Abstract
Background
In species across taxa, offspring have means to influence parental investment (PI). PI thus evolves as an interacting phenotype and indirect genetic effects may strongly affect the co-evolutionary dynamics of offspring and parental behaviors. Evolutionary theory focused on explaining how exaggerated offspring solicitation can be understood as resolution of parent-offspring conflict, but the evolutionary origin and diversification of different forms of family interactions remains unclear.
Methodology/Principal Findings
In contrast to previous theory that largely uses a static approach to predict how “offspring individuals” and “parental individuals” should interact given conflict over PI, we present a dynamic theoretical framework of antagonistic selection on the PI individuals obtain/take as offspring and the PI they provide as parents to maximize individual lifetime reproductive success; we analyze a deterministic and a stochastic version of this dynamic framework. We show that a zone for equivalent co-adaptation outcomes exists in which stable levels of PI can evolve and be maintained despite fast strategy transitions and ongoing co-evolutionary dynamics. Under antagonistic co-adaptation, cost-free solicitation can evolve as an adaptation to emerging preferences in parents.
Conclusions/Significance
We show that antagonistic selection across the offspring and parental life-stage of individuals favors co-adapted offspring and parental behavior within a zone of equivalent outcomes. This antagonistic parent-offspring co-adaptation does not require solicitation to be costly, allows for rapid divergence and evolutionary novelty and potentially explains the origin and diversification of the observed provisioning forms in family life.
Introduction
Offspring influences on parental investment (PI) are ubiquitous [1]–[4]. For instance, mammal and plant embryos release hormones to sequester resources from maternal tissues [5], [6], insect juveniles emit chemical cues to which caregivers respond [7]–[9], and bird chicks display their characteristic begging displays (calling, wing-flapping) and morphologies (gapes, ornamental plumage [10], [11]). The evolution of offspring characters that influence PI is traditionally understood as the phenotypic, and usually costly, manifestation of resolved conflict between parents and their offspring over PI [1]–[4], [12], driven either by sibling rivalry [13] or parental preference [14]. The major predictions of conflict resolution theory have received experimental support overall [e.g.], [12,15], but it is also becoming increasingly clear that it does not capture a number of critical aspect in the co-evolution of offspring and parental behaviors [15]. A repeatedly mentioned shortcoming of this theory [15]–[21] is its static approach of modeling how “offspring individuals” and “parental individuals” should interact given conflict [but see; 22], [23].
Quantitative genetic models of maternal effects and parent-offspring interactions represent an alternative approach focusing on the maintenance of heritable (co-)variation by modeling how offspring and parent behaviors interact to determine offspring (or parental) fitness [19], [24]–[26]. This approach makes predictions for patterns of co-adaptation in terms of genetic correlations between offspring and parental traits based on selection acting on offspring or on parents, but it has not yet been incorporated into an explicit functional context for PI [27].
Here, we develop a dynamic evolutionary model based on co-evolving behavioral reaction norms [27], [28] and the premise of antagonistic selection on the amount of PI individuals obtain/take as offspring and the PI they provide as parents. This model is evaluated both deterministically and stochastically. Antagonistic selection occurs because, under a trade-off between the number and quality of offspring [29], [30], obtained PI enhances survival of individuals when they are offspring, but provided PI per offspring reduces fecundity of individuals when they are parents. We show that this antagonistic selection across life-stages generates a neutral space of equivalent co-adapted strategy sets individuals may express as offspring and as parents. This outcome allows for evolutionary innovation in family interactions by maintaining cryptic heritable variation and ongoing evolutionary dynamics in the behaviors mediating PI.
The Model
We developed a co-adaptation model using both a deterministic analysis and stochastic simulations of antagonistic selection across life-stages. See Table 1 for a full list of definitions of model parameters and variables. The model assumes a trade-off between quality (survival) and number (fecundity) of offspring [29], [30], which necessarily implies that the amount of PI individuals obtain as an offspring tightly co-evolves with the amount provided as a parent (at equilibrium they have to be identical). In our model, selection on the offspring stage of generation t does not affect parental traits (generation t−1) because these individuals belong to different generations. Selection does however favor increased solicitation x in the offspring stage, which will increase the amount of obtained PI (fo) if, and only if, parents are sensitive. Conversely, selection on the parental stage of generation t can favor a reduction of provided PI (fp) in two ways: 1) by reducing the sensitivity a of a parent to offspring solicitation, or 2) by reducing a baseline amount b of PI. Differential selection on these two components of provided PI depends on the level of solicitation expressed in the offspring stage of generation t+1.
Table 1. Definitions of functions, model parameters and variables.
| Variable/Parameter | Definition |
| General | |
| fo | Amount of PI obtained as offspring |
| fp | Amount of PI provided as parent to each offspring |

|
Population mean amount of PI |
| t | Time index, in generations (t→focal generation) |
| S() | Offspring survival function |
| F() | Parental fecundity function |
| W() | Lifetime individual fitness function |
| H() | Heaviside function (step-function to set survival to 0 if fo<p min) |
| p min | Minimum amount of PI required for offspring survival (fitness parameter) |
| k | Shape parameter for offspring survival function (fitness parameter) |
| m | Total PI available for reproduction (fitness parameter) |
| a | Parental sensitivity to offspring solicitation (evolving trait) |
| x | Offspring solicitation level (evolving trait) |
| b | Baseline level of PI (evolving trait) |
| Deterministic model | |
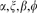
|
Population means (non-equilibrium) for parental sensitivity, offspring solicitation, baseline PI and total PI, respectively |

|
Equilibrium population means for sensitivity, solicitation, baseline PI and total PI, respectively |

|
Initial values (generation 0) for population mean sensitivity, solicitation and baseline PI, respectively, in numerical analyses |
| Stochastic simulation | |
| aj | Sensitivity alleles 1–5 (stochastic simulations); a 1→insensitive |
| xi | Solicitation alleles 1–5 (stochastic simulations); x 1→no solicitation |
| bconst | Baseline provided PI; fixed parameter |
Critically, a successful offspring solicitation strategy increases its representation in the parental stage of the current generation, but has yet to spread to the next generation. Thus, the spread of solicitation strategy x also depends on how the individual performs as a parent in terms of its fecundity and, hence, its sensitivity a and baseline PI b (Figure 1). Obviously, solicitation is not directly expressed during the parental life-stage, but, if the individual becomes a parent who is sensitive to offspring solicitation, there is an indirect genetic effect [31] of solicitation on fp [32] generating intergenomic (“social”) epistasis for fitness between offspring and parents [25], [33], [34]. Analogously, parental provisioning genes, despite only being expressed during the parental stage, must survive the offspring stage before they generate a fecundity pay-off during the stage at which they are expressed (Figure 1). Their evolutionary success depends on an interaction with the solicitation strategy x inherited to the offspring, to which they are also predictably associated within an individual (or genome). In summary, solicitation and provisioning co-evolve as interacting phenotypes [31], generate intergenomic (“social”) epistasis for fitness [25], [33], [34] and are under antagonistic selection across life-stages.
Figure 1. Transmission dynamics of provisioning genes (blue boxes) and solicitation genes (red boxes).
Filled boxes indicate the gene is expressed in that life-stage, hatched boxes that the gene in not expressed. Arrows depict the path through which genes get passed on to the next generation.
We assume throughout that a single parent interacts with a single offspring or, equivalently, with an arbitrary number of offspring that do not interact or compete. At evolutionary equilibrium, population means for fo and fp ( and
and 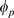 respectively), as mediated by the interaction of mean levels for solicitation
respectively), as mediated by the interaction of mean levels for solicitation  , sensitivity
, sensitivity  and baseline provisioning
and baseline provisioning 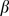 , must be identical (
, must be identical ( ). This equilibrium level of obtained/provided PI is denoted by
). This equilibrium level of obtained/provided PI is denoted by  (Table 1).
(Table 1).
Deterministic Model
The survival during the offspring stage in relation to fo of individuals from target generation t is determined by a diminishing returns function like in traditional life-history and conflict resolution models [3], [4], [12], [29]. Specifically,
| (1) |
where p
min is the minimal PI necessary for offspring survival and k is a ‘shape parameter’ determining the marginal returns on PI (Table 1). The Heaviside function (H) is a standard step-function used to set survival to zero if 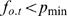 (i.e., to prevent negative fitness values). An important aspect of our model is that solicitation has no direct fitness cost during the offspring stage.
(i.e., to prevent negative fitness values). An important aspect of our model is that solicitation has no direct fitness cost during the offspring stage.
If an individual of target generation t survived the offspring stage, fecundity during the parental stage was defined as a decay function depicting a fecundity cost of increased provided PI
| (2) |
where m is a fixed amount of resources available for lifetime reproduction [3], [4]. The assumption of a fixed m implies that obtained PI affects only offspring survival, not the resources available for reproduction when the individual becomes a parent. See Figure 2 for a graphical illustration of the fitness model and its components.
Figure 2. Fitness model used throughout.
Panel A depicts how survival S is determined by the amount of obtained parental investment (fo,t) (for different shape parameters k), B how parental fecundity F changes with the amount of provided PI (fp,t) (for different total resources available for reproduction; parameter m), and C how individual lifetime fitness W changes in relation to PI when fo = fp and for different values of k.
We used a quantitative genetic model of trait expression, incorporating the cross-generational interaction between parents and their offspring as an indirect genetic effect [31], [35] mediated by an evolving linear behavioral reaction norm [27]. The standard assumptions of quantitative genetic models apply (normally distributed trait values, random mating, constant additive genetic (co-)variances, and weak selection). We focused on the directional selection driving antagonistic co-adaptation in terms of evolving population mean levels of offspring solicitation and parental provisioning. Non-linear selection affects the genetic variances and co-variances, an aspect studied in previous co-adaptation models [24], [25], but it has no direct effect on evolving mean trait values [36] and thus was not included in the present analyses.
For simplicity, we assume PI responds linearly to solicitation according to linear behavioral reaction norms [27], [28]: fo,t = at −1 xt+bt −1 and fp,t = at xt+ 1+bt (subscripts t−1 and t+1 refer to the previous and subsequent generation, respectively). To ensure behavioral stability and convergence of interacting offspring and parental behavioral reaction norms [37], and for tractability, we assume that a strategic change in provisioning has fitness consequences for, but no behavioral effect on, solicitation.
Fitness was calculated over both life-stages as the product of survival at the offspring stage and fecundity at the parental stage (i.e., W = S×F).
 |
(3) |
where Greek versions of letters reflect the population mean for a trait, and the 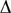 notation indicates the evolutionary change between generations t−1 and t. In order to get equation 3 we assume random mating, traits are additively inherited, that provisioning is performed uniparentally and the offspring is the caring parent's progeny. To calculate the average fitness function
notation indicates the evolutionary change between generations t−1 and t. In order to get equation 3 we assume random mating, traits are additively inherited, that provisioning is performed uniparentally and the offspring is the caring parent's progeny. To calculate the average fitness function 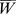 , we assumed that the per generation changes in baseline provisioning and parental sensitivity are small relative to the mean values of these traits (i.e.,
, we assumed that the per generation changes in baseline provisioning and parental sensitivity are small relative to the mean values of these traits (i.e.,  ).
).
Using the fitness function (W) from equation 3, we calculated the evolutionary dynamics of the system using the standard machinery of quantitative genetics [36]. In particular, we analyzed the dynamic system 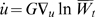 – where
– where 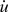 is the time derivative of u which is the trait vector {α, ξ, β} and
is the time derivative of u which is the trait vector {α, ξ, β} and 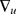 is the gradient operator – for equilibria and their stability. A first-order Taylor series approximation of W about the point
is the gradient operator – for equilibria and their stability. A first-order Taylor series approximation of W about the point 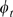 , the mean of fo,t (equation 1), was used to compute the average fitness function
, the mean of fo,t (equation 1), was used to compute the average fitness function
| (4) |
Because the evolutionary system is a gradient system, the single fitness maximum at  (Figure 2C) is an evolutionarily stable equilibrium of the system [36].
(Figure 2C) is an evolutionarily stable equilibrium of the system [36].
The time-dynamics of the system were numerically explored. Initial values for population mean parental sensitivity α 0, offspring begging ξ 0, and baseline provisioning β 0 for a starting population of interacting offspring and their parents were chosen such that the resulting level of PI was not too far from the equilibrium (±2 units of PI). This choice was made due to convergence problems when the dynamic system was started with more extreme values, and our analyses is therefore not for global stability (rather for an ‘attraction basin’); in practice, the only circumstance under which the system did not converge was when one of the three provisioning traits evolved to zero prior to achieving equilibrium, which was more likely to happen for extreme starting values. Numerical analyses were carried out using the software Mathematica (v. 6.0) [38].
Stochastic Simulations
To address the role of stochastic effects in the antagonistic co-adaptation process, we developed a stochastic, numerical analogue of our deterministic model that explicitly incorporated mutation, migration and environmental stochasticity. The simulations were set up as a discrete-time Monte Carlo simulation model in which each event (survival, reproduction and death) occurred probabilistically. Offspring solicitation and parental sensitivity were represented by two distinct loci with alleles determining the corresponding levels of behavior. We assumed solicitation (x) was governed by one locus with i alleles and parental sensitivity (a) by one locus with j alleles. We assumed equal allele numbers for the two loci, and present the results for i = j = 5 (qualitatively similar results were obtained with different allele numbers; SG and MK unpublished results). The baseline provisioning bconst was incorporated as a fixed parameter in the stochastic simulations. To parallel the analytical version of our model, we assumed PI responds linearly to solicitation. Each offspring genotype xi expressed a different level of solicitation, the lowest (x 1) always being “no solicitation”; similarly, each parental genotype aj expressed a different level of sensitivity to solicitation, the lowest (a 1) always being “no sensitivity”. The highest level (x 5) for an allele was calculated as the one that, combined with the highest parental sensitivity (a 5), would result in the upper limit for PI available to parents (parameter m). Alleles for intermediate solicitation levels (x 2–x 4) and intermediate sensitivity levels (a 2−a 4) were chosen to be evenly distributed between the minimum and maximum values.
We assumed a population composed of n/2 families (i.e., parent-offspring interactions) where n is the population size in terms of individuals present at any given moment in time; each family was composed of one parent and one offspring (i.e., individuals from successive generations). Individuals in a given life-stage have discrete and non-overlapping generations, but interact across life-stages and generations to determine PI. We further assumed the individuals are haploid and reproduce asexually. The haploid mode of inheritance was chosen for its simplicity. Nevertheless, it directly relates to the assumed additive mode of inheritance in the analytical version of the model because the expressed genetic variance under haploid inheritance is caused by additive components only [39, p. 92] (except for within-genomic epistatic interactions). To avoid some of the pitfalls of asexual reproduction in such simulations (e.g., lock-in of sub-optimal genotypes due to lack of genetic variability and sexual recombination), a relatively high mutation rate was chosen. Mutations caused a change of allelic state to another allele within the set of possible alleles. The mean±SE per-generation mutation rates over 1000 time-steps were 0.1004±0.0002 and 0.09873±0.0002 for the solicitation and sensitivity locus, respectively. The mutation rate was not supposed to reflect a biologically realistic rate, but rather to continuously regenerate heritable variation for parental sensitivity and offspring solicitation on which antagonistic selection could act in the absence of sexual recombination.
Similar to the analytical model, the success of an individual in generation t was the product of its survival S at the offspring stage and its fecundity F at the parent stage (see Table 1). Generation t was composed of two time-steps T in the simulation model (t = 2T), reflecting an offspring and parental life stage, respectively. The frequency of the solicitation genotype xi in the offspring subpopulation was computed at each time step T. Offspring survival/death happened with a likelihood proportional to S(fo,t). Specifically, the survival probability of an offspring carrying solicitation allele xi obtaining PI from its parent with sensitivity allele aj, was given by
where 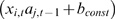 corresponds to the amount of obtained PI (fo,t). See table 1 for variable and parameter definitions. An offspring survived in the simulations if the value for S(fo,t) was larger than a random number drawn from a uniform distribution.
corresponds to the amount of obtained PI (fo,t). See table 1 for variable and parameter definitions. An offspring survived in the simulations if the value for S(fo,t) was larger than a random number drawn from a uniform distribution.
Equivalently, reproduction happened with a likelihood proportional to F(fp,t) the fecundity of a parent carrying sensitivity allele aj that provides PI to its offspring with solicitation allele xi. The frequency of the parental sensitivity genotype aj in the offspring sub-population of individuals from the next generation (t+1) was assumed to evolve according to
where  corresponds to provided PI (fp,t) as determined by the interaction of the sensitivity allele aj,t of the focal generation individuals in their parental stage, and the solicitation genotype of their offspring (xi,t
+1,). As before for survival, a parent reproduced if the value for F(fp,t) was larger than a random number drawn from a uniform distribution.
corresponds to provided PI (fp,t) as determined by the interaction of the sensitivity allele aj,t of the focal generation individuals in their parental stage, and the solicitation genotype of their offspring (xi,t
+1,). As before for survival, a parent reproduced if the value for F(fp,t) was larger than a random number drawn from a uniform distribution.
The population was assumed to be of constant size (in the examples shown, the population size n was equal 1000). To prevent population extinction we incorporated a stochastic immigration process that compensated for offspring mortality and reproductive failure. The genotypes of immigrant individuals were assumed to occur at a likelihood proportional to the genotype frequencies present in the resident population (i.e., we assumed that only genotypes similar to the ones established could successfully immigrate).
To test the prediction that the evolved offspring solicitation strategy should depend on the evolved parental sensitivity strategy (expressed during the adult stage), we ran simulations with fixed parental sensitivity strategies (a 1−a 5). To test stochastic influences for the evolution offspring solicitation and parental sensitivity, we ran simulations where offspring solicitation and parental sensitivity could both mutate and co-evolve. All simulations started with no genetic variability in the population; specifically all individuals carried the non-soliciting allele (x 1) for the offspring strategy and the insensitive allele (a 1) for the parental strategy. We used an experimental design with 100 replicate simulations per sensitivity level, and 1000 time steps (500 generations) per run.
Model Results
Deterministic model
In this model,  (equation 4) has one fitness-maximizing equilibrium with respect to PI at
(equation 4) has one fitness-maximizing equilibrium with respect to PI at 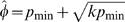 , which was found by setting
, which was found by setting 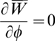 and solving for
and solving for 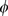 . This equilibrium can be shown to always be at least neutrally stable with respect to population means for parental sensitivity α, offspring solicitation ξ and baseline provisioning β, such that
. This equilibrium can be shown to always be at least neutrally stable with respect to population means for parental sensitivity α, offspring solicitation ξ and baseline provisioning β, such that
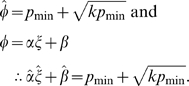 |
(5) |
This equilibrium defines combinations of parental sensitivity, baseline provisioning and offspring solicitation within individuals (genomes) due to antagonistic selection across life-stages, and we refer to it as a co-adaptation equilibrium (Figure 3). It can be further shown by numerical analysis that the dynamic system reaches the co-adaptation PI equilibrium ( ) irrespective of the structure of the genetic covariance matrix, i.e., also in the presence of genetic correlations between a, x and b (an analytical proof is available upon request as well).
) irrespective of the structure of the genetic covariance matrix, i.e., also in the presence of genetic correlations between a, x and b (an analytical proof is available upon request as well).
Figure 3. Co-adaptation equilibrium and neutral stability.
In A the fitness surface displaying the neutral curve for co-adapted associations of offspring solicitation and parental sensitivity for a fixed value of β is shown. B illustrates a typical dynamics of the system from two different sets of initial conditions (solid versus dashed lines). Red lines are for parental sensitivity α, green lines for offspring solicitation ξ and blue lines for the baseline provisioning β; clearly, under different initial conditions the system reaches different stable equilibria that are part of the stable equilibrium ‘curve’ in A.
The numerical analysis of the deterministic system was carried out systematically with parameter values p
min = 2, k = 2.5, m = 10, yielding an optimum level for 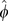 of approximately 4.2361. These are the same parameter values as those used in our stochastic simulations (see below). Different sets of genetic variances and genetic correlations among the traits were explored, with initial values (t = 0) for each trait (α
0, ξ
0, β
0) , genetic variances for, and correlations among traits chosen at random to numerically explore the stability of the co-adaptation equilibrium for different genetic covariance matrices. In all cases, the system evolved to the expected value for
of approximately 4.2361. These are the same parameter values as those used in our stochastic simulations (see below). Different sets of genetic variances and genetic correlations among the traits were explored, with initial values (t = 0) for each trait (α
0, ξ
0, β
0) , genetic variances for, and correlations among traits chosen at random to numerically explore the stability of the co-adaptation equilibrium for different genetic covariance matrices. In all cases, the system evolved to the expected value for 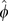 of 4.2361, irrespective of the genetic variances and correlations among the traits. This result holds for many more conditions of covariance matrices and initial trait values (BJR and MK unpublished results) as long as none of the three trait values evolved to zero prior to achieving equilibrium, a condition causing convergence problems.
of 4.2361, irrespective of the genetic variances and correlations among the traits. This result holds for many more conditions of covariance matrices and initial trait values (BJR and MK unpublished results) as long as none of the three trait values evolved to zero prior to achieving equilibrium, a condition causing convergence problems.
Stochastic Simulations
Based on the results of the analytical model (Figure 3), we predicted that 1) successful invasion and maintenance of offspring solicitation strategies should be critically determined by the provisioning strategy (sensitivity) expressed at the parental stage of the individuals in a population (co-adaptation), and 2) stochastic effects may lead to transitions in offspring solicitation and parental provisioning strategies within the limits set by the co-adaptation equilibrium without altering PI (neutral curve). Thus, a stable level of PI should be maintained despite ongoing changes at the genetic level for solicitation and sensitivity.
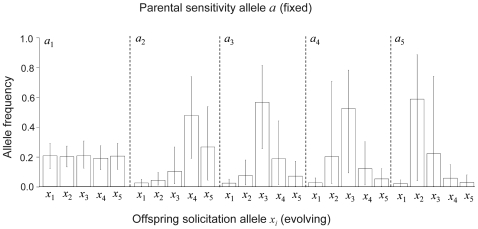
The first prediction was tested in simulations where the parental allele was fixed at different levels of sensitivity (a 1−a 5), and the solicitation locus was allowed to evolve and adapt to the fixed parental sensitivity. As predicted, the successful spread of different offspring solicitation strategies depended on the particular fixed sensitivity allele present in the parental stage of individuals of the population (ANOVA: interaction sensitivity allele x solicitation allele, F 16,2475 = 224.43, p<0.001; Figure 4). With insensitive parents, offspring solicitation is selectively neutral in our model and, correspondingly, solicitation strategies from low to high emerged and were maintained at low and similar frequencies. When a sensitive parental provisioning allele was fixed, a particular solicitation strategy quickly invaded and evolved to high frequency, but which solicitation allele took over depended on the parental sensitivity genotype. With high parental sensitivity, the low-solicitation alleles occurred at higher frequencies; similarly if parents were less sensitive, then high-solicitation alleles occurred at greater frequency (Figure 4).
Figure 4. Simulation outcomes with parental sensitivity fixed.
a 1 represents an insensitive parental strategy, and a 5 the most sensitive parental strategy. The solicitation locus is allowed to mutate among the five alleles and evolve. x 1 is a non-soliciting strategy, x 5 the highest solicitation strategy. and adapt to the parental sensitivity present in the parental sub-population. The mean and 95% percentiles of the distribution of evolutionary outcomes are shown for the frequencies of each solicitation allele after 500 generations.
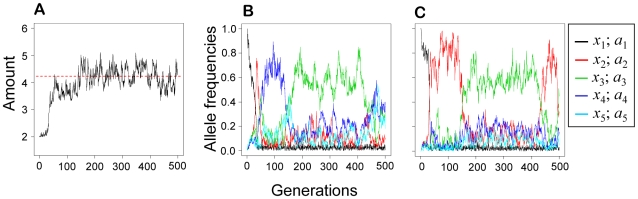
To test the second prediction, offspring and parental strategies were both allowed to evolve and co-adapt. As expected, co-evolutionary transitions sometimes occurred after PI reached the expected optimum (Figure 5B,C), and these transitions were barely detectable in terms of PI (Figure 5A). To quantitatively show evolutionary stasis in PI despite ongoing dynamics in offspring solicitation and parental sensitivity, we compared the coefficients of variation (C.V.) over time (second half of the simulations = 250 generations) between the evolving PI-level and the evolving solicitation and sensitivity allele-frequencies. The average CV and 95%-percentiles were computed across the 100 replicate runs. As expected, there was little variation in PI (less than 10%), but around 40% variation in solicitation and sensitivity allele-frequencies (Figure 6). This result confirms that a relatively stable optimal level of PI can be maintained by a range of co-adapted offspring and parental strategy sets that are equivalent in terms of lifetime fitness, just as our analytical result of neutral stability predicts.
Figure 5. Simulation outcome with offspring solicitation and parental sensitivity co-evolving.
Example of a single simulation run. Panel A) shows the evolution of the population mean level of PI (the dashed red line reflects the theoretical optimum level of PI for the chosen parameter values). B) the evolving frequencies of the five solicitation alleles, and C) the evolving frequencies of the five sensitivity alleles.
Figure 6. Time dynamics in the stochastic simulations.
The co-evolutionary time-dynamics for the level of PI, solicitation allele frequencies and sensitivity allele frequencies. Shown are the mean and 95% percentiles for the coefficients of variation (C.V.) calculated over the final 250 generations and across 100 simulation runs.
Discussion
Our model demonstrates that antagonistic selection across the offspring and parental life-stage of individuals favors the expression of co-adapted strategies that match to maximize an individual's lifetime fitness. In our model, offspring solicitation has no direct cost during the offspring stage and emerges from arbitrary cues as an adaptation to emerging parental sensitivities rather than as a resolution of parent-offspring conflict. We propose that under a trade-off between the number and quality of offspring (which is well supported experimentally; e.g. [40]) antagonistic co-adaptation is a critical component shaping offspring and parental strategies in the evolution of family interactions. It is an alternative, albeit not mutually exclusive, hypothesis for the evolution of offspring solicitation focused on co-evolving and interacting behavioral reaction norms [27], the resulting intergenomic epistasis for fitness and the co-adaptation of offspring and parental strategies within genomes.
The “possibility of coadaptive evolution”[16] in parent-offspring interactions was pointed out for the first time by Feldmann and Eshel [16], but explicit co-adaptation models were developed much later [24]–[26]. These previous co-adaptation models predict adaptive genetic correlation between offspring and parental traits due to maternal effects [24] or parent-offspring interactions [25] leading to the co-inheritance of offspring and parental behaviors. There is increasing experimental evidence supporting the prediction for co-inherited offspring and parental behaviors (see [25], [27], but see [41]), which implies that genes underlying offspring and parental behaviors may often segregate non-randomly and tend to be co-inherited. These models focused on the evolution of genetic correlations through linkage disequilibrium given offspring solicit and parents respond. They assumed selection to act either on offspring [24]–[26] or on parents [25], and that the interaction coefficients were evolutionarily fixed (i.e., constant strengths of indirect genetic effects). The present model differs from these previous co-adaptation models in that it derives a co-adaptation equilibrium in terms of population mean offspring and parental behaviors in an explicit functional context of PI (i.e., antagonistic selection across life-stages), tests the potential consequences of genetic correlation on the stability of this equilibrium and allows the parental behavioral reaction norm to evolve fully in terms of both sensitivity to solicitation and baseline provisioning.
The co-adaptation equilibrium 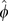 (equation 5) has several interesting properties. First, and similar to conflict resolution models, offspring solicitation, parental sensitivity, and baseline provisioning depend upon the marginal return on PI in terms of offspring survival (as determined by k) [4], [14]. Second, the genetic covariance between solicitation and sensitivity do not influence the equilibrium values for the strategies, and, contrary to expectations from previous suggestions [33], [42], [43], do not destabilize the equilibrium level of PI by, for example, triggering a runaway process. Third, the co-adaptation equilibrium is actually a ‘curve’ in space (Figure 3A) with many equilibria, including non-soliciting offspring and insensitive parents. Thus, the actual equilibrium trait values depend upon initial conditions of the system (Figure 3B) and, because the equilibrium is neutrally stable, the system can ‘drift’ to different points along the equilibrium curve. If the system is perturbed after reaching equilibrium it may not return to the identical equilibrium values depending on the extent of this perturbation (Figure 5). This last result suggests evolutionary robustness [44] in PI and an important role for stochastic evolutionary processes, such as mutation or migration, and environmental fluctuations facilitating the original evolution of offspring solicitation and parental sensitivity [15], [20]. Such events may represent instances of evolutionary innovation such as, for example, the origin of offspring solicitation. Thus, while PI itself is expected to be robust evolutionarily, the underlying traits used to mediate its provisioning should be highly labile and maintain substantial cryptic heritable variation and evolvability ([44], [45]; see also [24], [25]). As a result, populations and/or species may diverge and diversify quickly in how individuals solicit as offspring and respond as parents [46], despite similar levels of PI.
(equation 5) has several interesting properties. First, and similar to conflict resolution models, offspring solicitation, parental sensitivity, and baseline provisioning depend upon the marginal return on PI in terms of offspring survival (as determined by k) [4], [14]. Second, the genetic covariance between solicitation and sensitivity do not influence the equilibrium values for the strategies, and, contrary to expectations from previous suggestions [33], [42], [43], do not destabilize the equilibrium level of PI by, for example, triggering a runaway process. Third, the co-adaptation equilibrium is actually a ‘curve’ in space (Figure 3A) with many equilibria, including non-soliciting offspring and insensitive parents. Thus, the actual equilibrium trait values depend upon initial conditions of the system (Figure 3B) and, because the equilibrium is neutrally stable, the system can ‘drift’ to different points along the equilibrium curve. If the system is perturbed after reaching equilibrium it may not return to the identical equilibrium values depending on the extent of this perturbation (Figure 5). This last result suggests evolutionary robustness [44] in PI and an important role for stochastic evolutionary processes, such as mutation or migration, and environmental fluctuations facilitating the original evolution of offspring solicitation and parental sensitivity [15], [20]. Such events may represent instances of evolutionary innovation such as, for example, the origin of offspring solicitation. Thus, while PI itself is expected to be robust evolutionarily, the underlying traits used to mediate its provisioning should be highly labile and maintain substantial cryptic heritable variation and evolvability ([44], [45]; see also [24], [25]). As a result, populations and/or species may diverge and diversify quickly in how individuals solicit as offspring and respond as parents [46], despite similar levels of PI.
This co-adapted space arises because it constitutes “a collection of equivalent solutions to the same biological problem” [44; p. 195], the problem being in this case obtaining (as an offspring) and providing (as a parent) an amount of parental investment which maximizes lifetime fitness. All else being equal, lifetime fitness can be the same in rigid investment systems where parents simply provide an optimal baseline investment (i.e. 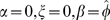 ) or communicative systems (
) or communicative systems (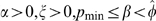 ). Only once parents evolve sensitivity to an otherwise selectively neutral offspring cue, can the evolution of an offspring signal originate [47]. Parental sensitivity may emerge purely stochastically as a sensory bias [48] (like in our model), or as an adaptation if sensitive parents provide resources more effectively [49], [50]. It will be interesting to extend our model to incorporate a direct benefit to sensitive parental strategies and a direct cost to offspring solicitation.
). Only once parents evolve sensitivity to an otherwise selectively neutral offspring cue, can the evolution of an offspring signal originate [47]. Parental sensitivity may emerge purely stochastically as a sensory bias [48] (like in our model), or as an adaptation if sensitive parents provide resources more effectively [49], [50]. It will be interesting to extend our model to incorporate a direct benefit to sensitive parental strategies and a direct cost to offspring solicitation.
We assumed throughout that soliciting is not costly to offspring. If antagonistic co-adaptation drives the evolution of offspring solicitation, begging costs during the offspring stage are not necessary for evolutionary stability, and the evolved (neutrally stable) solicitation level is determined by the sensitivity expressed in the parental stage. This stabilizing effect is due to the indirect genetic effect of solicitation on PI which leads to fecundity losses in individuals who are sensitive parents [32]. Thus, under antagonistic co-adaptation, intense solicitation does not automatically imply costliness or selfishness, which may help explain why many offspring cues or signals to which parents respond are subtle in nature [51], and begging costs to offspring – even for the vigorous bird begging displays – are often surprisingly low (see [11] and chapters therein).
The major conclusions of our model are that antagonistic co-adaptation is the direct consequence of an offspring quality-quantity trade-off generating selection on both solicitation and provisioning genes that temporarily share a genome when expressed in different life-stages of individuals, and interact across generations in a predictable manner (i.e., as determined by the degree of genetic relatedness). While our model is on the evolution of parent-offspring interactions and offspring solicitation, antagonistic selection across the offspring and parental life-stage can play an important role in life-history evolution in general, whenever trade-offs differentially affect fitness components to the offspring versus the parental life-stage. For instance, data from a long-term field study in soay sheep (Ovis aries) showed that selection favored singleton births at the offspring stage, but twin births at the parental stage [52]. Considering the consequences of antagonistic co-adaptation in the evolution of parent-offspring interactions might change predictions of conflict resolution theory, which assumed independent segregation of offspring and parental strategies [3], by, for example, constraining the evolutionary success of selfish and costly offspring strategies [16], [19], [32]. One recently suggested alternative possibility [26] is that different sets of genes may be shaped by co-adaptation and conflict. Genes under selection from co-adaptation would ensure the functional integrity of the parent-offspring interaction over the lifetime of individuals, while other loci under selection from conflict may lead to the evolutionary exaggeration of the signals due to a competitive advantage during the offspring life-stage. In future efforts to unify the evolutionary theory underlying family interactions, researchers need to clarify exactly how conflict resolution and co-adaptation relate to each other [27].
Acknowledgments
We thank E. D. Brodie III for stimulating discussions on conflict and coadaptation, and Arnon Lotem, Ralph Dobler, Flore Mas and two anonymous referees for valuable comments on earlier drafts of the manuscript. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position(s) of the funding agencies or the authors' institutions.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The study was financially supported by the Swiss National Science Foundation (grant no. PP00A-119190 to MK). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Trivers RL. Parent-offspring conflict. Am Zool. 1974;14:249–264. [Google Scholar]
- 2.Godfray HCJ. Evolutionary theory of parent-offspring conflict. Nature. 1995;376:133–138. doi: 10.1038/376133a0. [DOI] [PubMed] [Google Scholar]
- 3.Mock DW, Parker GA. The evolution of sibling rivalry. Oxford: Oxford University Press; 1997. [Google Scholar]
- 4.Parker GA, Royle NJ, Hartley IR. Intrafamilial conflict and parental investment: a synthesis. Phil Trans R Soc Lond B. 2002;357:295–307. doi: 10.1098/rstb.2001.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uma Shaanker R, Ganeshaiah KN, Bawa KS. Parent-offspring conflict, sibling rivalry, and brood size patterns in plants. Ann Rev Ecol Evol Syst. 1988;19:177–205. [Google Scholar]
- 6.Haig D. Genetic conflicts in human pregnancy. Q Rev Biol. 1993;68:495–519. doi: 10.1086/418300. [DOI] [PubMed] [Google Scholar]
- 7.Kölliker M, Chuckalovcak JP, Haynes KF, Brodie ED., III Maternal food provisioning in relation to condition-dependent offspring odours in burrower bugs (Sehirus cinctus). Proc R Soc Lond B. 2006;273:1523–1528. doi: 10.1098/rspb.2006.3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mas F, Kölliker M. Maternal care and offspring begging in social insects: chemical signalling, hormonal regulation and evolution. Anim Behav. 2008;76:1121–1131. [Google Scholar]
- 9.Mas F, Haynes KF, Kölliker M. A chemical signal of offspring quality affects maternal care in a social insect. Proc R Soc Lond B. 2009;276:2847–2853. doi: 10.1098/rspb.2009.0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kilner R, Johnstone RA. Begging the question: are offspring solicitation behaviours signals of need? Trends Ecol Evol. 1997;12:11–15. doi: 10.1016/s0169-5347(96)10061-6. [DOI] [PubMed] [Google Scholar]
- 11.Wright J, Leonard ML. The evolution of begging: competition, cooperation and communication. Dordrecht: Kluwer Academic Publishers; 2002. [Google Scholar]
- 12.Royle NJ, Hartley IR, Parker GA. Begging for control: when are offspring solicitation behaviours honest? Trends Ecol Evol. 2002;17:434–440. [Google Scholar]
- 13.Parker GA, Royle NJ, Hartley IR. Begging scrambles with unequal chicks: interactions between need and competitive ability. Ecol Letters. 2002;5:206–215. [Google Scholar]
- 14.Godfray HCJ. Signalling of need by offspring to their parents. Nature. 1991;352:328–330. [Google Scholar]
- 15.Godfray HCJ, Johnstone RA. Begging and bleating: the evolution of parent-offspring signalling. Phil Trans R Soc Lond B. 2000;355:1581–1591. doi: 10.1098/rstb.2000.0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feldman MW, Eshel I. On the theory of parent-offspring conflict: a two-locus genetic model. Am Nat. 1982;119:285–292. [Google Scholar]
- 17.Cheverud JM. Evolution by kin selection: a quantitative genetic model illustrated by maternal performance in mice. Evolution. 1984;38:766–777. doi: 10.1111/j.1558-5646.1984.tb00349.x. [DOI] [PubMed] [Google Scholar]
- 18.Lynch M. Evolution of intrafamilial interactions. Proc Natl Acad Sci USA. 1987;84:8507–8511. doi: 10.1073/pnas.84.23.8507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheverud JM, Moore AJ. Quantitative genetics and the role of the environment provided by relatives in behavioral evolution. In: Boake CRB, editor. Chicago, IL: Quantitative genetic studies of behavioral evolution; The University of Chicago Press; 1994. pp. 67–100. [Google Scholar]
- 20.Rodríguez-Gironés MA, Enquist M, Cotton PA. Instability of signaling resolution models of parent-offspring conflict. Proc Natl Acad Sci USA. 1998;95:4453–4457. doi: 10.1073/pnas.95.8.4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Price K, Ydenberg R, Daust D. State-dependent begging with asymmetries and costs: a genetic algorithm approach. In: Wright J, Leonard ML, editors. The evolution of begging. Dordrecht: Kluwer Academic Publishers; 2002. pp. 21–42. [Google Scholar]
- 22.Parker GA, Macnair MR. Models of parent-offspring conflict. I. Monogamy. Anim Behav. 1978;26:97–110. doi: 10.1016/0003-3472(78)90009-x. [DOI] [PubMed] [Google Scholar]
- 23.Parker GA, Macnair MR. Models of parent-offspring conflict. IV. Suppression: evolutionary retaliation by the parent. Anim Behav. 1979;27:1210–1235. [Google Scholar]
- 24.Wolf JB, Brodie ED., III The coadaptation of parental and offspring characters. Evolution. 1998;52:299–308. doi: 10.1111/j.1558-5646.1998.tb01632.x. [DOI] [PubMed] [Google Scholar]
- 25.Kölliker M, Brodie ED, III, Moore AJ. The coadaptation of parental supply and offspring demand. Am Nat. 2005;166:506–516. doi: 10.1086/491687. [DOI] [PubMed] [Google Scholar]
- 26.Wolf JB, Hager R. A maternal-offspring coadaptation theory for the evolution of genomic imprinting. PLoS Biology. 2006;4:0001–0006. doi: 10.1371/journal.pbio.0040380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smiseth PT, Wright J, Kölliker M. Parent-offspring conflict and co-adaptation: behavioural ecology meets quantitative genetics. Proc R Soc Lond B. 2008;275:1823–1830. doi: 10.1098/rspb.2008.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor PD, Day T. Stability in negotiation games and the emergence of cooperation. Proc R Soc Lond B. 2004;271:669–674. doi: 10.1098/rspb.2003.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith CC, Fretwell SD. The optimal balance between size and number of offspring. Am Nat. 1974;108:499–506. [Google Scholar]
- 30.Clutton-Brock TH. The evolution of parental care. Princeton, NJ: Princeton University Press; 1991. [Google Scholar]
- 31.Moore AJ, Brodie ED, III, Wolf JB. Interacting phenotypes and the evolutionary process: I. Direct and indirect genetic effects of social interactions. Evolution. 1997;51:1352–1362. doi: 10.1111/j.1558-5646.1997.tb01458.x. [DOI] [PubMed] [Google Scholar]
- 32.Alexander RD. The evolution of social behavior. Ann Rev Ecol Syst. 1974;5:325–383. [Google Scholar]
- 33.Wade MJ. The evolutionary genetics of maternal effects. In: Mousseau TA, Fox CW, editors. Maternal effects as adaptations. New York, NY: Oxford University Press; 1998. pp. 5–21. [Google Scholar]
- 34.Linksvayer TA. Ant species differences determined by epistasis between brood and worker genomes. PLoS ONE. 2007;2:e994. doi: 10.1371/journal.pone.0000994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolf JB, Brodie ED, III, Cheverud JM, Moore AJ, Wade MJ. Evolutionary consequences of indirect genetic effects. Trends Ecol Evol. 1998;13:64–69. doi: 10.1016/s0169-5347(97)01233-0. [DOI] [PubMed] [Google Scholar]
- 36.Lande R, Arnold SJ. The measurement of selection on correlated characters. Evolution. 1983;37:1210–1226. doi: 10.1111/j.1558-5646.1983.tb00236.x. [DOI] [PubMed] [Google Scholar]
- 37.Dobler R, Kölliker M. Behavioural attainability of evolutionarily stable strategies in repeated interactions. Anim Behav. 2009;77:1427–1434. [Google Scholar]
- 38.Wolfram S. Mathematica. Cambridge: Wolfram Media/Cambridge University Press; 1999. [Google Scholar]
- 39.Lynch M, Walsh B. Genetics and analysis of quantitative traits. Sunderland, MA: Sinauer Associates, Inc; 1998. [Google Scholar]
- 40.Stearns SC. The evolution of life histories. Oxford: Oxford University Press; 1992. [Google Scholar]
- 41.Dor R, Lotem A. Heritability of nestling begging intensity in the house sparrow (Passer domesticus). Evolution. 2009;63:738–748. doi: 10.1111/j.1558-5646.2008.00598.x. [DOI] [PubMed] [Google Scholar]
- 42.West-Eberhard MJ. Sexual selection, social competition, and speciation. Q Rev Biol. 1983;58:155–183. [Google Scholar]
- 43.Kölliker M, Brinkhof MWG, Heeb P, Fitze P, Richner H. The quantitative genetic basis of offspring solicitation and parental response in a passerine bird with biparental care. Proc R Soc Lond B. 2000;267:2127–2132. doi: 10.1098/rspb.2000.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wagner A. Robustness and evolvability in living systems. Princeton, NJ: Princeton University Press; 2005. [Google Scholar]
- 45.Houle D. Comparing evolvability and variability of quantitative traits. Genetics. 1992;130:195–204. doi: 10.1093/genetics/130.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qvarnström A, Vogel Kehlenbeck J, Wiley C, Svedin N, Sæther SA. Species divergence in offspring begging intensity: difference in need or manipulation of parents? Proc R Soc Lond B. 2007;274:1003–1008. doi: 10.1098/rspb.2006.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maynard Smith J, Harper D. Animal Signals. New York, NY: Oxford University Press; 2003. [Google Scholar]
- 48.Payne RJH, Rodríguez-Gironés MA. The origins of parent-offspring signalling. J theor Biol. 1998;195:273–279. doi: 10.1006/jtbi.1998.0798. [DOI] [PubMed] [Google Scholar]
- 49.Horn AG, Leonard ML. Efficacy and the design of begging signals. In: Wright J, Leonard ML, editors. The evolution of begging. Dordrecht: Kluwer Academic Publishers; 2002. pp. 127–141. [Google Scholar]
- 50.Grodzinski U, Lotem A. The adaptive value of parental responsiveness to nestling begging. Proc R Soc Lond B. 2007;274:2449–2456. doi: 10.1098/rspb.2007.0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bateson P. The dynamics of parent-offspring relationships in mammals. Trends Ecol Evol. 1994;9:399–403. doi: 10.1016/0169-5347(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 52.Wilson AJ, Pilkington JG, Pemberton JM, Coltman DW, Overall ADJ, et al. Selection on mothers and offspring: whose phenotype is it and does it matter? Evolution. 2005;59:451–463. [PubMed] [Google Scholar]