Abstract
A novel toxin, NetB, has recently been identified in virulent avian Clostridium perfringens isolates and shown to be an essential virulence factor in a clinical necrotic enteritis isolate. To assess whether NetB is more generally associated with avian necrotic enteritis isolates we have screened a range of C. perfringens strains from geographically diverse locations for both the presence and expression of the netB gene. Forty-four isolates were derived from necrotic enteritis disease cases from Australia, Belgium, Denmark and Canada and 55 isolates from healthy chickens from Australia and Belgium. The majority of strains isolated from necrotic enteritis-affected birds were netB positive (70%) and there was an absolute correlation between the presence of netB and in vitro expression of the NetB protein. Only two of the C. perfringens isolates from healthy chickens carried netB. Sequencing of the netB gene from 23 positive isolates showed that NetB is highly conserved, with only one predicted amino acid (A168T) difference, in six isolates, compared to the published sequence. This change did not alter the in vitro activity of the NetB toxin. The gene encoding the recently discovered TpeL toxin was also screened using PCR and only found in a small proportion of NetB-positive isolates from diseased birds. A selection of NetB-negative isolates, originating from diseased birds, was unable to cause disease in a necrotic enteritis induction model. This study provides further evidence that NetB is important in pathogenesis and advances our current understanding of C. perfringens virulence factors in avian necrotic enteritis.
Keywords: Clostridium perfringens, necrotic enteritis, NetB toxin, virulence factor, TpeL
1. INTRODUCTION
Necrotic enteritis in chickens is a common bacterial disease in the poultry industry and is caused by an increase in the population of pathogenic Clostridium perfringens in the chicken’s gastrointestinal tract [4, 5]. C. perfringens isolates are categorized into five toxin types: A, B, C, D and E, depending on the production of four major toxins: α, β, ε and ι toxins [18]. Type A strains produce α-toxin, but do not produce any of the other three typing toxins (β, ε, ι). In addition, all strains may produce other toxins such as enterotoxin (CPE) and β2-toxin. Type A strains of C. perfringens are the most common toxin type isolated from chickens suffering from necrotic enteritis [20, 22]. It was previously thought [1, 2, 9] that α-toxin was the major toxin involved in necrotic enteritis but we have shown that a α-toxin null mutant is still virulent [11].
Recently, we identified a new toxin, NetB, in avian C. perfringens type A strains. Initial studies indicated that NetB is only associated with strains isolated from chickens [12], although a recent study reported the recovery of a netB-positive C. perfringens isolate from a cow with liver abscesses [14]. NetB has limited sequence identity to the β-toxin from C. perfringens, which is associated with gastrointestinal syndromes in both humans and animals [23]. The importance of NetB in avian necrotic enteritis was demonstrated when a netB mutant did not cause necrotic enteritis in an experimental chicken model and virulence was restored when a functional netB gene was introduced back into the mutant strain [12]. This study demonstrated that NetB is an essential virulence factor, at least in one avian isolate.
Recent strain surveys in North America have found that the majority of C. perfringens isolates from chickens with clinical signs of necrotic enteritis carry netB whereas only a small percentage of isolates from healthy birds carry the gene [4, 14]. No surveys have yet investigated whether netB in each strain are functional and are expressed. This is an important issue since there is precedent for toxin genes being present but not expressed in C. perfringens [6].
Although we have clearly demonstrated that NetB is an essential virulence factor in strain EHE-NE18 [12], several studies have found that a minority of C. perfringens strains isolated from clear cases of necrotic enteritis do not carry netB [3, 12, 14]. The question therefore arises as to whether these netB-negative strains produce an alternative toxin that is responsible for necrotic enteritis. Another toxin, TpeL, has recently been identified in some chicken derived C. perfringens strains, although only in some netB-positive isolates [4].
In the work reported here the carriage of netB and tpeL and the production of NetB toxin have been assessed in geographically diverse C. perfringens necrotic enteritis isolates from Belgium, Denmark, Australia, and Canada, as well as isolates from healthy birds. In addition, we sequenced netB from many of these isolates to determine its sequence variation. Finally, we carried out virulence studies in chickens on netB-negative and netB-positive strains derived from diseased chickens. The results provide further evidence that NetB is important in the pathogenesis of avian necrotic enteritis.
2. MATERIALS AND METHODS
2.1. Bacterial isolates and growth conditions
The C. perfringens strains used in this study were isolated from chickens displaying clinical signs of necrotic enteritis (Tab. I) and from healthy chickens. C. perfringens were grown in tryptone-proteose peptone glucose (TPG) [13], fluid thioglycollate (FTG) (Becton, Dickinson and Company, NJ, USA) or TSC agar (Oxoid, Hampshire, UK). Agar cultures were grown at 37 °C in an atmosphere containing 10% H2, 10% CO2 and 80% N2.
Table I.
Necrotic enteritis C . perfringens strains and PCR, Western blotting and animal trial results.
| Strain | Sourcea | netBb | NetBc | netB sequenced | % disease inductione | Reference |
|---|---|---|---|---|---|---|
| NAG-NE1 | Aus | − | − | 0 | [19] | |
| EHE-NE3 | Aus | + | + | Con | [19] | |
| EHE-NE4 | Aus | + | + | Con | [19] | |
| EHE-NE5 | Aus | + | + | Con | 47 | [19] |
| EHE-NE7 | Aus | + | + | G769A (A168T) | [19] | |
| EHE-NE9 | Aus | + | + | G769A (A168T) | [19] | |
| EHE-NE13 | Aus | + | + | Con | 87 | [19] |
| EHE-NE14 | Aus | + | + | Con | [19] | |
| EUR-NE15 | Aus | + | + | Con | 75 | [19] |
| EHE-NE16 | Aus | + | + | Con | [19] | |
| EHE-NE17 | Aus | + | + | Con | [19] | |
| EHE-NE18 | Aus | + | + | Con | 100 | [19] |
| EHE-NE20 | Aus | + | + | Con | [11] | |
| EHE-NE21 | Aus | + | + | Con | [11] | |
| EHE-NE22 | Aus | + | + | Con | [11] | |
| NAG-NE23 | Aus | − | − | [19] | ||
| NAG-NE24 | Aus | − | − | 0 | [19] | |
| NAG-NE25 | Aus | − | − | 11 | [19] | |
| UNK-NE30 | Aus | + | NA | 154 | This study | |
| NAG-NE31f | Aus | + | NA | 68 | This study | |
| NAG-NE32f | Aus | + | NA | This study | ||
| BER-NE33 | Aus | − | 0 | This study | ||
| SOM-NE34 | Aus | + | NA | 46 | This study | |
| SOM-NE35 | Aus | + | NA | 77 | This study | |
| GrE ABAT | Can | + | Con | 77 | M. Boulianne, pers. com. | |
| 3 MB 2003 | Can | + | G769A (A168T) | M. Boulianne, pers. com. | ||
| 6 MB 2006 | Can | + | Con | 77 | M. Boulianne, pers. com. | |
| PF3 | Can | − | 0 | M. Boulianne, pers. com. | ||
| SHY07 383 | Can | − | M. Boulianne, pers. com. | |||
| ENV D4 | Can | − | M. Boulianne, pers. com. | |||
| R04-134T | Can | − | M. Boulianne, pers. com. | |||
| R04-104 | Can | − | M. Boulianne, pers. com. | |||
| R03-382 | Can | + | NA | M. Boulianne, pers. com. | ||
| 48 | Bel | − | − | 0 | [10] | |
| 67 | Bel | + | + | Con | [10] | |
| 56 | Bel | + | + | Con | 80 | [10] |
| 37 | Bel | + | + | G769A (A168T) | [10] | |
| 200302-1-1-Ba | Den | + | + | Con | L. Bjerrum, pers. com. | |
| 99.63206-34f | Den | + | + | G769A (A168T) | L. Bjerrum, pers. com. | |
| 97.78247-2 | Den | − | − | 0 | L. Bjerrum, pers. com. | |
| 75.65948-1 | Den | + | + | Con | L. Bjerrum, pers. com. | |
| 00.82196-2 | Den | − | − | L. Bjerrum, pers. com. | ||
| 98.78718-2 | Den | + | + | NA | L. Bjerrum, pers. com. | |
| 301001-1-B1f | Den | + | + | G769A (A168T) | L. Bjerrum, pers. com. |
Aus, Australia; Can, Canada; Bel, Belgium; Den, Denmark.
PCR analysis of C. perfringens strains, + indicates netB positive product of 383 bp was amplified, − indicates no amplification product.
Western blot results probing with rNetB anti-serum, + indicates product of 33 kDa, − indicates no product.
C. perfringens netB sequence, NA indicates the netB sequence was not determined, Con indicates EHE-NE18 consensus sequence (EU143239), G769A (A168T) indicates nucleotide change resulting in amino acid change. GenBank accession numbers for netB sequences are FJ189481-FJ189503.
Results of strain use in disease induction model. The results are derived from a series of independent trials. To allow a simple comparison of all the results, the average lesion score for each group was compared to the lesion score for EHE-NE18 challenge in the same trial (included in all trials as our standard challenge strain).
Indicates C. perfringens strains that are tpeL positive with a product size of 474 bp.
Indicates C. perfringens isolate from diseased turkey.
2.2. Detection of the netB and tpeL genes in various strains
The presence of netB and tpeL in C. perfringens strains was investigated by PCR. Clostridial genomic DNA was isolated as previously reported [17]. PCR amplification of the genomic DNA used Taq DNA polymerase (Roche) and 0.5 μM concentration of each primer. Denaturation (94 °C for 30 s), annealing (55 °C for 30 s), and extension (72 °C for 1 min) steps were performed for 35 cycles. Internal primer pairs AKP78 (5′GCTGGTGCTGGAATAAATGC3′) and AKP79 (5′TCGCCATTGAGTAGTTTCCC3′) and AKP80 (5′ATATAGAGTCAAGCAGTGGAG3′) and AKP81 (5′GGAATACCACTTGATATACCTG3′) were used to screen the C. perfringens strains for the presence of netB and tpeL, respectively. For sequencing of netB, the PCR and sequencing primers were JRP3943 (5′TTTTCTTTTAGACATGTCCATAGGC3′), which binds 268 bp upstream of the netB open reading frame (ORF), and JRP3944 (5′CCATCCCTTATTTCATCAGCATTTA3′), which binds 348 bp downstream of the netB ORF. The primers CPNE3416F (5′CACCATGAGTGAATTAAATGACATAAAC3′) and CPNE3416R (5′CAGATAATATTCTATTTTATGATCTTG3′) were also used to sequence the amplified PCR products. PCR products were purified using the QIAquick PCR purification kit before sequencing on an Applied Biosystems 3730S capillary sequencer.
2.3. Western blot analysis of C. perfringens isolates
Detection of NetB was performed as previously described [12]. Briefly, C. perfringens strains were grown in TPG broth to a turbidity at 600 nm of 0.6. Culture supernatants were obtained by centrifugation at 18 000 g for 10 min and separated by SDS-PAGE (NuPAGE® Novex 4–12% Bis-Tris gel, Invitrogen, CA, USA) in MES SDS running buffer (NuPAGE® MES SDS Running Buffer, Invitrogen). Proteins were transferred onto PDVF (PALL) membranes and probed with rabbit polyclonal anti-recombinant NetB (rNetB) anti-serum. Blots were developed with an ECL Western blotting kit (GE Healthcare Bio-sciences Pty. Ltd., Rydalmere, Australia) and the results recorded on autoradiographic film.
2.4. Cell line and cytotoxicity assay
Cytotoxicity assays were performed as previously described [12]. Volume normalized, dialyzed culture supernatant was added to the medium and incubated for 4 h at 37 °C. Lactate dehydrogenase (LDH) release into the supernatant was measured as an indicator of cytolysis using the Cyto-Tox (Promega, Alexandria, Australia) kit.
2.5. In vivo C. perfringens challenge model
The necrotic enteritis disease induction model was performed as previously described [11]. Briefly, commercial 1-day-old Ross 308 broiler chickens were fed an antibiotic-free chicken starter diet containing 20% protein for 13 days. On day 14 feed was changed to a wheat-based feed containing 50% fishmeal. On day 20, feed was withdrawn and each bird was orally challenged with 1.5 mL of C. perfringens culture (109 to 1010 CFU). On day 21 birds were again orally challenged and feed contaminated with C. perfringens was administered. C. perfringens strains were grown in FTG broth with the addition of 1.5% soluble starch and 2.0% thiopeptone and incubated at 37 °C for 14 h. Groups of 10 chickens were kept in adjacent separate pens in an animal isolation facility. On day 24, chickens were euthanased with inhaled carbon dioxide gas and their small intestines (duodenum to ileum) examined for gross necrotic lesions. The results for each challenge strain are expressed as a percentage lesion score compared with the standard challenge strain EHE-NE18. All animal experiments were assessed, approved and monitored by the Australian Animal Health Laboratory animal ethics committee.
3. RESULTS
3.1. Prevalence of netB and tpeL in C. perfringens isolates from chickens
PCR analysis of C. perfringens isolates from both healthy and diseased chickens was used to determine whether these isolates carry netB or tpeL. Positive C. perfringens strains gave a 383 bp and 474 bp PCR product from internal netB and tpeL sequences, respectively. The netB gene was present in 70% (31/44) of the strains isolated from diseased birds: 3 out of 4 isolates from Belgium were netB positive, 5 out of 7 from Denmark, 19 out of 24 Australian isolates and 4 out of 9 Canadian strains (Tab. I). The tpeL gene was only found in 9% (4/44) of isolates from diseased birds and was only present in netB-positive isolates. By contrast, only 2 of 55 isolates from healthy chickens from Australia and Belgium carried netB and none had tpeL.
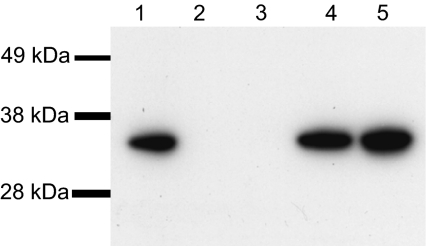
Western blot analysis was carried out using rabbit anti-serum against rNetB; for those strains positive for NetB toxin, a single immunoreactive band was present at approximately 33 kDa (Fig. 1). The results (Tab. I) showed that there was an absolute correlation between for the presence of the gene, as detected by PCR, and the ability to produce the NetB toxin.
Figure 1.
Western blot of NetB toxin from C. perfringens isolates from diseased chickens. Proteins were separated by 4–12% SDS-PAGE. SeeBlue Plus2 prestained marker (Invitrogen) was used as a size marker. Bands were transferred onto PVDF membranes and probed with rabbit polyclonal anti-rNetB antibody. Blots were developed with an ECL Western blotting kit and the results recorded on autoradiographic film. Strains: (1) EHE-NE18; (2) NAG-NE1; (3) 48; (4) 56; (5) 301001-1-B1.
3.2. The netB sequence is highly conserved
A PCR product encompassing the complete netB gene was amplified from 23 netB-positive strains and sequenced to determine the deduced amino acid sequence of the encoded 322 amino acid full-length NetB proteins. The toxins were all highly conserved in both nucleotide and amino acid sequence; only one different NetB sequence type was identified amongst these isolates, even though they represented several outbreaks of necrotic enteritis from different geographical locations (Tab. I). The netB nucleotide sequences of 15 of these strains were identical to the previously determined EHE-NE18 sequence [12], across the entire region analysed. The remaining 8 strains had very few nucleotide changes, which were represented in several strains from different countries. Only one change resulted in a NetB amino acid sequence change, altering the alanine residue at position 168 to threonine (A168T). The 6 strains that had this change included isolates from Australia, Canada, Denmark and Belgium (Tab. I). Finally, analysis of the non-coding regions surrounding the netB gene showed that they were very well conserved. Only three changes were observed, in a total of 5 strains. One of these changes involved an insertion of 10 additional nucleotides when compared to EHE-NE18. However, all changes are downstream of the netB ORF and are unlikely to alter gene expression.
3.3. NetB (A168T) retains cytotoxic activity
To determine if the single amino acid change at residue 168 of NetB affects its cytotoxic activity, culture supernatants from several strains producing the variant toxin were tested in the LMH cell cytotoxicity assay (Fig. 2). LDH release was used as a measure of cytotoxicity. The results showed that all five of the NetBA168T variants tested had similar toxicity for LMH cells as NetB from EHE-NE18. In addition, when supernatants from either NetB or NetBA168T derivatives were pre-incubated with anti-serum raised against the Escherichia coli-derived rNetB protein, a reduction in LDH release was observed. Incubation with pre-immune serum did not affect the cytotoxic activity of any of the NetB variants.
Figure 2.
Cytotoxic activity of NetB (A168T) supernatants on LMH cells. The LMH cells were grown to 70% confluence in a 96 well tissue culture plate and culture supernatant added to the medium for 4 h at 37 °C. Cytotoxicity was measured by release of LDH from the the LMH cells using the CytoTox kit (Promega). (A) EHE-NE18; (B) EHE-NE7; (C) EHE-NE9; (D) 3 MB-2003; (E) 37; (F) 301001-1-B1. (1) Dialyzed culture supernatant; (2) culture supernatant pre-incubated with pre-immune serum for 1 h at 37 °C; (3) culture supernatant pre-incubated with rNetB anti-serum for 1 h at 37 °C. The values are averages of triplicate assays with error bars representing SD.
3.4. NetB negative strains from diseased chickens do not cause necrotic enteritis in an experimental model
To investigate the role of NetB in necrotic enteritis a selection of netB positive and negative strains was tested in a necrotic enteritis induction model. All 11 strains tested that contained netB caused disease in the disease induction model (Tab. I). However, the 7 netB negative C. perfringens strains, all of which were derived from birds suffering from necrotic enteritis, induced little or no signs of disease when used to challenge these chickens. In the NAG-NE25 (netB-negative) challenge, the C. perfringens cells recovered from the few small lesions (approximately 1 mm in diameter) were clearly from a different source (presumably environmental) as PCR analysis showed that they carried netB.
4. DISCUSSION
In this study we found that 70% of C. perfringens isolates from chickens with necrotic enteritis carry netB. Importantly, all of the isolates that carried this gene also expressed the NetB protein in vitro. This result is in contrast to studies on β2 toxin, which recently showed that only 54.5% of the strains that were positive for its structural gene, cbp2, produced β2 toxin in vitro [6]. The C. perfringens isolates analysed in this study were sourced from a range of geographical locations, with NetB-positive strains detected from 4 countries, on 3 separate continents. There were insufficient numbers to determine if there was any statistically significant difference in netB carriage in these countries. Previous studies reported netB incidences of 7 out of 12 for isolates from the USA and a very high carriage rate of 92% for Canadian isolates [3, 14]. In agreement with our results, these strain surveys found a low incidence of netB in C. perfringens strains isolated from healthy birds.
These results, in conjunction with our previous finding that a netB mutant of a virulent isolate no longer causes disease [12], support the conclusion that NetB plays an important role in the pathogenesis of necrotic enteritis in chickens. However, recent studies have also found that there are some isolates from diseased birds that do not carry netB. There are several possible explanations for this finding, ranging from alternative virulence factors to the need for complex associations of other microflora required for disease production. In this study, 7 netB-negative strains isolated from diseased birds in all 4 countries were tested for their ability to induce disease in a standard disease induction model. No confirmed netB-negative isolates were able to induce disease whereas a range of netB-positive strains, from different geographical locations, were able to induce lesions typical of necrotic enteritis. These results strongly suggest that these isolates are not capable of causing disease without NetB. Of course, we cannot categorically rule out the possibility that these netB-negative isolates can cause disease in the field either by themselves or as part of a wider microbial consortium. The results reported here are consistent with our previous conclusion that NetB is important for virulence.
It is important when testing any isolates (e.g. netB-negative strains) in a disease model to test the genotype of isolates recovered from the lesions. The nature of the disease and the model systems are such that occasionally environmentally derived C. perfringens can initiate lesion formation and it is important to differentiate such lesions from lesions caused by the test organism. Other researchers have reported that some strains isolated from necrotic enteritis-affected chickens [4, 5] and strains from healthy birds [21], are not virulent in disease induction models, but the netB status of these strains was not reported.
Previous work has suggested that although different strains of C. perfringens can coexist in the cecum of healthy birds usually only one strain is involved in disease lesions in any particular bird [8, 16]. It would therefore seem unlikely that many of the apparently non-pathogenic isolates from diseased birds could result from mixed cultures of pathogenic and non-pathogenic strains coming from the sampled lesions. One possible explanation to consider is that the isolates have lost virulence during the in vitro culturing process that is necessary to derive a pure, defined culture from a lesion sample.
Other workers [3] surveyed C. perfringens strains from chickens and found that 11 out of 61 strains examined carried the tpeL gene, which encodes a potential glucosylating toxin. All of these tpeL positive strains also carried netB. Accordingly, we also screened for the tpeL gene, and obtained similar results. Only 4 out of 44 strains were tpeL positive, all of which also carried netB. Several strains that were tpeL negative caused typical disease in the disease induction model. It is clear from these studies that the putative TpeL toxin is not essential for necrotic enteritis in chickens.
Comparative sequence analysis of the netB genes revealed a very high degree of conservation with only one NetB variant detected (A168T) in 6 of the 23 genes sequenced. This difference was the result of the same single base substitution and the single amino acid change did not significantly alter the predicted physical properties of the encoded proteins (33.2 kDa, pI 6.14). All other netB-positive strains carried genes encoding the same protein as the prototype gene characterized in strain EHE-NE18 [12]. Alignment and structural modelling of NetB, in comparison to the related proteins, α-hemolysin from Staphylococcus aureus and β-toxin from C. perfringens, predicted that the A168T substitution occurs in a region that is expected to be within a membrane-spanning region [15]. The relatively conservative nature of the amino acid substitution would not be expected to significantly affect the tertiary structure of the protein and indeed the cytotoxicity assays showed that the variant protein was functional and had similar activity to the original NetB toxin.
The generally accepted model [22] for the development of necrotic enteritis in chickens proposes that C. perfringens normally resides in the gastrointestinal of birds, essentially behaving as a benign commensal organism. Some type of environmental or nutritional change or stress, results in overgrowth of the resident C. perfringens population, resulting in lesion formation. This study and other reports in the literature [3, 4, 7, 14] are giving a different picture of the disease process. It is now clear that the overall gastrointestinal population of C. perfringens in the normal healthy chicken is different to that of the organisms that cause disease. Whereas a mixed population of C. perfringens, containing a low percentage of NetB expressing clones, is present in healthy birds it has been found that a single clone tends to dominate the C. perfringens population in diseased birds. It is now clear that the clonal populations seen in diseased birds usually carry and express the netB gene. It now remains to be determined whether the increase in overall C. perfringens numbers that occurs during the disease process is due to a specific increase in the number of netB-positive organisms or whether there is a general overgrowth of all C. perfringens cells but only the netB-positive strains colonise, proliferate and cause cell and tissue damage.
In summary, we have shown that C. perfringens strains from chickens suffering necrotic enteritis produce highly conserved NetB toxins that closely resemble the NetB found in chicken isolate EHE-NE18. A high proportion of the C. perfringens strains from diseased birds were netB-positive, all of which were able to express NetB in vitro. Virulence testing of numerous strains in a standard disease induction model showed that only strains producing NetB were capable of producing disease. These results provide further evidence that NetB is an essential virulence factor in the pathogenesis of necrotic enteritis. Other toxin-encoding genes are present in some virulent strains but their role in virulence remains unclear. These results open up further studies aimed at the development of diagnostic tests and vaccines for the control and treatment of C. perfringens infections of commercial chickens; this work is currently being pursued in our laboratories.
Acknowledgments
This work was supported by a Ph.D. scholarship (A. Keyburn) and grants from the Australian Poultry Cooperative Research Centre and a Ph.D. scholarship (X. Yan) and research funding from the Australian Research Council Centre of Excellence in Structural and Functional Microbial Genomics. We thank M. Boulianne and L. Bjerrum for the supply of bacterial strains.
References
- 1.Al-Sheikhly F., Truscott R.B., The pathology of necrotic enteritis of chickens following infusion of broth cultures of Clostridium perfringens into the duodenum, Avian Dis. (1977) 21:230–240 [PubMed] [Google Scholar]
- 2.Al-Sheikhly F., Truscott R.B., The pathology of necrotic enteritis of chickens following infusion of crude toxins of Clostridium perfringens into the duodenum, Avian Dis. (1977) 21:241–255 [PubMed] [Google Scholar]
- 3.Chalmers G., Bruce H.L., Hunter D.B., Parreira V.R., Kulkarni R.R., Jiang Y.F., et al. , Multilocus sequence typing analysis of Clostridium perfringens isolates from necrotic enteritis outbreaks in broiler chicken populations, J. Clin. Microbiol. (2008) 46:3957–3964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chalmers G., Martin S.W., Hunter D.B., Prescott J.F., Weber L.J., Boerlin P., Genetic diversity of Clostridium perfringens isolated from healthy broiler chickens at a commercial farm, Vet. Microbiol. (2008) 127:116–127 [DOI] [PubMed] [Google Scholar]
- 5.Cooper K.K., Songer J.G., Necrotic enteritis in chickens: a paradigm of enteric infection by Clostridium perfringens type A, Anaerobe (2009) 15:55–60 [DOI] [PubMed] [Google Scholar]
- 6.Crespo R., Fisher D.J., Shivaprasad H.L., Fernandez-Miyakawa M.E., Uzal F.A., Toxinotypes of Clostridium perfringens isolated from sick and healthy avian species, J. Vet. Diagn. Invest. (2007) 19:329–333 [DOI] [PubMed] [Google Scholar]
- 7.Drigo I., Agnoletti F., Bacchin C., Bettini F., Cocchi M., Ferro T., et al. , Toxin genotyping of Clostridium perfringens field strains isolated from healthy and diseased chickens, Ital. J. Anim. Sci. (2008) 7:397–400 [Google Scholar]
- 8.Engstrom B.E., Fermer C., Lindberg A., Saarinen E., Baverud V., Gunnarsson A., Molecular typing of isolates of Clostridium perfringens from healthy and diseased poultry, Vet. Microbiol. (2003) 94:225–235 [DOI] [PubMed] [Google Scholar]
- 9.Fukata T., Hadate Y., Baba E., Uemura T., Arakawa A., Influence of Clostridium perfringens and its toxin in germ-free chickens, Res. Vet. Sci. (1988) 44:68–70 [PubMed] [Google Scholar]
- 10.Gholamiandekhordi A.R., Ducatelle R., Heyndrickx M., Haesebrouck F., Van Immerseel F., Molecular and phenotypical characterization of Clostridium perfringens isolates from poultry flocks with different disease status, Vet. Microbiol. (2006) 113:143–152 [DOI] [PubMed] [Google Scholar]
- 11.Keyburn A.L., Sheedy S.A., Ford M.E., Williamson M.M., Awad M.M., Rood J.I., Moore R.J., Alpha-toxin of Clostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens, Infect. Immun. (2006) 74:6496–6500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keyburn A.L., Boyce J.D., Vaz P., Bannam T.L., Ford M.E., Parker D., et al. , NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens , PLoS Pathog. (2008) 4:e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leslie D., Fairweather N., Pickard D., Dougan G., Kehoe M., Phospholipase C and haemolytic activities of Clostridium perfringens alpha-toxin cloned in Escherichia coli: sequence and homology with a Bacillus cereus phospholipase C, Mol. Microbiol. (1989) 3:383–392 [DOI] [PubMed] [Google Scholar]
- 14.Martin T.G., Smyth J.A., Prevalence of netB among some clinical isolates of Clostridium perfringens from animals in the United States, Vet. Microbiol. (2009) 136:202–205 [DOI] [PubMed] [Google Scholar]
- 15.Menestrina G., Serra M.D., Prevost G., Mode of action of beta-barrel pore-forming toxins of the staphylococcal alpha-hemolysin family, Toxicon (2001) 39:1661–1672 [DOI] [PubMed] [Google Scholar]
- 16.Nauerby B., Pedersen K., Madsen M., Analysis by pulsed-field gel electrophoresis of the genetic diversity among Clostridium perfringens isolates from chickens, Vet. Microbiol. (2003) 94:257–266 [DOI] [PubMed] [Google Scholar]
- 17.O’Connor J.R., Lyras D., Farrow K.A., Adams V., Powell D.R., Hinds J., et al. , Construction and analysis of chromosomal Clostridium difficile mutants, Mol. Microbiol. (2006) 61:1335–1351 [DOI] [PubMed] [Google Scholar]
- 18.Rood J.I., Virulence genes of Clostridium perfringens, Annu. Rev. Microbiol. (1998) 52:333–360 [DOI] [PubMed] [Google Scholar]
- 19.Sheedy S.A., Ingham A.B., Rood J.I., Moore R.J., Highly conserved alpha-toxin sequences of avian isolates of Clostridium perfringens, J. Clin. Microbiol. (2004) 42:1345–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Songer J.G., Clostridial enteric diseases of domestic animals, Clin. Microbiol. Rev. (1996) 9:216–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Timbermont L., Lanckriet A., Gholamiandehkordi A.R., Pasmans F., Martel A., Haesebrouck F., et al. , Origin of Clostridium perfringens isolates determines the ability to induce necrotic enteritis in broilers, Comp. Immunol. Microbiol. Infect. Dis. (2009) 32:503–512 [DOI] [PubMed] [Google Scholar]
- 22.Van Immerseel F., Rood J.I., Moore R.J., Titball R.W., Rethinking our understanding of the pathogenesis of necrotic enteritis in chickens, Trends Microbiol. (2009) 17:32–36 [DOI] [PubMed] [Google Scholar]
- 23.Vidal J.E., McClane B.A., Saputo J., Parker J., Uzal F.A., Effects of Clostridium perfringens beta-toxin on the rabbit small intestine and colon, Infect. Immun. (2008) 76:4396–4404 [DOI] [PMC free article] [PubMed] [Google Scholar]