Abstract
Rationale
Compounds that activate the 5-HT2A receptor, such as lysergic acid diethylamide (LSD), act as hallucinogens in humans. One notable exception is the LSD congener lisuride, which does not have hallucinogenic effects in humans even though it is a potent 5-HT2A agonist. LSD and other hallucinogens have been shown to disrupt prepulse inhibition (PPI), an operational measure of sensorimotor gating, by activating 5-HT2A receptors in rats.
Objective
We tested whether lisuride disrupts PPI in male Sprague–Dawley rats. Experiments were also conducted to identify the mechanism(s) responsible for the effect of lisuride on PPI and to compare the effects of lisuride to those of LSD.
Results
Confirming a previous report, LSD (0.05, 0.1, and 0.2 mg/kg, s.c.) reduced PPI, and the effect of LSD was blocked by pretreatment with the selective 5-HT2A antagonist MDL 11,939. Administration of lisuride (0.0375, 0.075, and 0.15 mg/kg, s.c.) also reduced PPI. However, the PPI disruption induced by lisuride (0.075 mg/kg) was not blocked by pretreatment with MDL 11,939 or the selective 5-HT1A antagonist WAY-100635 but was prevented by pretreatment with the selective dopamine D2/D3 receptor antagonist raclopride (0.1 mg/kg, s.c).
Conclusions
The effect of LSD on PPI is mediated by the 5-HT2A receptor, whereas activation of the 5-HT2A receptor does not appear to contribute to the effect of lisuride on PPI. These findings demonstrate that lisuride and LSD disrupt PPI via distinct receptor mechanisms and provide additional support for the classification of lisuride as a non-hallucinogenic 5-HT2A agonist.
Keywords: Hallucinogen, Rats, Prepulse inhibition, 5-HT2A, Lisuride, LSD
Considerable evidence demonstrates that the 5-HT2A receptor, which is coupled to Gq/11 and activates the phosphoinositide (PI) signaling pathway (Nichols and Nichols 2008), is largely responsible for mediating the effects of lysergic acid diethylamide (LSD) and other serotonergic hallucinogens. Phenylalkylamine and indoleamine hallucinogens bind to the 5-HT2A receptor with high affinity and act as agonists or partial agonists (Glennon 1990). It has been shown that hallucinogenic potency in humans and behavioral activity in laboratory animals are robustly and significantly correlated with 5-HT2A binding affinity (Glennon et al. 1984; Titeler et al. 1988; Sadzot et al. 1989). Studies in rats have consistently found that the behavioral effects of hallucinogens are blocked by the selective 5-HT2A antagonist M100907 (Schreiber et al. 1994; Sipes and Geyer 1995a; Krebs-Thomson et al. 1998; Vickers et al. 2001). Likewise, the ability of the hallucinogen 2,5-dimethoxy-4-iodoamphetamine (DOI) to induce head twitch response (HTR) and ear scratch and to increase locomotor activity is abolished in 5-HT2A −/− knockout mice (González-Maeso et al. 2007; Halberstadt et al. 2009). The selective 5-HT2 antagonist ketanserin blocks the hallucinogenic effects of the indoleamine psilocybin in human subjects (Vollenweider et al. 1998), providing strong support for the link between 5-HT2A receptor activation and hallucinogenesis. Such evidence has implications for the involvement of 5-HT2A receptor activation in the symptoms of schizophrenia and the mechanisms of action of atypical antipsychotic drugs (Geyer and Vollenweider 2008).
Lisuride hydrogen maleate is an isolysergic acid derivative that has been used clinically in the treatment of migraine (Herrmann et al. 1977; Somerville and Herrmann 1978), cluster headache (Raffaelli et al. 1983), acromegaly and hyperprolactinemia (Liuzzi et al. 1978; Verde et al. 1980), and Parkinson’s disease (Parkes et al. 1981; Lieberman et al. 1983). The structure and pharmacological activity of lisuride are strikingly similar to that of LSD. Lisuride and LSD bind non-selectively to a variety of serotonergic, dopaminergic, and adrenergic receptors (Leysen 1989; Piercey et al. 1996; Egan et al. 1998; Marona-Lewicka et al. 2002; Millan et al. 2002; Nichols et al. 2002). Furthermore, LSD and lisuride have high affinity for the 5-HT2A receptor, and both compounds act as partial agonists (Egan et al. 1998; Kurrasch-Orbaugh et al. 2003; Cussac et al. 2008). Nonetheless, despite the similar pharmacological properties of lisuride and LSD, lisuride is devoid of hallucinogenic effects when administered to humans at acute doses of up to 400 μg (Herrmann et al. 1977; Verde et al. 1980; Raffaelli et al. 1983; Beneš et al. 2006). This lack of effect contrasts with the potent hallucinogenic activity of LSD, which is psychoactive even at doses as low as 20–30 μg (Stoll 1949; Greiner et al. 1958).
Given the fact that lisuride is not hallucinogenic, many studies have compared the effects of LSD and lisuride directly in order to detect neurochemical differences that may explain why the latter drug is inactive. Toward this goal, lisuride has been tested in several animal behavioral paradigms known to be sensitive to the effects of hallucinogens. Jacobs et al. (1977) proposed that the ability of hallucinogens to induce limb flicks and abortive grooming in cats serves as an animal model of hallucinogen effects. However, lisuride also induces limb flicking and abortive grooming (Marini et al. 1981; White et al. 1981), and thus, this model cannot distinguish between LSD and lisuride. Drug discrimination studies have evaluated whether lisuride can evoke a hallucinogen-like discriminative stimulus, an effect known to be mediated by 5-HT2A receptors (Glennon et al. 1984; Fiorella et al. 1995a). There is some disagreement in the literature regarding the degree to which the stimulus effects of LSD, DOI, and 2,5-dimethoxy-4-methylamphetamine (DOM) generalize to lisuride, with some studies reporting full substitution of lisuride for those training drugs (White and Appel 1982; Glennon and Hauck 1985; Fiorella et al. 1995b) and other studies reporting only partial substitution (Holohean et al. 1982; Marona-Lewicka et al. 2002); nonetheless, it is clear that at least some similarity exists between the interoceptive stimulus cues evoked by hallucinogens and lisuride. Adams and Geyer (1985) compared the effects of lisuride and LSD on investigatory behavior and patterns of locomotor activity. The locomotor effects produced by lisuride were found to be distinct from those of LSD and closely resembled the effects of the dopamine (DA) agonist apomorphine.
Hallucinogens, including LSD, DOI, and DOM, induce the HTR in rats and mice, an effect that is mediated by activation of 5-HT2A receptors (Schreiber et al. 1995; Vickers et al. 2001; González-Maeso et al. 2007). Notably, despite the fact that lisuride is a 5-HT2A agonist, studies indicate that lisuride does not evoke the HTR (Gerber et al. 1985; González-Maeso et al. 2007). Based on the behavioral inactivity of lisuride in this paradigm, it appears that the HTR has utility as a behavioral screen that can distinguish hallucinogenic versus nonhallucinogenic 5-HT2A agonists. Unfortunately, the usefulness of the HTR as tool to study the effects of hallucinogens is diminished by the fact that the HTR is a behavioral effect that has no human counterpart, and thus, it is unclear how this behavior relates to the subjective effects of hallucinogens. By contrast, prepulse inhibition (PPI) of acoustic startle is a cross-species phenomenon that can be assessed in humans and animals using similar testing procedures. PPI refers to the fact that a weak prestimulus will attenuate the reaction to a subsequent startle-inducing stimulus and serves as an operational measure of sensorimotor gating. Hallucinogens affect PPI in rodents (Sipes and Geyer 1994; Johansson et al. 1995; Ouagazzal et al. 2001) and in humans (Vollenweider et al. 2007). Importantly, it has been shown that the decrease in PPI induced by LSD in rats is mediated by 5-HT2A receptors (Ouagazzal et al. 2001). The present studies were designed to test whether lisuride disrupts PPI in rats. Experiments were also conducted to identify the mechanism(s) responsible for the effect of lisuride on PPI and to compare the effects of lisuride to those of LSD.
Materials and methods
Animals
Male Sprague–Dawley rats (Harlan Industries, Indianapolis, IN, USA; initial weight 250–275 g) were housed in pairs in a temperature- and humidity-controlled vivarium under a 12-h reverse light–dark cycle (lights off at 0700 hours). Food and water were available ad libitum. Animals were acclimatized for approximately 1 week after arrival prior to behavioral testing and maintained in American Association for Accreditation of Laboratory Animal Care-approved facilities that meet all federal and state guidelines. Procedures were approved by the University of California San Diego institutional animal care and use committee. Principles of laboratory animal care were followed as well as specific laws of the USA.
Apparatus
Eight startle chambers (SR-LAB system, San Diego Instruments, San Diego, CA) were used to measure startle reactivity (Mansbach et al. 1988). The startle test chambers consist of a sound-attenuated, lighted, and ventilated enclosure holding a clear nonrestrictive cylindrical Plexiglas stabilimeter, 8.2 cm in diameter. A high-frequency loudspeaker mounted 24 cm above the Plexiglas cylinder produced all acoustic stimuli. The peak and average amplitudes of the startle response were detected by a piezoelectric accelerometer, digitized, and stored on disk. At the onset of the startling stimulus, 100 1-ms readings were recorded, and the average amplitude was used to determine the rat startle response. A dynamic calibration system was used to ensure comparable stabilimeter sensitivity across test chambers, and sound levels were measured using the dB(A) scale, as described previously (Mansbach et al. 1988).
Acoustic startle sessions
Acoustic startle test sessions consisted of startle trials (pulse-alone) and prepulse trials (prepulse + pulse). The pulse-alone trial consisted of a 40-ms 120-dB pulse of broadband white noise. Prepulse + pulse trials consisted of a 20-ms acoustic prepulse, an 80-ms delay, and then a 40-ms 120-dB startle pulse (100 ms onset–onset). There was an average of 15 s (range, 9–21 s) between trials. During each inter-trial interval, the movements of the rats were recorded once to measure responding when no stimulus was present (data not shown). Each startle session began with a 5-min acclimation period to a 65-dB broadband noise that was present continuously throughout the session. One week after arrival, animals were tested in a brief baseline startle/PPI session to create treatment groups matched for levels of startle and PPI.
For experiments with lisuride, the startle test session included three blocks. The first block tested acoustic startle response only and included four each of five different acoustic stimulus intensities: 80, 90, 100, 110, and 120 dB (unpublished data). The second block was designed to assess PPI; it contained 12 pulse-alone trials and 30 prepulse + pulse trials [ten prepulses each of 68, 71, and 77 dB (or 3, 6, and 12 dB above background)] presented in a pseudo-randomized order. The third block tested the effect of varying the inter-trial interval on PPI (unpublished data); it contained eight pulse-alone trials and 20 prepulse + pulse trials [77 dB prepulses (12 dB above background)]. Five inter-trial intervals (onset–onset) were used for the prepulse + pulse trials: 30, 60, 120, 240, or 2,000 ms. Five pulse-alone trials were presented at the beginning and the end of the test session but were not used in the calculation of PPI values.
For experiments with LSD, the startle test session included only one block. The test session contained 14 pulse-alone trials and 36 prepulse + pulse trials [12 prepulses each of 68, 71, and 77 dB (or 3, 6, and 12 dB above background)] presented in a pseudo-randomized order. Five pulse-alone trials were presented at the beginning and the end of the test session but were not used in the calculation of PPI values.
Data analysis
The amount of PPI was calculated as a percentage score for each prepulse + pulse trial type:%PPI = 100− {[(startle response for prepulse + pulse trial)/(startle response for pulse-alone trial)] × 100}. Startle magnitude was calculated as the average response to all of the pulse-alone trials. PPI data were analyzed with two- or three-factor analysis of variance (ANOVA) with pretreatment and/or treatment as between-subjects factors and trial type (prepulse intensity) as a repeated measure. For experiments in which there was no significant interaction between drug and prepulse intensity, PPI data were collapsed across prepulse intensity and the average PPI was used as the main dependent measure. Startle magnitude data were analyzed with one- or two-factor (pretreatment and/or treatment) ANOVA. Post-hoc analyses were carried out using Tukey’s test. The alpha level was set at 0.05.
Experimental design
Animals were placed in the startle chambers 10 min after treatment with lisuride or 5 min after treatment with LSD. In experiment 1, rats (n = 9–10, 38 total) were treated with vehicle, 37.5, 75, or 150 µg/kg lisuride. In experiment 2, rats (n = 10–12, 45 total) were treated with MDL 11,939 (vehicle or 0.3 mg/kg) 20 min before administration of lisuride (vehicle or 75 µg/kg). In experiment 3, rats (n = 11–12, 46 total) were treated with raclopride (vehicle or 0.1 mg/kg) 10 min before administration of lisuride (vehicle or 75 µg/kg). In experiment 4, rats (n = 12–13, 50 total) were treated with WAY-100635 (vehicle or 1.0 mg/kg) 20 min before administration of lisuride (vehicle or 75 µg/kg). In experiment 5, rats (n = 11–12, 47 total) were treated with vehicle, 50, 100, or 200 µg/kg LSD. In experiment 6, rats (n = 11–12, 46 total) were treated with MDL 11,939 (vehicle or 0.3 mg/kg) 25 min before administration of LSD (vehicle or 100 µg/kg).
Drugs
Drugs used were as follows: lisuride hydrogen maleate (purity 98.9%, IVAX Pharmaceuticals, Opava, Czech Republic); S-(−)-raclopride tartrate, N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexanecarboxamide maleate (WAY-100635; Sigma-Aldrich, St. Louis, MO, USA); α-phenyl-1-(2-phenylethyl)-4-piperidinemethanol (MDL 11,939; Tocris Bioscience, Ellisville, MO, USA); and (+)-lysergic acid diethylamide tartrate (LSD) (National Institute on Drug Abuse, Rockville, MD, USA). Drug doses are expressed as the salt form of the drug, with the exception of MDL 11,939, which refers to the freebase. Lisuride and LSD were dissolved in nitrogen-purged isotonic saline. MDL 11,939 was dissolved in saline (pH 5.0) containing 0.75% Tween 80. All other drugs were dissolved in isotonic saline. All drugs were administered subcutaneously in the nape of the neck in a volume of 1 ml/kg.
Results
Experiment 1: lisuride dose response
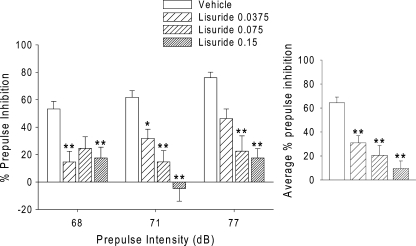
As shown in Fig. 1, administration of lisuride significantly reduced PPI [F(3,34) = 12.74, p < 0.0001]. Post hoc comparisons confirmed that all three doses of lisuride decreased PPI (p < 0.05, 0.01). There was a significant main effect of prepulse intensity [F(2,68) = 7.82, p = 0.0009; this effect was observed in all subsequent experiments], and there was a significant interaction between lisuride and prepulse intensity [F(6,68) = 3.11, p < 0.01]. Inspection of the data revealed significant effects of 37.5 µg/kg lisuride only for the 68- and 71-dB prepulse intensities (3 and 6 dB over background; p < 0.05, 0.01), whereas 75 µg/kg lisuride significantly decreased PPI only at the 71- and 77-dB prepulse intensities (p < 0.01). Administration of 75 µg/kg lisuride decreased PPI at the 68-dB prepulse intensity, and this effect approached but did not reach significance (p < 0.1). The highest dose of lisuride tested (150 µg/kg) decreased PPI at all three prepulse intensities (p < 0.01). There was an effect of lisuride on startle amplitude during block 2 [F(3,34) = 4.16, p < 0.02]. Post hoc comparisons revealed that 37.5 µg/kg lisuride significantly decreased startle magnitude (p < 0.05; Table 1). To confirm that the effect of lisuride on PPI is independent of changes in startle, we examined the effect of lisuride on PPI in subgroups of rats that were matched for block 2 startle magnitude (mean ± SEM = 320.32 ± 24.68, 261.72 ± 17.98, 257.28 ± 14.68, and 299.88 ± 21.23). Although lisuride had no effect on startle magnitude in those animals [F(3,16) = 2.32, p < 0.2 NS], PPI was still significantly reduced [F(3,16) = 4.97, p < 0.02].
Fig. 1.
Left panel Effects of lisuride (0.0375, 0.075, and 0.15 mg/kg, s.c.) on prepulse inhibition. Right panel Effects of lisuride averaged across the three prepulse intensities. Values represent mean ± SEM for each group. Drug doses are in milligram per kilogram. *p < 0.05, **p < 0.01, significantly different from vehicle control
Table 1.
Effect of drug treatment on startle magnitude
| Pretreatment (mg/kg) | Treatment (mg/kg) | n | Startle magnitude (mean ± SEM) |
|---|---|---|---|
| Vehicle | 9 | 457.95 ± 67.80 | |
| Lisuride 0.0375 | 10 | 221.68 ± 45.98* | |
| Lisuride 0.075 | 10 | 247.43 ± 36.41 | |
| Lisuride 0.15 | 9 | 389.55 ± 69.80 | |
| Vehicle | Vehicle | 11 | 275.30 ± 36.60 |
| MDL 11,939 0.3 | Vehicle | 12 | 300.94 ± 44.15 |
| Vehicle | Lisuride 0.075 | 10 | 178.96 ± 30.63 |
| MDL 11,939 0.3 | Lisuride 0.075 | 12 | 202.83 ± 29.89 |
| Vehicle | Vehicle | 12 | 437.63 ± 83.94 |
| Raclopride 0.1 | Vehicle | 11 | 260.07 ± 29.69 |
| Vehicle | Lisuride 0.075 | 11 | 262.48 ± 53.36 |
| Raclopride 0.1 | Lisuride 0.075 | 12 | 218.04 ± 43.22* |
| Vehicle | Vehicle | 12 | 397.60 ± 86.39 |
| WAY 100635 1.0 | Vehicle | 13 | 300.35 ± 56.09 |
| Vehicle | Lisuride 0.075 | 12 | 327.42 ± 46.89 |
| WAY 100635 1.0 | Lisuride 0.075 | 13 | 103.69 ± 12.20** |
| Vehicle | 12 | 543.67 ± 110.96 | |
| LSD 0.05 | 12 | 320.74 ± 64.62 | |
| LSD 0.1 | 12 | 287.22 ± 65.86 | |
| LSD 0.2 | 11 | 252.93 ± 40.19 | |
| Vehicle | Vehicle | 11 | 261.74 ± 34.75 |
| MDL 11,939 0.3 | Vehicle | 12 | 224.07 ± 26.19 |
| Vehicle | LSD 0.1 | 12 | 248.88 ± 22.57 |
| MDL 11,939 0.3 | LSD 0.1 | 11 | 262.89 ± 40.53 |
*p < 0.05, **p < 0.01 versus vehicle or vehicle–vehicle control
Experiment 2: lisuride versus the 5-HT2A antagonist MDL 11,939
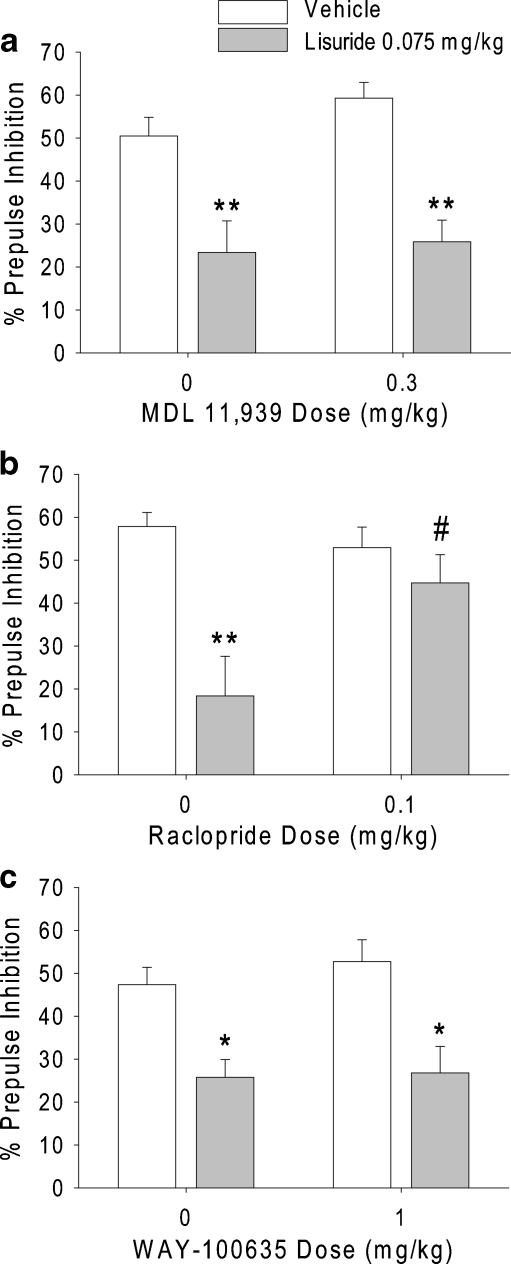
As expected, 75 µg/kg lisuride produced a significant decrease in PPI [F(1,41) = 34.62, p < 0.0001]. However, there was no significant interaction between pretreatment and treatment, indicating that the effect of lisuride was not blocked by pretreatment with 0.3 mg/kg MDL 11,939 (Fig. 2a). The dose of MDL 11,939 used was previously demonstrated to block 5-HT2A receptor-mediated behavioral effects completely (Halberstadt et al. 2008). Pretreatment with MDL 11,939 had no effect on PPI. Neither pretreatment nor treatment significantly affected startle magnitude (Table 1).
Fig. 2.
Effects of a the selective 5-HT2A antagonist MDL 11,939, b the D2/D3 antagonist raclopride, and c the 5-HT1A antagonist WAY-100635 on the disruption of PPI induced by lisuride. Values represent mean ± SEM for each group. *p < 0.05, **p < 0.01, significantly different from vehicle control, and # p < 0.05, significantly different from lisuride-treated animals
Experiment 3: lisuride versus the D2/3 antagonist raclopride
Administration of lisuride produced a significant decrease in PPI [F(1,42) = 14.39, p = 0.0005]. There was a significant interaction of pretreatment and treatment [F(1,42) = 6.18, p < 0.02]. As shown in Fig. 2b, 0.1 mg/kg raclopride blocked the reduction of PPI induced by 75 µg/kg lisuride (p < 0.05). There was a trend toward a main effect of pretreatment on PPI [F(1,42) = 2.90, p < 0.1], but this effect was not confirmed by post hoc analysis. There were also trends toward main effects of pretreatment [F(1,42) = 3.74, p < 0.06] and treatment [F(1,42) = 3.58, p < 0.07] on startle amplitude during block 2 (Table 1). However, there was no interaction between treatment and pretreatment for startle amplitude. To confirm that the ability of raclopride to block the lisuride-induced decrease in PPI is independent of changes in startle, we examined the effect of raclopride and lisuride on PPI in subgroups of rats that were matched for block 2 startle magnitude. Startle magnitude in that subset of animals was not affected by either pretreatment [F(1,20) = 0.00, NS] or treatment [F(1,20) = 0.63, NS], and there was no pretreatment × treatment interaction [F(1,20) = 1.39, NS]. Nonetheless, for PPI, there was still a significant effect of treatment [F(1,20) = 5.76, p < 0.03], and a significant interaction between pretreatment and treatment [F(1,20) = 6.68, p < 0.02].
Experiment 4: lisuride versus the 5-HT1A antagonist WAY-100635
There was a significant decrease in PPI after treatment with 75 µg/kg lisuride [F(1,46) = 22.75, p < 0.0001], but there was no significant interaction between pretreatment and treatment, indicating that the effect of lisuride was not blocked by pretreatment with 1.0 mg/kg WAY-100635 (Fig. 2c). There were main effects of pretreatment [F(1,46) = 8.37, p < 0.006] and treatment [F(1,46) = 5.78, p < 0.03] on startle amplitude, but no interaction between pretreatment and treatment. Post hoc comparisons demonstrated that startle amplitude was only significantly reduced in the WAY-100635–lisuride treatment group (Table 1).
Experiment 5: LSD dose response
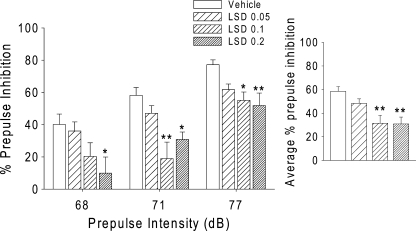
Confirming a previous report (Ouagazzal et al. 2001), there was a significant main effect of LSD on PPI [F(3,43) = 6.60, p = 0.0009]. There was no treatment × intensity interaction. Figure 3 shows that the two highest doses of LSD (100 and 200 µg/kg) significantly decreased PPI (p < 0.01). There was an overall effect of LSD on startle amplitude [F(3,43) = 2.99, p < 0.05]. Tukey’s test revealed that 100 and 200 µg/kg LSD induced a nonsignificant decrease in startle magnitude (p < 0.1). However, when subgroups of rats were matched for startle magnitude [F(3,20) = 0.57, NS], LSD was still capable of decreasing PPI [F(3,20) = 3.60, p < 0.04].
Fig. 3.
Left panel Effects of LSD (0.05, 0.1, and 0.2 mg/kg, s.c.) on prepulse inhibition. Right panel Effects of LSD averaged across the three prepulse intensities. Values represent mean ± SEM for each group. Drug doses are in milligram per kilogram. *p < 0.05, ** p < 0.01, significantly different from vehicle control
Experiment 6: LSD versus the 5-HT2A antagonist MDL 11,939
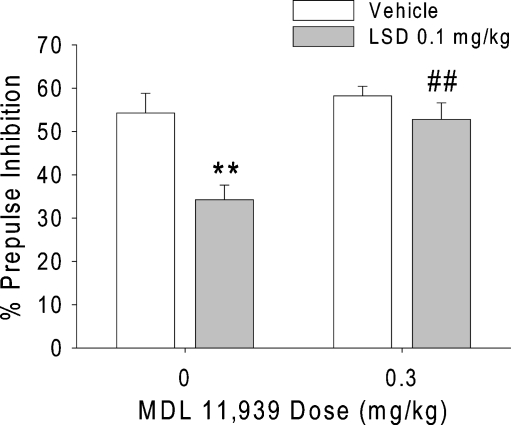
There was an interaction between pretreatment and treatment [F(1,42) = 4.23, p < 0.05]. Figure 4 shows that the disruption of PPI produced by 100 µg/kg LSD was significantly attenuated by 0.3 mg/kg MDL 11,939 (p < 0.01). As expected, there was a main effect of treatment [F(1,42) = 12.86, p = 0.0009]. Post hoc analysis revealed that 100 µg/kg LSD significantly decreased PPI (p < 0.01). There was also a main effect of pretreatment [F(1,42) = 10.05, p < 0.003], but this effect was not confirmed by post hoc analysis. There was no significant effect of either pretreatment or treatment on startle magnitude (Table 1).
Fig. 4.
Effect of the selective 5-HT2A antagonist MDL 11,939 on the disruption of PPI induced by LSD. Values represent mean ± SEM for each group. **p < 0.01, significantly different from vehicle control, and ## p < 0.01, significantly different from LSD-treated animals
Discussion
The present study demonstrated that lisuride produced a dose-dependent disruption of PPI in rats. The selective dopamine (DA) D2/D3 receptor antagonist raclopride blocked the lisuride-induced PPI disruption. Conversely, the selective 5-HT2A antagonist MDL 11,939 did not prevent the disruption of PPI by lisuride. Although lisuride is a potent and highly efficacious agonist at the 5-HT1A receptor (Marona-Lewicka et al. 2002) and activation of 5-HT1A receptors has previously been shown to disrupt PPI in rats (Sipes and Geyer 1995b), we were unable to block the effect of lisuride with the selective 5-HT1A antagonist WAY-100635. These results strongly indicate that the effect of lisuride on PPI is mediated by D2/D3 receptors and not by 5-HT2A or 5-HT1A receptors. We also tested the effect of LSD and found that LSD disrupts PPI, confirming a previous report (Ouagazzal et al. 2001). Ouagazzal et al. (2001) also reported that the ability of LSD to reduce PPI is blocked by pretreatment with the selective 5-HT2A antagonist M100907 but is unaffected by pretreatment with the D2/D3 antagonist haloperidol. Likewise, we found that MDL 11,939 blocked LSD-induced disruption of PPI. Thus, although LSD and lisuride both disrupt PPI, they do so by different receptor mechanisms.
The finding that lisuride disrupts PPI by activating D2/D3 receptors is consistent with previous evidence demonstrating that lisuride is a dopaminergic drug. Lisuride binds to D2 and D3 receptors with high affinity (K i values of 0.3 and 1.7 nM, respectively; Piercey et al. 1996) and acts as a partial agonist at recombinant D2 and D3 receptors (Newman-Tancredi et al. 2002a). Autoradiography has confirmed that [3H]lisuride binds to D2-like receptors in the striatum, nucleus accumbens, and olfactory tubercle in rat brain (Kimura et al. 1991). Lisuride has also been shown to directly inhibit the firing of A10 dopaminergic neurons in the ventral tegmental area (White and Wang 1983, 1984), indicating that it activates DA autoreceptors. Behavioral studies also demonstrate that lisuride has DA agonist activity. For example, lisuride induces contralateral turning in rats with unilateral 6-hydroxydopamine-induced lesions of the nigrostriatal DA pathway (Pieri et al. 1978), an effect that is consistent with direct activation of postsynaptic DA receptors. The effects of lisuride on locomotor activity are also similar to those of the DA agonist apomorphine and different from those of LSD (Adams and Geyer 1985). Furthermore, in drug discrimination studies, lisuride produces symmetrical generalization with the D2 agonists apomorphine and terguride (Holohean et al. 1982; Kimura et al. 1991; Yamaguchi et al. 1991).
LSD, like lisuride, is a dopaminergic agent. LSD binds to D1, D2, D3, D4, and D5 receptors (Nichols et al. 2002) and has been reported to act as a partial agonist at D1 receptors (Watts et al. 1995). Although we did not test whether raclopride blocks the effect of LSD on PPI, it was previously reported that haloperidol fails to attenuate the PPI-disruptive effect of LSD (Ouagazzal et al. 2001). Drug discrimination studies have also shown that dopaminergic activity does not contribute to the behavioral effects of LSD (Kuhn et al. 1978). However, recent evidence indicates that the dopaminergic effects of LSD may be time-dependent. Studies that have trained rats to discriminate LSD have typically used pretreatment times ranging from 15 to 30 min and have reliably shown that 5-HT2A antagonists block LSD-induced stimulus control. When a longer pretreatment time of 90 min is used, however, the resulting LSD cue is mediated by DA receptors rather than by 5-HT2A receptors (Marona-Lewicka et al. 2009). This finding raises the possibility that LSD may disrupt PPI via a dopaminergic mechanism if long pretreatment times are used.
As was noted earlier, lisuride has been tested in numerous clinical trials, but it has never been shown that the drug can produce hallucinogenic effects in normal individuals. Parkinsonism patients treated chronically with high daily doses of lisuride have been reported to experience CNS side effects, including confusion and hallucinations (Schachter et al. 1979; Lieberman et al. 1981; Gopinathan et al. 1981; Vaamonde et al. 1991). However, chronic administration of equivalent doses of lisuride to non-parkinsonian patients does not produce any significant CNS sequelae (Verde et al. 1980; Gillin et al. 1994; Schmidt et al. 2002). The DA agonist bromocriptine is also known to elicit hallucinations in Parkinsonism patients, an effect that is not observed when the drug is used to treat endocrine disorders (Vance et al. 1984). It is therefore likely that the ability of lisuride to induce hallucinations in Parkinsonism patients is due to its potent dopaminergic activity combined with underlying neuropathology, rather than to a LSD-like effect (Vaamonde et al. 1991).
Although both LSD and lisuride produce PPI disruption, we found that the receptors involved in the effects of these two drugs are distinct. Despite the fact that lisuride is a 5-HT2A agonist, it did not disrupt PPI via a mechanism involving 5-HT2A receptors, indicating that PPI can be used to differentiate hallucinogenic and non-hallucinogenic 5-HT2A agonists. This is an important finding because PPI is a cross-species behavioral paradigm that is altered by hallucinogens in animals and in humans. Tests with HTR have also demonstrated that there are behavioral differences between LSD and lisuride, but unfortunately HTR is not analogous to any behavior induced by hallucinogens in humans. The finding that the 5-HT2A receptor is not involved in the effect of lisuride on PPI provides additional support for the classification of lisuride as a nonhallucinogenic 5-HT2A agonist.
Several explanations have been proposed to account for the fact lisuride is not hallucinogenic. There has been speculation that the potent interaction of lisuride with 5-HT1A receptors may be acting to functionally antagonize the response to 5-HT2A receptor activation (Marona-Lewicka et al. 2002), but recent findings do not support this hypothesis (González-Maeso et al. 2007). Data have also been published, indicating that LSD is a 5-HT2C agonist, whereas lisuride acts as a 5-HT2C antagonist (Burris et al. 1991; Fitzgerald et al. 1999), and it has been proposed that lisuride may be non-hallucinogenic because it fails to activate the 5-HT2C receptor (Burris et al. 1991; Sanders-Bush 1994). However, other studies have demonstrated that lisuride acts as an agonist at the 5-HT2C receptor (Egan et al. 1998, 2000; Newman-Tancredi et al. 2002b; Marona-Lewicka et al. 2002; Cussac et al. 2008). It is therefore unlikely that differences between the effects of LSD and lisuride on 5-HT2C receptors account for the inactivity of the latter drug.
González-Maeso et al. (2007) suggested that differences in the behavioral effects of LSD and lisuride may be due to agonist-directed trafficking of 5-HT2A responses. Agonist-directed trafficking, or functional selectivity, refers to the phenomenon where receptors can couple independently to multiple effector mechanisms, and agonists may selectively activate a subset of the signaling pathways (Kenakin 1995). Both LSD and lisuride activate Gq/11 signaling via the 5-HT2A receptor. Conversely, LSD but not lisuride increases the cortical expression of egr-1 and egr-2 by activating pertussis toxin-sensitive Gi/o proteins and Src (González-Maeso et al. 2007). These workers also found that lisuride does not induce the HTR in mice and proposed that LSD and other hallucinogens are capable of inducing this behavioral response because they activate specific signaling mechanisms that are not recruited by lisuride.
Two key findings in the literature, however, do not support the agonist-directed trafficking hypothesis. First, although lisuride does not induce the HTR in rats (Gerber et al. 1985) or mice (González-Maeso et al. 2007), it does evoke the behavior in the least shrew (Cryptotis parva; Darmani et al. 1994). Thus, lisuride can induce head twitch under certain conditions (administration to a species that is highly sensitive to the behavioral effects of 5-HT2A agonists). Second, the ability of DOI to induce the HTR is markedly attenuated in Gq −/− knockout mice (Garcia et al. 2007), indicating that the Gq/11 pathway is involved in mediating the HTR to 5-HT2A activation. The latter finding is significant because both LSD and lisuride activate the Gq/11 pathway.
Recently, Cussac et al. (2008) compared the efficacies of LSD and lisuride for Gq/11 activation and calcium mobilization in CHO cells transfected with the human 5-HT2A receptor. LSD activated both pathways with high efficacy, whereas lisuride was less efficacious, having only 57% of the efficacy of LSD. Based on the fact that Gq plays a role in transducing the behavioral effects of 5-HT2A receptor activation (Garcia et al. 2007), these workers proposed that lisuride may have insufficient efficacy at the 5-HT2A receptor to induce HTR and other behavioral effects. Thus, lisuride may fail to recruit Gi/o not because of agonist-directed receptor trafficking but rather because it has very low intrinsic efficacy at the 5-HT2A receptor.
Results obtained using the drug discrimination paradigm are consistent with the hypothesis that lisuride fails to induce hallucinogenic effects because it has relatively weak efficacy at the 5-HT2A receptor in vivo. Even though the DOM stimulus completely generalizes to lisuride (Glennon and Hauck 1985; Fiorella et al. 1995b), DOM-induced stimulus control is attenuated when the training drug is co-administered with lisuride (Glennon 1991). Thus, the effects of lisuride in the drug discrimination paradigm are consistent with the behavior of a partial agonist. As would be expected for a partial agonist, lisuride is active when administered alone but acts as an antagonist when administered in combination with a more efficacious agonist (e.g., DOM). Indeed, using formation of [3H]inositol phosphates as a measure of 5-HT2A agonist efficacy, lisuride is only 20% as efficacious as DOM (Rabin et al. 2002).
Regardless of the underlying mechanism, there is substantial evidence that LSD and lisuride evoke distinct neurochemical and behavioral effects. It has been demonstrated that lisuride fails to mimic fully the effects of LSD on the activity of neurons in prefrontal cortex (Arvanov et al. 1999) and facial nucleus (McCall and Aghajanian 1980). Furthermore, there are marked differences in the effects of lisuride and LSD on gene expression (González-Maeso et al. 2003, 2007). Studies with head twitch indicate that this behavior is sensitive to LSD but not lisuride, at least in certain species. The present investigation extends those previous findings by demonstrating that different receptor mechanisms are responsible for the effects of LSD and lisuride on PPI. Further work is needed to clarify how these findings relate to the effects of LSD and lisuride in humans. Nevertheless, this study demonstrates that PPI can serve as a useful tool to compare hallucinogenic and non-hallucinogenic 5-HT2A agonists.
Acknowledgments
This work was supported by National Institute on Drug Abuse Awards DA002925 and DA025412 and the Veterans Affairs VISN 22 Mental Illness Research, Education, and Clinical Center. M.A. Geyer holds an equity interest in San Diego Instruments, Inc.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Adams LM, Geyer MA. Patterns of exploration in rats distinguish lisuride from lysergic acid diethylamide. Pharmacol Biochem Behav. 1985;23:461–468. doi: 10.1016/0091-3057(85)90022-X. [DOI] [PubMed] [Google Scholar]
- Arvanov VL, Liang X, Russo A, Wang R. LSD and DOB: interaction with 5-HT2A receptors to inhibit NMDA receptor-mediated transmission in the rat prefrontal cortex. Eur J NeuroSci. 1999;11:3064–3072. doi: 10.1046/j.1460-9568.1999.00726.x. [DOI] [PubMed] [Google Scholar]
- Beneš H, Deissler A, Rodenbeck A, Engfer A, Kohnen R. Lisuride treatment of Restless Legs Syndrome: first studies with monotherapy in de novo patients and in combination with levodopa in advanced disease. J Neural Transm. 2006;113:87–92. doi: 10.1007/s00702-005-0386-1. [DOI] [PubMed] [Google Scholar]
- Burris KD, Breeding M, Sanders-Bush E. (+)Lysergic acid diethylamide, but not its nonhallucinogenic congeners, is a potent serotonin 5-HT1C receptor agonist. J Pharmacol Exp Ther. 1991;258:891–896. [PubMed] [Google Scholar]
- Cussac D, Boutet-Robinet E, Ailhaud MC, Newman-Tancredi A, Martel JC, Danty N, Rauly-Lestienne I. Agonist-directed trafficking of signaling at serotonin 5-HT2A, 5-HT2B and 5-HT2C-VSV receptors mediated Gq/11 activation and calcium mobilisation in CHO cells. Eur J Pharmacol. 2008;594:32–38. doi: 10.1016/j.ejphar.2008.07.040. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Mock OB, Towns LC, Gerdes CF. The head twitch response in the least shrew (Cryptotis parva) is a 5-HT2- and not a 5-HT1C-mediated phenomenon. Pharmacol Biochem Behav. 1994;48:383–396. doi: 10.1016/0091-3057(94)90542-8. [DOI] [PubMed] [Google Scholar]
- Egan CT, Herrick-Davis K, Miller K, Glennon RA, Teitler M. Agonist activity of LSD and lisuride at cloned 5HT2A and 5HT2C receptors. Psychopharmacology. 1998;136:409–414. doi: 10.1007/s002130050585. [DOI] [PubMed] [Google Scholar]
- Egan C, Grinde E, Dupre A, Roth BL, Hake M, Teitler M, Herrick-Davis K. Agonist high and low state ratios predict drug intrinsic activity and a revised ternary complex mechanism at serotonin 5-HT2A and 5-HT2C receptors. Synapse. 2000;35:144–150. doi: 10.1002/(SICI)1098-2396(200002)35:2<144::AID-SYN7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Fiorella D, Rabin RA, Winter JC. The role of the 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs I: antagonist correlation analysis. Psychopharmacology. 1995;121:347–356. doi: 10.1007/BF02246074. [DOI] [PubMed] [Google Scholar]
- Fiorella D, Rabin RA, Winter JC. Role of 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs. II: reassessment of LSD false positives. Psychopharmacology. 1995;121:357–363. doi: 10.1007/BF02246075. [DOI] [PubMed] [Google Scholar]
- Fitzgerald LW, Conklin DS, Krause CM, Marshall AP, Patterson JP, Tran DP, Iyer G, Kostich WA, Largent BL, Hartig PR. High-affinity agonist binding correlates with efficacy (intrinsic activity) at the human serotonin 5-HT2A and 5-HT2C receptors: evidence favoring the ternary complex and two-state models of agonist action. J Neurochem. 1999;72:2127–2134. doi: 10.1046/j.1471-4159.1999.0722127.x. [DOI] [PubMed] [Google Scholar]
- Garcia EE, Smith RL, Sanders-Bush E. Role of Gq protein in behavioral effects of the hallucinogenic drug 1-(2, 5-dimethoxy-4-iodophenyl)-2-aminopropane. Neuropharmacology. 2007;52:1671–1677. doi: 10.1016/j.neuropharm.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber R, Barbaz BJ, Martin LL, Neale R, Williams M, Liebman JF. Antagonism of L-5-hydroxytryptophan-induced head-twitching in rats by lisuride: a mixed 5-hydroxytryptamine agonist-antagonist? Neurosci Lett. 1985;60:207–213. doi: 10.1016/0304-3940(85)90245-9. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Vollenweider FX. Serotonin research: contributions to understanding psychoses. Trends Pharmacol Sci. 2008;29:445–453. doi: 10.1016/j.tips.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Gillin JC, Pulvirenti L, Withers N, Golshan S, Koob G. The effects of lisuride on mood and sleep during acute withdrawal in stimulant abusers: a preliminary report. Biol Psychiatry. 1994;35:843–849. doi: 10.1016/0006-3223(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Glennon RA. Do classical hallucinogens act as 5-HT2 agonists or antagonists? Neuropsychopharmacology. 1990;3:509–517. [PubMed] [Google Scholar]
- Glennon RA. Discriminative stimulus properties of hallucinogens and related designer drugs. NIDA Res Monogr. 1991;116:25–44. [PubMed] [Google Scholar]
- Glennon RA, Hauck AE. Mechanistic studies on DOM as a discriminative stimulus. Pharmacol Biochem Behav. 1985;23:937–941. doi: 10.1016/0091-3057(85)90096-6. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Titeler M, McKenney JD. Evidence for 5-HT2 involvement in the mechanisms of action of hallucinogenic agents. Life Sci. 1984;35:2505–2511. doi: 10.1016/0024-3205(84)90436-3. [DOI] [PubMed] [Google Scholar]
- González-Maeso J, Yuen T, Ebersole BJ, Wurmbach E, Lira A, Zhou M, Weisstaub N, Hen R, Gingrich JA, Sealfon SC. Transcriptome fingerprints distinguish hallucinogenic and non-hallucinogenic 5-hydroxytryptamine 2A receptor agonist effects in mouse somatosensory cortex. J Neurosci. 2003;23:8836–8843. doi: 10.1523/JNEUROSCI.23-26-08836.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA. Hallucinogens recruit specific cortical 5-HT2A receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–452. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Gopinathan G, Teräväinen H, Dambrosia JM, Ward CD, Sanes JN, Stuart WK, Evarts EV, Calne DB. Lisuride in parkinsonism. Neurology. 1981;31:371–376. doi: 10.1212/wnl.31.4.371. [DOI] [PubMed] [Google Scholar]
- Greiner T, Burch NR, Edelberg R. Psychopathology and psychophysiology of minimal LSD-25 dosage; a preliminary dosage-response spectrum. AMA Arch Neurol Psychiatry. 1958;79:208–210. doi: 10.1001/archneurpsyc.1958.02340020088016. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL, Buell MR, Masten VL, Risbrough VB, Geyer MA. Modification of the effects of 5-methoxy-N, N-dimethyltryptamine on exploratory behavior in rats by monoamine oxidase inhibitors. Psychopharmacology. 2008;201:55–66. doi: 10.1007/s00213-008-1247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, van der Heijden I, Ruderman MA, Risbrough VB, Gingrich JA, Geyer MA, Powell SB. 5-HT2A and 5-HT2C receptors exert opposing effects on locomotor activity in mice. Neuropsychopharmacology. 2009;34:1958–1967. doi: 10.1038/npp.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann WM, Horowski R, Dannehl K, Kramer U, Lurati K. Clinical effectiveness of lisuride hydrogen maleate: a double-blind trial versus methysergide. Headache. 1977;17:54–60. doi: 10.1111/j.1526-4610.1977.hed1702054.x. [DOI] [PubMed] [Google Scholar]
- Holohean AM, White FJ, Appel JB. Dopaminergic and serotonergic mediation of the discriminable effects of ergot alkaloids. Eur J Pharmacol. 1982;81:595–602. doi: 10.1016/0014-2999(82)90349-1. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Trulson ME, Stern WC. Behavioral effects of LSD in the cats: proposal of an animal behavioral model for studying the actions of hallucinogenic drugs. Brain Res. 1977;132:301–314. doi: 10.1016/0006-8993(77)90423-1. [DOI] [PubMed] [Google Scholar]
- Johansson C, Jackson DM, Zhang J, Svensson L. Prepulse inhibition of acoustic startle, a measure of sensorimotor gating: effects of antipsychotics and other agents in rats. Pharmacol Biochem Behav. 1995;52:649–654. doi: 10.1016/0091-3057(95)00160-X. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Agonist-receptor efficacy. II. Agonist trafficking of receptor signals. Trends Pharmacol Sci. 1995;16:232–238. doi: 10.1016/S0165-6147(00)89032-X. [DOI] [PubMed] [Google Scholar]
- Kimura KK, Akai TT, Nakamura KK, Yamaguchi MM, Nakagawa H, Oshino NN. Dual activation by lisuride of central serotonin 5-HT1A and dopamine D2 receptor sites: drug discrimination and receptor binding studies. Behav Pharmacol. 1991;2:105–112. [PubMed] [Google Scholar]
- Krebs-Thomson K, Paulus MP, Geyer MA. Effects of hallucinogens on locomotor and investigatory activity and patterns: influence of 5-HT2A and 5-HT2C receptors. Neuropsychopharmacology. 1998;18:339–351. doi: 10.1016/S0893-133X(97)00164-4. [DOI] [PubMed] [Google Scholar]
- Kuhn DM, White FJ, Appel JB. The discriminative stimulus properties of LSD: mechanisms of action. Neuropharmacology. 1978;17:257–263. doi: 10.1016/0028-3908(78)90109-0. [DOI] [PubMed] [Google Scholar]
- Kurrasch-Orbaugh DM, Watts VJ, Barker EL, Nichols DE. Serotonin 5-hydroxytryptamine2A receptor-coupled phospholipase C and phospholipase A2 signaling pathways have different receptor reserves. J Pharrmacol Exp Ther. 2003;304:229–237. doi: 10.1124/jpet.102.042184. [DOI] [PubMed] [Google Scholar]
- Leysen JE. Use of 5-HT receptor agonists and antagonists for the characterization of their respective receptor sites. In: Boulton AA, Baker GB, Butterworth R, editors. Drugs as tools in neurotransmitter research. Neuromethods. Berlin: Springer; 1989. pp. 299–350. [Google Scholar]
- Lieberman A, Goldstein M, Neophytides A, Kupersmith M, Leibowitz M, Zasorin N, Walker R, Kleinberg D. Lisuride in Parkinson disease: efficacy of lisuride compared to levodopa. Neurology. 1981;31:961–965. doi: 10.1212/wnl.31.8.961. [DOI] [PubMed] [Google Scholar]
- Lieberman AN, Gopinathan G, Neophytides A, Leibowitz M, Walker R, Hiesiger E. Bromocriptine and lisuride in Parkinson disease. Ann Neurol. 1983;13:44–47. doi: 10.1002/ana.410130110. [DOI] [PubMed] [Google Scholar]
- Liuzzi A, Chiodini PG, Oppizzi G, Botalla L, Verde G, De Stefano L, Colussi G, Gräf KJ, Horowski R. Lisuride hydrogen maleate: evidence for a long lasting dopaminergic activity in humans. J Clin Endocrinol Metab. 1978;46:196–202. doi: 10.1210/jcem-46-2-196. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Geyer MA, Braff DL. Dopaminergic stimulation disrupts sensorimotor gating in the rat. Psychopharmacology. 1988;94:507–514. doi: 10.1007/BF00212846. [DOI] [PubMed] [Google Scholar]
- Marini JL, Jacobs BL, Sheard MH, Trulson ME. Activity of a non-hallucinogenic ergoline derivative, lisuride, in an animal behavior model for hallucinogens. Psychopharmacology. 1981;73:328–331. doi: 10.1007/BF00426460. [DOI] [PubMed] [Google Scholar]
- Marona-Lewicka D, Kurrasch-Orbaugh DM, Selken JR, Cumbay MG, Lisnicchia JG, Nichols DE. Re-evaluation of lisuride pharmacology: 5-hydroxytryptamine1A receptor-mediated behavioral effects overlap its other properties in rats. Psychopharmacology. 2002;164:93–107. doi: 10.1007/s00213-002-1141-z. [DOI] [PubMed] [Google Scholar]
- Marona-Lewicka D, Chemel BR, Nichols DE. Dopamine D4 receptor involvement in the discriminative stimulus effects in rats of LSD, but not the phenethylamine hallucinogen DOI. Psychopharmacology. 2009;203:265–277. doi: 10.1007/s00213-008-1238-0. [DOI] [PubMed] [Google Scholar]
- McCall RB, Aghajanian GK. Hallucinogens potentiate responses to serotonin and norepinephrine in the facial motor nucleus. Life Sci. 1980;26:1149–1156. doi: 10.1016/0024-3205(80)90654-2. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Maiofiss L, Cussac D, Audinot V, Boutin JA, Newman-Tancredi A. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. I. A multivariate analysis of the binding properties of 14 drugs at 21 native and cloned human receptor subtypes. J Pharmacol Exp Ther. 2002;303:791–804. doi: 10.1124/jpet.102.039867. [DOI] [PubMed] [Google Scholar]
- Newman-Tancredi A, Cussac D, Audinot V, Nicolas JP, De Ceuninck F, Boutin JA, Millan MJ. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. II. Agonist and antagonist properties at subtypes of dopamine D2-like receptor and alpha1/alpha2-adrenoceptor. J Pharmacol Exp Ther. 2002;303:805–814. doi: 10.1124/jpet.102.039875. [DOI] [PubMed] [Google Scholar]
- Newman-Tancredi A, Cussac D, Quentric Y, Touzard M, Verrièle L, Carpentier N, Millan MJ. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. III. Agonist and antagonist properties at serotonin, 5-HT1 and 5-HT2, receptor subtypes. J Pharmacol Exp Ther. 2002;303:815–822. doi: 10.1124/jpet.102.039883. [DOI] [PubMed] [Google Scholar]
- Nichols DE, Nichols CD. Serotonin receptors. Chem Rev. 2008;108:1614–1641. doi: 10.1021/cr078224o. [DOI] [PubMed] [Google Scholar]
- Nichols DE, Frescas S, Marona-Lewicka D, Kurrasch-Orbaugh DM. Lysergamides of isomeric 2, 4-dimethylazetidines map the binding orientation of the diethylamide moiety in the potent hallucinogenic agent N, N-diethyllysergamide (LSD) J Med Chem. 2002;45:4344–4349. doi: 10.1021/jm020153s. [DOI] [PubMed] [Google Scholar]
- Ouagazzal A, Grottick AJ, Moreau J, Higgins GA. Effect of LSD on prepulse inhibition and spontaneous behavior in the rat. A pharmacological analysis and comparison between two rat strains. Neuropsychopharmacology. 2001;25:565–575. doi: 10.1016/S0893-133X(01)00282-2. [DOI] [PubMed] [Google Scholar]
- Parkes JD, Schachter M, Marsden CD, Smith B, Wilson A. Lisuride in parkinsonism. Ann Neurol. 1981;9:48–52. doi: 10.1002/ana.410090109. [DOI] [PubMed] [Google Scholar]
- Piercey MF, Hoffmann WE, Smith MW, Hyslop DK. Inhibition of dopamine neuron firing by pramipexole, a dopamine D3 receptor-preferring agonist: comparison to other dopamine receptor agonists. Eur J Pharmacol. 1996;312:35–44. doi: 10.1016/0014-2999(96)00454-2. [DOI] [PubMed] [Google Scholar]
- Pieri M, Schaffner R, Pieri L, Da Prada M, Haefely W. Turning in MFB-lesioned rats and antagonism of neuroleptic-induced catalepsy after lisuride and LSD. Life Sci. 1978;22:1615–1622. doi: 10.1016/0024-3205(78)90057-7. [DOI] [PubMed] [Google Scholar]
- Rabin RA, Regina M, Doat M, Winter JC. 5-HT2A receptor-stimulated phosphoinositide hydrolysis in the stimulus effects of hallucinogens. Pharmacol Biochem Behav. 2002;72:29–37. doi: 10.1016/S0091-3057(01)00720-1. [DOI] [PubMed] [Google Scholar]
- Raffaelli E, Jr, Martins OJ, dos Santos P, Dãgua Filho A. Lisuride in cluster headache. Headache. 1983;23:117–121. doi: 10.1111/j.1526-4610.1983.hed2303117.x. [DOI] [PubMed] [Google Scholar]
- Sadzot B, Baraban JM, Glennon RA, Lyon RA, Leonhardt S, Jan C-R, Titeler M. Hallucinogenic drug interactions at human brains 5-HT2 receptor: implications for treating LSD-induced hallucinogenesis. Psychopharmacology. 1989;98:495–499. doi: 10.1007/BF00441948. [DOI] [PubMed] [Google Scholar]
- Sanders-Bush E. Neurochemical evidence that hallucinogenic drugs are 5-HT1C receptor agonists: what next? NIDA Res Monogr. 1994;146:203–213. [PubMed] [Google Scholar]
- Schachter M, Blackstock J, Dick JP, George RJ, Marsden CD, Parkes JD. Lisuride in Parkinson’s disease. Lancet. 1979;2(8152):1129. doi: 10.1016/S0140-6736(79)92523-6. [DOI] [PubMed] [Google Scholar]
- Schmidt LG, Kuhn S, Smolka M, Schmidt K, Rommelspacher H. Lisuride, a dopamine D2 receptor agonist, and anticraving drug expectancy as modifiers of relapse in alcohol dependence. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:209–217. doi: 10.1016/S0278-5846(01)00214-7. [DOI] [PubMed] [Google Scholar]
- Schreiber R, Brocco M, Millan MJ. Blockade of the discriminative stimulus effect of DOI by MDL 100, 907 and the ‘atypical’ antipsychotics, clozapine and risperidone. Eur J Pharmacol. 1994;264:99–102. doi: 10.1016/0014-2999(94)90643-2. [DOI] [PubMed] [Google Scholar]
- Schreiber R, Brocco M, Audinot V, Gobert A, Veiga S, Millan MJ. (1-(2, 5-Dimethoxy-4 iodophenyl)-2-aminopropane)-induced head-twitches in the rat are mediated by 5-hydroxytryptamine (5-HT) 2A receptors: modulation by novel 5-HT2A/2C antagonists, D1 antagonists and 5-HT1A agonists. J Pharmacol Exp Ther. 1995;273:101–112. [PubMed] [Google Scholar]
- Sipes TE, Geyer MA. Multiple serotonin receptor subtypes modulate prepulse inhibition of the startle response in rats. Neuropharmacology. 1994;33:441–448. doi: 10.1016/0028-3908(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Sipes TE, Geyer MA. 8-OH-DPAT disruption of prepulse inhibition in rats: reversal with (+)WAY 100, 135 and localization of site of action. Psychopharmacology. 1995;117:41–48. doi: 10.1007/BF02245096. [DOI] [PubMed] [Google Scholar]
- Sipes TE, Geyer MA. DOI disruption of prepulse inhibition of startle in the rat is mediated by 5-HT2A and not by 5-HT2C receptors. Behav Pharmacol. 1995;6:839–842. doi: 10.1097/00008877-199512000-00010. [DOI] [PubMed] [Google Scholar]
- Somerville BW, Herrmann WM. Migraine prophylaxis with Lisuride hydrogen maleate—a double blind study of Lisuride versus placebo. Headache. 1978;18:75–79. doi: 10.1111/j.1526-4610.1978.hed1802075.x. [DOI] [PubMed] [Google Scholar]
- Stoll WA. Ein neues, in sehr kleinen mengen wirksames phantastikum. Schweiz Arch neurol. 1949;64:483–484. [Google Scholar]
- Titeler M, Lyon RA, Glennon RA. Radioligand binding evidence implicates the brain 5-HT2 receptor as a site of action for LSD and phenylisopropylamine hallucinogens. Psychopharmacology. 1988;94:213–216. doi: 10.1007/BF00176847. [DOI] [PubMed] [Google Scholar]
- Vaamonde J, Luquin MR, Obeso JA. Subcutaneous lisuride infusion in Parkinson’s disease. Response to chronic administration in 34 patients. Brain. 1991;114(Pt 1B):601–617. doi: 10.1093/brain/114.1.601. [DOI] [PubMed] [Google Scholar]
- Vance ML, Evans WS, Thorner MO. Drugs five years later. Bromocriptine. Ann Intern Med. 1984;100:78–91. doi: 10.7326/0003-4819-100-1-78. [DOI] [PubMed] [Google Scholar]
- Verde G, Chiodini PG, Liuzzi A, Cozzi R, Favales F, Botalla L, Spelta B, Dalla Bonzana D, Rainer E, Horowski R. Effectiveness of the dopamine agonist lisuride in the treatment of acromegaly and pathological hyperprolactinemic states. J Endocrinol Invest. 1980;3:405–414. doi: 10.1007/BF03349379. [DOI] [PubMed] [Google Scholar]
- Vickers SP, Easton N, Malcolm CS, Allen NH, Porter RH, Bickerdike MJ, Kennett GA. Modulation of 5-HT2A receptor-mediated head-twitch behaviour in the rat by 5-HT2C receptor agonists. Pharmacol Biochem Behav. 2001;69:643–652. doi: 10.1016/S0091-3057(01)00552-4. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MFI, Bäbler A, Vogel H, Hell D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. NeuroReport. 1998;9:3897–3902. doi: 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Csomor PA, Knappe B, Geyer MA, Quednow BB. The effects of the preferential 5-HT2A agonist psilocybin on prepulse inhibition of startle in healthy human volunteers depend on interstimulus interval. Neuropsychopharmacology. 2007;32:1876–1887. doi: 10.1038/sj.npp.1301324. [DOI] [PubMed] [Google Scholar]
- Watts VJ, Lawler CP, Rox DR, Neve KA, Nichols DE, Mailman RB. LSD and structural analogs: pharmacological evaluation at D1 dopamine receptors. Psychopharmacology. 1995;118:401–409. doi: 10.1007/BF02245940. [DOI] [PubMed] [Google Scholar]
- White FJ, Appel JB. Lysergic acid diethylamide (LSD) and lisuride: differentiation of their neuropharmacological actions. Science. 1982;216:535–537. doi: 10.1126/science.7071600. [DOI] [PubMed] [Google Scholar]
- White FJ, Wang RY. Comparison of the effects of LSD and lisuride on A10 dopamine neurons in the rat. Neuropharmacology. 1983;22:669–676. doi: 10.1016/0028-3908(83)90089-8. [DOI] [PubMed] [Google Scholar]
- White FJ, Wang RY. Pharmacological characterization of dopamine autoreceptors in the rat ventral tegmental area: microiontophoretic studies. J Pharmacol Exp Ther. 1984;231:275–280. [PubMed] [Google Scholar]
- White FJ, Holohean AM, Appel JB. Lack of specificity of an animal behavior model for hallucinogenic drug action. Pharmacol Biochem Behav. 1981;14:330–343. doi: 10.1016/0091-3057(81)90400-7. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Kimura-Iwasaki K, Akai T, Nakada Y, Nakagawa H. Terguride, a dopamine D(2) partial agonist, as a discriminative stimulus in rats. Behav Pharmacol. 1991;2:233–240. doi: 10.1097/00008877-199106000-00007. [DOI] [PubMed] [Google Scholar]