Abstract
INTRODUCTION:
Common variable immunodeficiency is characterized by defective antibody production and recurrent pulmonary infections. Intravenous immunoglobulin is the treatment of choice, but the effects of Intravenous immunoglobulin on pulmonary defense mechanisms are poorly understood.
OBJECTIVE:
The aim of this study was to verify the impact of intravenous immunoglobulin on the physical properties of the sputum and on inflammatory alterations in the airways of patients with Common variable immunodeficiency associated with bronchiectasis.
METHOD:
The present study analyzed sputum physical properties, exhaled NO, inflammatory cells in the sputum, and IG titers in 7 patients with Common variable immunodeficiency and bronchiectasis with secretion, immediately before and 15 days after Intravenous immunoglobulin. A group of 6 patients with Common variable immunodeficiency and bronchiectasis but no sputum was also studied for comparison of the basal IgG level and blood count. The 13 patients were young (age=36±17 years) and comprised predominantly of females (n=11).
RESULTS:
Patients with secretion presented significantly decreased IgG and IgM levels. Intravenous immunoglobulin was associated with a significant decrease in exhaled NO (54.7 vs. 40.1 ppb, p<0.05), sputum inflammatory cell counts (28.7 vs. 14.6 cells/mm3, p<0.05), and a significant increase in respiratory mucus transportability by cough (42.5 vs. 65.0 mm, p < 0.05).
CONCLUSION:
We concluded that immunoglobulin administration in Common variable immunodeficiency patients results in significant improvement in indexes of inflammation of the airways with improvement in the transportability of the respiratory mucus by cough.
Keywords: IVIG, Airway inflammation, Pulmonary infection, Sputum, Pulmonary defense mechanisms
INTRODUCTION
Common Variable Immunodeficiency (CVID) is an immunological disorder characterized by defective antibody production1,2 and recurrent infections, most notably of the respiratory tract. Recurrent pulmonary infections can lead to chronic diseases such as bronchiectasis and chronic air flow limitation; such conditions predispose patients to recurrent or prolonged infections, leading to bronchial wall inflammation and destruction of the respiratory epithelium.3,4
The respiratory epithelium continuously secretes and eliminates mucus that traps potentially noxious particles and microorganisms. Mucociliary clearance is one of the most important defense mechanisms of the airways. Efficient mucociliary clearance is essential for the maintenance of the integrity of the respiratory system and depends on interactions between cilia, mucus, and periciliary fluid. Impairment of the mucociliary clearance increases the risk of infection and inflammation.5–9 Previous studies of bronchiectasic patients have shown marked alterations in mucus characteristics, including impairment in mucociliary transport,10 which may contribute to respiratory colonization and chronic inflammation.
Intravenous immunoglobulin (IVIG) is a blood product prepared from the serum of 1000 to 15000 donors per batch. It is the treatment of choice for patients with antibody deficiencies.11 IVIG (400 mg/kg body weight) is considered as a standard treatment in patients with CVID. This therapy has been shown to substantially reduce the number of sinopulmonary infections.12 IVIG is typically given monthly and has been primarily used as an antibody replacement therapy, however, a number of other clinical benefits of IVIG have been demonstrated, including anti-inflammatory and immunomodulatory effects.13 To the best of our knowledge, the effects of IVIG on respiratory markers of inflammation or on mucus properties have not been previously studied.
The present study evaluated the impact of IVIG on sputum airway inflammation, as well as, on the “in vitro” mucus properties of patients with CVID-related bronchiectasis with secretion, just prior to and 15 days after IVIG therapy. In addition, hemogram and IG titers were compared between secretive and non-secretive patients with CVID.
MATERIALS AND METHODS
For the present study we considered all patients with a diagnosis of CIVD associated with bronchiectasis who were followed regularly and were being treated once monthly with IVIG replacement at the Clinical Immunology and Allergy Division of the Hospital das Clínicas, Faculty of Medicine, University of São Paulo (HCFMUSP). CIVD was diagnosed by the presence of serum IgG and IgA levels that were two standard deviations below the average values for the patient’s age, a poor antibody response to vaccines, and the exclusion of other causes for hypogammaglobulinemia, as previously described1. Inclusion criteria also included the presence of bronchiectasis evidenced by computerized tomography (CT). To confirm the presence of bronchiectasis and CVID, the patients were thoroughly investigated using both CT examination and clinical criteria.14 Patients with evidence of acute respiratory infectious disease, as defined by fever, increased or altered aspects of respiratory secretion, and use of antibiotics over the 30 days before entry into or during the study period, were excluded from the study. All patients received all necessary information and signed a consent form that had been approved by the ethics committee of our Institution. Patients were divided according to the presence or absence of persistent sputum secretion. All patients were evaluated by hemogram and IG titers prior to the monthly gammaglobulin infusion. All patients with secretions were evaluated immediately before and 15 days after intravenous gammaglobulin infusion by each method described below. The IVIG was administered to these patient once a month, with an attack dose of 400 to 600 mg/kg of body weight and follow-up injections aimed at keeping the blood level of immunoglobulins above 400–500 mg/dL.
Exhaled Nitric Oxide (NO)
Exhaled NO was collected by a mouthpiece containing a 0.3 m3 bacterial HEPA filter (TROX Technik, Brazil).15 Subjects were required to inhale orally up to their total lung capacity (TLC) through the mouthpiece attached to the sampling kit (Sievers Instruments Inc.). This kit contains a pressure gauge and an inspiratory filter that reduces the NO level from the inhaled air to a very low concentration. Once the subject had inhaled to TLC, a gentle exhalation maneuver was performed through the sampling kit to the Mylar bag without any breath holding (mean oral NO). The patient was instructed to achieve a breath pressure of 12 cm H20, monitored by the pressure gauge, which resulted in a flow rate of 200 ml/s. During this expiratory time, the patient was instructed to depress a stainless steel valve attached to the sampling kit after 3 seconds of exhalation and to continue for about 2 seconds, setting a collected volume larger than 50 ml (three samples). The breathing sample was collected into a NO-impermeable reservoir bag (Mylar bag; Sievers Instruments Inc.) with a volume capacity of 1.5 L. The bags were sealed and subsequently analyzed for ENO by chemiluminescence (Sievers model NOA 280).15
Sputum collection
Sputum samples were collected through induced cough with expectoration into a sterile plastic container. All collection procedures were supervised by the researchers. Some patients failed to present any secretion samples. Respiratory mucus was visually separated from saliva and divided into 3 aliquots. One aliquot was processed for the total cell count, as described below, one was spread over glass slides, air-dried, fixed, and stained with Leishman stain for the differential cell count, and one was stored in eppendorf tubes immersed in liquid vaseline oil in sealed plastic containers at – 70°C for the analysis of mucus transportability by cilia, cough, and contact angle, as described below.
Total cell count in sputum
A phosphate-buffered saline solution was prepared with dithiothreitol (DTT) [Sigma-Aldrich, Brazil] at 0.1% concentrations, added to an equal volume of sputum, and the mixture was briefly stirred using a vortex mixer. DTT is a sulfhydryl reagent that causes mucolysis by breaking disulfide bonds that crosslink glycoprotein fibers. The sample was treated with 0.1% DTT phosphate-buffered solution up to a ratio of 1:4 by volume. The mixture was then vortexed and rocked for 20 minutes at 37°C. The clear cell suspension was filtered through 48-μm nylon gauze (BBSH Thompsom; Scarborough, ON, Canada) to remove debris and mucus. Samples were processed as soon as possible (within 2 hours) after collection. Total cell counts were evaluated using a hemocytometer (Neubauer Chamber).16
Differential cell count in sputum
The mucus sample was spread over glass slides, air-dried, fixed, and stained with Leishman stain. A differential cell count was performed using a light microscope at 1000X magnification.16 At least 200 cells were counted by two investigators who were blinded to the patient classification. Cells were classified as eosinophils, lymphocytes, neutrophils, or macrophages according to their morphology.17
In vitro mucociliary transport
The frog palate is a convenient system for studying mucociliary transport, since the frog’s palate epithelium is similar to that of the airways of vertebrates. Anesthetized frogs were decapitated, their jaws disarticulated, and the upper portion of the head was removed. The frog palate was kept in a refrigerator at 4°C for two days covered with plastic wrap in a humidified chamber to deplete the frog mucus. Ciliary activity was maintained under these experimental conditions. The transport of a mucus sample placed upon a mucus-depleted frog palate was determined using a stereomicroscope equipped with a reticulated eyepiece. The velocity of the mucus sample to be tested was compared to the transport speed of autologous frog mucus, and the results are expressed in terms of the relative speed. In the experimental situation, the frog palate epithelium was considered to be ideal, with only its physical properties influencing mucus transport.18
Cough transportability
In healthy subjects, respiratory mucus is cleared from the lung by ciliary transport, but in various respiratory diseases such as chronic bronchitis, bronchiectasis, cystic fibrosis, and asthma, the secretion of mucus occurs in association with impairment of mucociliary transport. In this situation, cough clearance assumes a central role in eliminating secretions.
In vitro cough experiments employ an apparatus called the “simulated cough machine” adapted from King et al., 1985.19 A compressed air cylinder with a pressure gauge serves as a gas supply. Gas release is controlled by a solenoid valve at the outflow port of the cylinder. This is followed by an “upstream resistance”, which serves to make the flow-time profile of the simulated cough comparable to that of human coughing. Mucus transport is calculated by determining the displacement of mucus with the aid of a millimeter ruler.18
Contact Angle
The wettability of a biological fluid characterizes its ability to spread when deposited onto a solid planar surface. This spreading occurs because a finite interaction exists between the solid surface and the molecules present in the liquid. The degree of wettability is characterized by the contact angle between the tangent to the liquid-air interface and the surface.18 The contact angle is visualized using a 25X magnification eyepiece with two movable arms (right and left, forward and backward). The eyepiece contains a goniometer with an angular scale of 0° to 180° that measures the angle between a mucus drop and the surface of a plate that has been treated with sulphochromic acid to remove the electric charges. A tempered-iron support with holes is placed under the plate, allowing humidification with vapor from a water bath kept at 37°C.18
Statistical Analyses
After descriptive analysis of the variables, the Kolmogorov-Smirnov test was used to assess the normal distributions of continuous variables. Differences among values were determined by the Student’s unpaired or paired t-test for independent comparisons or longitudinal observations of the same patients; the level of significance was p=0.05.
RESULTS
Thirty-five patients with a diagnosis of CVID associated with bronchiectasis who were regularly seen in the outpatient clinic were considered for the study; 18 of these patients were excluded because they did not agree to participate or had problems communicating. An additional 4 patients were excluded due to acute respiratory infection during the study period. Of the 13 patients studied, 7 presented secretion and 6 patients presented no secretion. The demographic characteristics of the 13 patients with and without secretion are described in Table I.
Table 1.
Demographic, clinical (time in IG therapy) and laboratorial characteristics (IG levels and differential leukocyte blood counts) of the 13 patients studied
| Total of patients (n= 13) | Secretive (n = 7) | No Sputum (n=6) | P | |
|---|---|---|---|---|
| Age (years) | 36 ± 17 | 29 ± 12 | 43 ± 19 | 0.153 |
| Gender, female (n) | 11 | 6 | 5 | - |
| BMI (Kg/m2) | 19.9 ± 1.9 | 19.8 ± 2.3 | 19.9 ± 1.6 | 0.976 |
| Time of IG therapy (years) | 4.6 ± 6.2 | 6.2 ± 7.6 | 2.7 ± 3.6 | 0.234 |
| IgG (mg/dL) | 429.4 ± 1 | 312 ± 186 | 556 ± 127 | 0.024 |
| IgA (mg/dL) | 47 ± 118 | 8.7 ± 4.0 | 91.3 ± 171 | 0.295 |
| IgM (mg/dL) | 6.7 ± 7.3 | 3.1 ± 2.1 | 11 ± 9.1 | 0.048 |
| Neutrophils/blood count (103céls/mm3) | 5 ± 2 | 5.1 ± 2.3 | 4.6 ± 1.4 | 0.651 |
| Limphocytes/blood count (103céls/mm3) | 2.7 ± 2.9 | 3.32 ± 4 | 1.81 ± 0.36 | 0.945 |
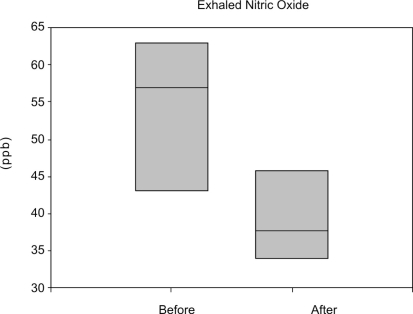
In the patients that presented secretion, we found elevated exhaled NO (54.7 × 9.8 ppb), and these levels decreased significantly 15 days after IVIG infusion (40.1 × 8.5 ppb) (Figure 1). Figure 2 shows the total number of mucus inflammatory cells present in these patients before and 15 days after IVIG infusion. Corroborating the results obtained by NO measurement, a marked decrease in the number of inflammatory cells was observed after IVIG infusion (p=0.016). A differential mucus inflammatory cell count was performed before and after intravenous immunoglobulin therapy. Although the total cell count was decreased after treatment, neutrophils remained the predominant cell type under both conditions (Figure 2B).
Figure 1.
The exhaled NO levels immediately before and 15 days after IVIG in seven secretive patients with CVID associated to bronchiectasis. Note the decrease in NO levels after the treatment (p<0.05)
Figure 2.
Total inflammatory cell counts (A) and the differential cell counts (B) (Neu=neutrophils, Maf=macrophages, Ly=Lymphocytes and Eo=eosinophils) in sputum before and after intravenous immunoglobulin
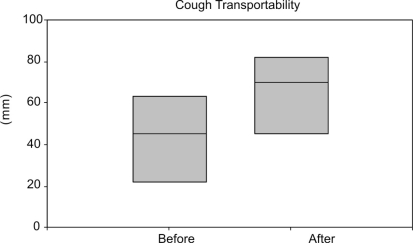
We observed no significant change in ciliary mucus transportability before and after IVIG (0.96 ± 0.28 and 0.92 ± 0.39, respectively, p=0.796) or in the mucus contact angle (50 ± 9 and 45 ± 9°, respectively, p=0.303). A significant increase in cough transportability after IVIG was observed, as shown in Figure 3 (p=0.048).
Figure 3.
Cough transportability data distribution before and after immunoglobulin infusion in these seven patients with secretion (p<0,05)
DISCUSSION
The present study showed that IVIG promotes a decrease in inflammation, as measured by decreases in exhaled NO and decreased numbers of mucus inflammatory cells, and is associated with an increase in mucus transportability by cough. These results suggest a correlation between inflammation and mucus physical properties, and this is, to our knowledge, the first study to address the importance of studying mucus properties in CVID.
In CVID patients with bronchiectasis, immunoglobulin replacement is an important tool to avoid the progression of the disease, reducing infectious episodes. IVIG is used every 3 to 4 weeks at a dose of 400 to 600 mg/kg of body weight; follow-up injections are aimed at keeping blood levels of immunoglobulins above 500 mg/dL.1,11,24–26 The mechanism of action of therapeutic immunoglobulin is complex. The objectives of immunoglobulin replacement are to diminish the frequency and severity of infectious episodes, to diminish the need for antibiotics, and to prevent the development of complications and sequelae. This treatment results in a reduction in the number of respiratory infections and a better quality of life for CVID patients.12,21,25 Nevertheless, its impact on the physical properties of mucus has not been previously addressed.
Analysis of exhaled NO and total inflammatory cells in induced sputum in secretory patients showed a marked reduction in these inflammatory parameters after IVIG infusion. It is known that the cellularity of sputum does not directly reflect the number of inflammatory cells present in the distal lung parenchyma; the latter would be better estimated from bronchoalveolar lavage (BAL) samples. Nevertheless, induced sputum has the advantage of being a less invasive technique. BAL and induced sputum are “photographs” of different compartments within the respiratory system; however, as substrates for measuring respiratory system inflammation, they show a direct correlation.27,28 IVIG propitiated an increase in mucus transportability by cough, probably by influencing the amount of inflammatory cells present in mucus with a consequent reduction in the solid component of the respiratory secretions, facilitating mucus transport by cough.8 Cough is characterized by inhaling approximately two liters of air, with a fast glottal closure and an increase in pleural pressure to 100 cm H2O or more. With glottal opening, a biphasic air flow expulsion occurs with a fast initial component lasting approximately 30–50 ms followed by a slower component, which travels along the partially collapsed trachea. The expired gas reaches the mucus layer and transfers part of its kinetic energy, moving the mucus towards the glottis. The in vitro analysis of transportation by cough and sneeze using the cough simulator utilizes a compressed air reservoir, which predicts air flow and is controlled by a solenoid valve, followed by a resistance, which provides a turbulent flow similar to that of cough and sneeze.18
Though the present study demonstrated a reduction of exhaled NO in patients treated with IVIG; exhaled NO was not reduced to normal levels in these patients. NO values as well as neutrophil counts remained high after intravenous immunoglobulin infusion. This finding reinforces the importance of avoiding mucus stasis and emphasizes that cough is the main and most efficient mechanism of clearing bronchial secretion.
In our study, secretive patients presented lower levels of all immunoglobulins measured (IgG, IgM, and IgA) compared to the group of patients without secretion on the date of IVIG infusion. This observation represents “a picture” of an acute moment in the natural history of the disease in these patients and may indicate an important topic to address, since the maintenance of normal levels of Ig may, in some patients, require more frequent IVIG infusions. It is interesting to note that no difference in blood cell counts was observed between patients with and without secretion. In contrast, levels of NO were significantly higher in patients with secretion, suggesting that these patients have increased inflammation in the lungs. The pathophysiology of the pulmonary alterations in CVID is complex and multifactorial. As a result, a multicentered approach must be taken in order to reduce the occurrence of bronchiectasis and the consequent loss of pulmonary function in these patients. Studies of inflammatory modulation and the physical properties of mucus in CVID can supply necessary information and help to improve treatment strategies for this disease.
CONCLUSION
Intravenous immunoglobulin therapy resulted in an improvement in inflammation of the airways fifteen days after infusion, reflecting an improvement of the transportability of mucus by cough and persistent secretion that was associated with lower levels of IgG among patients with CVID under IVIG.
REFERENCES
- 1.Kokron CM, Errante PR, Barros MT, Baracho GV, Camargo MM, Kalil J, et al. Clinical and laboratory aspects of common variable immunodeficiency. Anais da Academia Brasileira de Ciências. 2004;76:707–26. doi: 10.1590/s0001-37652004000400007. [DOI] [PubMed] [Google Scholar]
- 2.Yong PF, Tarzi M, Chua I, Grimbacher B, Chee R. Common variable immunodeficiency: an update on etiology and management. Immunol. Allergy Clin North Am. 2008;28:367–86. doi: 10.1016/j.iac.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 3.King P, Holdsworth S, Freezer N, Holmes P. Bronchietasis. Inter. Med. J. 2007;37:208–9. doi: 10.1111/j.1445-5994.2006.01219.x. [DOI] [PubMed] [Google Scholar]
- 4.Oksenhendler E, Gérard L, Fieschi C, Malphettes M, Mouillot G, Jaussaud R, et al. Infections in 252 patients with common variable immunodeficiency. Clinical Infect Dis. 2008;46:1547–54. doi: 10.1086/587669. [DOI] [PubMed] [Google Scholar]
- 5.Stannard W, O’Callaghan C. Ciliary function and the role of cilia in clearance. J Aerosol Med. 2006;19:110–5. doi: 10.1089/jam.2006.19.110. [DOI] [PubMed] [Google Scholar]
- 6.Willems T, Jorissen M. Correlations between ciliary structure and ciliary function. Acta Otorhinolaryngol Belg. 2000;54:299–308. [PubMed] [Google Scholar]
- 7.Lorenzi-Filho G, Bohm GM, Guimarães ET. Correlation between rheologic properties and in vitro ciliary transport of rat nasal mucus. Biorheology. 1992;29:433–40. doi: 10.3233/bir-1992-29406. [DOI] [PubMed] [Google Scholar]
- 8.Smith DJ, Gaffney EA, Blake JR. Modelling mucociliary clearance. Respir Physiol Neurobiol. 2008;163:178–88. doi: 10.1016/j.resp.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Del Donno M, Bittessnich D, Chetta A, Olivieri D, Lopez-Vidriero M. The effect of inflammation on mucociliary clearance in asthma: an overview. Chest. 2000;118:1142–9. doi: 10.1378/chest.118.4.1142. [DOI] [PubMed] [Google Scholar]
- 10.Wills P, Greenstone M. Inhaled hyperosmolar agents for bronchiectasis. Cochrane Database Syst Rev. 2006:CD002996. doi: 10.1002/14651858.CD002996.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Jolles S, Sewell WAC, Misbah SA. Clinical uses of intravenous immunoglobulin. Clinical and Experimental Immunology. 2005;142:1–11. doi: 10.1111/j.1365-2249.2005.02834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Busse PJ, Farzan S, Cunningham-Rundles C.Pulmonary complications of common variable immunodeficiency Ann Allergy Asthma Immunol 2007981–8.; quiz 8–11, 43 [DOI] [PubMed] [Google Scholar]
- 13.de Gracia J, Vendrell M, Alvarez A, Pallisa E, Rodrigo MJ, de la Rosa D, et al. Immunoglobulin Therapy to control lung damage in patients with common variable immunodeficiency. International Immunopharmacology. 2004;4:745–53. doi: 10.1016/j.intimp.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 14.King P, Holdsworth P, Freezer N, Holmes P. Bronchiectasis. Internal Medicine Journal. 2006;36:729–37. doi: 10.1111/j.1445-5994.2006.01219.x. [DOI] [PubMed] [Google Scholar]
- 15.Leme AS, Kasahara DI, Nunes MP, Martins MA, Vieira JA. Exhaled nitric oxide collected with two different mouthpieces: a study in asthmatic patients. Braz J Med Biol Res. 2002;35:1133–7. doi: 10.1590/s0100-879x2002001000004. [DOI] [PubMed] [Google Scholar]
- 16.Saraiva-Romanholo MB, Barnabé V, Carvalho AL, Martins MA, Saldiva PHN, Nunes MP. Comparison of three methods for differential cell count in induced sputum. Chest. 2003;124:1060–6. doi: 10.1378/chest.124.3.1060. [DOI] [PubMed] [Google Scholar]
- 17.Pizzichini E, Pizzichini MM, Efthimiadis A, Hargreave FE, Dolovich J. Measurement of inflammatory indices sputum: effects of selection of sputum to minimize salivary contamination. Eur Respir J. 1996;9:1174–80. doi: 10.1183/09031936.96.09061174. [DOI] [PubMed] [Google Scholar]
- 18.Trindade SHK, Mello Júnior JF, Mion OG, Lorenzi-Filho G, Macchione M, Guimarães ET, et al. Methods for studying mucociliary transport. Rev. Bras. Otorrinolaringol. 2007;73:704–12. doi: 10.1016/S1808-8694(15)30133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.King M. Clearance of mucus by simulated cough. J Appl Physiol. 1985;58:1776–82. doi: 10.1152/jappl.1985.58.6.1776. [DOI] [PubMed] [Google Scholar]
- 20.Pasteur MC, Helliwell SM, Houghton SJ, Webb SC, Foweraker JE, Coulden RA, et al. An investigation into causative factors in patients with bronchiectasis Am J Respir Crit Care Med 2000162(4Pt1):1277–84. [DOI] [PubMed] [Google Scholar]
- 21.Stead A, Douglas JG, Broadfoot CJ, Kaminski ER, Herriot RT. Humoral immunity and bronchiectasis. Clinical and Experimental Immunology. 2002;130:325–30. doi: 10.1046/j.1365-2249.2002.01974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daviskas E, Andreson SD, Gomes K. Inhaled mannitol for the treatment of mucociliary function in patients with bronchiectasis: effect on lung function, health status and sputum. Respirology. 2005;10:46–56. doi: 10.1111/j.1440-1843.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- 23.Barker AF. Medical progress: bronchiectasis. New England Journal of Medicine. 2002;346:1383–93. doi: 10.1056/NEJMra012519. [DOI] [PubMed] [Google Scholar]
- 24.Mouthon L, Kaveri SV, Spalter SH, Lacroix-Desmazes S, Lefranc C, Desai R, et al. Mechanisms of actions of intravenous immune globulin in immune-mediated diseases. Clin. Exp. Immunol. 1996;104(suppl.1):3–9. [PubMed] [Google Scholar]
- 25.Pourpak Z, Aghamohammadi A, Sedighipour L, Farhoudi A, Movahedi M, Gharagozlou M, et al. Effect of regular intravenous immunoglobulin therapy on prevention of pneumonia in patients with common variable immunodeficiency. J Microbiol Immunol Infect. 2006;39:114–20. [PubMed] [Google Scholar]
- 26.Mouthon L. Intravenous immunoglobulin therapy. Rev Prat. 2005;55:1049–56. [PubMed] [Google Scholar]
- 27.D’Ippolito R, Chetta A, Foresi A. Induced sputum and bronchoalveolar lavage from patients with hypersensitivity pneumonitis. Respir Med. 2004;98:977–83. doi: 10.1016/j.rmed.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Arjomandi M, Schmidlin I, Girling P, Boylen K, Ferrando R, Balmes J. Sputum induction and bronchoscopy for assessment of ozone-induced airway inflammation in asthma. Chest. 2005;128:416–23. doi: 10.1378/chest.128.1.416. [DOI] [PubMed] [Google Scholar]
- 29.Ziora D, Kaluska K, Rauer R, Kozielisk J. Concentration of nitric oxide exhaled air in patients with CPOD and bronchiectasis. Pneumol Alergol Pol. 2003;71:418–27. [PubMed] [Google Scholar]