Healthy subjects, heavy smokers, and subjects with severe obstructive airflow limitation tolerated 3He MR imaging, with no clinically significant effects on the parameters monitored.
Abstract
Purpose:
To evaluate the safety of hyperpolarized helium 3 (3He) magnetic resonance (MR) imaging.
Materials and Methods:
Local institutional review board approval and informed consent were obtained. Physiologic monitoring data were obtained before, during, and after hyperpolarized 3He MR imaging in 100 consecutive subjects (57 men, 43 women; mean age, 52 years ± 14 [standard deviation]). The subjects inhaled 1–3 L of a gas mixture containing 300–500 mL 3He and 0–2700 mL N2 and held their breath for up to 15 seconds during MR imaging. Heart rate and rhythm and oxygen saturation of hemoglobin as measured by pulse oximetry (Spo2) were monitored continuously throughout each study. The effects of 3He MR imaging on vital signs and Spo2 and the relationship between pulmonary function, number of doses, and clinical classification (healthy volunteers, patients with asthma, heavy smokers, patients undergoing lung volume reduction surgery for severe emphysema, and patients with lung cancer) and the lowest observed Spo2 were assessed. Any subjective symptoms were noted.
Results:
Except for a small postimaging decrease in mean heart rate (from 78 beats per minute ± 13 to 73 beats per minute ± 11, P < .001), there was no effect on vital signs. A mean transient decrease in Spo2 of 4% ± 3 was observed during the first minute after gas inhalation (P < .001) in 77 subjects who inhaled a dose of 1 L for 10 seconds or less, reaching a nadir of less than 90% at least once in 20 subjects and of less than 85% in four subjects. There was no correlation between the lowest Spo2 and pulmonary function parameters other than baseline Spo2 (r = 0.36, P = .001). The lowest mean Spo2 varied by 1% between the first and second and second and third doses (P < .001) and was unrelated to clinical classification (P = .40). Minor subjective symptoms were noted by 10 subjects. No serious adverse events occurred.
Conclusion:
Hyperpolarized 3He MR imaging can be safely performed in healthy subjects, heavy smokers, and those with severe obstructive airflow limitation, although unpredictable transient desaturation suggests that potential subjects should be carefully screened for comorbidities.
© RSNA, 2008
Magnetic resonance (MR) imaging of hyperpolarized helium 3 (3He) gas in the lungs can be used to evaluate the spatial and temporal distribution of ventilation, quantitatively assess air space size, and determine regional oxygen partial pressure in the lungs (1). Investigational and potential clinical uses of 3He MR imaging have included the characterization of lung microstructure (2,3), assessment of age-related changes in air space size (4–6), early detection and quantification of emphysema (7–11), evaluation of ventilation abnormalities in patients with asthma (12,13) and cystic fibrosis (14,15), and noninvasive early detection of chronic rejection in lung transplant recipients (16,17). Although inhalation of hyperpolarized 3He gas is generally considered to be safe, it is classified as an investigational contrast agent by the U.S. Food and Drug Administration and is not approved for clinical use. Despite this factor, potential safety concerns have received limited attention (18–20). Thus, our purpose in this study was to evaluate the safety of investigational 3He MR imaging by retrospectively analyzing the monitoring data collected in all subjects who underwent the procedure at our institution during the period of 2000–2006.
MATERIALS AND METHODS
Subjects
3He MR imaging was performed under an Investigational New Drug exemption from the U.S. Food and Drug Administration (Investigational New Drug authorization no. 59, 269). Local institutional review board approval was obtained for all 3He MR imaging studies, and written informed consent specific to 3He MR imaging was obtained from all subjects. Health Insurance Portability and Accountability Act guidelines were followed. A commercial polarizer, loaned by GE Healthcare (Princeton, NJ), was used to prepare gas for some of the studies. The authors had control of the data and the information submitted for publication.
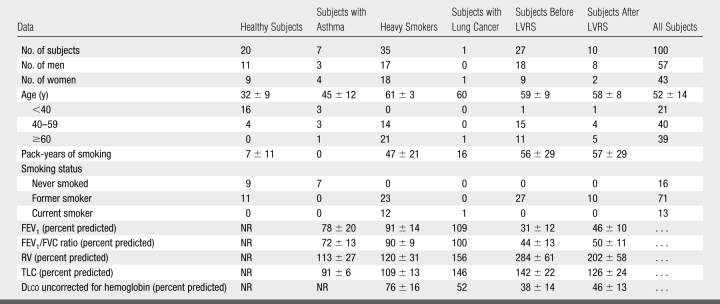
Safety monitoring data for the 100 consecutive individuals who had undergone investigational 3He MR imaging during the period 2000–2006 were retrospectively reviewed. The subjects comprised six distinct groups (Table 1): Healthy subjects were primarily members and contacts of the research team with no reported history of or symptoms of lung disease. Subjects with asthma were recruited for investigational 3He MR studies from the outpatient clinics of our institution by physicians specializing in asthma. Heavy smokers were participants in the National Lung Screening Trial (21) (http://www.cancer.gov/NLST), in which mortality rates of participants screened for lung cancer with either low-radiation-dose chest computed tomography (CT) or chest radiography are compared. The heavy smokers in our study had expressed interest in volunteering for a 3He MR imaging investigation unrelated to the National Lung Screening Trial at the time of their last of three annual screening CT examinations and had no coronary artery calcifications or CT findings requiring further evaluation. Requirements for National Lung Screening Trial enrollment included a smoking history of at least 30 pack-years and age of 55–74 years. Lung volume reduction surgery (LVRS) patients underwent investigational 3He MR imaging during their evaluation for LVRS for advanced emphysema. Some LVRS subjects also returned for 3He MR imaging at the time of their 6- or 12-month postoperative clinical evaluation. One patient with lung cancer underwent investigational 3He MR imaging before undergoing upper lobectomy of the right lung.
Table 1.
Clinical Data in Subjects
Note.—Values for nominal variables are frequencies; values for continuous variables are given as the mean ± standard deviation. Dlco = diffusing capacity of the lung for carbon monoxide, FEV1 = forced expiratory volume in 1 second, FVC = forced vital capacity, NR = not recorded, RV = residual volume, TLC = total lung capacity.
Most subjects had normal pulmonary function test results, but approximately one-third (the LVRS subjects) had advanced obstructive lung disease (Table 1). Twelve subjects, all belonging to the LVRS group, were receiving ambulatory supplemental oxygen. Because of the potential for transient hypoxia from inhalation of anoxic gases, exclusion criteria included persons who were clinically known to have or who were suspected of having cardiac or cerebrovascular disease and anemia and those who were known to be or who were suspected of being pregnant.
Hyperpolarized 3He MR Imaging Procedures
The 3He (Spectra Gases, Branchburg, NJ) was hyperpolarized with spin-exchange optical pumping (22) by using a custom-built laser apparatus or the commercial polarizer. MR imaging was conducted with the patient in the supine position by using a 1.5-T unit (Magnetom Vision or Sonata; Siemens, Erlangen, Germany) with custom-built radiofrequency coils positioned against the anterior and posterior chest wall. Conventional proton T1-weighted gradient-echo scout MR images were obtained first, while subjects held their breath with room air for the same period as or longer than the subsequent 3He MR imaging. For 3He imaging, a mouthpiece attached to a flexible ventilator tube (length, 30.5 cm; inner diameter, 2.54 cm; approximate volume, 150 mL) was inserted into the subject's mouth. Nose clips were applied to prevent mixing of room air oxygen with the hyperpolarized 3He gas, which would reduce the polarization and decrease image quality, during inhalation (23).
Before inhaling each dose, subjects exhaled to approximate functional residual capacity. A sealed polyethylene bag containing approximately 300–500 mL 3He at 20%–40% polarization and 0–2700 mL N2 was then connected to the flexible tube through which the subject inhaled. The 23 subjects studied through July 2001 inhaled to approximate total lung capacity and were administered up to 3 L of gas. For the next 77 subjects, studied from August 2001 through the end of 2006, a standard inhaled dose of 1 L (300–500 mL 3He) was adopted. Imaging commenced with the start of the breath hold, which was limited to 15 seconds or less. Subjects who required supplemental oxygen at rest breathed room air immediately before inhaling the 3He-N2 mixture, and supplemental O2 delivery was resumed after the 3He-N2 mixture was exhaled.
Subject Monitoring
The physiologic parameters measured and monitored were selected to help characterize baseline lung function, identify any effects of the overall 3He MR imaging procedure on basic cardiopulmonary vital signs, and assess the risks specific to inhalation of the anoxic gas mixture. Because helium is an inert simple asphyxiant with negligible solubility in water (24) and fat (25) and is used as a diluent for oxygen in deep sea diving and in the treatment of selected patients with upper airway obstruction and severe pulmonary disease (26), biochemical effects on blood chemistry and urinalysis were not expected or assessed.
Pre- and Postimaging Assessment
Pulmonary function test data were obtained before imaging in all subjects except those from the healthy group. As a surrogate functional assessment, the healthy subjects underwent a 6-minute walk test (27) before imaging to identify anyone with relatively advanced but undiagnosed lung disease who may have been unknowingly susceptible to desaturation. None were identified. Before placing the subject in the MR unit, the monitoring physician measured the subject's heart rate, blood pressure, respiratory rate, and oxygen saturation of hemoglobin as measured by pulse oximetry (Spo2) and reviewed a lead II electrocardiographic rhythm strip. These parameters were reassessed immediately after the imaging session was completed, and any symptoms were addressed. All subjects were contacted by telephone the following day (no later than 24 hours later) and asked to report any unusual symptoms or adverse events.
Subject Monitoring during Imaging
While the subject was in the imaging unit, a monitoring device (3150 MRI Physiologic Monitor; Invivo Research, Orlando, Fla) continuously displayed the subject's heart rate, lead II electrocardiographic rhythm strip, and Spo2 for the physician monitoring the study. When the patient inhaled each dose of the 3He-N2 mixture, the monitoring physician recorded the nadir Spo2 and the elapsed time after the commencement of the breath hold at which the nadir occurred (desaturation time). The elapsed time from the commencement of the breath hold until the return of the Spo2 back to baseline (recovery time) also was recorded.
Statistical Analysis
Values are presented as the mean ± standard deviation. Pre- and postimaging physiologic parameters were compared by using two-tailed paired t tests. The relationship between the lowest Spo2 observed after each subject's first dose to multiple physiologic parameters including baseline Spo2, spirometry, lung volumes, and diffusing capacity of lung for carbon monoxide was evaluated with the Pearson product moment correlation. Differences in lowest Spo2 for the first inhaled dose among the six investigational groups were tested for significance with analysis of variance. The effect of the first three doses was tested with repeated-measures analysis of variance. Differences with a P value of less than .05 were considered significant.
RESULTS
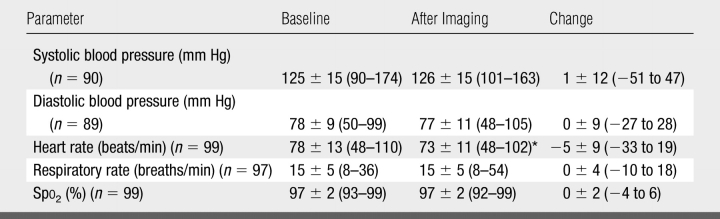
No change in Spo2 was seen from breath holding with room air during proton MR scout imaging. When data from all 100 subjects were combined, a mean decrease in heart rate of 5 beats per minute was found at the postimaging assessment compared with the preimaging assessment (P < .001) (Table 2). No change in heart rate, however, was seen in the healthy subject group. There was no change in mean blood pressure, respiratory rate, electrocardiographic rhythm, or Spo2 in any of the groups at the postimaging assessment.
Table 2.
Vital Signs and Values from Pulse Oximetry at Baseline and after Imaging in 100 Subjects
Note.—Data are the mean ± standard deviation. Numbers in parentheses are the ranges. Measurements of some parameters were not obtained in some subjects; those in whom both baseline and postimaging measurements were obtained are shown.
* P < .001 compared with baseline (two-tailed paired t test).
All subjects but one received more than one dose of the 3He-N2 mixture (mean, 2.92 doses per subject ± 0.69; range, 1–6 doses). Of the 292 total doses, 216 (73.9%) were administered at a volume of 1 L for a breath-hold time of 10 seconds or less (range, 5–10 seconds), 31 (10.6%) were administered at a volume of more than 1 L (maximum, 3 L) for 10 seconds or less (range, 7–10 seconds), seven (2.4%) were administered at a volume of 1 L or less (minimum, 0.4 L) for more than 10 seconds (range, 11–15 seconds), and seven (2.4%) were administered at a volume of more than 1 L (maximum, 3 L) for more than 10 seconds (range, 11–15 seconds). For the remaining 11 doses (3.8%), imaging was performed continuously during inhalation and exhalation, without breath holding. Dose data were incomplete for 20 (6.9%) doses in 16 subjects.
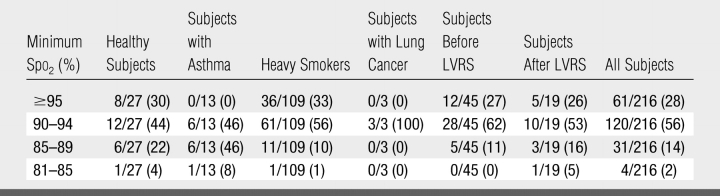
A total of 77 subjects inhaled the 216 doses of 1 L for 10 seconds or less. The nadir Spo2 remained at least 90% for 84% of these 216 doses (Table 3) and for all doses in 57 (74%) of these 77 subjects. In the other 20 subjects (26%), the nadir Spo2 decreased to less than 90% for at least one dose. The nadir was less than 85% in four (5%) of the 77 subjects and was as low as 81% in one subject, 83% in two subjects, and 84% in one subject and was less than 90% for all doses in four subjects.
Table 3.
Frequency of Desaturation Levels with 216 Doses of Inhaled Gas Volume of 1 L and a Breath Hold of 10 Seconds or Less in 77 Subjects
Note.—Values are fraction of doses associated with each minimum Spo2 range. Numbers in parentheses are percentages.
For the 77 subjects who inhaled a 1-L dose for 10 seconds or less, the nadir Spo2 after the first dose correlated only weakly with the baseline Spo2 (r = 0.36, P = .001). For 67 of these subjects who underwent pulmonary function testing (four with asthma, 35 heavy smokers, 18 before LVRS, nine after LVRS, and one with lung cancer), the correlations with the first-dose nadir Spo2 were low (r = −0.21 to 0.15, P = .1–.91) for all parameters tested, which included the percent predicted values for forced expiratory volume in one second, forced vital capacity, ratio of forced expiratory volume in 1 second to forced vital capacity, residual volume, total lung capacity, ratio of residual volume to total lung capacity, diffusing capacity of the lung for carbon monoxide, alveolar volume, and diffusing capacity of the lung for carbon monoxide corrected for alveolar volume. No significant difference in the first-dose nadir Spo2 of the six different investigational groups was seen (P = .4, analysis of variance).
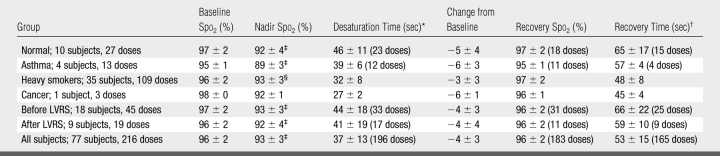
Transient desaturation from baseline by an absolute amount of 3% or greater during the 1st minute after 3He-N2 inhalation was observed in 130 (60%) of 216 1-L doses administered for a breath hold of 10 seconds or less, with a mean transient decrease in Spo2 of 4% (P < .001) (Table 4). A significant difference (P < .001) in nadir Spo2 was found for the different doses (first dose vs second dose vs third dose), but the differences between the mean nadir Spo2 for each dose were minimal (93% vs 92% vs 93%, respectively). The changes in Spo2 were similar with the other combinations of dose and breath-hold time. For example, the mean nadir for the seven subjects (four healthy subjects and three before LVRS) who inhaled more than 1 L for more than 10 seconds was 95% (range, 87%–98%); one of these subjects had desaturation to 87%, whereas the level in the remaining six subjects remained at more than 92%.
Table 4.
Baseline and Nadir Spo2 for Inhaled Gas Volume of 1 L and Breath Hold of 10 Seconds or Less
Note.—Values are the mean ± standard deviation. Where complete data were not available for each dose, the number of doses is shown in parentheses.
* Desaturation time was the time elapsed from the start of imaging to the nadir Spo2.
† Recovery time was the time elapsed from the start of imaging until the return of Spo2 to the baseline level.
‡ P < .001, compared with baseline Spo2 (two-tailed paired t test).
§ P < .05, compared with baseline Spo2 (two-tailed paired t test).
When transient desaturation occurred, a consistent temporal pattern was seen. The decrease in Spo2 began approximately 20–30 seconds after initiation of the breath hold, after the subjects had resumed breathing room air (or their supplemental O2). The Spo2 decrease continued for approximately 10–15 seconds until it reached its nadir, where it remained for no more than 10 seconds. The Spo2 then returned to the preinhalation baseline values after about 10–15 more seconds.
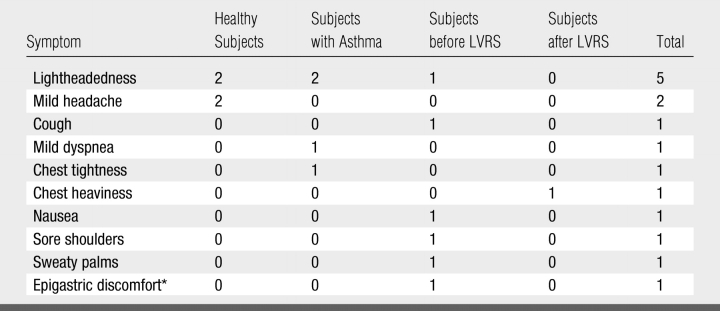
Transient subjective symptoms were reported by 10 (10%) subjects (Table 5), nine before discharge and one during telephone follow-up. All symptoms resolved during or immediately after the imaging sessions. No medical intervention was required, although resolution of mild dyspnea, chest tightness, and lightheadedness that developed after the first 3He dose in a subject with asthma followed inhalation of albuterol by means of a metered-dose inhaler, administered as part of a protocol in which pre- and postbronchodilator imaging was performed; asthma symptoms in this subject were known to be induced by stress, cold, and allergens.
Table 5.
Summary of Symptoms That Occurred during or after 3He MR Imaging
Note.—Symptoms were reported by 10 subjects; three subjects reported more than one symptom.
* Reported at telephone follow-up on the following day.
DISCUSSION
Since initial reports in 1996 (28,29), hyperpolarized 3He MR imaging has been performed in more than 1000 human subjects (30). Although subject characteristics and study protocols varied, the inhaled gas compositions, volumes, and breath-hold times used have been similar. To our knowledge, the only serious adverse event reported was an uncontrolled cough necessitating treatment in a patient with atypical asthma characterized by severe, chronic cough (18). However, objective and ongoing evaluation of the theoretic and known risks is important, not only to protect the safety of subjects but also to guide investigators and institutional review boards in assessing the safety of research protocols involving 3He inhalation.
Both normal subjects and subjects with severe obstructive airflow limitation tolerated 3He MR imaging, with no clinically important effects on the parameters monitored. It is unlikely that the small decrease in mean heart rate (5 beats per minute) observed after the imaging session is attributable to inhalation of the 3He-N2 mixture. The factors most likely to account for this postimaging decrease are the effects of lying for nearly 1 hour at rest in the imaging unit and, possibly, relief of apprehension about imaging. The latter possibility is supported by the observation that the subjects in the healthy group, who were most familiar with the study personnel and procedures, did not have a decrease in heart rate.
Our analysis documents the frequency and degree of transient hypoxemia with 3He MR imaging in both healthy subjects and those with obstructive airflow limitation. The nadir Spo2 was only weakly correlated to the baseline Spo2 and did not correlate significantly with pulmonary function parameters. These findings indicate that the results of pulmonary function tests cannot help predict the degree of transient desaturation or whether the Spo2 will decrease with subsequent doses or in other subjects with similar clinical characteristics. It is possible that physiologic factors such as differences in the basal metabolic rate, unventilated dead space, or ventilation-perfusion mismatch contributed to some of the variability in the degree of desaturation. Regardless of the mechanism, the observation that Spo2 may decrease to as low as 80%–85% with relatively small inhaled volumes and short breath-hold times emphasizes that transient hypoxemia is a risk that investigators should be aware of and attempt to minimize. Thus, monitoring of Spo2 during 3He MR studies seems prudent.
Temporal changes in Spo2 during 3He MR imaging similar to the changes in our study have been reported in a study of five subjects who had undergone single-lung transplantation for pulmonary fibrosis (31) and in a study of 18 children with cystic fibrosis (32). Other studies (8–20 subjects in each) have reported observing a decrease in Spo2 of 5% or less (33,34), a decrease no greater than 10% (12), and a nadir less than 90% for no more than 10 seconds (7), without specifying details. Reasons for any differences from our experience are uncertain but may be due to differences in protocols and subject populations studied. We note that other studies have shown that breath holding with smaller lung volumes or lower initial alveolar Po2 results in a faster decrease in alveolar Po2 (35,36). Thus, we expect that larger inhaled volumes of anoxic gas, smaller lung volumes during the breath hold, and longer breath-hold periods would result in greater desaturation. The number of observations with the nonstandard inhaled volumes and breath-hold periods used in our cohort, however, was too small to determine these effects.
Transitory and mild adverse events were noted by 10% of our subjects, and this finding was similar to the experience of other investigators (18,37). Some of these symptoms, such as sweaty palms, shoulder soreness, and epigastric discomfort, were probably unrelated to 3He-N2 inhalation. Other symptoms conceivably could have been induced by the 3He-N2 inhalation or by anxiety from participating in a research study. Although no serious adverse events requiring physician intervention were encountered, it is possible that albuterol administration would have been needed to relieve the symptoms of mild dyspnea, chest tightness, and lightheadedness that occurred in the subject with asthma had it not been administered for postbronchodilator imaging as planned.
Several limitations of this study should be recognized. Because subjects were excluded from 3He MR imaging for conditions in which even transient desaturation may be medically inadvisable, safety in those with known cardiovascular or cerebrovascular disease or anemia could not be evaluated. Because only those physiologic parameters considered clinically relevant were monitored and analyzed, there could be risks of which we are unaware or of such low frequency that exposure of much larger numbers of subjects would be required for their identification. Finally, the effects of the procedure on subjects with other pulmonary diseases, such as interstitial lung disease in which gas exchange is impaired, were not evaluated and may be different.
In summary, our data support the safety of hyperpolarized 3He MR imaging. Healthy subjects, heavy smokers, and subjects with severe obstructive airflow limitation tolerated 3He MR imaging, with no clinically significant effects on the parameters monitored. However, desaturation to the 80%–90% range may briefly occur in a minority of both healthy subjects and subjects with disease with use of typical inhalation and imaging protocols. Because most subjects develop transient decreases in Spo2 after inhalation of the 3He-N2 mixture, screening for relevant medical comorbidities, minimizing the breath-hold time and inhaled gas volume, and monitoring Spo2 levels are advisable. Methods with inhalation of room air after that of pure 3He gas have been reported to provide an acceptable signal-to-noise ratio (2) and may attenuate oxyhemoglobin desaturation (20), but the full effect of this factor on Spo2 levels, 3He gas distribution, and diffusivity measurements remains to be determined.
Advances in Knowledge.
There were no clinically important effects of 3He MR imaging on vital signs.
A mean transient oxyhemoglobin desaturation of 4% occurred when 1-L doses were inhaled for 10 seconds or less, with a nadir of less than 90% saturation after at least one dose in 26% of subjects and a nadir of 81%–84% after at least one dose in 5% of subjects.
Baseline oxyhemoglobin saturation, pulmonary function tests, clinical status, or number of doses administered do not appear to be of practical value in predicting individual desaturation.
Implications for Patient Care.
Potential subjects for 3He MR imaging should be carefully screened for conditions in which even transient oxyhemoglobin desaturation would be inadvisable.
Oxygen saturation of hemoglobin as measured by pulse oximetry should be monitored during 3He MR imaging.
Received October 21, 2007; revision requested January 10, 2008; revision received January 24; accepted February 27; final version accepted March 4.
Funding: This work was supported by the National Institutes of Health (grants R01-HL72369 and R01-HL70037).
See Materials and Methods for pertinent disclosures.
J.D.C. receives royalties from Synovis, is a paid consultant for Emphasys Medical and Broncus Technologies, and owns Broncus stock. Supported by National Institutes of Health grants R01 HL72369 and R01 HL70037, and contract N01 CN25516.
Abbreviations:
- LVRS
- lung volume reduction surgery
- Spo2
- oxygen saturation of hemoglobin as measured by pulse oximetry
References
- 1.Hopkins SR, Levin DL, Emami K, et al. Advances in magnetic resonance imaging of lung physiology. J Appl Physiol 2007;102:1244–1254 [DOI] [PubMed] [Google Scholar]
- 2.Schreiber WG, Morbach AE, Stavngaard T, et al. Assessment of lung microstructure with magnetic resonance imaging of hyperpolarized helium-3. Respir Physiol Neurobiol 2005;148:23–42 [DOI] [PubMed] [Google Scholar]
- 3.Yablonskiy DA, Sukstanskii AL, Leawoods JC, et al. Quantitative in vivo assessment of lung microstructure at the alveolar level with hyperpolarized 3He diffusion MRI. Proc Natl Acad Sci U S A 2002;99:3111–3116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altes TA, Mata J, de Lange EE, Brookeman JR, Mugler JP 3rd.Assessment of lung development using hyperpolarized helium-3 diffusion MR imaging. J Magn Reson Imaging 2006;24:1277–1283 [DOI] [PubMed] [Google Scholar]
- 5.Fain SB, Altes TA, Panth SR, et al. Detection of age-dependent changes in healthy adult lungs with diffusion-weighted 3He MRI. Acad Radiol 2005;12:1385–1393 [DOI] [PubMed] [Google Scholar]
- 6.Waters B, Owers-Bradley J, Silverman M.Acinar structure in symptom-free adults by helium-3 magnetic resonance. Am J Respir Crit Care Med 2006;173:847–851 [DOI] [PubMed] [Google Scholar]
- 7.Fain SB, Panth SR, Evans MD, et al. Early emphysematous changes in asymptomatic smokers: detection with 3He MR imaging. Radiology 2006;239:875–883 [DOI] [PubMed] [Google Scholar]
- 8.Saam BT, Yablonskiy DA, Kodibagkar VD, et al. MR imaging of diffusion of (3)He gas in healthy and diseased lungs. Magn Reson Med 2000;44:174–179 [DOI] [PubMed] [Google Scholar]
- 9.Salerno M, de Lange EE, Altes TA, Truwit JD, Brookeman JR, Mugler JP 3rd.Emphysema: hyperpolarized helium 3 diffusion MR imaging of the lungs compared with spirometric indexes—initial experience. Radiology 2002;222:252–260 [DOI] [PubMed] [Google Scholar]
- 10.Swift AJ, Wild JM, Fichele S, et al. Emphysematous changes and normal variation in smokers and COPD patients using diffusion 3He MRI. Eur J Radiol 2005;54:352–358 [DOI] [PubMed] [Google Scholar]
- 11.Woods JC, Choong CK, Yablonskiy DA, et al. Hyperpolarized 3He diffusion MRI and histology in pulmonary emphysema. Magn Reson Med 2006;56:1293–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altes TA, Sureda A.Hyperpolarized 3He MR lung ventilation imaging in asthmatics: preliminary findings. J Magn Reson Imaging 2001;13:378–384 [DOI] [PubMed] [Google Scholar]
- 13.de Lange EE, Altes TA, Patrie JT, et al. Evaluation of asthma with hyperpolarized helium-3 MRI: correlation with clinical severity and spirometry. Chest 2006;130:1055–1062 [DOI] [PubMed] [Google Scholar]
- 14.Koumellis P, van Beek EJ, Woodhouse N, et al. Quantitative analysis of regional airways obstruction using dynamic hyperpolarized 3He MRI: preliminary results in children with cystic fibrosis. J Magn Reson Imaging 2005;22:420–426 [DOI] [PubMed] [Google Scholar]
- 15.Mentore K, Froh DK, de Lange EE, Brookeman JR, Paget-Brown AO, Altes TA.Hyperpolarized HHe 3 MRI of the lung in cystic fibrosis: assessment at baseline and after bronchodilator and airway clearance treatment. Acad Radiol 2005;12:1423–1429 [DOI] [PubMed] [Google Scholar]
- 16.Gast KK, Viallon M, Eberle B, et al. MRI in lung transplant recipients using hyperpolarized 3He: comparison with CT. J Magn Reson Imaging 2002;15:268–274 [DOI] [PubMed] [Google Scholar]
- 17.McAdams HP, Palmer SM, Donnelly LF, Charles HC, Tapson VF, MacFall JR.Hyperpolarized 3He-enhanced MR imaging of lung transplant recipients: preliminary results. AJR Am J Roentgenol 1999;173:955–959 [DOI] [PubMed] [Google Scholar]
- 18.Altes TA, Gersbach JC, Mata JF, Mugler JP, 3rd, Brookeman JR, De Lange EE.Evaluation of the safety of hyperpolarized helium-3 gas as an inhaled contrast agent for MRI [abstr]. In: Proceedings of the Fifteenth Meeting of the International Society for Magnetic Resonance in Medicine. Berkeley, Calif: International Society for Magnetic Resonance in Medicine, 2007; 1305 [Google Scholar]
- 19.De Lange EE, Altes TA, Wright CM, Mata JF, Harding DA, Harrell FE, 525 Hyperpolarized gas MR imaging of the lung: safety assessment of inhaled helium-3 [abstr]. In: Radiological Society of North America Scientific Assembly and Annual Meeting Program. Oak Brook, Ill: Radiological Society of North America, 2003. . [Google Scholar]
- 20.Woodhouse N, Wild JM, Mills GH, Fleming S, Fichele S, Van Beek EJ.Comparison of hyperpolarized 3helium administration methods in healthy and diseased subjects [abstr]. In: Proceedings of the Fourteenth Meeting of the International Society for Magnetic Resonance in Medicine. Berkeley, Calif: International Society for Magnetic Resonance in Medicine, 2006; 1288 [Google Scholar]
- 21.Church TR.Chest radiography as the comparison for spiral CT in the National Lung Screening Trial. Acad Radiol 2003;10:713–715 [DOI] [PubMed] [Google Scholar]
- 22.Leawoods JC, Yablonskiy DA, Saam B, Gierada DS, Conradi MS.Hyperpolarized 3He gas production and MR imaging of the lung. Concepts Magn Reson 2001;13:277–293 [Google Scholar]
- 23.Saam B, Happer W, Middleton H.Nuclear relaxation of 3He in the presence of O2. Phys Rev A 1995;52:862–865 [DOI] [PubMed] [Google Scholar]
- 24.Braker W, Mossman AL, eds. Gas data book Lyndhurst, NJ: Matheson, 1980. [Google Scholar]
- 25.Dawe RA, Miller KW, Smith EB.Solubility relations of fluorine compounds and inert gas narcosis [letter]. Nature 1964;204:789 14235681 [Google Scholar]
- 26.Gainnier M, Forel JM.Clinical review: use of helium-oxygen in critically ill patients. Crit Care 2006;10:241–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111–117 [DOI] [PubMed] [Google Scholar]
- 28.Ebert M, Grossmann T, Heil W, et al. Nuclear magnetic resonance imaging with hyperpolarised helium-3. Lancet 1996;347:1297–1299 [DOI] [PubMed] [Google Scholar]
- 29.MacFall JR, Charles HC, Black RD, et al. Human lung air spaces: potential for MR imaging with hyperpolarized He-3. Radiology 1996;200:553–558 [DOI] [PubMed] [Google Scholar]
- 30.van Beek EJ, Wild JM.Hyperpolarized 3-helium magnetic resonance imaging to probe lung function. Proc Am Thorac Soc 2005;2:528–532, 510 [DOI] [PubMed] [Google Scholar]
- 31.Markstaller K, Kauczor HU, Puderbach M, et al. 3He-MRI-based vs. conventional determination of lung volumes in patients after unilateral lung transplantation: a new approach to regional spirometry. Acta Anaesthesiol Scand 2002;46:845–852 [DOI] [PubMed] [Google Scholar]
- 32.van Beek EJ, Hill C, Woodhouse N, et al. Assessment of lung disease in children with cystic fibrosis using hyperpolarized 3-helium MRI: comparison with Shwachman score, Chrispin-Norman score and spirometry. Eur Radiol 2007;17:1018–1024 [DOI] [PubMed] [Google Scholar]
- 33.de Lange EE, Mugler JP 3rd, Brookeman JR, et al. Lung air spaces: MR imaging evaluation with hyperpolarized 3He gas. Radiology 1999;210:851–857 [DOI] [PubMed] [Google Scholar]
- 34.McMahon CJ, Dodd JD, Hill C, et al. Hyperpolarized 3helium magnetic resonance ventilation imaging of the lung in cystic fibrosis: comparison with high resolution CT and spirometry. Eur Radiol 2006;16:2483–2490 [DOI] [PubMed] [Google Scholar]
- 35.Findley LJ, Ries AL, Tisi GM, Wagner PD.Hypoxemia during apnea in normal subjects: mechanisms and impact of lung volume. J Appl Physiol 1983;55:1777–1783 [DOI] [PubMed] [Google Scholar]
- 36.Kryger MH.Respiratory failure 1: oxygen In: Kryger MH, ed. Introduction to respiratory medicine. 2nd ed. New York, NY: Churchill Livingstone, 1990; 169–205 [Google Scholar]
- 37.Fain SB, Korosec FR, Holmes JH, O'Halloran R, Sorkness RL, Grist TM.Functional lung imaging using hyperpolarized gas MRI. J Magn Reson Imaging 2007;25:910–923 [DOI] [PubMed] [Google Scholar]