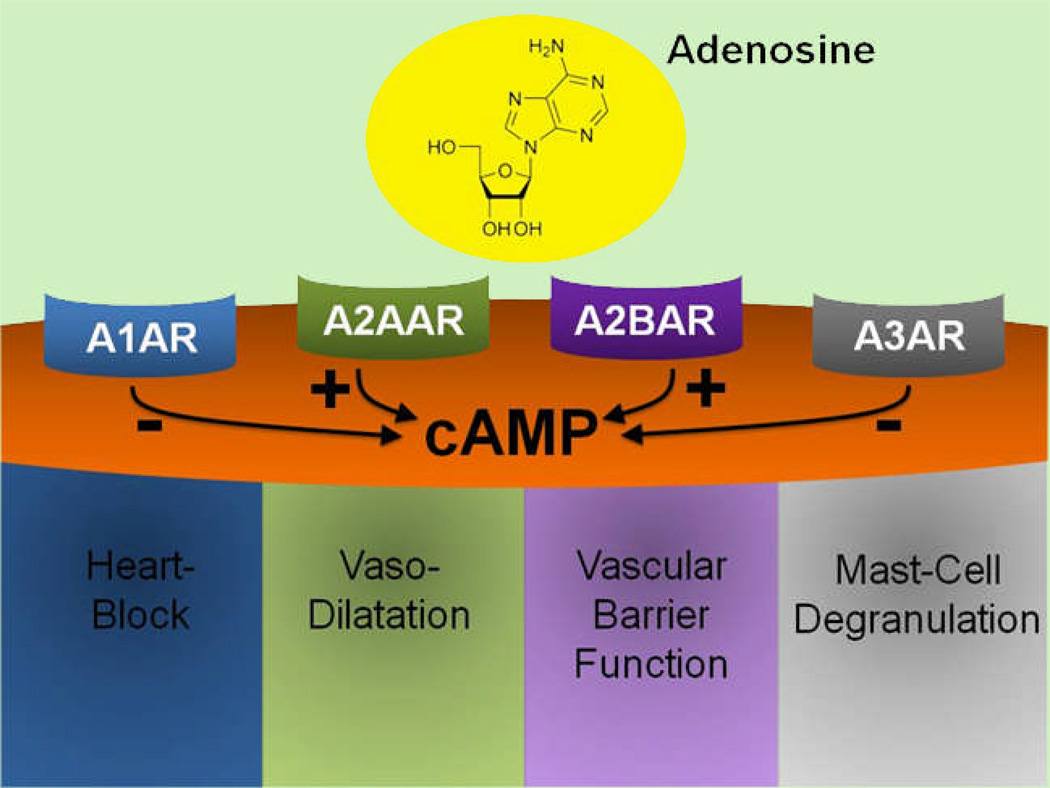
Figure 2. Extracellular Adenosine Signaling.
Extracellular adenosine exerts its biological effects through activation of adenosine receptors (ARs). At present, four different ARs have been identified: A1AR, A2AAR, A2BAR and A3AR. ARs are G-protein coupled receptors that utilize cyclic adenosine monophosphate (cAMP) as a second messenger. While signaling events through the A1 or A3AR dampen intracellular cAMP levels, activation of the A2A or A2BAR elevates cAMP levels. Biological examples for AR signaling events include adenosine-elicited heart-block for activation of the A1, vaso-dilation for the A2A, vascular barrier function for the A2B or murine mast-cell degranulation for the A3AR.
AR signalling occurs through the changes in adenylyl cyclase activity, resulting in subsequent alteration of intracellular cAMP levels as second messenger.17 Based on their ability to elevate or to attenuate cAMP, ARs were initially classified as A1ARs (attenuation of cAMP) or A2ARs (elevation of cAMP), respectively.62 However, subsequent studies have refined the classification of A2ARs into two sub-groups, A2AARs with a high affinity, and A2BARs with a low affinity for adenosine.63 More recently, the A3AR was discovered as fourth AR. Similar to the A1AR, the A3AR signaling is associated with attenuation of intracellular cAMP levels.56 Examples for typical physiological responses associated with the activation of individual ARs include adenosine-mediated bradycardia via activation of the A1AR,64 arterial vasodilatation or inhibition of platelet aggregation via activation of the A2AAR,11,65–67 ischemic preconditioning of different organs via activation of the A2BAR,44,54,55 or rodent mast-cell degranulation through A3AR-dependent attenuation of intracellular cAMP concentrations (fig. 2).68 Taken together, extracellular adenosine mainly exerts its biological actions through activation of four adenosine receptors (ARs). While activation of the A1AR or the A3AR leads to attenuation of intracellular cAMP levels, activation of the high affinity A2AAR or the low affinity A2BAR are associated with elevation of cAMP levels (fig. 2).
Extracellular Adenosine Uptake
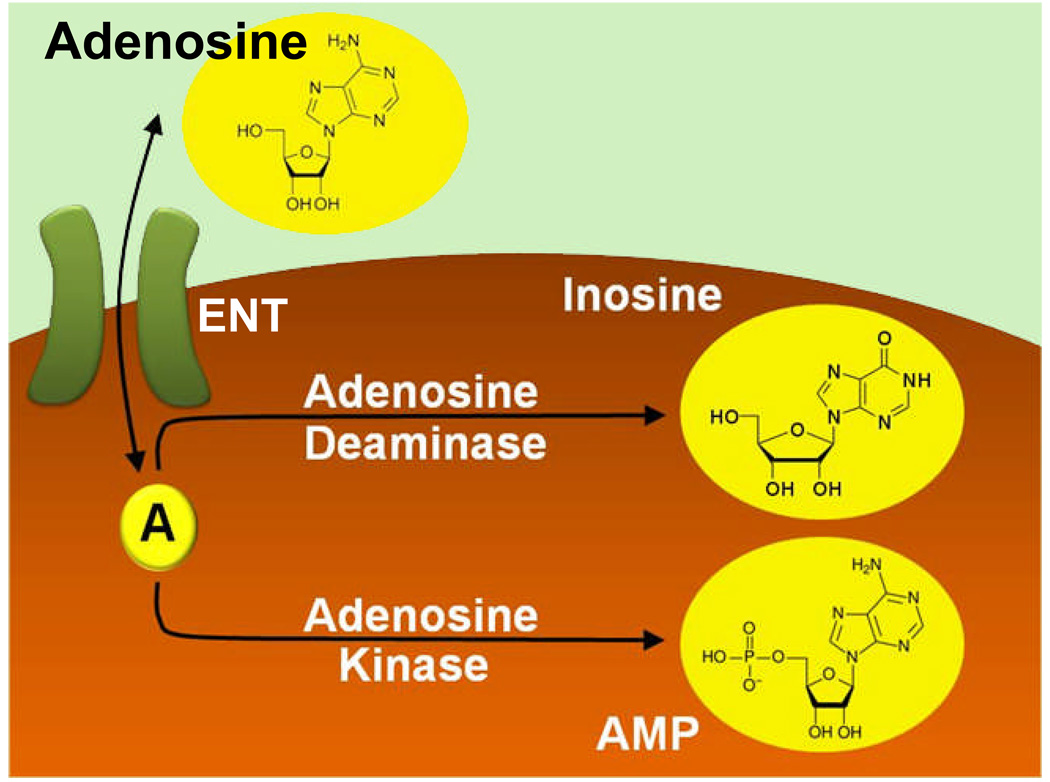
When using intravenous adenosine in the treatment of perioperative cardiac arrhythmias, anesthesiologists rely heavily on the short half-life of adenosine. In fact, the heart-block induced in patients treated with a rapid intravenous bolus of adenosine is terminated due to swift decline of plasma adenosine concentrations. Thus, the adenosine-induced heart block typically lasts for a period of only 5–10 seconds. The main mechanism responsible for the fast decline of vascular adenosine levels following intravenous injection is uptake of adenosine from the extracellular to the intracellular compartment,69 followed by rapid intracellular metabolism via the adenosine deaminase (conversion to inosine) or adenosine kinase (conversion to AMP).70–72 Adenosine can traverse the cell membrane through concentrative or equilibrative nucleoside transporters. Equilibrative nucleoside transporters (ENT) 1 and ENT2 are functionally most relevant adenosine transporters (fig. 3).60,69 Equilibrative nucleoside transporters represent channels that allow adenosine to freely cross the cell membrane following a concentration gradient.69 Under physiological conditions, differences between intra- or extracellular adenosine concentrations are very small. Therefore, net flow through ENTs is minimal under normal circumstances (fig. 4).69 This is different following intravenous application of an adenosine bolus. In this setting, extracellular adenosine concentrations rise substantially, and flow through ENTs is directed from the extracellular compartment towards the intracellular space, resulting in swift uptake of adenosine. ENTs are widely expressed, including vascular endothelia, epithelia, erythrocytes or inflammatory cells. Rapid adenosine transport as described above is the main mechanism for the prompt decline in adenosine plasma concentrations following an IV adenosine bolus, and is responsible for the swift termination of adenosine-induced heart block.
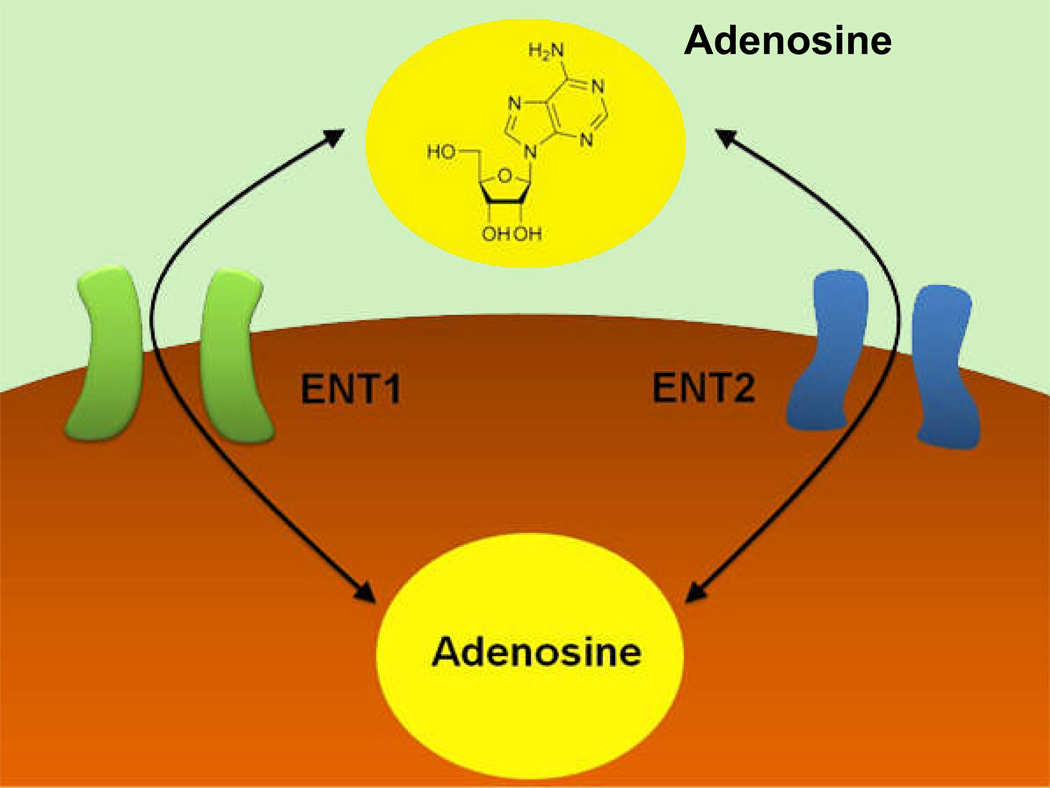
Figure 3. Extracellular Adenosine Uptake.
Extracellular adenosine is taken up from the extracellular to the intracellular space via nucleoside transporters. Functionally, extracellular adenosine uptake is mainly achieved through “equilibrative nucleoside transporters”, ENT1 and ENT2. These transporters represent diffusion-limited channels that allow adenosine to freely cross the cell membrane following a concentration gradient.
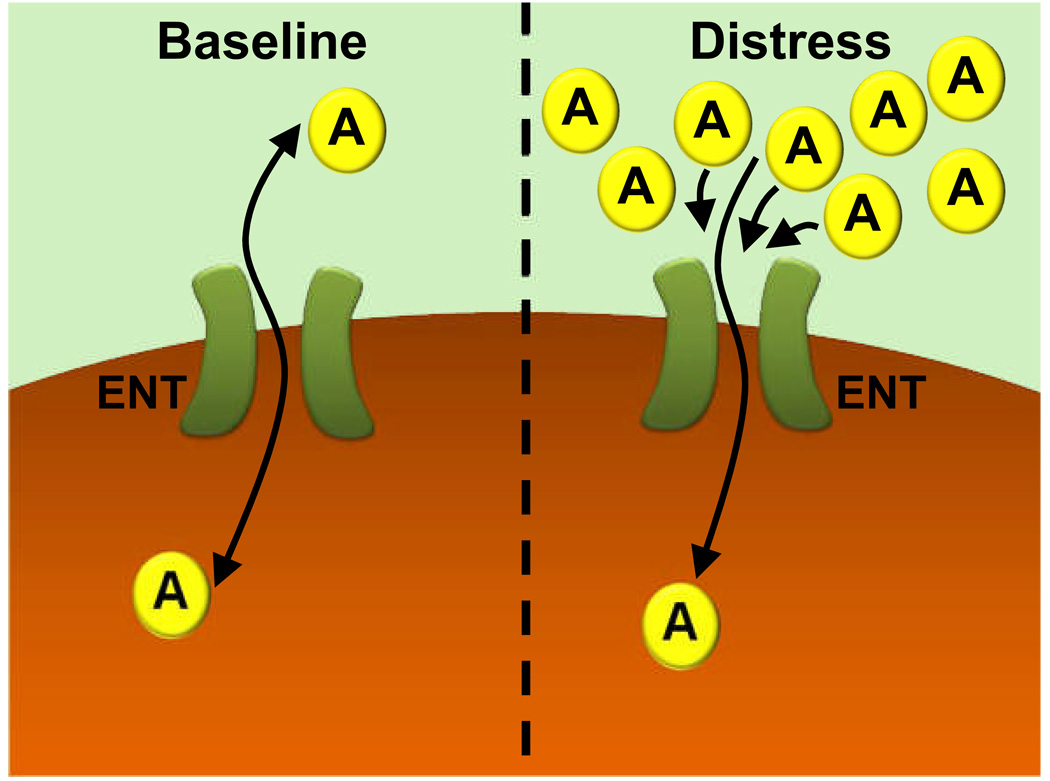
Figure 4. Extracellular Adenosine Uptake at “Baseline” or during “Distress.”.
Adenosine (A) can freely cross the cell membrane via equilibrative nucleoside transporters (ENTs). ENTs represent diffusion limited channels that allow free passage of adenosine through the cell membrane. During baseline conditions, only a small concentration gradient for adenosine across the cell membrane is present. Therefore, flux through ENTs is minimal. In contrast, extracellular adenosine concentrations are elevated during conditions of hypoxia, ischemia, or inflammation (“distress”). Under these conditions, adenosine flux through ENTs is directed mainly from the extracellular space towards the intracellular compartment. As long as flux through ENTs is directed from the outside towards the inside of the cell, inhibitors of ENTs (such as dipyridamole) or transcriptional mechanisms that repress ENTs will attenuate adenosine transport, and result in increased extracellular adenosine concentrations and signaling effects.
Similar transport phenomena become important during conditions when extracellular adenosine levels are elevated as a response to hypoxia, ischemia, inflammation or other injurious conditions (“distress”, fig. 4). Again, these conditions cause adenosine transport through ENTs to be directed from the extracellular towards the intracellular space (fig. 4). Taken together, extracellular adenosine signaling is terminated by rapid adenosine transport through diffusion limited channels across the cell membrane. These transporters (mainly ENT1 and ENT2) are placed in an anatomically ideal position to modulate extracellular adenosine levels and signaling events. Therefore, pharmacological inhibition of adenosine transporters (e.g., with dipyridamole) or transcriptional mechanisms to repress ENT-expression result in prolonged actions of extracellular adenosine. 73As such, dipyridamole treatment will enhance extracellular adenosine signaling events, as long as the transport of adenosine is directed from the outside towards the inside of the cell (e.g., during hypoxia, ischemia, inflammation etc., fig. 4).60,69,74
Intracellular Adenosine Metabolism
Following uptake into the intracellular compartment, adenosine is rapidly metabolized. Two alternative metabolic pathways compete for the intracellular fate of adenosine. Adenosine can either be converted to inosine through enzymatic activity of the adenosine deaminase (fig. 5).71,72,75,76 Alternatively, adenosine can be converted by the adenosine kinase to AMP (fig. 5).17,70 It is important to point out that intracellular adenosine metabolism represents an additional mechanism to modulate extracellular adenosine levels and signaling events. For example, genetic deletion of the adenosine deaminase in mice is associated with dramatic increases in extracellular adenosine levels, and a phenotype that is characterized by pulmonary adenosine toxicity. 76,77 Similarly, inhibition of adenosine kinase is associated with increases in extracellular adenosine signaling, which can be beneficial to attenuate the detrimental effects of hypoxia or ischemia. 70,78
Figure 5. Intracellular Adenosine Metabolism.
Following adenosine uptake via equilibrative nucleoside transporters (ENTs), intracellular adenosine is rapidly metabolized. Two competing pathways exist. Intracellular adenosine can be metabolized to inosine by the adenosine deaminase. Alternatively, adenosine is phosphorylated by the adenosine kinase to 5’-adenosine monophosphate (AMP).
Extracellular Adenosine and Hypoxia
Modulation of Extracellular Adenosine Signaling Events by Hypoxia
Extracellular adenosine plays a critical role in tissue adaptation to limited oxygen availability (hypoxia).20–22 Along these lines, several studies have found that extracellular adenosine levels are elevated during conditions of hypoxia. For example, one study exposed human volunteers to normoxia or moderate hypoxia (oxygen saturation of 80% over 20 min). Following hypoxia exposure, adenosine levels increased from approximately 20 nM to over 50 nm concentrations.79 Moreover, adenosine tissue concentrations in hearts32,44 or kidneys31,45 exposed to ischemia increase approximately 5-fold. Similar studies with mice deficient in extracellular adenosine generation suggest that ischemia-induced increases of extracellular adenosine occur mainly in the extracellular space.31,32,44,45
Over the past decade, convincing evidence has demonstrated a central role of hypoxia-inducible factor (HIF) in mammalian oxygen homeostasis.23,80 Therefore, it is not surprising that HIF is central in the transcriptional coordination of hypoxia-elicited adenosine responses.21,48,54,60,61,70,74 The transcription factor HIF-1 is composed of two subunits: constitutively expressed HIF-1β and oxygen-regulated HIF-1α.80 Under normoxic conditions, HIF-1α is subjected to hydroxylation on proline residues, resulting in proteasomal degradation.81 Under hypoxic conditions, hydroxylation is inhibited, allowing HIF to be active and to stimulate the transcriptional activation of HIF-dependent genes.54 Transcriptional induction of HIF-target genes is tailored towards adapting the cells to limited oxygen availability (e.g., by inducing a switch from aerobic metabolism to anaerobic glycolysis)82 or towards restoring adequate tissue oxygen levels (e.g., induction of erythropoietin resulting in enhanced erythropoiesis).81
Acute hypoxia elicited changes of extracellular adenosine result in increased adenosine signaling events. Several steps of this transcriptionally controlled pathway are coordinated by HIF. As first step, hypoxia coordinates increases in extracellular adenosine production. This is achieved by a transcriptional induction of extracellular enzymes that produce extracellular adenosine. While CD39 transcription during hypoxia is driven by the transcription factor Sp1,39 hypoxia-induced stabilization of HIF profoundly enhances CD73 transcription, translation and surface activity.48 As such, studies of the CD73 promoter revealed a binding site for HIF-1, and additional studies with promoter constructs, including site-directed mutagenesis of the HIF-binding site or HIF-loss and gain of function studies confirmed HIF-1 in the transcriptional induction of CD73 during hypoxia.48,54
Secondly, studies investigated the consequences of hypoxia or ischemia on extracellular adenosine signaling events. Such studies revealed a selective induction of the A2BAR with ambient hypoxia.24,61 Similarly to the studies performed with CD73, strong evidence supports a critical role for HIF-1α in the transcriptional induction of the A2BAR with hypoxia or ischemia.54,61
Thirdly, studies of extracellular adenosine uptake indicate that HIF-1 coordinates transcriptional repression of the adenosine transporters ENT160 and ENT2,74 resulting in attenuated uptake of extracellular adenosine, and enhanced extracellular adenosine signaling events. Finally, HIF also coordinates transcriptional changes in intracellular adenosine metabolism.83 As such, acute hypoxia results in HIF-dependent transcriptional repression of adenosine kinase, attenuated adenosine metabolism, and eventually in enhanced extracellular adenosine signaling events. Taken together, extracellular adenosine signaling events are enhanced during hypoxia by a series of steps mainly coordinated by the transcription factor HIF-1. Hypoxia-induced increases in adenosine signaling are critical to counterbalance the deleterious effects of acute hypoxia, including attenuation of hypoxia-induced vascular leakage,49,58,70 ischemia-associated organ dysfunction 46,51 or hypoxia-induced inflammation. 18,23,24
Adenosine Signaling during Hypoxia-Induced Inflammation
Sites of acute inflammation are characterized by shifts in the supply and demand of metabolites that result in limited oxygen availability (inflammation-associated hypoxia).20,21,23 However, studies of ambient hypoxia provided strong evidence that hypoxia itself represents an inflammatory stimulus.84,85 For example, ambient hypoxia exposure causes activation of the transcription factor nuclear factor NF-κB, which activates transcription of genes encoding pro-inflammatory molecules.84–86 Moreover, exposure of mice to ambient hypoxia (e.g., 8% oxygen over 4–8 h) induces increased leakage through epithelial or endothelial barriers, and inflammatory cell accumulation in mucosal organs.18,24,48,58,60,70 Tissue inflammation caused by limited oxygen availability plays an important role in several human clinical conditions including solid organ transplantation (e.g., lung or liver), when limited oxygen availability caused by graft ischemia is associated with increased inflammation, ischemia-reperfusion injury, and early organ failure.87,88 Similarly, hypoxia-associated inflammation strongly influences the clinical outcome of organ ischemia, and antiinflammatory therapeutic approaches have been proposed for myocardial,32,44,54,89 renal,55 hepatic,47 or intestinal ischemia.46,51 Due to their large surface areas, mucosal organs such as the lungs and the intestine are particularly prone to hypoxia-induced inflammation.23,46,51
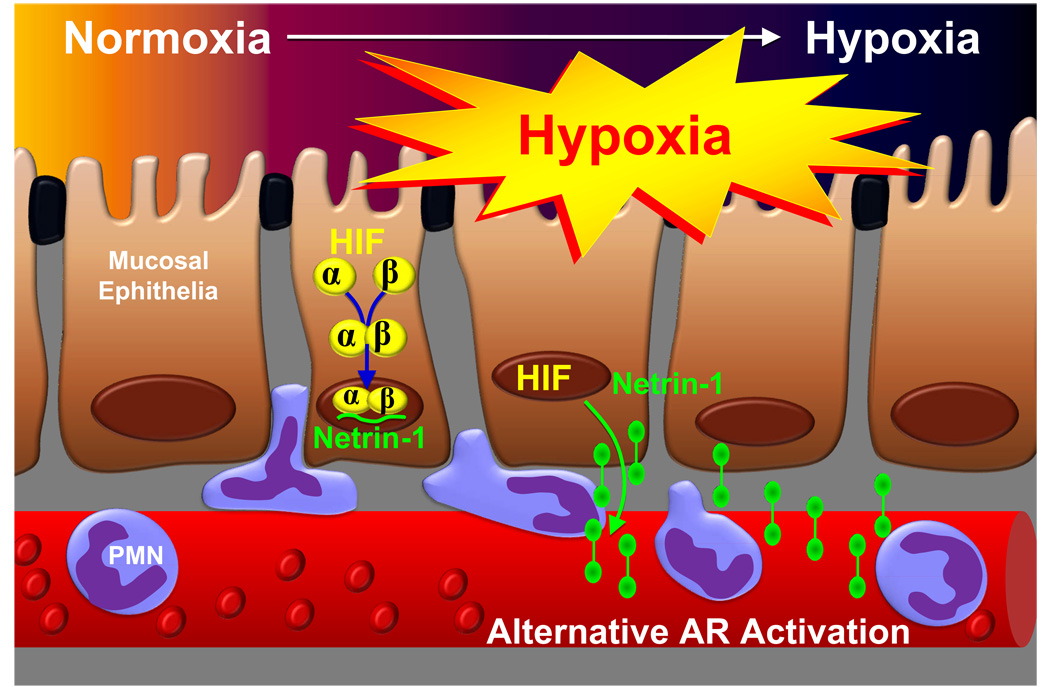
Whereas tissue hypoxia induces hypoxia-induced inflammation, hypoxia also drives hypoxia-associated antiinflammatory responses, particularly through changes in gene-expression coordinated by the transcription factor HIF-1.20,21 As outlined above, HIF-1 coordinates the metabolism and signaling properties of extracellular adenosine, 21,54,60,70 as important antiinflammatory agent. HIF-1 also induces metabolic changes in immune cells by switching from aerobic metabolism to glycolysis, and thereby markedly affects immune responses.20 HIF-1α is stabilized in inflamed90,91 or infected tissues92, and some data suggest an antiinflammatory and tissue protective role of HIF-1α signaling during acute inflammation20,21,54,60,70,90–93 or bacterial infections.92,94 In this context, a recent study identified an important role of the neuronal guidance molecule netrin-1 in enhancing extracellular adenosine signaling pathways. Given that mucosal surfaces are particularly prone to hypoxia-elicited inflammation, this study sought to determine the role of netrin-1 in hypoxia-induced inflammation. The authors detected HIF-1α-dependent induction of netrin-1 gene (Ntn1) expression in hypoxic epithelia. Neutrophil transepithelial migration studies showed that by engaging A2BARs on neutrophils, netrin-1 attenuates neutrophil transmigration. Exogenous netrin-1 suppressed hypoxia-elicited inflammation in wild-type, but not A2BAR-deficient mice, and inflammatory hypoxia was enhanced in Ntn1+/− compared to Ntn1+/+ mice. Taken together, these studies demonstrate that HIF-1α-dependent induction of netrin-1 attenuates hypoxia-elicited inflammation at mucosal surfaces by enhancing extracellular adenosine signaling events (fig. 6).
Figure 6. Netrin-1 Dampens Hypoxia-Induced Inflammation by Enhancing Extracellular Adenosine Signaling.
During hypoxia-elicited inflammation of mucosal organs such as the lungs or the intestine, the transcription factor hypoxia-inducible factor (HIF) coordinates the induction of netrin-1. While originally described as neuronal guidance molecule, recent studies implicate netrin-1 in the regulation of inflammatory responses. Here, epithelial-released netrin-1 dampens neutrophil accumulation in the hypoxic mucosa. This process involves activation of A2B adenosine receptor (AR)-dependent signaling pathways of neutrophils (“alternative AR activation”).
Utilizing Adenosine Signaling Pathways for Perioperative Medicine
The field of extracellular adenosine signaling has rapidly expanded over the past years. This goes hand in hand with the recent availability of gene-targeted mice that allow for studies of specific alterations in adenosine generation, signaling or metabolism in a large array of disease models. Therefore, the following examples for potential therapeutic applications of extracellular adenosine are not meant to be a complete list, but resemble therapeutic examples that could potentially be applied to the setting of perioperative medicine. Based on the biologic effects of acute hypoxia on enhancing extracellular adenosine effects, many of these examples include conditions of limited oxygen availability where adenosine signaling is pivotal to tissue adaptation and attenuation of the deleterious effects of hypoxia-associated inflammation.
Acute Lung Injury
Acute lung injury (ALI) is a syndrome consisting of acute hypoxemic respiratory failure with bilateral pulmonary infiltrates, not attributable to left heart failure.95 Despite optimal management consisting of aggressive treatment of the initiating cause, vigilant supportive care, and the prevention of nosocomial infections, mortality ranges between 35 and 60%.95 The pathogenesis of ALI is characterized by the influx of a protein-rich edema fluid into the interstitial and intraalveolar spaces as a consequence of increased permeability of the alveolar–capillary barrier. The importance of endothelial injury and increased vascular permeability to the formation of pulmonary edema in this disorder has been well established 95. Nevertheless, molecular details of how pulmonary capillary leakage is caused or maintained during ALI are largely unknown and studies linking its mechanisms with mechanical ventilation are currently areas of intense investigation.
Despite the large impact of ALI on morbidity and mortality in critically ill patients,95 many episodes of ALI are self-limiting, and resolve spontaneously through unknown mechanisms. For example, patients undergoing major surgery requiring prolonged mechanical ventilation have an overall incidence of ALI between 0.2 and 5%, depending on the kind of surgery.96–98 Based on the rare occurrence of clinically relevant ALI in patients requiring mechanical ventilation, recent studies found that extracellular adenosine production via CD39 and CD73 are enhanced during cyclic mechanical stretch in vitro, or during ventilator-induced lung injury (VILI) in vivo. In fact, cd39−/− or cd73−/− mice experience enhanced lung inflammation and pulmonary edema in different models of ALI.36,41,99 Further studies examined the contribution of endogenous adenosine signaling to attenuation of VILI or endotoxin induced lung injury.53 Initial profiling studies using gene-targeted mice for the A1, A2A, A2B or A3AR revealed that genetic deletion of the A2BAR was specifically associated with reduced survival-time and increased pulmonary albumin leakage (5.3±0.15-fold) during VILI. Studies in wild-type mice showed that treatment with A2BAR-selective antagonist PSB1115 resulted in enhanced pulmonary inflammation, edema and attenuated gas-exchange, while treatment with the A2BAR-agonist BAY 60–6583 attenuated VILI. Studies in bone-marrow chimeric A2BAR mice demonstrated pulmonary A2BARs in VILI-induced albumin leakage and edema, while increases in pulmonary inflammation were, at least in part, bone-marrow mediated. Measurement of alveolar fluid clearance indicated that A2BAR signaling enhanced amiloride-sensitive fluid transport via elevation of pulmonary cAMP levels - similarly to beta-adrenergic agonist stimulation - suggesting that A2BAR-agonist treatment protects by drying out the lungs during VILI.53 Taken together, such studies demonstrate that extracellular adenosine production via CD39 and CD73, in conjunction with A2BAR signaling represents in endogenous pathway to protect the lungs from pulmonary edema and excessive inflammation.41,53 Moreover, the A2BAR represents a potential therapeutic target for enhancing fluid transport and attenuating pulmonary edema and lung inflammation during ALI.53 Other studies implicated A2BAR signaling in attenuating acute lung inflammation during hypoxia by dampening proinflammatory signaling pathways in the lungs, involving A2BAR-mediated cullin-1 deneddylation.100 It is important to point out that while extracellular adenosine signaling appears to be protective during acute forms of lung injury, adenosine signaling may enhance aspects of chronic forms of lung injury, such as pulmonary fibrosis.76 For example, adenosine-deaminase-deficient mice develop signs of chronic pulmonary injury in association with chronically elevated pulmonary adenosine levels. In fact, adenosine-deaminase-deficient mice die within weeks after birth from severe respiratory distress,76 and recent studies suggest that attenuation of adenosine signaling may reverse the severe pulmonary phenotypes in adenosine-deaminase-deficient mice, suggesting that chronic adenosine elevation can affect signaling pathways that mediate aspects of chronic lung disease.76,101
Iatrogenic Hyperoxia
Other studies have indicated a protective role of signaling through the A2AAR during inflammatory conditions, including different forms of ALI.19,22,102 As such, a recent study by Thiel et al., in a team led by Michail Sitkovsky, tested the hypothesis that oxygenation weakens a tissue-protecting mechanism triggered by hypoxia. Similar to signaling through the A2BAR,53 hypoxia also triggers a signaling pathway mediated by the A2AAR that attenuates lung inflammation and tissue damage.22 This hypoxia-driven pathway protects the lungs from the toxic effects of overactive immune cells such as neutrophils. Using a mouse model of acute lung injury induced by bacterial infection, Thiel et al. exposed one group of mice to 100% oxygen, mimicking therapeutic oxygenation, and left another group at normal ambient levels (21% oxygen).22 Five times more mice died after receiving 100% oxygen than died breathing normal oxygen levels. Mice given 60% oxygen—considered clinically safe—got worse, but didn't die. Hypoxia protects against lung damage, the authors conclude, by working through the A2AAR signaling pathway to control inflammation. Above-normal oxygen levels interrupt this anti-inflammatory pathway, paving the way for further lung injury.103 Taken together, such studies indicate that high levels of inspired oxygen - as maybe required to provide sufficient tissue oxygenation in patients suffering for ALI - may weaken the local tissue hypoxia-driven and AR-mediated antiinflammatory mechanism and thereby further exacerbate lung injury. 22
Vascular Leakage during Hypoxia
Changes in vascular barrier function closely coincide with tissue injury of many etiologies, and result in fluid loss, edema, and organ dysfunction. Particularly during conditions of limited oxygen availability such as occurs during lung injury, sepsis, or a systemic inflammatory responses syndrome, the vascular barrier becomes leaky.72 The predominant barrier (~90%) to movement of macromolecules across a blood vessel wall is presented by the vascular endothelium. As outlined above, extracellular nucleotide metabolites – particularly adenosine - may function as an endogenous protective mechanism during hypoxia and ischemia and could counterbalance hypoxia-induced increases in vascular leakage. As such, the vascular endothelium is the primary interface between a hypoxic insult and the surrounding tissues. This critical anatomic location places vascular endothelial cells in an ideal position to coordinate extracellular metabolic events important to endogenous responses to hypoxia. The pacemaker enzyme of extracellular adenosine generation is the ecto-5'-nucleotidase (CD73, extracellular conversion of AMP to adenosine). Endothelial cells of many origins express constitutive CD73. Thus, extracellular nucleotides are metabolized to adenosine by CD73 and subsequent activation of surface adenosine receptors have been shown to regulate endothelial barrier function. In vivo studies in models of murine whole-body hypoxia revealed that hypoxia-induced CD73 is critical for protecting the endothelial barrier, since cd73−/− mice show increased vascular permeability and profound pulmonary edema upon hypoxia exposure (8% oxygen over 4 h).49 . In vitro studies of endothelial permeability suggested, that activation of endothelial adenosine receptors leads to a barrier resealing response following neutrophil transmigration.104 In fact, all four ARs are expressed on vascular endothelia.24 To identify the role of individual ARs in attenuating hypoxia-induced vascular leakage, a recent study found that small-interfering--RNA-mediated repression of the A2BAR selectively increased endothelial leak in response to hypoxia in vitro.58 In parallel, vascular permeability was significantly increased in vascular organs of A2BAR−/−-mice subjected to ambient hypoxia (8% oxygen, 4 h). By contrast, hypoxia-induced vascular leak was not accentuated in A1AR−/−-, A2AAR−/−-, or A3AR−/−-deficient mice, suggesting a degree of specificity for the A2BAR. Further studies in wild type mice revealed that the selective A2BAR antagonist PSB1115 resulted in profound increases in hypoxia-associated vascular leakage while A2BAR agonist (BAY60–6583) treatment was associated with almost complete reversal of hypoxia-induced vascular leakage. Taken together, these studies indicate extracellular adenosine production and signaling as central control point for hypoxia-associated vascular leak.
Myocardial Ischemia
Myocardial ischemia is among the leading causes of morbidity and mortality in surgical patients.105 Current therapeutic interventions for myocardial ischemia focus mainly on early and persistent coronary reperfusion. However, percutaneous coronary intervention in combination with anticoagulation and platelet inhibitors may not be suitable in the perioperative settings due to risk of bleeding from the surgical site.105 Therefore, it is not surprising that the search for novel therapeutic approaches to prevent or treat perioperative myocardial ischemia is currently an area of intense investigation. A powerful strategy for cardioprotection would be to pharmacologically recapitulate the consequences of ischemic preconditioning (IP), where short and repeated episodes of ischemia and reperfusion prior to myocardial infarction result in attenuation of infarct sizes.73,106 Despite multiple attempts to identify the underlying molecular mechanisms, pharmacological strategies utilizing such pathways have yet to be further defined and introduced into clinical practice. Extracellular adenosine generation has been studied for its role in IP-responses and cardioprotection from ischemia for many years.107 An important insight was gained from studies measuring cardiac adenosine levels following preconditioning. These studies revealed an about 5-fold increase of cardiac adenosine levels immediately following preconditioning. In contrast, adenosine levels derived from preconditioned myocardium in mice deficient of extracellular adenosine generation (cd39−/− or cd73−/− mice) was similar to un-preconditioned wild-type mice.32,44,54 These studies indicate that extracellular adenosine levels are dramatically elevated with preconditioning.
Functional studies revealed that pharmacological inhibition or genetic deletion of extracellular adenosine production is associated with abolished cardioprotection by IP-treatment.32,44 As all four ARs have been associated with tissue protection in different settings, the question which AR mediates IP-dependent cardioprotection is controversial. While some studies found a critical role of the A1108 or the A3AR,109 a recent study compared IP responses in gene-targeted mice for all 4 ARs.44 While IP-dependent cardioprotection was attenuated in different mice, including A1AR−/− mice,108 complete loss of IP-dependent cardioprotection was observed only in A2BAR−/− mice.44 Moreover, treatment with a specific A2BAR resulted in a robust reduction of infarct size in wild-type mice, but not in A2BAR−/− mice.44 Nevertheless, it is important to point out that also other AR signaling pathways have been associated with cardioprotection from ischemia, including the A1AR108 or the A2AAR.89 In addition, other studies have found critical roles for adenosine signaling in ischemic postconditioning110 - a recently described cardioprotective modality against reperfusion injury, through series of brief reflow interruptions applied at the very onset of reperfusion - including signaling events involving the A1AR,111,112 A2AAR113, A2BAR114 or A3AR.110 Taken together, these studies indicate cardioprotection from myocardial ischemia through CD39- or CD73-dependent generation of extracellular adenosine and signaling via ARs. 32,44,54
Attenuation of Ischemia Reperfusion Injury in other Organs
In addition to its role in cardioprotection, 32,44,54 extracellular adenosine generation and signaling has also been implicated in protection from ischemia-reperfusion injury in other organ systems. For example, different studies pointed out a protective role of extracellular adenosine generation31,45 and signaling through the A2AAR115 or the A2BAR55 in reno-protection from acute ischemic renal failure. Considerable progress has been made by studies in gene-targeted mice that only express a specific adenosine receptor on renal tissues or inflammatory cells. This can be achieved by irridation of gene-targeted mice for a specific adenosine receptor, followed by bone marrow transplantation from wild-type animals, and vice versa. Studies in these “bone marrow chimeric mice” demonstrated that A2AARs expressed on inflammatory cells,115,116 and A2BARs expressed on renal tissues55 attenuate renal ischemia reperfusion injury. Such studies indicate a potential cross-talk and/or functional compensation among the subtypes of adenosine receptors.
Other studies found protection from intestinal46,51 or hepatic ischemia reperfusion injury47 by adenosine generation or signaling.47 For example, a very elegant study demonstrated that adenosine-dependent attenuation of hepatic ischemia reperfusion injury involves activation of A2AARs localized on immune cells.117 Specifically, this study found a surprising role for adenosine-dependent inhibition of natural killer T-cells, a sub-population of lymphocytes representing about 0.2% of peripheral blood T-cells. Natural killer T-cells recognize the nonpolymorphic CD1d molecule, an antigen-presenting molecule that binds self- and foreign lipids and glycolipids. This study provides strong evidence that the activation of natural killer T-cells by a CD1d-dependent mechanism play a central role in initiating the inflammatory cascade responsible for reperfusion injury in the liver and that these cells are key targets of A2AAR agonist-protection in hepatic ischemia-reperfusion injury.117 Moreover, extracellular adenosine production appears to play a critical role in hepatic regeneration as observed following partial hepatectomy. As such recent studies demonstrate that regulated phosphohydrolysis of extracellular nucleotides by CD39 coordinates both hepatocyte and endothelial cell proliferation following partial hepatectomy. Lack of CD39 activity is associated with decreased hepatic regeneration and failure of vascular reconstitution.42
Other Medical Applications for Adenosine Signaling Pathways
Many other treatment modalities have utilized adenosine-dependent signaling pathways. For example, experimental studies suggest a protective role of extracellular adenosine signaling in models of sepsis and acute inflammation, particularly through signaling events involving the A2AAR.19 Similarly, a protective and antiinflammatory role of A2BAR signaling has been found during vascular inflammation118 or vascular injury.119 Other studies found a protective role of extracellular adenosine generation and signaling during murine colitis.50,120 As such, mice deficient in the A2BAR show increased sensitivity to chemically induced intestinal inflammation.120 Other studies found a protective role of A2BAR agonist treatment in intestinal ischemia, highlighting a tissue protective and antiinflammatory role of intestinal A2BAR signaling.51 Similarly, gene-targeted mice for the A2BAR show dramatically increased responses to immunoglobulin E-elicited mast-cell activation, indicating that A2BAR functions as a critical regulator of signaling pathways within the mast cell, which act in concert to limit the magnitude of mast cell responsiveness when antigen is encountered.121 However, there may be different regulatory mechanisms involved in human mast cell degranulation (e.g., the A2BAR).122 Other studies identified a critical role of adenosine in stimulating angiogenesis.123 While this is not the focus of the present review, it is important to point out that adenosine signaling also plays a role as central nervous system signaling molecule. For example, signaling through the A1AR has been implicated in reducing hypersensitivity following peripheral nerve injury or following surgery.66,124,125
Summary and Future Challenges
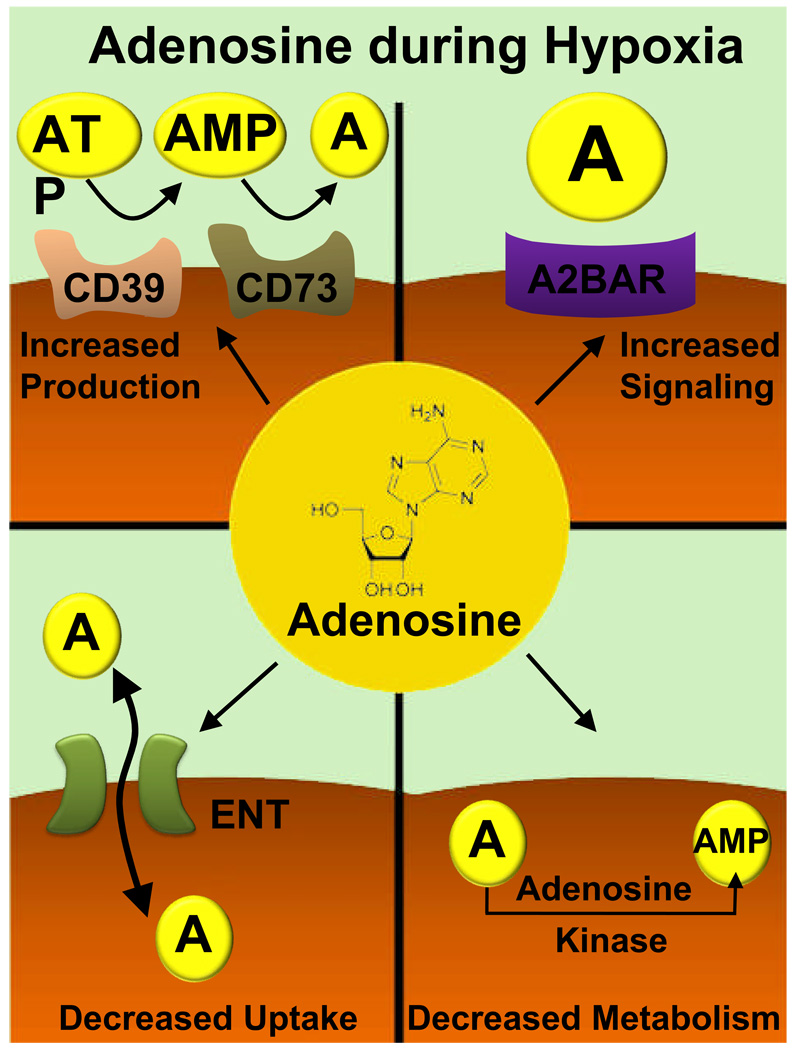
Adenosine has been used in the perioperative setting for the treatment of supraventricular tachycardia for many decades. More recently, research with specific AR agonists or antagonists in conjunction with studies in genetic models for adenosine generation or signaling have identified a rapidly expanding field of biomedical roles and potential therapeutic applications of extracellular adenosine signaling. Particularly during conditions of limited oxygen availability as occurs during acute inflammation, organ ischemia or acute lung injury, several pathways synergize to elevate extracellular adenosine levels and increase adenosine signaling effects. Such changes include increased extracellular adenosine production, increased expression patterns of specific adenosine receptors, and decreased adenosine uptake and intracellular metabolism (fig. 7). In this context, pharmacological strategies to enhance extracellular adenosine production (e.g., treatment with apyrase or nucleotidase) or specific AR agonist appears to be particularly important to counterbalance the deleterious effects of hypoxia, such as for the treatment of hypoxia-induced vascular leakage, excessive inflammation, pulmonary edema or ischemia-reperfusion injury.
Figure 7. Consequences of Hypoxia on Adenosine Signaling Pathways.
During situations of cellular distress (acute hypoxia, inflammation, ischemia reperfusion injury), hypoxia coordinates changes that lead to increases in extracellular signaling effects. These changes involve four mechanisms. First, extracellular adenosine production is enhanced through the transcriptional induction of the adenosine-producing enzymes ecto-apyrase (CD39; conversion of adenosine tri-phosphate, ATP, to adenosine mono-phosphate, AMP) and5’-ecto-nucleotidase (CD73; conversion of AMP to adenosine). In addition, adenosine effects are also enhanced on the receptor level. As such, hypoxia coordinates the selective induction of the A2B adenosine receptor (A2BAR). Moreover, hypoxia leads to transcriptional repression of equilibrative nucleoside transporters (ENTs), resulting in attenuated adenosine uptake, enhanced extracellular adenosine concentration and signaling. Finally, hypoxia also causes transcriptional repression of the adenosine kinase, the main enzyme for intracellular adenosine metabolism. Adenosine kinase catalyzes phosphorylation of adenosine to adenosine monophosphate (AMP). Hypoxia-dependent repression of adenosine kinase represents an additional hypoxia-elicited mechanism that enhances extracellular adenosine concentration and signaling during hypoxia.
Most of the studies that were discussed in the present review were performed in murine models. While we are presently at a stage, where specific adenosine receptor agonists are explored in human volunteers or patients,126 most of the studies discussed in the present review will require translation from murine models into a clinical setting. Moreover, specific side-effects and long-term safety of pharmacological agents utilizing adenosine signaling pathways have to be further defined. For example, it will be important to define the hemodynamic consequences of specific AR agonists. Also, it will critical to address the question if long-term use of AR agonists may be associated with fibrotic changes of the liver or the lungs. Such observations may limit these drugs for a short-term use in an acute setting. Moreover, specific sub-sets of patients who may require exclusion from therapy will have to be defined.127,128
Acknowledgement
I would like to acknowledge Shelley A. Eltzschig, B.S.B.A., Artist, Mucosal Inflammation Program, University of Colorado Denver, Denver, Colorado, for the artwork included in this manuscript.
Sources of Funding: The present review is supported by United States National Institutes of Health (Bethesda, Maryland) grant R01-HL092188 and Foundation for Anesthesia Education and Research (Rochester, Minnesota) grants to HKE.
References
- 1.Drury AN, Szent-Gyorgyi A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J Physiol. 1929;68:213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belhassen B, Pelleg A. Electrophysiologic effects of adenosine triphosphate and adenosine on the mammalian heart: Clinical and experimental aspects. J Am Coll Cardiol. 1984;4:414–424. doi: 10.1016/s0735-1097(84)80233-8. [DOI] [PubMed] [Google Scholar]
- 3.Delacretaz E. Clinical practice. Supraventricular tachycardia. N Engl J Med. 2006;354:1039–1051. doi: 10.1056/NEJMcp051145. [DOI] [PubMed] [Google Scholar]
- 4.Blomstrom-Lundqvist C, Scheinman MM, Aliot EM, Alpert JS, Calkins H, Camm AJ, Campbell WB, Haines DE, Kuck KH, Lerman BB, Miller DD, Shaeffer CW, Jr, Stevenson WG, Tomaselli GF, Antman EM, Smith SC, Jr, Faxon DP, Fuster V, Gibbons RJ, Gregoratos G, Hiratzka LF, Hunt SA, Jacobs AK, Russell RO, Jr, Priori SG, Blanc JJ, Budaj A, Burgos EF, Cowie M, Deckers JW, Garcia MA, Klein WW, Lekakis J, Lindahl B, Mazzotta G, Morais JC, Oto A, Smiseth O, Trappe HJ. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias--executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Supraventricular Arrhythmias) Circulation. 2003;108:1871–1909. doi: 10.1161/01.CIR.0000091380.04100.84. [DOI] [PubMed] [Google Scholar]
- 5.Stemp LI, Roy WL. Adenosine for the cardioversion of supraventricular tachycardia during general anesthesia and open heart surgery. Anesthesiology. 1992;76:849–852. doi: 10.1097/00000542-199205000-00024. [DOI] [PubMed] [Google Scholar]
- 6.Trohman RG. Supraventricular tachycardia: Implications for the intensivist. Crit Care Med. 2000;28:N129–N135. doi: 10.1097/00003246-200010001-00004. [DOI] [PubMed] [Google Scholar]
- 7.Robins K, Lyons G. Supraventricular tachycardia in pregnancy. Br J Anaesth. 2004;92:140–143. doi: 10.1093/bja/aeh004. [DOI] [PubMed] [Google Scholar]
- 8.Plaschke K, Bockler D, Schumacher H, Martin E, Bardenheuer HJ. Adenosine-induced cardiac arrest and EEG changes in patients with thoracic aorta endovascular repair. Br J Anaesth. 2006;96:310–316. doi: 10.1093/bja/ael002. [DOI] [PubMed] [Google Scholar]
- 9.Fang TD, Lippmann M, Kakazu C, Donayre CE, Bui H, Kopchok GE, White RA. High-dose adenosine-induced asystole assisting accurate deployment of thoracic stent grafts in conscious patients. Ann Vasc Surg. 2008;22:602–607. doi: 10.1016/j.avsg.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Picano E, Trivieri MG. Pharmacologic stress echocardiography in the assessment of coronary artery disease. Curr Opin Cardiol. 1999;14:464–470. doi: 10.1097/00001573-199911000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Hart ML, Kohler D, Eckle T, Kloor D, Stahl GL, Eltzschig HK. Direct treatment of mouse or human blood with soluble 5'-nucleotidase inhibits platelet aggregation. Arterioscler Thromb Vasc Biol. 2008;28:1477–1483. doi: 10.1161/ATVBAHA.108.169219. [DOI] [PubMed] [Google Scholar]
- 12.Sacco RL, Diener HC, Yusuf S, Cotton D, Ounpuu S, Lawton WA, Palesch Y, Martin RH, Albers GW, Bath P, Bornstein N, Chan BP, Chen ST, Cunha L, Dahlof B, De Keyser J, Donnan GA, Estol C, Gorelick P, Gu V, Hermansson K, Hilbrich L, Kaste M, Lu C, Machnig T, Pais P, Roberts R, Skvortsova V, Teal P, Toni D, Vandermaelen C, Voigt T, Weber M, Yoon BW. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359:1238–1251. doi: 10.1056/NEJMoa0805002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halker RB, Demaerschalk BM, Wellik KE, Wingerchuk DM, Rubin DI, Crum BA, Dodick DW. Caffeine for the prevention and treatment of postdural puncture headache: debunking the myth. Neurologist. 2007;13:323–327. doi: 10.1097/NRL.0b013e318145480f. [DOI] [PubMed] [Google Scholar]
- 14.Hampl KF, Schneider MC, Ruttimann U, Ummenhofer W, Drewe J. Perioperative administration of caffeine tablets for prevention of postoperative headaches. Can J Anaesth. 1995;42:789–7892. doi: 10.1007/BF03011178. [DOI] [PubMed] [Google Scholar]
- 15.Weber JG, Klindworth JT, Arnold JJ, Danielson DR, Ereth MH. Prophylactic intravenous administration of caffeine and recovery after ambulatory surgical procedures. Mayo Clin Proc. 1997;72:621–626. doi: 10.1016/S0025-6196(11)63567-2. [DOI] [PubMed] [Google Scholar]
- 16.Fanta CH. Asthma. N Engl J Med. 2009;360:1002–1014. doi: 10.1056/NEJMra0804579. [DOI] [PubMed] [Google Scholar]
- 17.Fredholm BB. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 2007;14:1315–1323. doi: 10.1038/sj.cdd.4402132. [DOI] [PubMed] [Google Scholar]
- 18.Eltzschig HK, Thompson LF, Karhausen J, Cotta RJ, Ibla JC, Robson SC, Colgan SP. Endogenous adenosine produced during hypoxia attenuates neutrophil accumulation: Coordination by extracellular nucleotide metabolism. Blood. 2004;104:3986–3992. doi: 10.1182/blood-2004-06-2066. [DOI] [PubMed] [Google Scholar]
- 19.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 20.Sitkovsky M, Lukashev D. Regulation of immune cells by local-tissue oxygen tension: HIF1 alpha and adenosine receptors. Nat Rev Immunol. 2005;5:712–721. doi: 10.1038/nri1685. [DOI] [PubMed] [Google Scholar]
- 21.Sitkovsky MV, Lukashev D, Apasov S, Kojima H, Koshiba M, Caldwell C, Ohta A, Thiel M. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- 22.Thiel M, Chouker A, Ohta A, Jackson E, Caldwell C, Smith P, Lukashev D, Bittmann I, Sitkovsky MV. Oxygenation inhibits the physiological tissue-protecting mechanism and thereby exacerbates acute inflammatory lung injury. PLoS Biol. 2005;3:e174. doi: 10.1371/journal.pbio.0030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenberger P, Schwab JM, Mirakaj V, Masekowsky E, Mager A, Morote-Garcia JC, Unertl K, Eltzschig HK. Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat Immunol. 2009;10:195–202. doi: 10.1038/ni.1683. [DOI] [PubMed] [Google Scholar]
- 24.Eltzschig HK, Ibla JC, Furuta GT, Leonard MO, Jacobson KA, Enjyoji K, Robson SC, Colgan SP. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: Role of ectonucleotidases and adenosine A2B receptors. J Exp Med. 2003;198:783–796. doi: 10.1084/jem.20030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eltzschig HK, Weissmuller T, Mager A, Eckle T. Nucleotide metabolism and cell-cell interactions. Methods Mol Biol. 2006;341:73–87. doi: 10.1385/1-59745-113-4:73. [DOI] [PubMed] [Google Scholar]
- 26.Faigle M, Seessle J, Zug S, El Kasmi KC, Eltzschig HK. ATP release from vascular endothelia occurs across Cx43 hemichannels and is attenuated during hypoxia. PLoS ONE. 2008;3:e2801. doi: 10.1371/journal.pone.0002801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 28.Eltzschig HK, Eckle T, Mager A, Kuper N, Karcher C, Weissmuller T, Boengler K, Schulz R, Robson SC, Colgan SP. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res. 2006;99:1100–1108. doi: 10.1161/01.RES.0000250174.31269.70. [DOI] [PubMed] [Google Scholar]
- 29.Weissmuller T, Campbell EL, Rosenberger P, Scully M, Beck PL, Furuta GT, Colgan SP. PMNs facilitate translocation of platelets across human and mouse epithelium and together alter fluid homeostasis via epithelial cell-expressed ecto-NTPDases. J Clin Invest. 2008;118:3682–3692. doi: 10.1172/JCI35874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robson SC, Wu Y, Sun X, Knosalla C, Dwyer K, Enjyoji K. Ectonucleotidases of CD39 family modulate vascular inflammation and thrombosis in transplantation. Semin Thromb Hemost. 2005;31:217–233. doi: 10.1055/s-2005-869527. [DOI] [PubMed] [Google Scholar]
- 31.Grenz A, Zhang H, Hermes M, Eckle T, Klingel K, Huang DY, Muller CE, Robson SC, Osswald H, Eltzschig HK. Contribution of E-NTPDase1 (CD39) to renal protection from ischemia-reperfusion injury. FASEB J. 2007;21:2863–2873. doi: 10.1096/fj.06-7947com. [DOI] [PubMed] [Google Scholar]
- 32.Kohler D, Eckle T, Faigle M, Grenz A, Mittelbronn M, Laucher S, Hart ML, Robson SC, Muller CE, Eltzschig HK. CD39/ectonucleoside triphosphate diphosphohydrolase 1 provides myocardial protection during cardiac ischemia/reperfusion injury. Circulation. 2007;116:1784–1794. doi: 10.1161/CIRCULATIONAHA.107.690180. [DOI] [PubMed] [Google Scholar]
- 33.Enjyoji K, Sevigny J, Lin Y, Frenette PS, Christie PD, Esch JS, 2nd, Imai M, Edelberg JM, Rayburn H, Lech M, Beeler DL, Csizmadia E, Wagner DD, Robson SC, Rosenberg RD. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med. 1999;5:1010–1017. doi: 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- 34.Pinsky DJ, Broekman MJ, Peschon JJ, Stocking KL, Fujita T, Ramasamy R, Connolly ES, Jr, Huang J, Kiss S, Zhang Y, Choudhri TF, McTaggart RA, Liao H, Drosopoulos JH, Price VL, Marcus AJ, Maliszewski CR. Elucidation of the thromboregulatory role of CD39/ectoapyrase in the ischemic brain. J Clin Invest. 2002;109:1031–1040. doi: 10.1172/JCI10649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qawi I, Robson SC. New developments in anti-platelet therapies: Potential use of CD39/vascular ATP diphosphohydrolase in thrombotic disorders. Curr Drug Targets. 2000;1:285–296. doi: 10.2174/1389450003349173. [DOI] [PubMed] [Google Scholar]
- 36.Reutershan J, Vollmer I, Stark S, Wagner R, Ngamsri KC, Eltzschig HK. Adenosine and inflammation: CD39 and CD73 are critical mediators in LPS-induced PMN trafficking into the lungs. FASEB J. 2009;23:473–482. doi: 10.1096/fj.08-119701. [DOI] [PubMed] [Google Scholar]
- 37.Friedman DJ, Rennke HG, Csizmadia E, Enjyoji K, Robson SC. The vascular ectonucleotidase ENTPD1 is a novel renoprotective factor in diabetic nephropathy. Diabetes. 2007;56:2371–2379. doi: 10.2337/db06-1593. [DOI] [PubMed] [Google Scholar]
- 38.Dwyer KM, Robson SC, Nandurkar HH, Campbell DJ, Gock H, Murray-Segal LJ, Fisicaro N, Mysore TB, Kaczmarek E, Cowan PJ, d’Apice AJ. Thromboregulatory manifestations in human CD39 transgenic mice and the implications for thrombotic disease and transplantation. J Clin Invest. 2004;113:1440–1446. doi: 10.1172/JCI19560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eltzschig HK, Kohler D, Eckle T, Kong T, Robson SC, Colgan SP. Central role of Sp1-regulated CD39 in hypoxia/ischemia protection. Blood. 2009;113:224–232. doi: 10.1182/blood-2008-06-165746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marcus AJ, Broekman MJ, Drosopoulos JH, Islam N, Pinsky DJ, Sesti C, Levi R. Metabolic control of excessive extracellular nucleotide accumulation by CD39/ecto-nucleotidase-1: Implications for ischemic vascular diseases. J Pharmacol Exp Ther. 2003;305:9–16. doi: 10.1124/jpet.102.043729. [DOI] [PubMed] [Google Scholar]
- 41.Eckle T, Fullbier L, Wehrmann M, Khoury J, Mittelbronn M, Ibla J, Rosenberger P, Eltzschig HK. Identification of ectonucleotidases CD39 and CD73 in innate protection during acute lung injury. J Immunol. 2007;178:8127–8137. doi: 10.4049/jimmunol.178.12.8127. [DOI] [PubMed] [Google Scholar]
- 42.Beldi G, Wu Y, Sun X, Imai M, Enjyoji K, Csizmadia E, Candinas D, Erb L, Robson SC. Regulated catalysis of extracellular nucleotides by vascular CD39/ENTPD1 is required for liver regeneration. Gastroenterology. 2008;135:1751–1760. doi: 10.1053/j.gastro.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Linden J. Molecular approach to adenosine receptors: Receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol. 2001;41:775–787. doi: 10.1146/annurev.pharmtox.41.1.775. [DOI] [PubMed] [Google Scholar]
- 44.Eckle T, Krahn T, Grenz A, Kohler D, Mittelbronn M, Ledent C, Jacobson MA, Osswald H, Thompson LF, Unertl K, Eltzschig HK. Cardioprotection by ecto-5'-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–1590. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- 45.Grenz A, Zhang H, Eckle T, Mittelbronn M, Wehrmann M, Kohle C, Kloor D, Thompson LF, Osswald H, Eltzschig HK. Protective role of ecto-5’-nucleotidase (CD73) in renal ischemia. J Am Soc Nephrol. 2007;18:833–845. doi: 10.1681/ASN.2006101141. [DOI] [PubMed] [Google Scholar]
- 46.Hart ML, Henn M, Kohler D, Kloor D, Mittelbronn M, Gorzolla IC, Stahl GL, Eltzschig HK. Role of extracellular nucleotide phosphohydrolysis in intestinal ischemia-reperfusion injury. FASEB J. 2008;22:2784–2797. doi: 10.1096/fj.07-103911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hart ML, Much C, Gorzolla IC, Schittenhelm J, Kloor D, Stahl GL, Eltzschig HK. Extracellular adenosine production by ecto-5’-nucleotidase protects during murine hepatic ischemic preconditioning. Gastroenterology. 2008;135:1739–1750. doi: 10.1053/j.gastro.2008.07.064. e3. [DOI] [PubMed] [Google Scholar]
- 48.Synnestvedt K, Furuta GT, Comerford KM, Louis N, Karhausen J, Eltzschig HK, Hansen KR, Thompson LF, Colgan SP. Ecto-5’-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thompson LF, Eltzschig HK, Ibla JC, Van De Wiele CJ, Resta R, Morote-Garcia JC, Colgan SP. Crucial role for ecto-5’-nucleotidase (CD73) in vascular leakage during hypoxia. J Exp Med. 2004;200:1395–1405. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Louis NA, Robinson AM, MacManus CF, Karhausen J, Scully M, Colgan SP. Control of IFN-alphaA by CD73: Implications for mucosal inflammation. J Immunol. 2008;180:4246–4255. doi: 10.4049/jimmunol.180.6.4246. [DOI] [PubMed] [Google Scholar]
- 51.Hart ML, Jacobi B, Schittenhelm J, Henn M, Eltzschig HK. Cutting edge: A2B adenosine receptor signaling provides potent protection during intestinal ischemia/reperfusion injury. J Immunol. 2009;182:3965–3968. doi: 10.4049/jimmunol.0802193. [DOI] [PubMed] [Google Scholar]
- 52.Chen H, Yang D, Carroll SH, Eltzschig HK, Ravid K. Activation of the macrophage A2b adenosine receptor regulates tumor necrosis factor-alpha levels following vascular injury. Exp Hematol. 2009;37:533–538. doi: 10.1016/j.exphem.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eckle T, Grenz A, Laucher S, Eltzschig HK. A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J Clin Invest. 2008;118:3301–3315. doi: 10.1172/JCI34203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eckle T, Kohler D, Lehmann R, El Kasmi KC, Eltzschig HK. Hypoxia-inducible factor-1 is central to cardioprotection: A new paradigm for ischemic preconditioning. Circulation. 2008;118:166–175. doi: 10.1161/CIRCULATIONAHA.107.758516. [DOI] [PubMed] [Google Scholar]
- 55.Grenz A, Osswald H, Eckle T, Yang D, Zhang H, Tran ZV, Klingel K, Ravid K, Eltzschig HK. The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Medicine. 2008;5:e137. doi: 10.1371/journal.pmed.0050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hasko G, Linden J, Cronstein B, Pacher P. Therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7:759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fredholm BB, Irenius E, Kull B, Schulte G. Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem Pharmacol. 2001;61:443–448. doi: 10.1016/s0006-2952(00)00570-0. [DOI] [PubMed] [Google Scholar]
- 58.Eckle T, Faigle M, Grenz A, Laucher S, Thompson LF, Eltzschig HK. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood. 2008;111:2024–2035. doi: 10.1182/blood-2007-10-117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baraldi PG, Romagnoli R, Preti D, Fruttarolo F, Carrion MD, Tabrizi MA. Ligands for A2B adenosine receptor subtype. Curr Med Chem. 2006;13:3467–3482. doi: 10.2174/092986706779010306. [DOI] [PubMed] [Google Scholar]
- 60.Eltzschig HK, Abdulla P, Hoffman E, Hamilton KE, Daniels D, Schonfeld C, Loffler M, Reyes G, Duszenko M, Karhausen J, Robinson A, Westerman KA, Coe IR, Colgan SP. HIF-1-dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. J Exp Med. 2005;202:1493–14505. doi: 10.1084/jem.20050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kong T, Westerman KA, Faigle M, Eltzschig HK, Colgan SP. HIF-dependent induction of adenosine A2B receptor in hypoxia. Faseb J. 2006;20:2242–2250. doi: 10.1096/fj.06-6419com. [DOI] [PubMed] [Google Scholar]
- 62.van Calker D, Muller M, Hamprecht B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J Neurochem. 1979;33:999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]
- 63.Bruns RF, Lu GH, Pugsley TA. Characterization of the A2 adenosine receptor labeled by [3H]NECA in rat striatal membranes. Mol Pharmacol. 1986;29:331–346. [PubMed] [Google Scholar]
- 64.Prystowsky EN, Niazi I, Curtis AB, Wilber DJ, Bahnson T, Ellenbogen K, Dhala A, Bloomfield DM, Gold M, Kadish A, Fogel RI, Gonzalez MD, Belardinelli L, Shreeniwas R, Wolff AA. Termination of paroxysmal supraventricular tachycardia by tecadenoson (CVT-510), A novel A1-adenosine receptor agonist. J Am Coll Cardiol. 2003;42:1098–1102. doi: 10.1016/s0735-1097(03)00987-2. [DOI] [PubMed] [Google Scholar]
- 65.Ledent C, Vaugeois JM, Schiffmann SN, Pedrazzini T, El Yacoubi M, Vanderhaeghen JJ, Costentin J, Heath JK, Vassart G, Parmentier M. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature. 1997;388:674–678. doi: 10.1038/41771. [DOI] [PubMed] [Google Scholar]
- 66.Obata H, Li X, Eisenach JC. Spinal adenosine receptor activation reduces hypersensitivity after surgery by a different mechanism than after nerve injury. Anesthesiology. 2004;100:1258–12562. doi: 10.1097/00000542-200405000-00030. [DOI] [PubMed] [Google Scholar]
- 67.Hansen PB, Schnermann J. Vasoconstrictor and vasodilator effects of adenosine in the kidney. Am J Physiol Renal Physiol. 2003;285:F590–F599. doi: 10.1152/ajprenal.00051.2003. [DOI] [PubMed] [Google Scholar]
- 68.Jin X, Shepherd RK, Duling BR, Linden J. Inosine binds to A3 adenosine receptors and stimulates mast cell degranulation. J Clin Invest. 1997;100:2849–2857. doi: 10.1172/JCI119833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Loffler M, Morote-Garcia JC, Eltzschig SA, Coe IR, Eltzschig HK. Physiological roles of vascular nucleoside transporters. Arterioscler Thromb Vasc Biol. 2007;27:1004–1013. doi: 10.1161/ATVBAHA.106.126714. [DOI] [PubMed] [Google Scholar]
- 70.Morote-Garcia JC, Rosenberger P, Kuhlicke J, Eltzschig HK. HIF-1-dependent repression of adenosine kinase attenuates hypoxia-induced vascular leak. Blood. 2008;111:5571–5580. doi: 10.1182/blood-2007-11-126763. [DOI] [PubMed] [Google Scholar]
- 71.Eltzschig HK, Faigle M, Knapp S, Karhausen J, Ibla J, Rosenberger P, Odegard KC, Laussen PC, Thompson LF, Colgan SP. Endothelial catabolism of extracellular adenosine during hypoxia: The role of surface adenosine deaminase and CD26. Blood. 2006;108:1602–1610. doi: 10.1182/blood-2006-02-001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Van Linden A, Eltzschig HK. Role of pulmonary adenosine during hypoxia:Extracellular generation, signaling and metabolism by surface adenosine deaminase/CD26. Expert Opin Biol Ther. 2007;7:1437–1447. doi: 10.1517/14712598.7.9.1437. [DOI] [PubMed] [Google Scholar]
- 73.Eckle T, Grenz A, Kohler D, Redel A, Falk M, Rolauffs B, Osswald H, Kehl F, Eltzschig HK. Systematic evaluation of a novel model for cardiac ischemic preconditioning in mice. Am J Physiol Heart Circ Physiol. 2006;291:H2533–H2540. doi: 10.1152/ajpheart.00472.2006. [DOI] [PubMed] [Google Scholar]
- 74.Morote-Garcia JC, Rosenberger P, Nivillac NM, Coe IR, Eltzschig HK. Hypoxia-inducible factor-dependent repression of equilibrative nucleoside transporter 2 attenuates mucosal inflammation during intestinal hypoxia. Gastroenterology. 2009;136:607–618. doi: 10.1053/j.gastro.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 75.Lloyd HG, Fredholm BB. Involvement of adenosine deaminase and adenosine kinase in regulating extracellular adenosine concentration in rat hippocampal slices. Neurochem Int. 1995;26:387–395. doi: 10.1016/0197-0186(94)00144-j. [DOI] [PubMed] [Google Scholar]
- 76.Blackburn MR, Volmer JB, Thrasher JL, Zhong H, Crosby JR, Lee JJ, Kellems RE. Metabolic consequences of adenosine deaminase deficiency in mice are associated with defects in alveogenesis, pulmonary inflammation, and airway obstruction. J Exp Med. 2000;192:159–170. doi: 10.1084/jem.192.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Blackburn MR. Too much of a good thing: Adenosine overload in adenosinedeaminase-deficient mice. Trends Pharmacol Sci. 2003;24:66–70. doi: 10.1016/S0165-6147(02)00045-7. [DOI] [PubMed] [Google Scholar]
- 78.Decking UKM, Schlieper G, Kroll K, Schrader J. Hypoxia-Induced Inhibition of adenosine kinase potentiates cardiac adenosine release. Circ Res. 1997;81:154–164. doi: 10.1161/01.res.81.2.154. [DOI] [PubMed] [Google Scholar]
- 79.Saito H, Nishimura M, Shinano H, Makita H, Tsujino I, Shibuya E, Sato F, Miyamoto K, Kawakami Y. Plasma concentration of adenosine during normoxia and moderate hypoxia in humans. Am J Respir Crit Care Med. 1999;159:1014–1018. doi: 10.1164/ajrccm.159.3.9803100. [DOI] [PubMed] [Google Scholar]
- 80.Semenza GL. Life with oxygen. Science. 2007;318:62–64. doi: 10.1126/science.1147949. [DOI] [PubMed] [Google Scholar]
- 81.Semenza GL. O2 sensing: Only skin deep? Cell. 2008;133:206–208. doi: 10.1016/j.cell.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 82.Semenza GL. HIF-1, O(2), and the 3 PHDs: How animal cells signal hypoxia to the nucleus. Cell. 2001;107:1–3. doi: 10.1016/s0092-8674(01)00518-9. [DOI] [PubMed] [Google Scholar]
- 83.Sitkovsky MV. Damage control by hypoxia-inhibited AK. Blood. 2008;111:5424–5425. doi: 10.1182/blood-2008-03-143990. [DOI] [PubMed] [Google Scholar]
- 84.Cummins EP, Berra E, Comerford KM, Ginouves A, Fitzgerald KT, Seeballuck F, Godson C, Nielsen JE, Moynagh P, Pouyssegur J, Taylor CT. Prolyl hydroxylase-1 negatively regulates IkappaB kinase-beta, giving insight into hypoxia-induced NFkappaB activity. Proc Natl Acad Sci U S A. 2006;103:18154–18159. doi: 10.1073/pnas.0602235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Taylor CT. Interdependent roles for hypoxia inducible factor and nuclear factor-kappaB in hypoxic inflammation. J Physiol. 2008;586:4055–4059. doi: 10.1113/jphysiol.2008.157669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG, Karin M. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.De Perrot M, Sekine Y, Fischer S, Waddell TK, McRae K, Liu M, Wigle DA, Keshavjee S. Interleukin-8 release during early reperfusion predicts graft function in human lung transplantation. Am J Respir Crit Care Med. 2002;165:211–215. doi: 10.1164/ajrccm.165.2.2011151. [DOI] [PubMed] [Google Scholar]
- 88.Naka Y, Toda K, Kayano K, Oz MC, Pinsky DJ. Failure to express the P-selectin gene or P-selectin blockade confers early pulmonary protection after lung ischemia or transplantation. Proc Natl Acad Sci U S A. 1997;94:757–761. doi: 10.1073/pnas.94.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang Z, Day YJ, Toufektsian MC, Ramos SI, Marshall M, Wang XQ, French BA, Linden J. Infarct-sparing effect of A2A-adenosine receptor activation is due primarily to its action on lymphocytes. Circulation. 2005;111:2190–2197. doi: 10.1161/01.CIR.0000163586.62253.A5. [DOI] [PubMed] [Google Scholar]
- 90.Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest. 2004;114:1098–1106. doi: 10.1172/JCI21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Robinson A, Keely S, Karhausen J, Gerich ME, Furuta GT, Colgan SP. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology. 2008;134:145–155. doi: 10.1053/j.gastro.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Peyssonnaux C, Datta V, Cramer T, Doedens A, Theodorakis EA, Gallo RL, Hurtado-Ziola N, Nizet V, Johnson RS. HIF-1{alpha} expression regulates the bactericidal capacity of phagocytes. J Clin Invest. 2005;115:1806–1815. doi: 10.1172/JCI23865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cummins EP, Seeballuck F, Keely SJ, Mangan NE, Callanan JJ, Fallon PG, Taylor CT. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology. 2008;134:156–165. doi: 10.1053/j.gastro.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 94.Kempf VA, Lebiedziejewski M, Alitalo K, Walzlein JH, Ehehalt U, Fiebig J, Huber S, Schutt B, Sander CA, Muller S, Grassl G, Yazdi AS, Brehm B, Autenrieth IB. Activation of hypoxia-inducible factor-1 in bacillary angiomatosis: Evidence for a role of hypoxia-inducible factor-1 in bacterial infections. Circulation. 2005;111:1054–1062. doi: 10.1161/01.CIR.0000155608.07691.B7. [DOI] [PubMed] [Google Scholar]
- 95.Ware LB, Matthay MA. The Acute Respiratory Distress Syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 96.Licker M, de Perrot M, Spiliopoulos A, Robert J, Diaper J, Chevalley C, Tschopp JM. Risk factors for acute lung injury after thoracic surgery for lung cancer. Anesth Analg. 2003;97:1558–1565. doi: 10.1213/01.ANE.0000087799.85495.8A. [DOI] [PubMed] [Google Scholar]
- 97.Milot J, Perron J, Lacasse Y, Letourneau L, Cartier PC, Maltais F. Incidence and predictors of ARDS after cardiac surgery. Chest. 2001;119:884–888. doi: 10.1378/chest.119.3.884. [DOI] [PubMed] [Google Scholar]
- 98.Shorr AF, Abbott KC, Agadoa LY. Acute respiratory distress syndrome after kidney transplantation: Epidemiology, risk factors, and outcomes. Crit Care Med. 2003;31:1325–1330. doi: 10.1097/01.CCM.0000053645.38356.A6. [DOI] [PubMed] [Google Scholar]
- 99.Volmer JB, Thompson LF, Blackburn MR. Ecto-5’-nucleotidase (CD73)-mediated adenosine production is tissue protective in a model of bleomycin-induced lung injury. J Immunol. 2006;176:4449–4458. doi: 10.4049/jimmunol.176.7.4449. [DOI] [PubMed] [Google Scholar]
- 100.Khoury J, Ibla JC, Neish AS, Colgan SP. Antiinflammatory adaptation to hypoxia through adenosine-mediated cullin-1 deneddylation. J Clin Invest. 2007;117:703–711. doi: 10.1172/JCI30049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sun CX, Zhong H, Mohsenin A, Morschl E, Chunn JL, Molina JG, Belardinelli L, Zeng D, Blackburn MR. Role of A2B adenosine receptor signaling in adenosine-dependent pulmonary inflammation and injury. J Clin Invest. 2006;116:2173–2182. doi: 10.1172/JCI27303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reutershan J, Cagnina RE, Chang D, Linden J, Ley K. Therapeutic anti-inflammatory effects of myeloid cell adenosine receptor A2a stimulation in lipopolysaccharide-induced lung injury. J Immunol. 2007;179:1254–12563. doi: 10.4049/jimmunol.179.2.1254. [DOI] [PubMed] [Google Scholar]
- 103.Hypoxia to the rescue: When oxygen therapies backfire. PLoS Biology. 2005;3:e211. [Google Scholar]
- 104.Weissmuller T, Eltzschig HK, Colgan SP. Dynamic purine signaling and metabolism during neutrophil-endothelial interactions. Purinergic Signal. 2005;1:229–239. doi: 10.1007/s11302-005-6323-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof E, Fleischmann KE, Freeman WK, Froehlich JB, Kasper EK, Kersten JR, Riegel B, Robb JF, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Buller CE, Creager MA, Ettinger SM, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Lytle BW, Md RN, Ornato JP, Page RL, Tarkington LG, Yancy CW. ACC/AHA 2007 Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): Developed in Collaboration With the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. Circulation. 2007;116:1971–1996. doi: 10.1161/CIRCULATIONAHA.107.185700. [DOI] [PubMed] [Google Scholar]
- 106.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 107.Downey JM, Davis AM, Cohen MV. Signaling pathways in ischemic preconditioning. Heart Fail Rev. 2007;12:181–188. doi: 10.1007/s10741-007-9025-2. [DOI] [PubMed] [Google Scholar]
- 108.Lankford AR, Yang JN, Rose'Meyer R, French BA, Matherne GP, Fredholm BB, Yang Z. Effect of modulating cardiac A1 adenosine receptor expression on protection with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2006;290:H1469–H1473. doi: 10.1152/ajpheart.00181.2005. [DOI] [PubMed] [Google Scholar]
- 109.Armstrong S, Ganote CE. Adenosine receptor specificity in preconditioning of isolated rabbit cardiomyocytes: Evidence of A3 receptor involvement. Cardiovasc Res. 1994;28:1049–1056. doi: 10.1093/cvr/28.7.1049. [DOI] [PubMed] [Google Scholar]
- 110.Kin H, Zatta AJ, Lofye MT, Amerson BS, Halkos ME, Kerendi F, Zhao ZQ, Guyton RA, Headrick JP, Vinten-Johansen J. Postconditioning reduces infarct size via adenosine receptor activation by endogenous adenosine. Cardiovasc Res. 2005;67:124–133. doi: 10.1016/j.cardiores.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 111.Donato M, D'Annunzio V, Berg G, Gonzalez G, Schreier L, Morales C, Wikinski RL, Gelpi RJ. Ischemic postconditioning reduces infarct size by activation of A1 receptors and K+(ATP) channels in both normal and hypercholesterolemic rabbits. J Cardiovasc Pharmacol. 2007;49:287–292. doi: 10.1097/FJC.0b013e31803c55fe. [DOI] [PubMed] [Google Scholar]
- 112.Xi L, Das A, Zhao ZQ, Merino VF, Bader M, Kukreja RC. Loss of myocardial ischemic postconditioning in adenosine A1 and bradykinin B2 receptors gene knockout mice. Circulation. 2008;118:S32–S37. doi: 10.1161/CIRCULATIONAHA.107.752865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Morrison RR, Tan XL, Ledent C, Mustafa SJ, Hofmann PA. Targeted deletion of A2A adenosine receptors attenuates the protective effects of myocardial postconditioning. Am J Physiol Heart Circ Physiol. 2007;293:H2523–H2529. doi: 10.1152/ajpheart.00612.2007. [DOI] [PubMed] [Google Scholar]
- 114.Philipp S, Yang XM, Cui L, Davis AM, Downey JM, Cohen MV. Postconditioning protects rabbit hearts through a protein kinase C-adenosine A2b receptor cascade. Cardiovasc Res. 2006;70:308–314. doi: 10.1016/j.cardiores.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 115.Day YJ, Huang L, McDuffie MJ, Rosin DL, Ye H, Chen JF, Schwarzschild MA, Fink JS, Linden J, Okusa MD. Renal protection from ischemia mediated by A2A adenosine receptors on bone marrow-derived cells. J Clin Invest. 2003;112:883–891. doi: 10.1172/JCI15483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Day YJ, Huang L, Ye H, Li L, Linden J, Okusa MD. Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: the role of CD4+ T cells and IFN-gamma. J Immunol. 2006;176:3108–3114. doi: 10.4049/jimmunol.176.5.3108. [DOI] [PubMed] [Google Scholar]
- 117.Lappas CM, Day YJ, Marshall MA, Engelhard VH, Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med. 2006;203:2639–2648. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang D, Zhang Y, Nguyen HG, Koupenova M, Chauhan AK, Makitalo M, Jones MR, St Hilaire C, Seldin DC, Toselli P, Lamperti E, Schreiber BM, Gavras H, Wagner DD, Ravid K. The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. J Clin Invest. 2006;116:1913–1923. doi: 10.1172/JCI27933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang D, Koupenova M, McCrann DJ, Kopeikina KJ, Kagan HM, Schreiber BM, Ravid K. The A2b adenosine receptor protects against vascular injury. Proc Natl Acad Sci U S A. 2008;105:792–796. doi: 10.1073/pnas.0705563105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Frick JS, MacManus CF, Scully M, Glover LE, Eltzschig HK, Colgan SP. Contribution of adenosine A2B receptors to inflammatory parameters of experimental colitis. J Immunol. 2009;182:4957–4964. doi: 10.4049/jimmunol.0801324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hua X, Kovarova M, Chason KD, Nguyen M, Koller BH, Tilley SL. Enhanced mast cell activation in mice deficient in the A2b adenosine receptor. J Exp Med. 2007;204:117–128. doi: 10.1084/jem.20061372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hasko G, Csoka B, Nemeth ZH, Vizi ES, Pacher P. A(2B) adenosine receptors in immunity and inflammation. Trends Immunol. 2009 May;6 doi: 10.1016/j.it.2009.04.001. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Clark AN, Youkey R, Liu X, Jia L, Blatt R, Day YJ, Sullivan GW, Linden J, Tucker AL. A1 adenosine receptor activation promotes angiogenesis and release of VEGF from monocytes. Circ Res. 2007;101:1130–1138. doi: 10.1161/CIRCRESAHA.107.150110. [DOI] [PubMed] [Google Scholar]
- 124.Zhang Y, Conklin DR, Li X, Eisenach JC. Intrathecal morphine reduces allodynia after peripheral nerve injury in rats via activation of a spinal A1 adenosine receptor. Anesthesiology. 2005;102:416–420. doi: 10.1097/00000542-200502000-00027. [DOI] [PubMed] [Google Scholar]
- 125.Chiari AI, Eisenach JC. Intrathecal adenosine: interactions with spinal clonidine and neostigmine in rat models of acute nociception and postoperative hypersensitivity. Anesthesiology. 1999;90:1413–1421. doi: 10.1097/00000542-199905000-00026. [DOI] [PubMed] [Google Scholar]
- 126.Hendel RC, Bateman TM, Cerqueira MD, Iskandrian AE, Leppo JA, Blackburn B, Mahmarian JJ. Initial clinical experience with regadenoson, a novel selective A2A agonist for pharmacologic stress single-photon emission computed tomography myocardial perfusion imaging. J Am Coll Cardiol. 2005;46:2069–2075. doi: 10.1016/j.jacc.2005.05.097. [DOI] [PubMed] [Google Scholar]
- 127.Leaker BR, O'Connor B, Hansel TT, Barnes PJ, Meng L, Mathur VS, Lieu HD. Safety of regadenoson, an adenosine A2A receptor agonist for myocardial perfusion imaging, in mild asthma and moderate asthma patients: A randomized, double-blind, placebo-controlled trial. J Nucl Cardiol. 2008;15:329–336. doi: 10.1016/j.nuclcard.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 128.Laighold S, Druz R. Initial clinical experience with a selective A2A receptor agonist, regadenoson, in a patient with end-stage renal disease on hemodialysis. J Nucl Cardiol. 2009;16:478–480. doi: 10.1007/s12350-008-9043-z. [DOI] [PubMed] [Google Scholar]