Abstract
INTRODUCTION:
Visual analysis is widely used to interpret regional cerebral blood flow (rCBF) SPECT images in clinical practice despite its limitations. Automated methods are employed to investigate between-group rCBF differences in research studies but have rarely been explored in individual analyses.
OBJECTIVES:
To compare visual inspection by nuclear physicians with the automated statistical parametric mapping program using a SPECT dataset of patients with neurological disorders and normal control images.
METHODS:
Using statistical parametric mapping, 14 SPECT images from patients with various neurological disorders were compared individually with a databank of 32 normal images using a statistical threshold of p<0.05 (corrected for multiple comparisons at the level of individual voxels or clusters). Statistical parametric mapping results were compared with visual analyses by a nuclear physician highly experienced in neurology (A) as well as a nuclear physician with a general background of experience (B) who independently classified images as normal or altered, and determined the location of changes and the severity.
RESULTS:
Of the 32 images of the normal databank, 4 generated maps showing rCBF abnormalities (p<0.05, corrected). Among the 14 images from patients with neurological disorders, 13 showed rCBF alterations. Statistical parametric mapping and physician A completely agreed on 84.37% and 64.28% of cases from the normal databank and neurological disorders, respectively. The agreement between statistical parametric mapping and ratings of physician B were lower (71.18% and 35.71%, respectively).
CONCLUSION:
Statistical parametric mapping replicated the findings described by the more experienced nuclear physician. This finding suggests that automated methods for individually analyzing rCBF SPECT images may be a valuable resource to complement visual inspection in clinical practice.
Keywords: Brain SPECT, ECD, Statistical parametric mapping, Cerebral blood flow, Dementia
INTRODUCTION
The technique of single photon emission computed tomography (SPECT) is widely used to evaluate regional cerebral blood flow (rCBF) patterns in subjects with neuropsychiatric disorders. It can be used in neurological practice as a valuable tool for different clinical situations, such as locating the epileptic focus,1 providing diagnostic support for dementia,2 evaluating closed cranial trauma3 and assessing cerebrovascular disease.4
In clinical practice, visual inspection has been the method most used for analyzing cerebral SPECT images. In this approach, the nuclear clinician needs to decide whether the image is normal or abnormal, which structures are affected, whether the detected abnormality relates to hypo- or hyperperfusion, and the extent and severity of the damage. This assessment is essentially carried out subjectively based on the reference frame of the individual examining the images. Such individual points of reference will generally have been acquired through the individual’s own practical experience over the course of his/her career.
However, when visual evaluation is insufficient, particularly for cases of borderline findings from cerebral SPECT images, a semiquantitative means of evaluation based on superposing the regions of interest (ROIs) on predefined cerebral structures can be used.5,6 Through this approach, mean counts for radioactive substrate uptake in the cerebral structures under analysis can be obtained. These counts can be compared with the uptake in other brain structures taken as normal references, which are chosen because they are presumed not to be affected by the disease under investigation by cerebral SPECT examination (for instance, the cerebellum or occipital cortex would be used as a reference). However, analysis by means of ROIs presents a variety of methodological problems, such as laboriousness, dependence on the observer who delineates the ROIs, the impossibility of distinguishing between different activity patterns within portions of the same ROI and the induced errors that occur when small ROIs are delineated (which may lead to failure in identifying foci of abnormalities that might not fall within the area delimited by the ROI).
In recent years, there has been great interest in methodologies that allow quantification of cerebral SPECT data used to evaluate rCBF patterns more objectively and rapidly than ROI-based methods.7,8 The automated method for statistical analysis of SPECT images that has been rated highest is statistical parametric mapping (SPM).9 This method was originally designed for use with positron emission tomography (PET) images and was subsequently adapted for processing cerebral SPECT data. SPM has been widely used in studies in which cerebral images from groups of patients with a specific diagnosis are compared with images from groups of normal volunteers who have been matched for demographic variables. For each voxel of brain volume, this comparison between groups tests the differences between the means of the two sets of data while taking the variance within groups into account.10 With SPM, statistical comparisons between different subgroups of patients can also be made (for example, with different types of dementia).11 In addition, regional cerebral function patterns among samples of patients can be correlated with the severity of specific symptoms by quantifying each voxel using standardized scales.12 These statistical analyses are computed through several stages of image preprocessing including realignment, automatic spatial normalization and smoothing. Following these preprocessing stages, statistical brain mapping is compiled and evaluated. The result of these analyses shows significant foci in a standardized anatomical space.9,13
Despite the good results that have been obtained using SPM for comparisons between groups in neuroscience studies involving the PET and cerebral SPECT techniques,14,15 only a few studies have evaluated the usefulness of SPM for evaluating individual cases in daily practice within nuclear medicine. Automatic analysis methods have already been incorporated into the clinical routine within nuclear medicine and in other medical fields of medical knowledge, specifically nuclear cardiology. However, this type of analysis continues to be minimally explored in clinical practice within neurology and psychiatry. In the first study that evaluated the use of an automated analysis method, Signorini et al.16 (1999) measured cerebral glucose metabolism using PET after administering fluorodeoxyglucose ([18F]FDG) to six patients with different brain-based neurological disorders (ND). The images from each patient were statistically contrasted with images from a pool of nineteen normal controls to generate individual statistical maps showing the locations of the cerebral metabolic abnormalities in each patient. Subsequent studies further demonstrated potential clinical usefulness of SPM for objective evaluation of the locations of epileptic foci in SPECT (rCBF)17,18 and PET19 images, as well as for evaluation of patients with closed cranial trauma using cerebral SPECT.20
One particularly important but still little-investigated issue is the verification of the degree of concordance between visual assessments made by nuclear medicine clinicians and the results obtained automatically using SPM to evaluate individual PET or cerebral SPECT examinations.16,21,22 For example, no cerebral SPECT study has yet investigated whether any differences exist in the degree of concordance between visual assessments and the statistical map generated by SPM in individual cases. Furthermore, no study has addressed this question in relation to the degree of experience of the nuclear medicine clinician performing the visual inspection. Demonstrating differences in concordance between clinicians with different levels of experience and the automated method might strengthen the usefulness of SPM for assisting general clinicians within nuclear medicine.
In the present study, we analyzed images from patients who had undergone evaluations using cerebral SPECT to support diagnoses of ND. We used automated SPM evaluations and visual assessments of two clinicians with different degrees of experience in inspecting cerebral SPECT data. The two aims of this study were (1) to ascertain the frequency of concordance between visual and automated evaluations so as to demonstrate the degree of usefulness of SPM for representing perfusion abnormalities and (2) to evaluate the possible differences in the degree of concordance between SPM and the two clinicians. Our working hypothesis was that the automated method would present a greater degree of concordance with the visual assessment of the more experienced clinician.
MATERIAL AND METHODS
SPECT images from the group of patients with neurological disorders
Fourteen cerebral SPECT images were selected from a database of patients who had been referred to the Nuclear Medicine Service of the Heart Hospital of Associação do Sanatório Sírio, in São Paulo, Brazil (HCor). The images were from 4 men and 10 women of ages ranging from 37 to 87 years [mean = 66.5 years; standard deviation (SD) = 10.33]. These patients had been referred by experienced clinical neurologists and neuropsychiatrists in the city of São Paulo who requested the image evaluation as complementary support for the clinical diagnosis. In most cases (n=4 subjects), the suspicion that motivated the request for SPECT examination was a condition of dementia, generally Alzheimer’s disease. Among the remaining images (n= 5 subjects), the suspected diagnoses were fronto-temporal dementia (n=1), neurocysticercosis (n=1), cerebral tumor (n=1) and stroke (n=2).
SPECT images from the control group
A database of 32 cerebral SPECT images was created from individuals who were presumed not to have cerebral abnormalities. The images were from 15 men and 17 women of ages ranging from 26 to 84 years, with a mean age of 44.5 years (SD = 16.004). Two different sources were used to compile this database. The first source was a control group of healthy volunteers (n = 26) who were recruited for a SPECT study conducted by Busatto et al.14 (2000). From this group, 21 individuals over the age of 20 years were selected to approximately match the age groups of subjects with ND described above. Before undergoing the SPECT examination, all subjects in this control group underwent a detailed assessment, including general medical anamnesis and the structured clinical interview for DSM-IV diagnosis of psychiatric disorders (SCID23), to rule out the presence of any neurological and/or psychiatric disorders. Moreover, these individuals showed no signs of gross brain abnormalities, as assessed by structural magnetic resonance scans that were visually inspected by two radiologists independently from each other. The second source for the control database was individuals who had been examined at the Nuclear Medicine Service of HCor to clarify the diagnosis in cases of complaints of headache or temporary memory deficit. Eleven cerebral SPECT images from these subjects were selected for the control data set. These had been assessed as showing a normal visual appearance, without any abnormalities, by a nuclear clinician who was highly experienced in evaluating cerebral SPECT examinations (C.A.B.).
Protocol for acquisition and processing of cerebral SPECT images
The protocols used for acquisition and processing of cerebral SPECT data were similar for all subjects in the above groups. After venous puncture in a superficial vein of the arm, the individuals remained at rest for 20 minutes and then received a dose of 740 MBq of 99mTc-ECD. Image acquisition began after another 30 minutes. All examinations were performed using a device with two detectors and high-resolution collimators (OPTIMA NX, General Electric, Milwaukee, USA). The images were acquired using the step-and-shoot method. A matrix of 128 X 128 was used, and 128 projections were acquired (20 seconds per projection).
The orbital-meatal line was used for reconstruction of the SPECT images, which was done using the filtered back-projection method with attenuation correction in accordance with Chang’s algorithm (μ=0.12 cm−1). All images were reconstructed with 2.25 mm pixels and a tenth-order Butterworth filter with Nyquist frequency of 0.57. Transverse sections through the reconstructed images were selected for the subsequent stages of the analysis.
Protocol for visual inspection of the SPECT images
For the visual analysis, two nuclear clinicians with different experience levels from recognized institutions in the city of São Paulo were invited to participate. One of these physicians (clinician A) was highly experienced in analyzing neurofunctional images acquired using the SPECT method, while the other (clinician B) was experienced in general nuclear medicine, without specific expertise in brain SPECT image analysis. The clinicians were positioned in front of a computer monitor and the images (both normal and abnormal) were shown without identification and randomly. The clinicians remained blind to the clinical data related to the images under examination and were asked to record whether the image was normal or abnormal. If the image was considered to show any rCBF abnormality, the clinicians were asked to indicate its location, severity (mild, moderate or severe), extent of damage (partial or complete) and any involvement of adjacent cerebral structures.
Generation of individualized statistical parametric maps showing rCBF abnormalities
Individualized statistical parametric maps for each subject were produced using the SPM program, version 1999, executed in MATLAB 4.2. First, SPECT images of all patients with ND and controls were spatially normalized with linear 12-point affine transformations to an anatomical template provided by the SPM program, which approximates the stereotaxic space of the Talairach-Tournoux24 atlas. Next, images were re-sliced using bi-linear interpolation to a final voxel size of 2 × 2 × 2 mm3 and smoothed with an isotropic Gaussian filter (12 mm full-width at half maximum) to improve the signal-to-noise ratio and to reduce errors attributed to inter-individual variations in gyral and sulcal anatomy. For each of the subjects with ND (n=14), a voxel-by-voxel t-test map was obtained by comparison against the pool of controls (n=32). For the purpose of accounting for inter-individual differences in overall cerebral blood flow, the regional 99mTc-ECD uptake was standardized to the mean overall uptake using proportional scaling. The measure of total brain radioactive uptake was obtained automatically from the SPM program in terms of the mean counts of all voxels included in the SPECT volume of each subject, after the spatial transformations described above. Given the significant difference between the mean age of the control group (44.5 +/− 16 y) and that of the ND group (66.5 +/− 10.33 y), age was used as a confounding covariate in the individual analyses conducted for all patients. To reduce the number of statistical comparisons, only voxels with signal intensities above 50% of the mean overall value were entered into each analysis. The individualized SPM{t} maps for each subject were transformed to a normal distribution (Z scores), thresholded at 3.09 (corresponding to ρ<0.001, uncorrected for multiple comparisons) and displayed as statistical parametric maps in standard space. The same procedure was carried out to provide individualized statistical parametric maps of rCBF abnormalities for the control subjects by comparing each control individual against the remaining 31 subjects.
Clusters of rCBF abnormalities in the individualized maps were then examined in terms of size (k) and peak height (u) and were considered significant only if they retained statistical significance after correction for multiple comparisons based on Gaussian random field theory (p<0.05), either at the level of individual voxel height or at the level of clusters25.
Criteria for evaluating the concordance between the clinicians and SPM
For each image evaluated by the two clinicians, criteria were established for applying concordance scores ranging from 0 to 3. A score of 0 (complete disagreement) was applied if one of the evaluators considered the image to be normal while the other found abnormalities of any nature in the rCBF profile. A score of 1 was given if the evaluators agreed that the image was abnormal, but disagreed about which cerebral structures were affected. A score of 2 was given for other situations of partial agreement (they agreed about which region was affected but also noted another different region or they disagreed on the extent of the abnormality or the hemisphere). Finally, a score of 3 indicated complete agreement regarding the location, extent and severity of the rCBF abnormalities detected by the two evaluators. Based on the scores obtained using these criteria, the general percent concordance between the two clinicians was calculated both for the entire sample of patients with ND and control individuals, and separately for these two groups of subjects. The same criteria were applied to evaluate the percent concordance between each of the two clinicians separately and the individualized statistical maps obtained using the SPM software.
Finally, with the aim of investigating possible statistically significant differences in the degree of concordance that the evaluators had with SPM, comparisons between the respective scores were made using the chi-square test (at a significance level of p < 0.05). These analyses were performed using the scores obtained for the entire sample of patients with ND and control individuals, and separately for these two groups.
RESULTS
Automated analysis using SPM
Among the images from the control group, 4 of the 32 subjects presented at least one region of the brain with abnormal rCBF according to the analyses carried out using SPM (in individualized analyses comparing one subject with all others) using a threshold of statistical significance defined for this study (p < 0.05; corrected for multiple comparisons at individual voxel level or cluster level). Three of these images came from “source 1”, which consisted of young adults who had been screened prospectively to exclude general medical conditions and neuropsychiatric disorders.
With the automated analysis using SPM to compare each of the 14 ND subjects with the above pool of normal controls, statistically significant foci for rCBF abnormalities were identified in 13 cases. Nine of these abnormalities met a significant threshold of p<0.05 and were corrected for multiple comparisons at individual voxel level or cluster level, while 4 were significant only at the cluster level (Table 1).
Table 1.
Results from individual analyses using the statistical parametric mapping (SPM) method for patients with neurological disorders (n = 14)
| Subjects | Clinical diagnostic hypothesis | Age | Z max | P corrected to individual voxel level | P corrected to cluster level | Number of voxels | Location |
|---|---|---|---|---|---|---|---|
| 1 | CVD | 80 | 3.92 | 0.453 | 0.03 | 401 | Middle occipital |
| 2 | AD | 58 | 6.0 | 0.000 | 0.000 | 6086 | Right posterior parietal |
| 3 | CVD | 65 | 4.06 | 0.318 | 0.263 | Superior frontal | |
| 4 | AD | 64 | 6.07 | 0.000 | 0.000 | 3156 | Right middle temporal |
| 5 | AD | 73 | 5.07 | 0.01 | 0.000 | 5067 | Right inferior temporal |
| 6 | AD | 66 | 4.93 | 0.013 | 0.055 | 1242 | Right inferior temporal |
| 7 | CVD+AD | 68 | 7.42 | 0.000 | 0.000 | 2789 | Right central posterior parietal |
| 8 | AD | 69 | 7.11 | 0.000 | 0.000 | 1351 | Right parietal |
| 9 | FTD | 77 | 3.47 | 0.09 | 0.510 | 136 | Right middle frontal |
| 10 | NC | 37 | 4.85 | 0.021 | 0.002 | 918 | Left occipital |
| 11 | AD | 65 | 5.99 | 0.000 | 0.000 | 4603 | Right middle temporal |
| 12 | AD | 61 | 3.91 | 0.459 | 0.035 | Superior parietal | |
| 13 | TD | 79 | 6.39 | 0.000 | 0.000 | 4699 | Left inferior frontal |
| 14 | PAD | 70 | 5.80 | 0.000 | 0.000 | 968 | Right inferior temporal |
AD: Alzheimer’s disease; FTD: fronto-temporal dementia; CVD: cerebrovascular disease; TD: tumoral disease; NC: neurocysticercosis
Frequency of concordance in visual inspection between the two nuclear medicine clinicians
Table 2 lists the findings regarding agreement and disagreement in visual inspection between the two nuclear medicine clinicians for examinations of previously identified subjects with ND (n=14). In this group, the two clinicians agreed completely on 7 assessments (50%), partially agreed on 4 (28.7%) and disagreed on 3 (21.4%). For examinations of the control group (n=32), the two clinicians agreed completely on 25 of the 32 subjects (75%). Clinician A considered 27 SPECT images to show no rCBF abnormalities, while clinician B interpreted 23 of the same images as normal (Table 2).
Table 2.
Concordance between the findings of visual inspection by clinician a (more experienced in neurology) and clinician b (general medical experience)
| Groups | Complete agreement n (%) | Partial agreement n (%) | Complete disagreement n (%) | Σ n (%) |
|---|---|---|---|---|
| Patients with neurological disorders | 7 (50.0) | 4 (28.7) | 3 (21.4) | 14 (100) |
| Control group | 24 (75.0) | – (–) | 7 (25.0) | 32 (100) |
Frequency of concordance between the visual inspection of the two clinicians and the automated analysis using SPM
As shown in Table 3, the visual assessment of clinician A showed complete agreement with the result from the individual maps generated by SPM for 27 of the 32 images from the control group (84.37%), and disagreed for 5 of the 32 images. Clinician A indicated that 29 were normal. In contrast, the visual inspection of clinician B agreed with the analysis using SPM to a lesser degree in the control group, agreeing for only 23 of the 32 subjects (71.18%) (Table 3) and disagreeing for 9 subjects. Clinician B found that 26 of the images were normal.
Table 3.
Concordance between the findings from visual inspection by clinicians a and b and the results from individualized maps generated by the spm software
| Group of images | Complete agreement (n) | Partial agreement (n) | Complete disagreement (n) | % concordance | S images | P(χ2) |
|---|---|---|---|---|---|---|
| Control group | ||||||
| Clinician A versus SPM | 27 | 0 | 5 | 84.37 | 32 | 0.364 |
| Clinician B versus SPM | 23 | 0 | 9 | 71.18 | 32 | |
| Patients with neurological disorders | ||||||
| Clinician A versus SPM | 9 | 2 | 3 | 64.28 | 14 | 0.315 |
| Clinician B versus SPM | 5 | 4 | 5 | 35.71 | 14 |
Among the group of patients with ND, there was complete agreement between the visual inspection of clinician A and the automated analysis using SPM in 9 cases, with 2 partial agreements and 3 disagreements (Table 3; Figure 1). Between clinician B and SPM, there were 5 complete agreements, 4 partial agreements and 5 disagreements (Table 3). Thus, the visual inspection of clinician A agreed more frequently with the analysis using SPM than did the visual inspection of clinician B (64.28% concordance with SPM for clinician A versus 35.71% for clinician B).
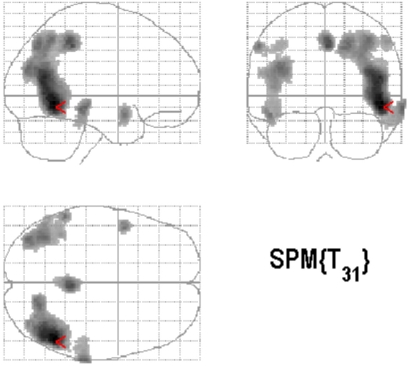
Figure 1A.
Statistical parametric maps on three axes showing cerebral areas of hypoperfusion in a 65 year-old patient with a diagnostic hypothesis of Alzheimer’s Disease in comparison with the pool of 32 control subjects using a statistical threshold of p < 0.001
The statistical evaluation of the frequencies of concordance between the clinicians and SPM using the chi-square test showed no statistically significant differences when considering the entire pool of images (n = 46 with p = 0.192) or when considering either the patients with ND (n = 14 with p = 0.315) or the control group (n = 32 with p = 0.364).
DISCUSSION
The results reported in this study confirm previous findings that it is possible to use automated methods for voxel-by-voxel analysis of rCBF abnormalities in SPECT images to evaluate individual cases in clinical practice.16,21,22 This conclusion is based on the high frequency with which our automated analyses using SPM software reproduced the rCBF abnormalities detected by the nuclear medicine clinicians in patients with ND and also on the low frequency with which functional brain abnormalities were observed for individual statistical parametric maps in the control group subjects.
To date, few studies on cerebral SPECT have explored the question of the degree of concordance between visual evaluations by nuclear medicine clinicians and results from statistical maps obtained using automated software like SPM. In one of the few studies in which SPM was used to evaluate individual images, Barnes et al.21 (2000) investigated the impact of adding individual maps generated with SPM to clinicians’ decision-making processes after these clinicians had visually inspected cerebral SPECT images from patients with suspected dementia (n = 9). That study demonstrated that the clinicians did not modify their initial reports after having had access to the results from SPM. Moreover, the degree of concordance between the visual evaluations and SPM was only marginal (Barnes et al., 2000). In another study in which 15 patients with Alzheimer’s disease and 20 with Lewy body dementia were evaluated, the individual maps generated using SPM were shown to be significantly more accurate than visual analysis for both diagnostic categories. SPM detected more areas with rCBF abnormalities, particularly in the precuneus and medial temporal cortex.22
In contrast to the previous investigations cited above, the frequency of concordance between the visual assessments by clinicians and the results from maps obtained using the SPM software was high in our study. This was especially true for evaluation by the nuclear medicine clinician with greater neuropsychiatry experience. These results indicate that automated methods like SPM for rapid objective analysis of rCBF abnormalities in SPECT images have clinical validity. However, it should be considered that manipulation of the SPM package demands basic knowledge about image processing methods and routines of the MATLAB program and, therefore, should preferentially be used under the supervision of trained computer scientists. The need for such specialized staff may prevent more disseminated use of SPM in busy nuclear medicine services mainly dedicated to clinical work.
The difference in the degree of concordance of the visual assessments with SPM between the two clinicians was consistent with the hypothesis of our study; we predicted that clinician A (with greater experience) would produce a greater proportion of concordant findings, which is what we found. This demonstration of differences in concordance between clinicians with different backgrounds and the automated method emphasizes the usefulness of automated methods like SPM as an auxiliary tool for general clinicians within nuclear medicine. However, possibly because of the size of the sample, the better performance by clinician A did not result in statistically significant differences according to the chi-square test.
SPM on neurofunctional images of individual cases is an approach of interest because automated methods are already widely used in other specialties. For example, within nuclear cardiology, this method contributes to the interpretation of myocardial perfusion examinations.26 Paralleling the application of SPM in routine clinical practice within nuclear cardiology, our study used this technique to evaluate a wide spectrum of brain perfusion abnormalities, which implies that the method reproduced the abnormalities seen by clinician A for many different brain perfusion abnormalities. Therefore, this method has significant potential in practical application to clinical practice when evaluating subjects with a large variety of perfusion abnormalities.
One methodological feature of interest was that a minority of the subjects in the control group presented foci of rCBF abnormalities on the maps generated using SPM. Among the four images from the control image database that presented some type of abnormality, the first three belonged to “source 1”, which was the pool of images from screened normal volunteers who were selected through rigorous interviews and clinical assessments.14 It should be emphasized that all individuals in this group also underwent structural magnetic resonance examination and no anatomical abnormalities were found after evaluation by two radiologists. In view of these screening conditions, it is tempting to consider that the rCBF abnormalities observed on the statistical maps generated for these normal individuals might be related to artifacts in the data acquisition or analysis process and not to cerebral functional abnormalities. Signorini et al.16 (1999) sought to validate the use of SPM for individual analysis of FDG-PET images and also observed abnormalities on maps generated for some of the normal volunteers included in the control group. These artifacts may appear as widening of the cortical sulcus or asymmetry between the brain hemispheres. In cerebral functional images from elderly individuals, it has been demonstrated that maps generated using SPM may show artifactual abnormalities that are possibly related to localized cerebral atrophy.27–29 This type of finding indicates that nuclear medicine clinicians need to be alert to the possibility of artifactual rCBF abnormalities in cerebral SPECT images, even in normal individuals. The data from the present study, along with previous investigations in the literature,16 indicate that the SPM method is sensitive towards detecting this type of abnormality in asymptomatic individuals. This observation reinforces the argument that SPM, like other automated methods for analyzing brain images, should not be used for more than indicating which structures on the maps have a greater likelihood of presenting some type of functional abnormality. It is the clinician’s responsibility, after careful analysis of the region that has been indicated as abnormal and correlation with the clinical condition, to decide how such abnormalities should be interpreted.
It should be noted that construction of individual statistical maps using SPM is not the only methodological strategy for using automated methods to analyze cerebral SPECT data in clinical practice. For instance, alternative analysis methods have been developed to support the diagnosis of neuropsychiatric conditions, enabling fully automated categorization of individual structural or functional brain images based on machine-learning techniques such as support vector machines (SVMs).30,31 The SVM approach is a supervised classification method, including a ‘training step’ on differences between the groups to be classified. Individual scans are then compared with trained datasets to be categorized as member of a particular group. Recent studies have demonstrated the reliability and validity of SVM-based techniques as well as their good diagnostic performance in discriminating, for instance, between Alzheimer’s disease patients and healthy control individuals.31
One important limitation of the present study is that we did not use a design that afforded information on the differences between the capacity of physicians A versus B to reach the correct diagnoses of the diseases investigated, with or without SPM support.21,32 Due to that limitation, it is not possible to ascertain whether use of the automated approach helped physician B reach the correct diagnoses to a greater extent than physician A. Future studies should evaluate this issue to further ascertain the clinical implications of incorporating automated brain image analysis methods to the clinical repertoire of nuclear medicine physicians.
In conclusion, the results of the present study indicate that the use of automated methods to perform statistical comparisons between multiple-detector SPECT data from a single subject and a control group of healthy volunteers presented a good degree of concordance with the visual assessment by a clinician highly experienced in the evaluation of brain images, both for detecting abnormalities among patients with ND and for inspecting SPECT images without apparent abnormalities. This observation suggests that statistical maps based on voxel-by-voxel analysis methods may have practical application in routine clinical practice within nuclear neurology, thereby benefitting clinicians with less experience in interpreting neurofunctional images.
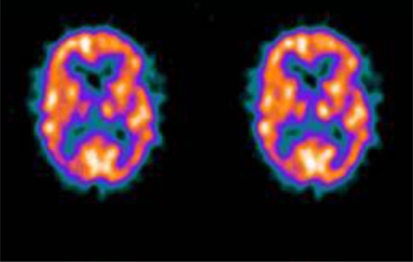
Figure 1B.
Cerebral SPECT image from the same patient showing biparietal-temporal perfusion deficits similar to the result from the SPM map. From the visual inspection, the two evaluators reported the same findings as seen on the map produced by the SPM software
REFERENCES
- 1.Grunwald F, Menzel C, Pavics L, Bauer J, Hufnagel A, Reichmann K, et al. Ictal and inter-ictal brain SPECT using tecnhnetium-99mECD. J. Nucl. Med. 1994;35:1896–901. [PubMed] [Google Scholar]
- 2.Bonte FJ, Tintner R, Weiner MF, Bigio EH, White CL., 3rd Brain-blood flow in the dementias: SPECT with hystopathologic correlation. Radiology. 1993;186:361–5. doi: 10.1148/radiology.186.2.8421735. [DOI] [PubMed] [Google Scholar]
- 3.Bavetta S, Nimmon CC, White J, McCabe J, Huneidi AH, Bomanji J, et al. A prospective study comparing SPECT with MRI and CT as prognostic indicators following severe closed head injury. Nucl. Med. Commun. 1994;15:961–8. doi: 10.1097/00006231-199412000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Kim JS, Monn DH, Kim GE, Cho PC, Kim JS, Ryu JS, et al. Acetazolamide stress brain-perfusion SPECT predicts the need for carotid shunting during carotid endarterectomy. J. Nucl. Med. 2000;4:1836–41. [PubMed] [Google Scholar]
- 5.Clauss JJ, van Harskamp K, Breteler MM, Krenning EP, Van Der Cammen TJ, Hofman A, et al. Assessment of cerebral perfusion with single-photon emission tomography in normal subjects and in patients with Alzheimer’s disease effects of region of interest selection. Eur. J. Nucl. Med. 1994;21:1044–51. doi: 10.1007/BF00181058. [DOI] [PubMed] [Google Scholar]
- 6.Liu HG, Mountz JM, Inampudi C, San Pedro EC, Deutsch G. A semiquantitative cortical circumferential normalization method for clinical evaluation of rCBF brain SPECT. Clin Nuc Med. 1997;22:596–604. doi: 10.1097/00003072-199709000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Woods RP. Modeling for intergroup comparisons of imaging data. Neuroimage. 1996;4:S84–S94. doi: 10.1006/nimg.1996.0058. [DOI] [PubMed] [Google Scholar]
- 8.Van Horn JD, Ellmore TM, Esposito G, Berman KF. Mapping voxel-based statistical power on parametric images. Neuroimage. 1998;7:97–107. doi: 10.1006/nimg.1997.0317. [DOI] [PubMed] [Google Scholar]
- 9.Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Comparing functional (PET) images: the assessment of significant change. J. Cereb. Blood Flow Metab. 1991;11:690–9. doi: 10.1038/jcbfm.1991.122. [DOI] [PubMed] [Google Scholar]
- 10.Chaim TM, Duran FL, Uchida RR, Périco CA, de Castro CC, Busatto GF.Volumetric reduction of the corpus callosum in Alzheimer’s disease in vivo as assessed with voxel-based morphometry Psychiatry Res 200715154:59–68. [DOI] [PubMed] [Google Scholar]
- 11.Chou YY, Leporé N, Avedissian C, Madsen SK, Parikshak N, Hua X, et al. Mapping correlations between ventricular expansion and CSF amyloid and tau biomarkers in 240 subjects with Alheimer’s disease, mild cognitive impairment and elderly controls. Neuroimage. 2009;46:394–410. doi: 10.1016/j.neuroimage.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stein DJ, Arya M, Pietrini P, Rapoport JL, Swedo SE. Neurocircuitry of disgust and anxiety in obsessive-compulsive disorder : a positron emission tomography. Metab Brain Dis. 2006;21:267–77. doi: 10.1007/s11011-006-9021-6. [DOI] [PubMed] [Google Scholar]
- 13.Stamatakis EA, Glabus MF, Wyper DJ, Barnes A, Wilson JT. Validation of statistical parametric mapping in assessing cerebral lesions: a simulation study. Neuroimage. 1999;10:397–07. doi: 10.1006/nimg.1999.0477. [DOI] [PubMed] [Google Scholar]
- 14.Busatto GF, Zamignani DR, Buchpiguel CA, Garrido GE, Glabus MF, Rocha ET, et al. A voxel-based investigation of regional cerebral blood flow abnormalities in obsessive-compulsive disorder using single photon emission computed tomography (SPECT) Psychiatry Res. 2000;99:15–27. doi: 10.1016/s0925-4927(00)00050-0. [DOI] [PubMed] [Google Scholar]
- 15.Ebmeier KP, Glabus MF, Prentice N, Ryman A, Goodwin GM. A voxel-based analysis of cerebral perfusion in dementia and depression of old age. Neuroimage. 1998;7:199–08. doi: 10.1006/nimg.1998.0321. [DOI] [PubMed] [Google Scholar]
- 16.Signorini M, Paulesu E, Friston K, Perani D, Colleluori A, Lucignani G, et al. Rapid assessment of regional cerebral metabolic abnormalities in single subjects with quantitative and non-quantitative 18F-FDG PET: a clinical validation of statistical parametric mapping. Neuroimage. 1999;9:63–80. doi: 10.1006/nimg.1998.0381. [DOI] [PubMed] [Google Scholar]
- 17.Lee JD, Kim HJ, Lee BI, Kim OJ, Jeon TJ, Kim MJ. Evaluation of ictal brain SPECT using statistical parametric mapping in temporal lobe epilepsy. Eur. J. Nucl. Med. 2000;27:1658–65. doi: 10.1007/s002590000364. [DOI] [PubMed] [Google Scholar]
- 18.Chang DJ, Zubal IG, Gottschalk C, Necochea A, Stokking R, Studholme C, et al. Comparison of statistical parametric mapping and SPECT difference imaging in patients with temporal lobe epilepsy. Epilepsia. 2002;43:68–4. doi: 10.1046/j.1528-1157.2002.21601.x. [DOI] [PubMed] [Google Scholar]
- 19.Ohta Y, Nariai T, Ishii K, Ishiwata K, Mishina M, Senda M, et al. Voxel- and ROI-based statistical analyses of PET parameters for guidance in the surgical treatment of intractable mesial temporal lobe epilepsy. Ann Nuc Med. 2008;22:495–503. doi: 10.1007/s12149-008-0140-5. [DOI] [PubMed] [Google Scholar]
- 20.Stamatakis EA, Wilson JT, Hadley DM, Wyper DJ. SPECT imaging in head injury interpreted with statistical parametric mapping. J. Nucl. Med. 2002;43:476–83. [PubMed] [Google Scholar]
- 21.Barnes A, Lusman D, Patterson J, Brown D, Wyper D. The use of statistical parametric mapping (SPM96) as a decision aid in the differential diagnosis of dementia using 99mTc-HMPAO SPECT. Behav. Neurol. 2000;12:77–86. doi: 10.1155/2000/482606. [DOI] [PubMed] [Google Scholar]
- 22.Kemp PM, Hoffmann SA, Holmes C, Bolt L, Ward T, Holmes RB, et al. The contribution of statistical parametric mapping in the assessment of precuneal and medial temporal lobe perfusion by 99mTc-HMPAO SPECT in mild Alzheimer’s and Lewy body dementia. Nucl Med Commun. 2005;26:1099–106. doi: 10.1097/00006231-200512000-00009. [DOI] [PubMed] [Google Scholar]
- 23.First MB, Spitzer RL, Willians JBW, Gibbons M.Structures Clinical Interview for DSM-IV – Patients EditiinSCIDP. American Psychiatric Press; Washington, DC: 1995 [Google Scholar]
- 24.Talairach J, Tournoux P. Coplanar stereotaxic atlas of the human brain. New Y, NY: Thieme Medical; 1998. [Google Scholar]
- 25.Friston KJ, Holmes A, Poline JB, Price CJ, Frith CD. Detecting activations in PET and FMRI: levels of inference and power. Neuroimage. 1996;4:223–35. doi: 10.1006/nimg.1996.0074. [DOI] [PubMed] [Google Scholar]
- 26.Kang X, Berman DS, Van Train KF, Amanullah AM, Areeda J, Friedman JD. Clinical validation of automatic quantitative defect size in rest technetium-99m-sestamibi myocardial perfusion SPECT. J Nuc Med. 1997;38:1441–6. [PubMed] [Google Scholar]
- 27.Ishii K, Willoch F, Minoshima S, Drzezga A, Ficaro EP, Cross DJ, et al. Statistical brain mapping of 18F-FDG PET in Alzheimer’s disease: validation of anatomic standardization for atrophied brains. J. Nucl. Med. 2001;42:548–57. [PubMed] [Google Scholar]
- 28.Hosaka K, Ishii K, Sakamoto S, Sadato N, Fukuda H, Kato T, et al. Validation of anatomical standardization of FDG PET images of normal brain: comparison of SPM and NEUROSTAT. Eur J Nucl Med Mol Imaging. 2005;32:92–7. doi: 10.1007/s00259-004-1576-z. [DOI] [PubMed] [Google Scholar]
- 29.Thompson PM, Schwartz C, Lin RT, Khan AA, Toga AW. Three-dimensional statistical analysis of sulcal variability in the human brain. J. Neurosci. 1996;16:4261–74. doi: 10.1523/JNEUROSCI.16-13-04261.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalatzis I, Pappas D, Piliouras N, Cayouras D. Support vector machines based analysis of brain SPECT images for determining cerebral abnormalities in asymptomatic diabetic patients. Med Inform Internet Med. 2003;28:221–30. doi: 10.1080/14639230310001613449. [DOI] [PubMed] [Google Scholar]
- 31.Fung Glenn, Stoeckel Jonathan. SVM feature selection for classification of SPECT images of Alzheimer’s disease using spatial information. Knowledge and Information Systems. Springer. 2007;2:243–58. [Google Scholar]
- 32.Dougall N, Nobili F, Ebmeier KP. Predicting the accuracy of a diagnosis of Alzheimer’s disease with 99mTc HMPAO single photon emission computed tomography. Psychiatry Res. 2004;131:157–68. doi: 10.1016/j.pscychresns.2003.11.001. [DOI] [PubMed] [Google Scholar]