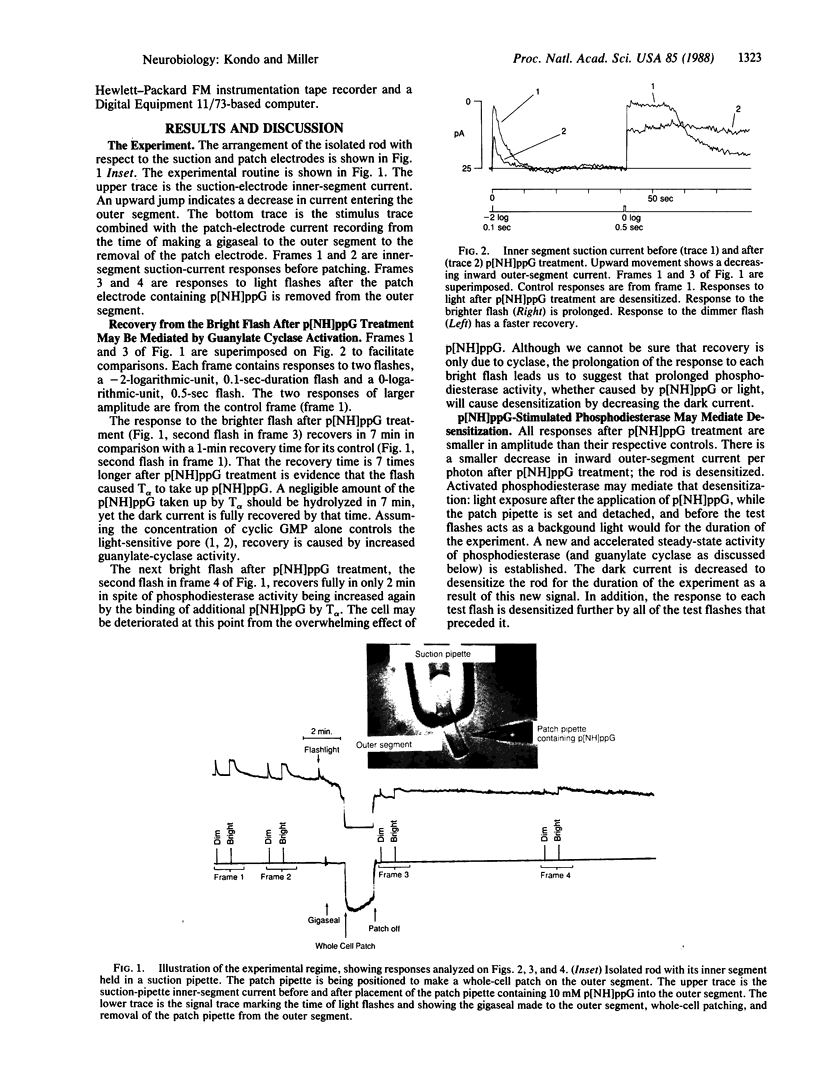
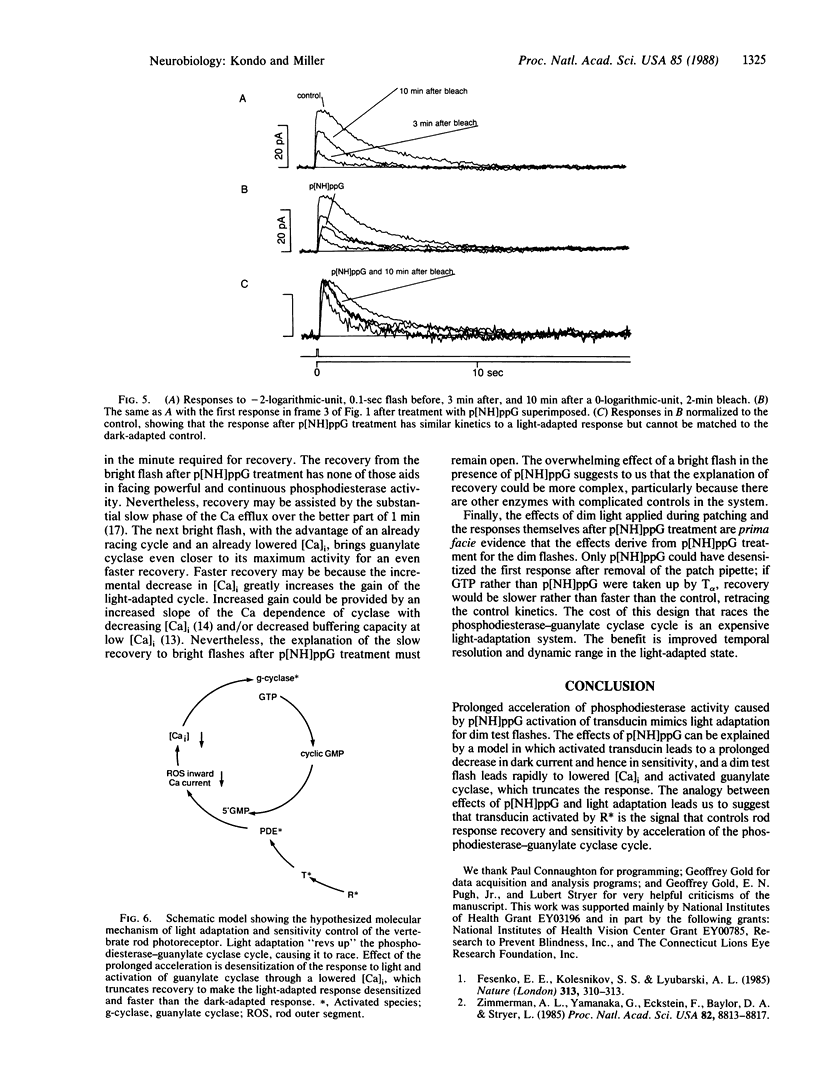
Abstract
We compare the retinal rod photocurrent before and after introduction of an hydrolysis-resistant analog of GTP into the outer segment by the whole-cell patch technique. Others have shown that GTP bound to transducin leads to the hydrolysis of cyclic GMP, causing the response to light--a decrease in dark current. The hydrolysis-resistant GTP analog prolongs the response to a bright flash, which leads us to suggest that prolonged transducin activation by bright light desensitizes the rod by a prolonged decrease in dark current. Recovery from the response to a bright flash does occur after introduction of the analog; that recovery requires acceleration of cyclase activity rather than inhibition of phosphodiesterase. The analog mimics light adaptation by desensitizing the rod and speeding the recovery from a dim flash. The analog plus light or light adaptation prolongs the activities of transducin and phosphodiesterase (oligonucleate 5'-nucleotidohydrolase, EC 3.1.4.1) to mediate desensitization by reducing the dark current. Hence, this faster recovery from a dim flash would be by increased activity of guanylate cyclase [GTP pyrophosphate-lyase (cyclizing), EC 4.6.1.2] rather than by inhibited phosphodiesterase. Accelerated activity of guanylate cyclase may speed recovery by response truncation. We conclude that transducin, activated by photolyzed rhodopsin, may lead to increased activity of both phosphodiesterase and guanylate cyclase to mediate the desensitization and the faster recovery of the light-adapted response.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett N., Dupont Y. The G-protein of retinal rod outer segments (transducin). Mechanism of interaction with rhodopsin and nucleotides. J Biol Chem. 1985 Apr 10;260(7):4156–4168. [PubMed] [Google Scholar]
- Cobbs W. H., Pugh E. N., Jr Cyclic GMP can increase rod outer-segment light-sensitive current 10-fold without delay of excitation. Nature. 1985 Feb 14;313(6003):585–587. doi: 10.1038/313585a0. [DOI] [PubMed] [Google Scholar]
- Fesenko E. E., Kolesnikov S. S., Lyubarsky A. L. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985 Jan 24;313(6000):310–313. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- Gold G. H. Plasma membrane calcium fluxes in intact rods are inconsistent with the "calcium hypothesis". Proc Natl Acad Sci U S A. 1986 Feb;83(4):1150–1154. doi: 10.1073/pnas.83.4.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg N. D., Ames A. A., 3rd, Gander J. E., Walseth T. F. Magnitude of increase in retinal cGMP metabolic flux determined by 18O incorporation into nucleotide alpha-phosphoryls corresponds with intensity of photic stimulation. J Biol Chem. 1983 Aug 10;258(15):9213–9219. [PubMed] [Google Scholar]
- Kwok-Keung Fung B., Stryer L. Photolyzed rhodopsin catalyzes the exchange of GTP for bound GDP in retinal rod outer segments. Proc Natl Acad Sci U S A. 1980 May;77(5):2500–2504. doi: 10.1073/pnas.77.5.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. The role of metarhodopsin in the generation of spontaneous quantum bumps in ultraviolet receptors of Limulus median eye. Evidence for reverse reactions into an active state. J Gen Physiol. 1985 Feb;85(2):171–187. doi: 10.1085/jgp.85.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W. H. Physiological evidence that light-mediated decrease in cyclic GMP is an intermediary process in retinal rod transduction. J Gen Physiol. 1982 Jul;80(1):103–123. doi: 10.1085/jgp.80.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepe I. M., Panfoli I., Cugnoli C. Guanylate cyclase in rod outer segments of the toad retina. Effect of light and Ca2+. FEBS Lett. 1986 Jul 14;203(1):73–76. doi: 10.1016/0014-5793(86)81439-9. [DOI] [PubMed] [Google Scholar]
- Pugh E. N., Jr The nature and identity of the internal excitational transmitter of vertebrate phototransduction. Annu Rev Physiol. 1987;49:715–741. doi: 10.1146/annurev.ph.49.030187.003435. [DOI] [PubMed] [Google Scholar]
- Stryer L. Cyclic GMP cascade of vision. Annu Rev Neurosci. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]
- Torre V., Matthews H. R., Lamb T. D. Role of calcium in regulating the cyclic GMP cascade of phototransduction in retinal rods. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7109–7113. doi: 10.1073/pnas.83.18.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilden U., Hall S. W., Kühn H. Phosphodiesterase activation by photoexcited rhodopsin is quenched when rhodopsin is phosphorylated and binds the intrinsic 48-kDa protein of rod outer segments. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1174–1178. doi: 10.1073/pnas.83.5.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Light-induced reduction of cytoplasmic free calcium in retinal rod outer segment. Nature. 1985 Feb 14;313(6003):579–582. doi: 10.1038/313579a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman A. L., Yamanaka G., Eckstein F., Baylor D. A., Stryer L. Interaction of hydrolysis-resistant analogs of cyclic GMP with the phosphodiesterase and light-sensitive channel of retinal rod outer segments. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8813–8817. doi: 10.1073/pnas.82.24.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]




