Abstract
Aims/hypothesis
Microalbuminuria is common in type 1 diabetes and is associated with an increased risk of renal and cardiovascular disease. We aimed to develop and validate a clinical prediction rule that estimates the absolute risk of microalbuminuria.
Methods
Data from the European Diabetes Prospective Complications Study (n = 1115) were used to develop the prediction rule (development set). Multivariable logistic regression analysis was used to assess the association between potential predictors and progression to microalbuminuria within 7 years. The performance of the prediction rule was assessed with calibration and discrimination (concordance statistic [c-statistic]) measures. The rule was validated in three other diabetes studies (Pittsburgh Epidemiology of Diabetes Complications [EDC] study, Finnish Diabetic Nephropathy [FinnDiane] study and Coronary Artery Calcification in Type 1 Diabetes [CACTI] study).
Results
Of patients in the development set, 13% were microalbuminuric after 7 years. Glycosylated haemoglobin, AER, WHR, BMI and ever smoking were found to be the most important predictors. A high-risk group (n = 87 [8%]) was identified with a risk of progression to microalbuminuria of 32%. Predictions showed reasonable discriminative ability, with c-statistic of 0.71. The rule showed good calibration and discrimination in EDC, FinnDiane and CACTI (c-statistic 0.71, 0.79 and 0.79, respectively).
Conclusions/interpretation
We developed and validated a clinical prediction rule that uses relatively easily obtainable patient characteristics to predict microalbuminuria in patients with type 1 diabetes. This rule can help clinicians to decide on more frequent check-ups for patients at high risk of microalbuminuria in order to prevent long-term chronic complications.
Electronic supplementary material
The online version of this article (doi:10.1007/s00125-009-1585-3) contains a list of the members of the EURODIAB, EDC, FinnDiane and CACTI Study Groups and other contributors to the study, which is available to authorised users.
Keywords: Microalbuminuria risk, Prediction model, Progression, Type 1 diabetes
Introduction
Microalbuminuria is common in type 1 diabetes. Of patients with type 1 diabetes, 30 to 60% progress to microalbuminuria within 10 to 20 years [1, 2]. Various risk factors have been found for the progression to microalbuminuria including: hyperglycaemia, hyperlipidaemia, (central) obesity, hypertension and smoking [2–5].
Patients with microalbuminuria have elevated risks of diabetic renal disease [6, 7] and cardiovascular disease [8]. Timely prediction of progression to microalbuminuria in patients with type 1 diabetes is therefore of major importance. Accurate identification of patients at high risk of microalbuminuria at an early stage can guide disease management in order to prevent further complications. Intervention in high-risk patients may be beneficial, although data to support this are inconclusive.
Prediction of microalbuminuria progression is rarely studied [2, 5]. Only one prediction rule has been proposed before [5]. This rule uses arbitrary dichotomisation of various continuous predictor values (HbA1c and AER). Further, selection of the predictors was based on a p value of 0.05, which is considered conservative in prediction modelling. Both strategies potentially lead to an optimistic or overfitted prediction rule [9, 10]. Moreover, the prediction rule was not validated in an independent sample.
Using state of the art methods in prediction modelling, we therefore developed and externally validated a clinical prediction rule for estimating risk of progression to microalbuminuria in patients with type 1 diabetes. We used data from large prospective cohort studies of type 1 diabetes. The rule was developed with data from the European Diabetes Prospective Complications Study (EURODIAB PCS) [11] and validated in US patients treated in a similar time period (Pittsburgh Epidemiology of Diabetes Complications [EDC]) study [1]. Since treatment policies have changed over the years, we also studied the validity of the rule in two more contemporary studies, i.e. the Finnish Diabetic Nephropathy study (FinnDiane) [12] and the Coronary Artery Calcification in Type 1 Diabetes study (CACTI) [13].
Methods
Patients
We used data from the EURODIAB PCS to develop the prediction rule (development set). Full details of the design, methods and recruitment have been published elsewhere [11]. In brief, the study was designed to investigate risks and determinants of microvascular complications in type 1 diabetes patients. The EURODIAB PCS cohort included patients from 31 centres in 16 European countries.
Type 1 diabetes was defined as a diagnosis made before the age of 36 with continuous insulin treatment within 1 year of diagnosis. Patient measurements were taken at baseline (1990–1991) and at 7 years follow-up (1997–1999). Patients were included in the present analysis if they had a normal AER, i.e. below 20 μg/min, at baseline and a normal AER or microalbuminuria (AER between 20 and 200 μg/min) at follow-up.
We used data from three studies to externally validate the prediction rule (validation sets): the EDC study, FinnDiane and CACTI. The EDC study is a hospital-based incidence cohort from the Children’s Hospital of Pittsburgh, USA, set up to investigate risk factors for complications of these patients [1]. After the baseline examination (1986–1988), patients were re-examined biennially. The FinnDiane study is a nationwide, multicentre study with the aim to identify genetic and clinical risk factors for diabetic nephropathy in type 1 diabetes [12]. Patients have been followed since the baseline examination (1998). The CACTI study is a prospective cohort study designed to assess risk factors associated with the development and progression of subclinical coronary artery disease [13]. Baseline examination was performed between 2000 and 2002. All three studies are ongoing. For the present analysis, follow-up measurements 7 years after inclusion were taken to assess the development of microalbuminuria. The same inclusion criteria were used as for the development set.
Outcome
Microalbuminuria (yes/no) was determined 7 years after inclusion and defined as an AER between 20 and 200 μg/min. In EURODIAB PCS, the average of two 24 h urine samples was used for classification. Albumin concentrations were assessed with an immunoturbidimetric [14] method that included goat anti-human albumin antisera (Sanofi Diagnostics Pasteur, Chaska, MN, USA) and human serum albumin standards (ORHA 20/21 grade HAS; Behring Diagnostics, Hoechst UK, Hounslow, UK). Proteinuria due to urinary tract infection was excluded.
In the EDC study, an immuno-nephelometric method was used [15] and the median AER was calculated on the basis of three urine samples (24 h, overnight and 4 h post-clinic) or the average AER if only two samples were available. In the FinnDiane study, AER was calculated from a single 24 h urine collection, which was measured by immunoturbidimetry. The actual classification of patients was based on two out of three consecutive urine samples. In the CACTI study, AER was calculated from urinary albumin measured using RIA in two timed overnight urine samples with the results from the two nights being averaged.
Candidate predictors
The patient characteristics available in the EURODIAB PCS dataset were critically reviewed using the literature and clinical expertise. The following characteristics were considered to be possibly predictive for microalbuminuria: age, sex, duration of diabetes, HbA1c, AER, fasting triacylglycerol, non-HDL- and LDL-cholesterol, WHR, BMI, pulse pressure, hypertension and smoking. HbA1c was measured with an enzyme immunoassay using a monoclonal antibody. The HbA1c values obtained were converted to DCCT values [16]. One 24 h urine sample was analysed to determine the AER. Serum lipids were measured with standard enzymatic methods. Non-HDL-cholesterol was calculated as the total cholesterol level minus the HDL level. Height, weight, and waist and hip circumference were measured in a standardised way to calculate BMI and WHR. Blood pressure was measured by a random zero sphygmomanometer and the mean of two measurements taken. Pulse pressure was calculated as systolic minus diastolic pressure. Hypertension was defined as a systolic pressure of 140 mmHg or more, a diastolic pressure of 90 mmHg or more, or the current use of blood-pressure-lowering medication. Smoking status was dichotomised into ever vs never smokers.
Statistical analysis
In the EURODIAB PCS, 355 patients had values missing for one or more predictors. The average percentage of missing values per predictor was 3%. We imputed missing data using the linear-regression method with addition of a random-error term (single imputation) available in SPSS software (version 12.0; SPSS, Chicago, IL, USA).
Logistic regression was used to estimate univariable and multivariable regression coefficients, and odds ratios with 95% CIs for each predictor. The nature of the association between continuous predictors and risk of microalbuminuria was studied with restricted cubic splines with three knots (two df). The restricted cubic splines were plotted and approximated with simple transformations. The simplest transformation is a linear term. Other possible transformations included the natural logarithm, square and square root.
A full multivariable model was fitted that included all candidate predictors with chosen transformations. The number of predictors was reduced with backward stepwise selection. We applied Akaike’s information criterion [17] for predictor selection, which corresponds to a p value of 0.157 for a predictor with one regression coefficient. Interaction terms between predictors were assessed, but not included because their predictive effects were very limited. The regression coefficients in the final model were multiplied with a shrinkage factor, which was estimated with bootstrapping [9, 18]. Shrinkage is applied to obtain accurate predictions for new patients; without shrinkage, predictions are in general too extreme, resulting in low predictions being too low and high predictions too high.
The described analytical strategy to develop the prediction rule is in accordance with the current state of the art [9]. The strategy aims to develop a prediction rule that can provide accurate predictions for future patients rather than predictions that are correct for patients of the development dataset. Therefore, the selection of predictors in the model is based on multivariable modelling only with a backward selection procedure and liberal p value (0.157 in this study). Use of higher p values results in inclusion of relatively weaker predictors in the model at the cost of possible selection of a nuisance variable. Such a model performs well in new patients [19]. Further, the backward selection procedure starts with a limited number of candidate predictors with continuous variables studied in their original form (i.e. continuously). Dichotomising or categorising continuous variables may be seen as introducing an extreme form of rounding, with an inevitable loss of information [20].
The final model with the shrunken regression coefficients was presented as a score chart to facilitate clinical application. Scores were calculated by dividing the products of regression coefficients and predictor values by the smallest product. A constant was subtracted or added to rescale the scores in positive integers. The sum scores were then related to predicted risks.
The performance of the prediction rule was studied in terms of calibration (i.e. agreement between predicted risks and observed proportions of microalbuminuria) and discrimination. Discrimination was expressed with a concordance statistic (c-statistic), which is similar to the area under the receiver-operating characteristic curve when dichotomous outcomes are considered [21]. The performance was studied in 100 bootstrap samples (internal validation) and in the independent validation sets of the EDC, FinnDiane and CACTI studies (external validation). Internal validation gives an impression of how much the performance of the prediction rule may deteriorate when applied to a new patient sample from the same population. External validation indicates how generalisable the prediction rule is to similar patients from other populations.
Results
Development of prediction rule
The EURODIAB PCS cohort contained 1,115 patients with normal AER at baseline and normal AER or microalbuminuria at follow-up. Microalbuminuria was found in 143 (13%) patients after a mean follow-up of 7.3 years. The mean age at baseline was 33 years, 49% of the patients were male and mean duration of diabetes was 14 years (Table 1).
Table 1.
Characteristics of patients in the development and validation sets
| EURODIAB PCS (1990–1991) | EDC (1986–1988) | FinnDiane (1998) | CACTI (2000–2002) | |
|---|---|---|---|---|
| Participants (n) | 1,115 | 190 | 1,248 | 331 |
| Age (years) | 33 (9.6) | 26 (8.1) | 36 (12) | 37 (9) |
| Male sex, n (%) | 542 (49) | 82 (43) | 591 (47) | 147 (44) |
| Diabetes duration (years) | 14 (8.9) | 18 (7.3) | 16 (8.6) | 23 (9) |
| HbA1c (%) | 8.0 (1.7) | 8.3 (1.3) | 8.2 (1.4) | 7.8 (1.2) |
| AER (μg/min)a | 8.2 (0.91–20) | 8.5 (0.83–20) | 5.2 (0.30–20) | 5.0 (0.5–20) |
| Fasting triacylglycerol (mmol/l)a | 0.88 (0.30–5.1) | 0.77 (0.35–4.8) | 0.93 (0.32–10) | 0.80 (0.30–4.1) |
| Non-HDL (mmol/l) | 3.6 (1.0) | 3.1 (0.72) | 3.5 (0.91) | 3.0 (0.80) |
| LDL (mmol/l) | 3.2 (0.90) | 2.7 (0.62) | 3.0 (0.80) | 2.5 (0.72) |
| WHR, men | 0.88 (0.07) | 0.86 (0.04) | 0.89 (0.07) | 0.86 (0.06) |
| WHR, women | 0.79 (0.10) | 0.77 (0.05) | 0.80 (0.06) | 0.78 (0.07) |
| BMI, men (kg/m2) | 24 (2.6) | 23 (3.3) | 25 (3.1) | 26 (3.4) |
| BMI, women (kg/m2) | 23 (2.9) | 24 (3.5) | 25 (3.7) | 26 (4.9) |
| Diastolic pressure (mmHg) | 73 (11) | 68 (8.7) | 78 (9.0) | 77 (8.4) |
| Systolic pressure (mmHg) | 118 (15) | 108 (11) | 129 (16) | 115 (13) |
| Pulse pressure (mmHg) | 44 (12) | 40 (9.0) | 51 (13) | 39 (11) |
| Hypertension, n (%) | 148 (13) | 2 (1) | 176 (14) | 107 (32) |
| Ever smoked, n (%) | 498 (45) | 52 (27) | 507 (41) | 65 (20) |
| Outcome at follow-up | ||||
| Microalbuminuria, n (%) | 143 (13) | 34 (18) | 96 (7.7) | 20 (6.0) |
Values are the mean (SD) unless otherwise noted
aMedian (range)
Table 2 shows the univariable associations of the studied patient characteristics and progression to microalbuminuria. Continuous predictor values for this table were dichotomised at clinically relevant cut-offs or at the medians, in order to show interpretable odds ratios. Note that the multivariable analysis was performed with the continuous variables. Progression to microalbuminuria was associated with HbA1c, AER, all lipids, WHR, BMI and smoking.
Table 2.
Univariable associations of patient characteristics assessed at baseline with progression to microalbuminuria
| Patient characteristic | Normo-albuminuria | Micro-albuminuria | OR | 95% CI | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Age | ||||||
| <30 years | 435 | 87 | 63 | 13 | ||
| ≥30 years | 537 | 87 | 80 | 13 | 1.0 | 0.72–1.5 |
| Sex | ||||||
| Men | 469 | 86 | 73 | 14 | ||
| Women | 503 | 88 | 70 | 12 | 0.89 | 0.63–1.3 |
| Duration | ||||||
| <15 years | 610 | 87 | 91 | 13 | ||
| ≥15 years | 362 | 87 | 52 | 13 | 1.0 | 0.67–1.4 |
| HbA1c | ||||||
| <8.0% | 558 | 92 | 52 | 8 | ||
| ≥8.0% | 414 | 82 | 91 | 18 | 2.4 | 1.6–3.4 |
| AER | ||||||
| <8 μg/min | 491 | 91 | 46 | 9 | ||
| ≥8 μg/min | 481 | 83 | 97 | 17 | 2.2 | 1.5–3.1 |
| Fasting triacylglycerol | ||||||
| <1.0 mmol/l | 643 | 90 | 75 | 10 | ||
| ≥1.0 mmol/l | 329 | 83 | 68 | 17 | 1.8 | 1.2–2.5 |
| Non-HDL | ||||||
| <3.5 mmol/l | 498 | 90 | 56 | 10 | ||
| ≥3.5 mmol/l | 474 | 84 | 87 | 16 | 1.6 | 1.1–2.3 |
| LDL | ||||||
| <3.0 mmol/l | 449 | 90 | 50 | 10 | ||
| ≥3.0 mmol/l | 523 | 85 | 93 | 15 | 1.6 | 1.1–2.3 |
| WHR | ||||||
| Men | ||||||
| <0.95 | 417 | 87 | 62 | 13 | ||
| ≥0.95 | 52 | 82 | 11 | 18 | 1.4 | 0.70–2.9 |
| Women | ||||||
| <0.85 | 425 | 89 | 54 | 11 | ||
| ≥0.85 | 78 | 83 | 16 | 17 | 1.6 | 0.88–3.0 |
| BMI | ||||||
| Men | ||||||
| <25 kg/m2 | 347 | 89 | 43 | 11 | ||
| ≥25 kg/m2 | 122 | 80 | 30 | 20 | 2.0 | 1.2–3.3 |
| Women | ||||||
| <25 kg/m2 | 389 | 89 | 49 | 11 | ||
| ≥25 kg/m2 | 114 | 84 | 21 | 16 | 1.5 | 0.84–2.5 |
| Pulse pressure | ||||||
| <40 mmHg | 368 | 86 | 58 | 14 | ||
| ≥40 mmHg | 604 | 88 | 85 | 12 | 0.89 | 0.62–1.3 |
| Hypertension | ||||||
| No | 844 | 87 | 123 | 13 | ||
| Yes | 128 | 86 | 20 | 14 | 1.1 | 0.65–1.8 |
| Smoking | ||||||
| Never | 550 | 89 | 67 | 11 | ||
| Ever | 422 | 85 | 76 | 15 | 1.5 | 1.0–2.1 |
| Total | 972 | 87 | 143 | 13 | ||
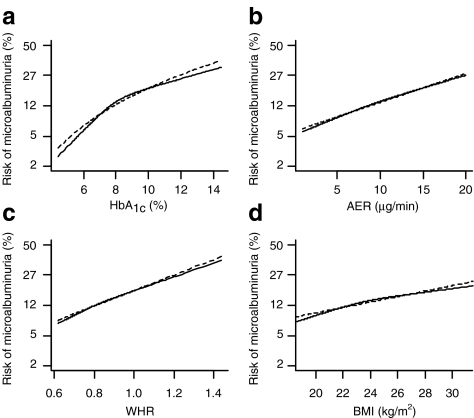
The multivariable analysis indicated that HbA1c, AER, WHR, BMI and smoking were the strongest predictors of progression to microalbuminuria (Table 3). The best transformation for HbA1c was the natural logarithm (Fig. 1a). As a consequence, this variable was transformed into loge (HbA1c-value). Linear associations with risk of microalbuminuria were observed for AER, WHR and BMI (Fig. 1b–d). The regression coefficients in the final model were multiplied with the estimated shrinkage factor of 0.88 (Table 3).
Table 3.
Multivariable associations of selected predictors for progression to microalbuminuria
| Predictor | OR | 95% CI | β coefficienta |
|---|---|---|---|
| HbA1c (%), transformedb | 1.26 | 1.09, 1.45 | 0.206 |
| AER (μg/min) | 1.09 | 1.07, 1.11 | 0.076 |
| WHR, transformedc | 1.20 | 0.90, 1.61 | 0.163 |
| BMI (kg/m2) | 1.07 | 1.03, 1.10 | 0.059 |
| Ever smoking | 1.40 | 1.16, 1.69 | 0.300 |
| Intercept | −9.883 | ||
| c-statisticd | 0.71 | 0.69 |
a β coefficients were multiplied by a shrinkage factor of 0.88 to improve predictions in future patients
bLoge(HbA1c) × 10
cWHR × 10
dEstimated in the development set and corrected for optimism after bootstrapping
Fig. 1.
Nature of the univariable associations of the continuous predictors a HbA1c, b AER, c WHR and d BMI with the risk of microalbuminuria. Continuous lines, restricted cubic splines with three knots; dotted lines, the chosen transformations. Transformations: a loge, b–d linear. The deviant scaling on y-axes corresponds to the logodds or logit of microalbuminuria, which is the adequate scale to study the shape of the association
We distinguished four risk groups that were based on the sum scores (Table 4). The observed proportions and mean predicted risks were in good agreement across the four risk groups. The two highest risk groups together (sum scores 16–20 and ≥21) contained 77% 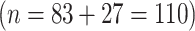 of all 143 microalbuminuric patients of the EURODIAB PCS; the low-risk group (sum scores 2–10) contained only 1% (n = 2) of microalbuminuric patients. The c-statistic was 0.71, indicating reasonable discriminative ability. Internal validation reduced the c-statistic to 0.69 indicating minor optimism in the modelling procedure.
of all 143 microalbuminuric patients of the EURODIAB PCS; the low-risk group (sum scores 2–10) contained only 1% (n = 2) of microalbuminuric patients. The c-statistic was 0.71, indicating reasonable discriminative ability. Internal validation reduced the c-statistic to 0.69 indicating minor optimism in the modelling procedure.
Table 4.
Observed proportions and predicted risks of microalbuminuria among four risk groups in the development set (EURODIAB PCS) and validation sets (EDC, FinnDiane and CACTI)
| Sum score | EURODIAB PCS | EDC | FinnDiane | CACTI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Observed proportion | Mean predicted risk | Observed proportion | Mean predicted risk | Observed proportion | Mean predicted risk | Observed proportion | Mean predicted risk | |||||
| n/N | % | % | n/N | % | % | n/N | % | % | n/N | % | % | |
| 2–10 | 2/73 | 3 | 3 | 0/5 | 0 | 4 | 1/61 | 2 | 4 | 1/22 | 5 | 4 |
| 11–15 | 31/470 | 7 | 8 | 7/88 | 8 | 8 | 14/564 | 3 | 8 | 3/196 | 2 | 8 |
| 16–20 | 83/485 | 17 | 16 | 22/83 | 27 | 15 | 47/509 | 9 | 15 | 12/93 | 13 | 15 |
| ≥21 | 27/87 | 31 | 32 | 5/14 | 36 | 31 | 34/114 | 30 | 32 | 4/20 | 20 | 31 |
External validation of the prediction rule
The EDC patients were slightly younger than patients of the development set (mean age 26 years compared with 33 years). The FinnDiane and CACTI patients were slightly older (36 and 37). Occurrence of hypertension varied between 1% (EDC) and 32% for the validation sets, with 13% in the development set. Progression to microalbuminuria occurred in 18% (EDC), 8% (FinnDiane) and 6% (CACTI) of patients (Table 1).
Most of the microalbuminuric patients were categorised in one of the two groups with the highest risk: 27/34 (79%) for EDC, 81/96 (84%) for FinnDiane and 16/20 (80%) for CACTI (Table 4), which is similar to the results found in the development set. The discriminative ability of the prediction rule was similar to that in the development set for the EDC patients with c-statistic = 0.72. The discriminative ability was even better in the FinnDiane and CACTI patients (c-statistic = 0.79 in both).
The mean predicted risks in the two datasets with recently treated patients (FinnDiane and CACTI) were higher than the observed proportions of microalbuminuria (13% predicted risk and 8% observed in FinnDiane, 11% and 6% in CACTI). We adjusted the intercept of the prediction rule in such a way that the mean predicted risk agrees with the observed proportion of microalbuminuria (intercept changes from −9.88 to −10.14).
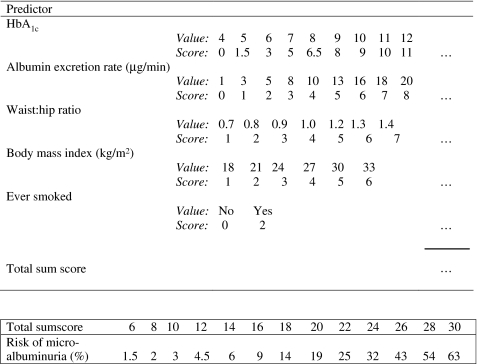
Figure 2 shows a score chart that corresponds to the adjusted prediction rule. The score chart facilitates calculation of the predicted risk of microalbuminuria for an individual patient who is currently treated. Values for continuous predictors are given in small steps. An individual sum score consists of the sum of all scores. The lower part of the figure shows the predicted risks that correspond to the sum scores.
Fig. 2.
Score chart to predict risk of microalbuminuria in patients with type 1 diabetes. The scores are derived from the prediction rule that contain the shrunken regression coefficients of the final model and updated intercept: 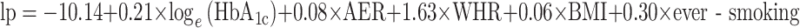 , where loge(HbA1c) is the natural logarithm of HbA1c, AER is expressed in μg/min, BMI is expressed in kg/m2 and ever-smoking is set at 1 if true and 0 if false.
, where loge(HbA1c) is the natural logarithm of HbA1c, AER is expressed in μg/min, BMI is expressed in kg/m2 and ever-smoking is set at 1 if true and 0 if false. 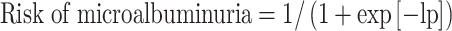 . A hypothetical patient with HbA1c of 6% (3 points), AER of 5 μg/min (2 points), WHR of 0.9 (3 points), BMI of 24 (3 points) and who has never smoked (0 points) has a sum score of 11, which corresponds to a risk of 4%
. A hypothetical patient with HbA1c of 6% (3 points), AER of 5 μg/min (2 points), WHR of 0.9 (3 points), BMI of 24 (3 points) and who has never smoked (0 points) has a sum score of 11, which corresponds to a risk of 4%
Discussion
We developed and validated a clinical prediction rule that can be used to predict progression from normal AER to microalbuminuria in type 1 diabetes patients. First, we identified a series of predictors for the risk of progression to microalbuminuria within a period of 7 years. The following five characteristics predominantly contributed to prediction in a multivariable logistic regression model: HbA1c, AER, WHR, BMI and ever smoking. These predictors can be relatively easily obtained by physical examination and laboratory tests. External validation of the prediction rule in three independent patient samples showed good performance.
HbA1c and baseline AER have consistently been shown to be important predictors of microalbuminuria [2–5, 22] and were also included in a previous model developed by Rossing and co-workers [5]. Smoking was also included in both models. In contrast, BMI and WHR were included in the present model, but were not considered in the development of the Rossing model, which did, however, include retinopathy. We found with the EURODIAB data that the present model discriminated patients with microalbuminuria from those with normal AER better than the Rossing model, which included retinopathy (c-statistics 0.69 and 0.65 respectively). A model that included the four predictors of the Rossing model (HbA1c, AER, current smoking and any retinopathy) fitted with the EURODIAB PCS data showed a c-statistic of 0.66. The additive predictive value for WHR in a model that already includes BMI indicates that central obesity contains different information than general obesity. This is confirmed by the relatively low correlation between WHR and BMI (Pearson correlation r = 0.21). The differences in effect of central and general obesity have also been described for the risk of cardiovascular events [23].
The predictors hyperglycaemia and central obesity are part of the insulin resistance syndrome [24]. Insulin resistance is also a risk factor for the development of diabetic complications. We further studied the added value of insulin sensitivity with the amount of fasting insulin (per kg body weight). However, this factor did not have added value in our model (p = 0.53).
Continuous predictors are best included in a model as such and not categorised [20]. In this way, all information is used for the prediction. Continuous variables do not necessarily have to be included as linear terms. We found that logarithmic transformation of HbA1c predicts the risk of microalbuminuria better than the frequently used linear values. The nature of the relation between AER and risk of microalbuminuria was linear, although a logarithmic transformation of AER is frequently used [3, 5]. Apparently, a logarithmic transformation of AER is only necessary to estimate correct values of the mean and standard deviation, given the skewed distribution of AER.
We studied the generalisability of our prediction rule with respect to place (EDC), time (FinnDiane) and time and place (CACTI). The US cohort (EDC) with patients treated in the same time period as the patients from the development set (the 1990s) showed similar discriminative ability of the prediction rule (c-statistic = 0.71 compared with 0.69 in the development set). C-statistic values around 0.7 indicate reasonable discriminative ability for prognostic models. Prediction of an event later in time (here 7 years) is more difficult than prediction of an event shortly after the baseline measurement. Furthermore, the predicted risks were reasonably in agreement with the observed proportions of microalbuminuria (Table 4).
It was particularly important to study the generalisability of the prediction rule in time, since treatment of type 1 diabetes patients with normal AER has changed. Nowadays, ACE inhibitors and statins are prescribed more frequently to lower blood pressure and lipid plasma concentrations respectively. The use of these medications has been shown to affect the transition from normo- to microalbuminuria in type 1 diabetes patients [25, 26], as was also apparent from our data. The incidence of microalbuminuria was only 7.7% in FinnDiane and 6.0% in CACTI compared with 13% in the earlier development sample. Indeed, predicted risks were too high for the patients from the FinnDiane and CACTI studies. However, the model was well able to discriminate between patients with and without microalbuminuria. Therefore, a simple recalibration step was sufficient to make the rule valid for recently treated patients.
The clinical implications of our study are that risk of microalbuminuria can be established at an early stage and patient management tailored to risk levels. We categorised the patients into four risk groups with scores 2 to 10, 11 to 15, 16 to 20, and 21 and higher. The highest two risk groups together contained 77% (110/143) of all microalbuminuric patients of the development set and 79%, 80% and 84% of all microalbuminuric patients of the validation sets. We would advocate offering these high-risk patients more frequent check-ups than once a year, perhaps together with the quarterly routine visit and with an overnight or 24 h urine collection to measure the albumin:creatinine ratio. Current surveillance protocols recommend follow-up of patients more frequently than once a year only after an abnormal surveillance result. Under this protocol, a considerable proportion of new microalbuminuria cases will be recognised later than necessary. The identification, by our prediction rule, of a group of patients with normal AER and at high risk of progressing to microalbuminuria might facilitate the early introduction of ACE inhibitor or angiotensin receptor blocker therapy, if trials currently under way suggest benefit. Glycaemic control could also be intensified and other risk factors for microalbuminuria, e.g. smoking, BMI and WHR could be more strictly controlled than is usual. It is currently unknown whether such intervention strategies based on our prediction rule would be of benefit; this should be the focus of future research.
Unfortunately, the model predicts for one occasion only, i.e. after 7 years of follow-up. The assessment of the outcome only after 7 years has two implications. First, we do not have information on time of onset of microalbuminuria. Time-to-event analysis, e.g. with Cox proportional hazards regression analysis, was therefore not possible. Second, we do not know how many patients remain microalbuminuric and how many regress. Several studies have shown regression of microalbuminuria in type 1 diabetes patients [2, 27, 28]. Cumulative incidences of regression vary between 13% and 56% and are mainly induced by intensive therapy. Only few patients with microalbuminuria undergo spontaneous regression that is permanent (around 15%) [2]. Consequently, our rule may only slightly overestimate a patient’s risk of progression to microalbuminuria.
In conclusion, we developed a prediction rule to estimate the risk of progressing to microalbuminuria in individual type 1 diabetes patients. The rule was developed in a European cohort and externally validated in two US cohorts and another European cohort. We believe that this prediction rule could be used to divide patients into different risk categories. Such risk categories could guide surveillance recommendations and ultimately improve the prevention of long-term chronic complications.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(PDF 46 kb)
Acknowledgements
We would like to thank all study participants who volunteered their time, all the staff involved in the EURODIAB PCS, EDC, FinnDiane and CACTI studies, and all investigators and consultants. EURODIAB PCS was financially supported by the Wellcome Trust, the European Community and Diabetes UK. The EDC study was supported by National Institutes of Health Grant R01 DK34818. The FinnDiane Study was supported by the Folkhälsan Research Foundation, Wilhelm and Else Stockmann Foundation, Liv och Hälsa Foundation, Signe and Ane Gyllenberg Foundation, Sigrid Juselius Foundation and the European Commission (QLG2-CT-2001-01669; LSHB-CT-2003-503364 and LSHB-CT-2006-037681). Support for the CACTI study was provided by the National Institutes of Health grants R01 HL61753 and R01 HL079611, American Diabetes Association grant 7-09-CVD-06 and Diabetes Endocrinology Research Center Clinical Investigation Core P30 DK57516. The study was performed at the Adult General Clinical Research Center at the University of Colorado Denver Anschutz Medical Center (supported by NIH grant M01 RR000051) and at the Barbara Davis Center for Childhood Diabetes. Y. Vergouwe and K. G. M. Moons were supported by the Netherlands Organization for Scientific Research Grant ZON-MW 917.46.360.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- CACTI
Coronary Artery Calcification in Type 1 Diabetes
- c-statistic
Concordance statistic
- EDC
Epidemiology of Diabetes Complications
- EURODIAB PCS
European Diabetes Prospective Complications Study
- FinnDiane
Finnish Diabetic Nephropathy study
References
- 1.Orchard TJ, Dorman JS, Maser RE, et al. Prevalence of complications in IDDM by sex and duration. Pittsburgh Epidemiology of Diabetes Complications Study II. Diabetes. 1990;39:1116–1124. doi: 10.2337/diabetes.39.9.1116. [DOI] [PubMed] [Google Scholar]
- 2.Hovind P, Tarnow L, Rossing P, et al. Predictors for the development of microalbuminuria and macroalbuminuria in patients with type 1 diabetes: inception cohort study. BMJ. 2004;328:1105. doi: 10.1136/bmj.38070.450891.FE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coonrod BA, Ellis D, Becker DJ, et al. Predictors of microalbuminuria in individuals with IDDM. Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes care. 1993;16:1376–1383. doi: 10.2337/diacare.16.10.1376. [DOI] [PubMed] [Google Scholar]
- 4.Group MCS Predictors of the development of microalbuminuria in patients with type 1 diabetes mellitus: a seven-year prospective study. Diabet Med. 1999;16:918–925. doi: 10.1046/j.1464-5491.1999.00182.x. [DOI] [PubMed] [Google Scholar]
- 5.Rossing P, Hougaard P, Parving HH. Risk factors for development of incipient and overt diabetic nephropathy in type 1 diabetic patients: a 10-year prospective observational study. Diabetes care. 2002;25:859–864. doi: 10.2337/diacare.25.5.859. [DOI] [PubMed] [Google Scholar]
- 6.Mogensen CE, Christensen CK. Predicting diabetic nephropathy in insulin-dependent patients. N Engl J Med. 1984;311:89–93. doi: 10.1056/NEJM198407123110204. [DOI] [PubMed] [Google Scholar]
- 7.Almdal T, Norgaard K, Feldt-Rasmussen B, Deckert T. The predictive value of microalbuminuria in IDDM. A five-year follow-up study. Diabetes care. 1994;17:120–125. doi: 10.2337/diacare.17.2.120. [DOI] [PubMed] [Google Scholar]
- 8.Krolewski AS, Kosinski EJ, Warram JH, et al. Magnitude and determinants of coronary artery disease in juvenile-onset, insulin-dependent diabetes mellitus. Am J Cardiol. 1987;59:750–755. doi: 10.1016/0002-9149(87)91086-1. [DOI] [PubMed] [Google Scholar]
- 9.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 10.Steyerberg EW, Eijkemans MJC, Harrell FE, Jr, Habbema JDF. Prognostic modelling with logistic regression analysis: a comparison of selection and estimation methods in small data sets. Stat Med. 2000;19:1059–1079. doi: 10.1002/(SICI)1097-0258(20000430)19:8<1059::AID-SIM412>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 11.Group EICS Microvascular and acute complications in IDDM patients. Diabetologia. 1994;37:278–285. doi: 10.1007/BF00398055. [DOI] [PubMed] [Google Scholar]
- 12.Thorn LM, Forsblom C, Fagerudd J, et al. Metabolic syndrome in type 1 diabetes: association with diabetic nephropathy and glycemic control (the FinnDiane study) Diabetes care. 2005;28:2019–2024. doi: 10.2337/diacare.28.8.2019. [DOI] [PubMed] [Google Scholar]
- 13.Dabelea D, Kinney G, Snell-Bergeon JK, et al. Effect of type 1 diabetes on the gender difference in coronary artery calcification: a role for insulin resistance? The Coronary Artery Calcification in Type 1 Diabetes (CACTI) Study. Diabetes. 2003;52:2833–2839. doi: 10.2337/diabetes.52.11.2833. [DOI] [PubMed] [Google Scholar]
- 14.Kearney EM, Mount JN, Watts GF, Slavin BM, Kind PR. Simple immunoturbidimetric method for determining urinary albumin at low concentrations using Cobas-Bio centrifugal analyser. J Clin Pathol. 1987;40:465–468. doi: 10.1136/jcp.40.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellis D, Buffone GJ. New approach to evaluation of proteinuric states. Clin Chem. 1977;23:666–670. [PubMed] [Google Scholar]
- 16.Chaturvedi N, Sjoelie AK, Porta M, et al. Markers of insulin resistance are strong risk factors for retinopathy incidence in type 1 diabetes. Diabetes Care. 2001;24:284–289. doi: 10.2337/diacare.24.2.284. [DOI] [PubMed] [Google Scholar]
- 17.Atkinson AC. A note on the generalized information criterion for choice of a model. Biometrika. 1980;67:413–418. doi: 10.1093/biomet/67.2.413. [DOI] [Google Scholar]
- 18.Efron B, Tibshirani RJ. An introduction to the bootstrap. London: Chapman and Hall; 1993. [Google Scholar]
- 19.Steyerberg EW, Eijkemans MJ, Habbema JD. Stepwise selection in small data sets: a simulation study of bias in logistic regression analysis. J Clin Epidemiol. 1999;52:935–942. doi: 10.1016/S0895-4356(99)00103-1. [DOI] [PubMed] [Google Scholar]
- 20.Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. 2006;25:127–141. doi: 10.1002/sim.2331. [DOI] [PubMed] [Google Scholar]
- 21.Harrell FE, Jr, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA. 1982;247:2543–2546. doi: 10.1001/jama.247.18.2543. [DOI] [PubMed] [Google Scholar]
- 22.Chaturvedi N, Bandinelli S, Mangili R, Penno G, Rottiers RE, Fuller JH. Microalbuminuria in type 1 diabetes: rates, risk factors and glycemic threshold. Kidney Int. 2001;60:219–227. doi: 10.1046/j.1523-1755.2001.00789.x. [DOI] [PubMed] [Google Scholar]
- 23.Kannel WB, Cupples LA, Ramaswami R, Stokes J, 3rd, Kreger BE, Higgins M. Regional obesity and risk of cardiovascular disease; the Framingham Study. J Clin Epidemiol. 1991;44:183–190. doi: 10.1016/0895-4356(91)90265-B. [DOI] [PubMed] [Google Scholar]
- 24.Williams KV, Erbey JR, Becker D, Arslanian S, Orchard TJ. Can clinical factors estimate insulin resistance in type 1 diabetes? Diabetes. 2000;49:626–632. doi: 10.2337/diabetes.49.4.626. [DOI] [PubMed] [Google Scholar]
- 25.Group TES Randomised placebo-controlled trial of lisinopril in normotensive patients with insulin-dependent diabetes and normoalbuminuria or microalbuminuria. Lancet. 1997;349:1787–1792. doi: 10.1016/S0140-6736(96)10244-0. [DOI] [PubMed] [Google Scholar]
- 26.Douglas K, O'Malley PG, Jackson JL. Meta-analysis: the effect of statins on albuminuria. Ann Intern Med. 2006;145:117–124. doi: 10.7326/0003-4819-145-2-200607180-00009. [DOI] [PubMed] [Google Scholar]
- 27.Giorgino F, Laviola L, Cavallo Perin P, Solnica B, Fuller J, Chaturvedi N. Factors associated with progression to macroalbuminuria in microalbuminuric type 1 diabetic patients: the EURODIAB Prospective Complications Study. Diabetologia. 2004;47:1020–1028. doi: 10.1007/s00125-004-1413-8. [DOI] [PubMed] [Google Scholar]
- 28.Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med. 2003;348:2285–2293. doi: 10.1056/NEJMoa021835. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 46 kb)