Abstract
One of the obstacles to AIDS vaccine development is the variability of HIV-1 within individuals and within infected populations, enabling viral escape from highly specific vaccine induced immune responses. An understanding of the different immune mechanisms capable of inhibiting HIV infection may be of benefit in the eventual design of vaccines effective against HIV-1 variants. To study this we first compared the immune responses induced in Rhesus monkeys by using two different immunization strategies based on the same vaccine strain of HIV-1. We then utilized a chimeric simian/HIV that expressed the envelope of a dual tropic HIV-1 escape variant isolated from a later time point from the same patient from which the vaccine strain was isolated. Upon challenge, one vaccine group was completely protected from infection, whereas all of the other vaccinees and controls became infected. Protected macaques developed highest titers of heterologous neutralizing antibodies, and consistently elevated HIV-1-specific T helper responses. Furthermore, only protected animals had markedly increased concentrations of RANTES, macrophage inflammatory proteins 1α and 1β produced by circulating CD8+ T cells. These results suggest that vaccine strategies that induce multiple effector mechanisms in concert with β-chemokines may be desired in the generation of protective immune responses by HIV-1 vaccines.
An understanding of the types of immune responses important for protection from HIV-1 infection and the development of AIDS may possibly facilitate development of safe, effective AIDS vaccines (1, 2). Clues into the complex nature of protective immunity to HIV infection have been emerging from preclinical subunit HIV-1 vaccine efficacy studies in nonhuman primates, and from multiply exposed uninfected high-risk individuals (3–5). In early HIV-1 subunit vaccine efficacy studies in chimpanzees, neutralizing antibodies (NA) have been found to correlate with protection from homologous (6–8) laboratory-adapted T cell tropic isolates, but not heterologous isolates (9–11). In two recently published studies in chimpanzees immune correlates were either not found (12) or merely suggestive as in the case of cytotoxic T lymphocyte (CTL) responses in one animal (13) when challenged with the SF2 isolate. Subunit HIV-1 vaccine efficacy studies with macrophage or dual tropic clinical isolates remain to be undertaken. In other settings where more individuals are available for study certain immune correlates of protection become more evident. In the simian immunodeficiency virus (SIV) macaque model different studies have implicated the role of cytotoxic T lymphocytes (CTL) in protection from infection and reduction of virus load (14, 15). In exposed uninfected humans, studies have suggested the role of type-1 like T helper (Th1) responses (4) as well as CTL (16) in protection to HIV-1 infection. Elevated levels of the β-chemokines RANTES, macrophage inflammatory protein 1α (MIP-1α), and MIP-1β, which inhibit macrophage or dual tropic HIV-1 variants, were observed to be produced at higher levels in certain uninfected high risk individuals (17, 18). In addition, recent findings in the SIV model have suggested a role of these β-chemokines in vaccine induced protective immunity to SIV (19).
One of the most critical problems remaining to be overcome in the development of an effective HIV-1 vaccine is the problem of the virus variability, cell tropism and immune escape by variants of HIV-1. To address this issue we designed a study in which rhesus monkeys (Macaca mulatta) were immunized with antigens of a particular T cell tropic HIV-1 vaccine strain and then challenged with a chimeric virus expressing the envelope of a closely related but dual tropic variant HIV-1SF13 (20, 21). After the challenge we determined if protection was obtained and which immune responses correlated with protection from infection.
METHODS
Macaques, Immunizations, and Humoral Responses.
All protocols for the use of animals (mature outbred M. mulatta) in this study were approved by the institution’s animal care and use committee according to standard international scientific and ethical guidelines. A light anesthesia of 10 mg/kg ketamine-HCl was used for all procedures. Animals were captive bred and housed at the Biomedical Primate Research Centre with no prior immunizations and were confirmed negative for SIV, simian T- lymphotrophic virus, and simian retrovirus type D infection before study entry. A total of 12 animals (4 HIV-1 immune stimulating complex (ISCOM), 4 controls, and 4 HIV-1 FP) were immunized at 0, 6 and 16 weeks. Four animals received HIV-1 ISCOM preparations whereas two controls received PR8-Flu ISCOM. Two additional controls received wild-type fowlpox (FP) vaccine preparation to give a total of four controls. Four animals received HIV-1SF2 glycoprotein of Mr 160,000 (gp160) chimeric FP vaccines as previously prepared and administered (22). The ISCOMs made for this study, in contrast to previous studies in primates, were prepared with the defined Quillaja components QH-A and QH-C in the proportion of 7:3 (Iscoprep 703) kindly supplied by Iscotec (Uppsala, Sweden). This Quillaja product is free of toxic components and intended for human use in clinical trials. The first two immunizations at weeks 0 and 6 consisted of glycosylated Chinese hamster ovary-expressed monomeric HIV-1SF2 gp120 (13) and p24 incorporated into ISCOMs. At weeks 6 and 16 ISCOMs covalently coupled with the synthetic peptides IRDKIQKENALFRNLC (representing the V2 region) and NNNTRKSIYIGPGRAC (representing the V3 region) coupled to PR8-Flu-ISCOMs were also administered (23). The HIV-1gp120 specific antibody titers were determined by ELISA (24, 25), and virus neutralization assays for HIV-1SF2 and HIV-1SF13 were performed as described (23).
Cell-Mediated Immune Responses.
At weeks 0, 6, 8, 12, and 18 freshly isolated peripheral blood mononuclear cells (PBMCs) from each monkey were assayed for antigen specific T cell responses by enzyme-linked immunosorbent spot assay. Enumeration of antigen specific cytokine [interferon γ (IFN-γ)-, interleukin 2 (IL-2)-, and IL-4]-secreting cells as well as antigen specific lymphocyte proliferation assays were performed as previously reported by this and other laboratories (26–28). CTL responses were assayed as described in rhesus monkeys (14), using overlapping 15-mer peptides homologous for the entire gp120 and gag p24. Peptide pools used to analyze specific cytotoxicity were grouped as follows: A and B, gag p24; C–F, gp120; G, V2 and V3 regions and CD4 binding region of gp120. Pool A contained the peptides 1–12 and pool B the peptides 13–23 of gag p24 of HIV-1SF2. Pool C contained peptides 5–16, pool D the peptides 17–28, pool E the peptides 29–40, pool F the peptides 41–52 (25), pool G the peptides MM403 (V2 neutralization region), MM404 (V3 neutralizing region), CM332 (T1 peptide, amino acid sequence 416–430), CM333 (T2 peptide, amino acid sequence 110–120), CM334 (P18 peptide, amino acid sequence 306–320); and pool H, the peptides 54–86 of env of HIV-1SF2 (24). Assays were based on the use of at least three different effector–target ratios. Positive responses were scored when the specific lysis detected was greater than 10% and scored together with proportional changes in effector–target ratios (14).
Simian/HIV (SHIV)SF13 Challenge and Follow-Up.
The SHIVSF13 molecular clone (generously donated by C. Cheng-Mayer, Aaron Diamond, AIDS Research Center, New York) was derived from the SIVmac239 molecular clone in which the HIV-1SF13 envelope was cloned in place of the SIV envelope (21). A rhesus PBMC stock was propagated in vitro and titrated in vivo in mature outbred M. mulatta to determine the ID50 (29). After the immunization protocol all twelve monkeys were simultaneously challenged one month after the last immunization with 30 ID50 i.v. Samples were taken at 2-week intervals for quantitative virus isolation and nested DNA PCR as described (28).
Chemokine Production by CD8+ T Cells and Inhibition.
Without exception, assays were performed as according to standardized protocols as described previously (19). RANTES, MIP-1α, MIP-1β, and macrophage chemotactic protein were assayed in the CD8+ cell culture supernatants generated by in vivo immunization by using specific ELISA kits (R & D Systems). Optimum conditions were established with the CD8+ cell culture supernatant diluted at 1:8 and all the results were corrected for the dilution factor and presented in pg/ml.
Inhibition assays were performed on primary phytohemagglutinin-stimulated CD4+ enriched rhesus PBMC. Cells were incubated with differing concentrations (pg/ml) of each of the β-chemokines, RANTES, MIP-1α, MIP-1β, or the control MCP-1 (R & D Systems) 30 min before infection with the challenge virus. After incubation for 2 hr, infected cells were plated out (2 × 105/100 μl/well) into 96-well culture plates and 0.25–250 ng/ml of the β-chemokine at the start of the culture and every 2 days thereafter. Virus replication was tested 7 days after infection by measurement of reverse transcriptase in culture supernatant. Data were calculated as the mean ± SEM and were analyzed by either the Studentized range test, the Kruskal-Walus nonparametric or Wilcoxon statistical tests depending on the comparisons made as indicated.
RESULTS
Vaccine Protection and Correlation with Immune Responses.
Upon challenge all HIV-1 ISCOM immunized animals remained negative for virus by all parameters, demonstrating that “sterilizing immunity” was obtained. All HIV-1 FP immunized animals became infected, although quantitative virus isolation suggested a possible reduction in virus load (Table 1). To determine which immune responses correlated with protection from infection, we undertook a comparison of prechallenge immune responses in all animals. Prechallenge humoral immune responses assessed included gp120 antibody titers, homologous NA to the vaccine strain HIV-1SF2 as well as heterologous NA titers to the escape variant HIV-1SF13. Antibody responses to gp120 were highest in the HIV-1 ISCOM immunized group before challenge (Table 1). Furthermore, animals immunized with HIV-1 ISCOMs had higher NA titers to the vaccine strain as well as to the challenge variant (ranging from 160 to 320). In contrast, although they mounted homologous neutralizing titers in most cases the HIV-1 FP immunized animals had undetectable or low (titers of 20) heterologous NA titers (Table 1). This lack of significant (P < 0.05) heterologous NA in HIV-1 FP immunized animals is most likely the single most important reason why they were not protected from infection from this cell-free challenge. Notably, this group had lower virus loads than controls that in itself was indicative that HIV-1 specific immunity was induced and in the absence of significant NA, was most likely cell mediated in nature.
Table 1.
Individual immune responses immediately before challenge and virus status post challenge
| Animal vaccine | Immune responses prechallenge*
|
Postchallenge virology†
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| VNT‡
|
gp120‡ | LP§
|
CTL¶
|
PCR (vi) (week)
|
|||||
| SF2 | SF13 | titers | SI (cpm) | Pep. pools (% lysis) | 2 | 4 | 6 | Status | |
| HIV-I ISCOM | |||||||||
| 9263 | 400 | 320 | 2,500 | 4.1 (5554) | – | −(0) | −(0) | −(0) | protected |
| BB85 | 200 | 160 | 2,500 | 4.3 (441) | 41–52 (16.4%) and V2, V3 (19%) | −(0) | −(0) | −(0) | protected |
| 9251 | 200 | 160 | 2,500 | 2.8 (1985) | 1–12 (8.0%) and 13–24 (10.4%) | −(0) | −(0) | −(0) | protected |
| 9111 | 400 | 160 | 2,500 | 2.6 (436) | – | −(0) | −(0) | −(0) | protected |
| Controls | |||||||||
| 9258 | – | – | 0.8 (196) | – | +(270) | +(270) | +(25) | infected | |
| BB70 | – | – | 1.3 (144) | – | +(90) | +(30) | +(12.5) | infected | |
| BB102 | – | – | 1.0 (608) | – | +(90) | +(270) | +(12.5) | infected | |
| 9219 | – | – | 0.6 (235) | – | +(810) | +(810) | +(50) | infected | |
| HIV-1 fowlpox | |||||||||
| 4044 | >160 | 20 | 500 | 1.8 (548) | – | +(30) | +(90) | +(16.7) | infected |
| 9264 | >80 | 20 | 100 | 0.9 (172) | – | +(30) | +(10) | +(1.0) | infected |
| BB98 | – | – | – | 1.2 (170) | – | +(90) | +(90) | +(25) | infected |
| BB108 | 160 | – | 100 | 5.4 (689) | – | +(270) | +(30) | +(25) | infected |
| Statistics | |||||||||
| Kruskal Wallis‖ | 5.69–7.54 | n.a. | 8.77 | 6.04 (3.04) | 8.16 | 8.54 | 7.42 | ||
| P value | <0.049 | n.a. | <0.008 | 0.049 > P > 0.011 (> 0.104) | <0.008 | <0.008 | 0.049 > P > 0.011 | ||
| ISCOMS VS FOWLPOX: | |||||||||
| Wilcoxon** | >1 | 0 | 0 | 2 (−) | 0 | 0 | 0 | ||
| P value | NS | <0.05 | <0.05 | NS (−) | <0.05 | <0.05 | <0.05 | ||
SI, stimulation index; NS, not significant; n.a., not applicable.
Immune responses are from samples taken two weeks after the third immunization.
Post challenge virology was based on DNA-PCR (28) as represented as positive (+) or negative (−), and quantitative virus isolation (vi) given as the number of virus producing cells in 1 × 107 PBMC (36, 37). Animals negative by both assays after more than five consecutive samplings were classified as protected.
LP given as stimulation index, SI or cpm.
CTL responses performed as described (14); CTL based on >10% specific lysis at two or more E:T ratios (specific lysis is shown at an E:T ratio of 5:1) on autologous peptide pulsed target cells are represented by the letter of the peptide pools (overlapping 15 mers) giving positive responses.
Statistical analysis to determine if there was a significant difference between the three groups.
To determine if there was statistical significance between the two HIV-1 vaccine groups.
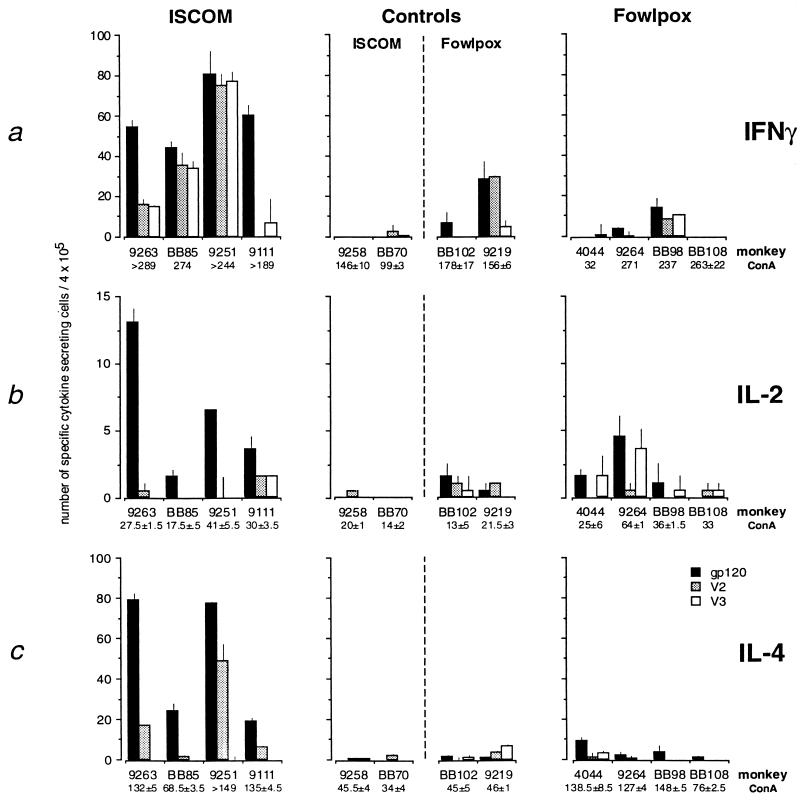
Subsequently, we set out to determine if preferred Th1 or Th2 responses were associated with protective immunity and the generation of heterologous NA responses. Two weeks after the last immunization the number of gp120, V2, and V3 peptide-specific as well as mitogen-stimulated cytokine (IFN-γ, IL-2, and IL-4)-secreting PBMCs measured by using an enzyme-linked immunosorbent spot assay (26, 27) were compared. The number of antigen specific IFN-γ (Fig. 1a), IL-2 (Fig. 1b) and IL-4 (Fig. 1c)-secreting cells in all animals 2 weeks before challenge were proportionally higher to gp120 in the protected vaccinated animals. In addition the number of V2 and V3 peptide-specific IFN-γ-secreting cells were elevated, and to a lesser extent IL-2 and IL-4 in HIV-1 ISCOM-vaccinated animals. In contrast, in FP-immunized animals background was sporadic and at 2 weeks before challenge in one control animal background responses were higher than in the HIV-1 FP vaccines. These IFN-γ background responses were only found occasionally in those animals receiving the live chimeric FP virus vaccines and were likely generated by host responses to infection with the FP vaccine. Such background responses were not observed in any of the animals that received the subunit vaccines. Without exception, HIV-1 ISCOM immunized animals had more antigen specific cytokine-secreting cells in circulation than HIV-1 FP vaccinees and controls. The same general trend observed with Th cytokine responses was supported in part when gp120 specific lymphocyte proliferation assays were measured (Table 1). A notable exception was one HIV-1 FP immunized animal BB108 that had the highest stimulation index.
Figure 1.
Individual HIV-1gp120, V2-, and V3- specific IFN-γ (a), IL-2 (b) and IL-4 (c) cytokine-secreting cells per 4 × 105 PBMC (26). Controls (n = 2 FluISCOM plus n = 2 wtFP) grouped together with HIV-1 ISCOM and HIV-1FP groups, respectively. The y axis for each cytokine is used based on the optimal working range for each cytokine. IFN-γ (0 -100), IL-2 (0–15), and IL-4 (0–100), respectively.
We then examined the ability of CD8+ T lymphocytes from vaccinated monkeys to lyse autologous lymphocytes pulsed with peptides consisting of overlapping 15 mers spanning the entire gp120. Pools of overlapping 15-mer peptides were used to identify CTL in bulk culture as we have previously described for SIV-vaccinated macaques (14). Similar to our previous findings in outbred M. mulatta, CTL responses (greater than 10% specific release scored as positive) were found to some peptide pools at different time points. These responses were observed only in the HIV-1 ISCOM-vaccinated animals but not in all of the animals in this group. In this study CTL responses were detected 2 weeks after the second and third immunizations in animal BB85 to peptide pool 29–40 (16.4% specific lysis at effector–target of 5:1) and to peptide pool of V2 and V3 peptides 18, 34, 43/44, 12, 32/33 (19% at 5:1) Similarly animal 9251 had CTLs to peptide pools 1–12 (8% at 5:1) and to pool 13–24 (10.4% at 5:1), Table 1. Interestingly, the same two animals had consistent and strong IFN-γ responses to HIV-1 peptides (Fig. 1a). The presence of detectable CTL responses was not a necessary correlate with protection, because reproducible responses were found in only two of four protected animals (Table 1). We then turned to an anti-HIV immune response more recently described as being mediated predominantly by CD8+ T cells (17).
Chemokines and Cytokines.
Because macrophage and dual tropic HIV-1 isolates use the β-chemokine receptor CCR5 as a coreceptor for entry into CD4+ cells (30–34), and β-chemokines have been implicated in blocking infection of these HIV-1 variants (17, 18), we examined the possibility that β-chemokines might be significantly elevated in protected HIV-1 vaccinees. We first confirmed the ability of SF13 and SHIVSF13 to utilize the CCR5 coreceptor by limiting dilutions of virus on U87 cells transfected with different chemokine receptors (data not shown). Subsequently, enriched CD8+ PBMC taken 2 weeks before challenge from each of the animals were assayed independently and blindly for production of RANTES, MIP-1α, and MIP-1β, with MCP-1 as a control (19). Remarkably, there was a clear significant correlation with those HIV-1 vaccinees protected from infection and prechallenge levels with each of the β-chemokines, RANTES (P < 0.008), MIP-1α (P < 0.011), MIP-1β (0.049 > P > 0.011) (Kruskal-Wallis nonparametric test), but not MCP-1 (Fig. 2). Furthermore, control animals that received Flu-Pr8 ISCOMs had significantly lower concentrations of these three chemokines, suggesting a specific response induced by the HIV-1 ISCOM preparation, and not a nonspecific ISCOM adjuvant related phenomenon. To determine if the increases in these β-chemokines were linked to either a type-1- or type-2-like HIV-1 specific T helper cytokine response, we plotted the levels of RANTES, MIP-1α, and MIP-1β against either the number of HIV-specific IFN-γ or IL-4 responses of each animal. No correlation with a preferred Th1-or a Th2-type response was observed, but rather the levels of these chemokines were related to the number of both IFN-γ- and IL-4-secreting cells (Fig. 3).
Figure 2.
Concentration of β-chemokines produced by CD8+ enriched T cells from animals 2 weeks before challenge. Values expressed as pg/ml from each individual animal plotted per group; HIV-1 vaccinees (HIV-1 ISCOM) protected, HIV-1 vaccinees (HIV-1 FP) not protected, and controls (two Flu Pr8 ISCOM, two FP wild type). (a) Concentrations of RANTES produced per individual animal plotted per group. (b) Concentrations of MIP-1α produced per individual animal plotted per group. (c) Concentrations of MIP-1β produced per individual animal plotted per group. (d) Concentrations of the control chemokine MCP-1 produced per individual animal plotted per group.
Figure 3.
Correlation of the concentration of β-chemokines with type-1 (a; IFN-γ) or type-2 (b; IL-4) T helper responses. In animals protected from vaccine challenge a correlation of β-chemokines produced just before challenge (RANTES, MIP-1α, and MIP-1β) was found with both the number of IFN-γ- and IL-4-secreting cells. Protected animals have high numbers of all three β-chemokines produced in relationship with both the number of gp120-specific IFN-γ- and IL-4-secreting cells.
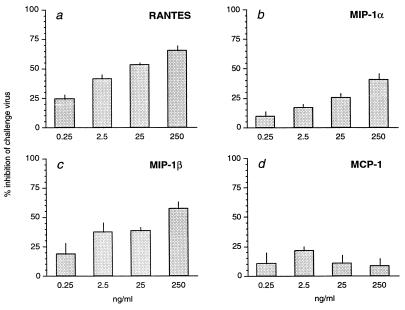
Finally, to confirm that the three β-chemokines found to be elevated in the protected vaccinees could have a specific inhibitory effect on the challenge virus, a dose dependent inhibition assay was performed (Fig. 4). The results revealed that, independent of each other, the three β-chemokines, RANTES, MIP-1α, and MIP-1β, but not MCP-1, could specifically inhibit the SHIVSF13 challenge virus in primary CD4+ rhesus PBMC at increasing concentrations. Although the concentrations observed to inhibit the CCR5 utilizing SHIVSF13 were slightly higher than reported with human cells, this is due to the use of the recombinant human β-chemokines in primary rhesus PBMC. In each case when inhibition was observed the effect was clearly dose dependent.
Figure 4.
β-chemokine inhibition of infection of rhesus PBMCs with the challenge virus SHIVSF13. Inhibitory concentrations (ng/ml) of (a) RANTES; (b) MIP-1α, and (c) MIP-1β. Lack of inhibition of SHIVSF13 infection with the control C-C chemokine MCP-1 (d).
DISCUSSION
We demonstrate that a subunit HIV-1 ISCOM vaccine is capable of inducing sterilizing immunity by protecting rhesus monkeys from infection with a chimeric virus bearing the envelope from a dual tropic HIV-1 variant that uses the CCR5 coreceptor. Protection from infection was correlated with certain immune responses including heterologous neutralizing antibodies, the intensity and quality of both Th1 and Th2 responses and the striking production of RANTES, MIP-1α, MIP-1β before challenge. Neither a preferred Th1- nor a Th2-like, but rather a balanced and strong T helper response was associated with vaccine-induced immunity. The production of β-chemokines was not associated with a particular type of T helper response. Furthermore, we confirmed that the challenge virus uses CCR5 and could be inhibited by each of these three β-chemokines independently in a dose dependent fashion.
It is noteworthy that the HIV-1 FP immunized animals did not develop increased β-chemokines suggesting that the nature of HIV-1 antigen presentation such as that induced by this ISCOM strategy was critical in inducing these responses. Until more detailed immunization studies in this model can be carried out, it can only be suggested that the HIV-1 ISCOM immunization protocol that included boosting with ISCOM coupled V3 loop peptides may have been involved in inducing these responses. This hypothesis is supported by data demonstrating V3 loop specificity in HIV-1 envelope interaction with the β-chemokine CCR5 receptor (33, 35). The N-terminal V3 peptide used to boost animals in this study lacks the arginine (R) at position 306 in V3, thus resembling an NSI motif, possibly having CCR5 specificity.
Despite the fact that HIV-1SF2-specific immune responses, in particular homologous neutralizing antibody, were induced by the HIV-1 FP immunized animals, neither β-chemokines nor heterologous neutralizing antibodies were induced by this strategy. All animals in this group became infected. The ability to induce the high level of β-chemokines and heterologous neutralizing antibodies may in part be dependent on the ability of the vaccinees to generate potent T helper responses. The responses generated by this HIV-1 ISCOM strategy were characterized by persistent IFN-γ as well as strong IL-4 responses, likely driving both humoral as well as cellular effector mechanisms. Although we have yet to determine the breadth of the immunity achieved by this strategy we were able to induce sterilizing immunity against a closely related variant of HIV-1SF2 with clearly different tropism (i.e., macrophage) than the parental vaccines strain (20, 21). Studies are needed to determine if immunity can be generated against more divergent HIV-1 isolates more representative of those encountered in the field. Furthermore, studies are underway to determine if the immune responses observed would be sufficient to protect from more virulent challenge strains administered by different routes. The SHIV model used here will undoubtedly be an important asset in evaluating the multiple variables that need to be addressed to develop a working HIV-1 vaccine. The results observed in this study provide a successful basis on which future HIV-1 subunit vaccine approaches could be built on.
The immune responses observed in the macaques protected from infection may be of importance with regard to understanding protective immunity in the context of HIV-1 vaccine development. Current evidence suggests that sexual transmission of HIV-1 involves macrophage or dual tropic variants that may preferentially use the CCR5 and related coreceptors. Either their susceptibility to blocking by β-chemokines, and/or the induction of immune responses associated with these β-chemokines may be exploitable strategies for the design of more effective HIV-1 vaccines. As with antiviral chemotherapy, multiple immune mechanisms capable of blocking HIV at different stages of infection, combined with the production of β-chemokines may be the most effective type of vaccine induced immunity for the prevention of HIV-1 infection.
Acknowledgments
We thank Drs. W. Paul, J. W. Eichberg, and B. Rosenwirth for constructive comments, P. ten Haaft for PCR, H. van Westbroek for figures and J. Schouw for word processing. P. van der Meide supplied us with the enzyme-linked immunosorbent spot reagents. We are grateful to K. Steimer and G. Ott of Chiron Corporation for their enthusiasm, encouragement and generous support. Dr. C. Cheng-Mayer very kindly provided us with the SHIVSF13 molecular clone and helpful discussions. This project was supported by the European Community Centralized Facility program for HIV-1 vaccine development (BMH4-CT95-0206 and BMH4-CT97-2067) and was carried out at the Biomedical Primate Research Centre under strict international ethical guidelines. This paper is dedicated in memory to the late Kathy Steimer.
ABBREVIATIONS
- NA
neutralizing antibodies
- MIP-1α
macrophage inflammatory protein 1α
- SHIV
simian/HIV
- SIV
simian immunodeficiency virus
- CTL
cytotoxic T lymphocytes
- IL
interleukin
- IFN
interferon
- Th1
type 1-like T helper
- PBMC
peripheral blood mononuclear cells
- ISCOM
immune stimulating complex
- FP
fowlpox
References
- 1.Bloom B R. Science. 1996;272:1888–1890. doi: 10.1126/science.272.5270.1888. [DOI] [PubMed] [Google Scholar]
- 2.Haynes B F, Pantaleo G, Fauci A S. Science. 1996;271:324–328. doi: 10.1126/science.271.5247.324. [DOI] [PubMed] [Google Scholar]
- 3.Heeney J L, Bruck C, Goudsmit J, Montagnier L, Schultz A, Tyrrell D, Zolla-Pazner S. Immunol Today. 1997;18:4–8. doi: 10.1016/s0167-5699(97)80005-9. [DOI] [PubMed] [Google Scholar]
- 4.Shearer G M, Clerici M. Immunol Today. 1996;17:21–24. doi: 10.1016/0167-5699(96)80564-0. [DOI] [PubMed] [Google Scholar]
- 5.Heeney J L. AIDS. 1996;10:S115–S122. doi: 10.1097/00002030-199601001-00016. [DOI] [PubMed] [Google Scholar]
- 6.Berman P W, Gregory T J, Riddle L, Nakamura G R, Champe M A, Porter J P, Wurm F M, Hershberg R D, Cobb E K, Eichberg J W. Nature (London) 1990;345:622–625. doi: 10.1038/345622a0. [DOI] [PubMed] [Google Scholar]
- 7.Girard M, Kieny M P, Pinter A, Barre-Sinoussi F, Nara P, Kolbe H, Kusumi K, Chaput A, Reinhart T, Muchmore E, et al. Proc Natl Acad Sci USA. 1991;88:542–546. doi: 10.1073/pnas.88.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruck C, Thiriart C, Fabry L, Francotte M, Pala P, Van-Opstal O, Culp J, Rosenberg M, De-Wilde M, Heidt P, et al. Vaccine. 1994;12:1141–1148. doi: 10.1016/0264-410x(94)90185-6. [DOI] [PubMed] [Google Scholar]
- 9.Berman P W, Murthy K K, Wrin T, Vennari J C, Cobb E K, Eastman D J, Champe M, Nakamura G R, Davison D, Powell M F, et al. J Infect Dis. 1996;173:52–59. doi: 10.1093/infdis/173.1.52. [DOI] [PubMed] [Google Scholar]
- 10.Girard M, Meignier B, Barre-Sinoussi F, Kieny M P, Matthews T, Muchmore E, Nara P L, Wei Q, Rimsky L, Weinhold K, Fultz P. J Virol. 1995;69:6239–6248. doi: 10.1128/jvi.69.10.6239-6248.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girard M, Yue L, Barre-Sinoussi F, van-der-Ryst E, Meignier B, Muchmore E, Fultz P N. J Virol. 1996;70:8229–8233. doi: 10.1128/jvi.70.11.8229-8233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyer J D, Ugen K E, Wang B, Agadjanyan M, Gilbert L, Bagarazzi M L, Chattergoon M, Frost P, Javadian A, Williams W V, et al. Nat Med. 1997;3:526–532. doi: 10.1038/nm0597-526. [DOI] [PubMed] [Google Scholar]
- 13.Lubeck M D, Natuk R, Myagkikh M, Kalyan N, Aldrich K, Sinangil F, Alipanah S, Murthy S C S, Chanda P K, Nigida S M, et al. Nat Med. 1997;3:651–658. doi: 10.1038/nm0697-651. [DOI] [PubMed] [Google Scholar]
- 14.Heeney J L, van-Els C, de-Vries P, ten-Haaft P, Otting N, Koornstra W, Boes J, Dubbes R, Niphuis H, Dings M, et al. J Exp Med. 1994;180:769–774. doi: 10.1084/jem.180.2.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallimore A, Cranage M, Cook N, Almond N, Bootman J, Rud E, Silvera P, Dennis M, Corcoran T, Stott J, et al. Nat Med. 1995;1:1167–1173. doi: 10.1038/nm1195-1167. [DOI] [PubMed] [Google Scholar]
- 16.Rowland-Jones S L, Sutton J, Ariyoshi K, Dong T, Gotch F, McAdam S, Whitby D, Sabally S, Gallimore A, Corrah T, et al. Nat Med. 1995;1:59–64. doi: 10.1038/nm0195-59. [DOI] [PubMed] [Google Scholar]
- 17.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 18.Paxton W A, Martin S R, Tse D, O’Brien T R, Skurnick J, VanDevanter N L, Padian N, Braun J F, Kotler D P, Wolinsky S M, et al. Nat Med. 1996;2:412–417. doi: 10.1038/nm0496-412. [DOI] [PubMed] [Google Scholar]
- 19.Lehner T, Wang Y, Cranage M, Bergmeier L A, Mitchell E, Tao L, Hall G, Dennis M, Cook N, Brookes R, et al. Nat Med. 1996;2:767–775. doi: 10.1038/nm0796-767. [DOI] [PubMed] [Google Scholar]
- 20.Cheng-Mayer C, Seto D, Tateno M, Levy J A. Science. 1988;240:80–82. doi: 10.1126/science.2832945. [DOI] [PubMed] [Google Scholar]
- 21.Cheng-Mayer C, Shioda T, Levy J A. J Virol. 1991;65:6931–6941. doi: 10.1128/jvi.65.12.6931-6941.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radaelli A, De-Giuli-Morghen C. Vaccine. 1994;12:1101–1109. doi: 10.1016/0264-410x(94)90180-5. [DOI] [PubMed] [Google Scholar]
- 23.Davis D, Morein B, Åkerblom L, Van Gils M E, Bogers W, Teeuwsen V, Heeney J L. Vaccine. 1997;15:1661–1669. doi: 10.1016/s0264-410x(97)00084-4. [DOI] [PubMed] [Google Scholar]
- 24.Davis D, Chaudhri B, Stephens D M, Carne C A, Willers C, Lachmann P J. J Gen Virol. 1990;71:1975–1983. doi: 10.1099/0022-1317-71-9-1975. [DOI] [PubMed] [Google Scholar]
- 25.Davis D, Stephens D M, Willers C, Lachmann P J. J Gen Virol. 1990;71:2889–2898. doi: 10.1099/0022-1317-71-12-2889. [DOI] [PubMed] [Google Scholar]
- 26.van der Meide P H, Groenestein R J, de Labie M C D C, Heeney J L, Pala P, Slaoui M. J Med Primatol. 1995;24:271–281. doi: 10.1111/j.1600-0684.1995.tb00181.x. [DOI] [PubMed] [Google Scholar]
- 27.Forsthuber T, Yip H C, Lehmann P V. Science. 1996;271:1728–1730. doi: 10.1126/science.271.5256.1728. [DOI] [PubMed] [Google Scholar]
- 28.Mooij P, Van der Kolk M, Bogers W M J M, ten Haaft P J F, Van der Meide P, Almond N, Stott J, Deschamps M, Labbe D, Momin P, et al. AIDS. 1998;12:F1–F8. doi: 10.1097/00002030-199805000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Bogers W M J M, Dubbes R, ten Haaft P, Niphuis H, Cheng-Mayer C, Stahl-Hennig C, Hunsmann G, Kuwata T, Hayami M, Jones S, et al. Virology. 1997;236:110–117. doi: 10.1006/viro.1997.8744. [DOI] [PubMed] [Google Scholar]
- 30.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, et al. Nature (London) 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 31.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di-Marzio P, Marmon S, Sutton R E, Hill C M, et al. Nature (London) 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 32.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 33.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 34.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 35.Cocchi F, DeVico A L, Garzino-Demo A, Cara A, Gallo R C, Lusso P. Nat Med. 1996;2:1244–1247. doi: 10.1038/nm1196-1244. [DOI] [PubMed] [Google Scholar]
- 36.ten Haaft P J F, Cornelissen M, Goudsmit J, Koornstra W, Dubbes R, Niphuis H, Peeters M, Thiriart C, Bruck C, Heeney J L. J Gen Virol. 1995;76:1015–1020. doi: 10.1099/0022-1317-76-4-1015. [DOI] [PubMed] [Google Scholar]
- 37.Bogers W M, Niphuis H, ten-Haaft P, Laman J D, Koornstra W, Heeney J L. AIDS. 1995;9:F13–F18. [PubMed] [Google Scholar]