Abstract
Nonalcoholic fatty liver disease (NAFLD), ranging from simple steatosis to nonalcoholic steatohepatitis (NASH), and eventually cirrhosis and liver failure, is seen to be increasing amongst Western children. NAFLD rates are rising in parallel with the epidemic of childhood obesity, and in particular, fatty liver evolves more easily in NASH when poor dietary habits and sedentary lifestyle are combined. In fact, its general prevalence in the child population varies between 2.6% and 10%, but increases up to 80% in obese children. Since NASH is expected to become the most common cause of pediatric chronic liver disease in the near future, there is broad interest amongst clinical researchers to move forward, both in diagnosis and treatment. Unfortunately, to date, the expensive and invasive procedure of liver biopsy is seen as the gold standard for NASH diagnosis and few noninvasive diagnostic methods can be applied successfully. Moreover, there are still no approved pharmacological interventions for NAFLD/NASH. Therefore, current management paradigms are based upon the presence of associated risk factors and aims to improve an individual’s quality of life, thus reducing NAFLD-associated morbidity and mortality. Today, lifestyle intervention (diet and exercise) is the treatment of choice for NAFLD/NASH. Thus far, no study has evaluated the potential preventive effect of lifestyle intervention on children at risk of NAFLD/NASH. Future studies will be required in this area with the perspective of developing a national program to promote nutrition education and increase physical activity as means of preventing the disease in individuals at risk. Here, we outline the clinical course, pathogenesis and management of NAFLD in children, highlighting the preventive and therapeutic value of lifestyle intervention.
Keywords: Fatty liver, Children, Lifestyle, Diet, Exercise, Prevention
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD), a worldwide health problem principally affecting millions of people in Western countries, ranges from simple steatosis to nonalcoholic steatohepatitis (NASH), and eventually cirrhosis and liver failure. A few population-based studies have reported that NAFLD/NASH prevalence has been increasing over the past three decades, both in children and adolescents, presenting a worldwide problem[1-3]. The rate of prevalence, ranging from 2.6% to 10%, increases with age and the number of risk factors associated with NAFLD. The most prominent risk factor for NAFLD/NASH is overweight/obesity and the disease is most common in male adolescents[4,5]. Studies of the prevalence of NAFLD in overweight/obese children have reported values which range from 8% to 80%[2,4]. Discrepancies in prevalence data reported in these studies depend on the methods used for diagnosis[6-11]. In fact, although definitive diagnosis of NASH and fibrosis staging and grading requires liver biopsy, most studies have been limited to the use of indirect measures, such as elevated serum alanine transaminase (ALT) and ultrasound to predict histological outcome[4,12].
The clinical course of NAFLD, as well as the management of fatty liver and steatohepatitis, is complicated by limited knowledge of the natural history and pathogenesis of the disease, and the paucity of safe and effective treatment modalities[1,13]. Most NAFLD children are asymptomatic and the few signs and symptoms that are observed are often nonspecific. NAFLD is a multifactorial disease that clinically may or may not comprise elevated serum ALT levels, hyperlipidemia, hyperglycemia and insulin resistance, associated with increased body weight and echogenic liver that is suggestive of hepatic steatosis[13-15]. Over the initial period of disease, many patients progress to the more advanced form of NAFLD, which combines the mentioned dysmetabolic pattern with severe liver injury, including steatohepatitis, necro-inflammation and fibrosis[16].
In contrast to the past, there is today, a widespread and growing recognition of the disease, but some aspects of its pathogenesis and multi-causality still remain obscure[17,18]. As a consequence, currently, it is only the presence of associated risk factors that contributes to updating the management program of pediatric NAFLD, which is essentially focused on improving the individual’s quality of life, thus reducing NAFLD-associated morbidity and mortality. In this management program, lifestyle intervention (diet and exercise) is the best choice of treatment for NAFLD/NASH in children[19,20]. However, the potential preventive effect of lifestyle intervention on children at risk of NAFLD/NASH has still not been evaluated.
Here, we outline the clinical course, pathogenesis and management of pediatric NAFLD in children, and highlight the preventive and therapeutic impact of lifestyle intervention. In addition, we suggest further studies in the area of NAFLD prevention in children, but we also presume that soon a national program will be undertaken to promote nutrition education and increase the physical activity for preventing the disease in individuals at risk.
CLINICAL COURSE AND PATHOGENESIS OF PEDIATRIC NAFLD
Pediatric NAFLD, as in adults, is defined as fat accumulation in the liver that exceeds 5%-10% by wet weight, in the absence of excessive alcohol consumption[21]. Although, the natural history of NAFLD is poorly understood in children, it is a multifactorial liver disease that comprises a large spectrum of clinical features: simple fatty liver accumulation (hepatic steatosis); steatosis accompanied by inflammation and other evidence of cellular injury, including various degrees of fibrosis (NASH); and end-stage liver disease, such as rare cases of cirrhosis and hepatocellular carcinoma[12,22].
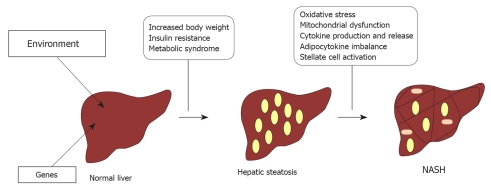
All genetic and environmental factors responsible for fatty liver and its progression to NASH are still obscure. The most widely accepted model is a “multiple hits” process (Figure 1), during which a first hit induces accumulation of fat in the liver, which causes hepatic steatosis, and renders hepatocytes more susceptible to additional cofactors (i.e. oxidative stress, mitochondrial dysfunction, overproduction and release of pro-inflammatory cytokines, adipocytokine imbalance, and stellate cell activation), which induce persistent liver injury that leads to NASH[17,23,24].
Figure 1.
Nonalcoholic fatty liver disease (NAFLD)/nonalcoholic steatohepatitis (NASH) pathogenesis.
Causes of hepatic steatosis
Hepatic steatosis is caused by imbalance between the delivery of fat in the liver and its subsequent secretion or metabolism. Fat accumulates in the liver for different reasons, in particular because of: excessive intake of dietary free fatty acids (FFAs), de novo hepatic lipogenesis, and great liver FFA influx caused by insulin resistance[25,26]. Interestingly, Donnelly et al[27] have demonstrated that liver FFA accumulation in NAFLD subjects derives from non-esterified fatty acids for about 60%-80%; de novo lipogenesis for 26%, and originates from diet for about 15%. These findings reinforce the hypothesis that several intracellular pathways may contribute to the accumulation of hepatic fat in NAFLD. These pathways include deregulation of β oxidation, decreased hepatic lipid export via very low-density lipoproteins, increased lipogenesis due to insulin-resistance-dependent activation of sterol regulatory element-binding protein (SREBP-1c), and glucose-regulated activation of carbohydrate response element-binding protein (ChREBP)[28].
Steatohepatitis and fibrosis
Several factors play central roles in the second-hit progression from simple steatosis to NASH. Various mechanisms have been proposed, including increased oxidative stress, inflammation, hepatocellular apoptosis and fibrogenesis[17,29,30]. There is accumulating evidence that oxidative stress and mitochondrial dysfunction are relevant in the pathogenesis of steatohepatitis, whatever its initial cause[31,32]. Moreover, oxidative stress and mitochondrial dysfunction, with insulin resistance, form a complex network of interactions, which promotes progressive liver injury (fibrosis), which causes chronic accumulation of liver FFA, antioxidant depletion, enhanced cytokine-mediated hepatoxicity, and promotion of stellate cell activation and proliferation[33-35]. This last event ultimately results in increased inflammation, apoptosis and liver fibrosis[36].
MANAGEMENT OF PEDIATRIC NAFLD/NASH: FIRST-LINE TREATMENT AND PROMISING THERAPEUTIC AGENTS
Significantly high levels of triglycerides, glucose, insulin, serum ALT, increased body mass index (BMI) and waist circumference (central adiposity) are all possible clinical features of pediatric NAFLD, which suggests that interventions on these variables can help to cure fatty liver, as well as to prevent progression to NASH[37]. On the other hand, resolution of histological abnormalities revealed by liver biopsy, is, at this time, the main target of NASH treatment[20,38].
Several recent studies have been carried out to examine the effects of dietary composition on NAFLD/NASH in children[39-41]. However, some others have also looked at the effects of energy restriction combined with physical activity and pharmaceutical treatments (i.e. vitamin E)[42,43].
Diet and exercise
As most NAFLD children are overweight/obese, weight loss may help to reduce pediatric NAFLD prevalence. Weight loss can be achieved through diet and proper exercise, it leads to significant improvement in serum ALT and liver histology in adults with NAFLD[44,45]. Studies on pediatric subjects have shown that moderate weight loss can improve serum BMI and levels of ALT, and reduce fatty liver infiltration and necro-inflammation, although no change has been demonstrated in degree of fibrosis[43,46].
Based upon knowledge of NAFLD pathogenesis, a proper diet might be a low-glycemic index diet; in fact, a similar diet may lead to reduction in serum ALT and hepatic steatosis[47,48]. Nevertheless, a rapid and excessive caloric restriction and weight loss is not recommended because it might potentially increase dysmetabolism and liver injury[12].
Vitamin E
As oxidative stress play a pivotal role in NASH pathogenesis, the use of natural antioxidants, such as vitamin E, is under investigation as a therapeutic approach for NASH patients. Vitamin E has been shown to improve ALT and liver histology in adults with NAFLD[49]. However, only one open-label study has demonstrated that 2-4 mo treatment with vitamin E normalizes serum ALT in obese children[50]. The efficacy of vitamin E is currently under investigation in a histology-based, double-blind, randomized, placebo-controlled study conducted by NASH Clinical Research Network, and its results will be available in 2010 (ClinicalTrials.gov Identifier: NCT00063635).
Insulin sensitizers
Most pediatric NAFLD patients present with insulin resistance, therefore, another approach to decreasing dysmetabolic and histological features associated with this liver disease is treatment with insulin-sensitizing agents. Metformin, a biguanide, is the only insulin-sensitizing agent that has been evaluated for the treatment of pediatric NAFLD. Metformin seems safe and effective in treating type 2 diabetes in children[51,52]. In addition, metformin has been evaluated in several pilot studies, which have demonstrated a significant improvement in ALT and hepatic steatosis[3]. A pediatric, randomized controlled trial with metformin as a monotherapy in NAFLD is now underway (ClinicalTrials.gov Identifier: NCT00063635). Also, thiazolidinediones, such as pioglitazone and rosiglitazone, have been used successfully for improving insulin resistance and possibly liver histology in adults, but their use in children still requires an accurate control study before they can be considered for use in clinical practice[53-55].
Ursodeoxycholic acid (UDCA)
UDCA may act as a cytoprotective and antioxidant agent. It is able to reduce ALT levels and improve liver histology in adults with NAFLD[56]. However, in a randomized control trial, Vajro et al[57] have demonstrated by ultrasound that UDCA is ineffective in improving serum ALT or steatosis, both alone and in combination with diet. On the other hand, another randomized controlled trial has shown that UDCA in combination with vitamin E may improve serum ALT and liver histology, but also decrease hepatocellular apoptosis and restore serum levels of adiponectin[58,59].
PREVENTIVE AND THERAPEUTIC EFFECTS OF LIFESTYLE INTERVENTION ON SEVERITY AND OUTCOME OF NAFLD
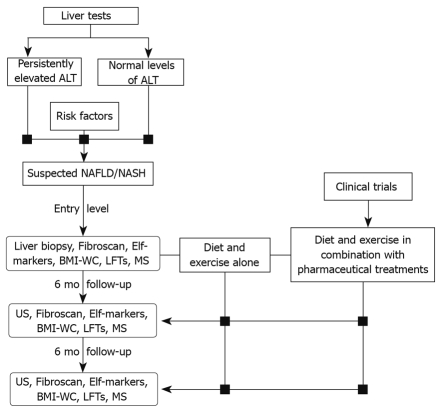
Patients suffering early liver dysfunction, such as simple hepatic steatosis, or at risk of developing a severe disease, including NASH and cirrhosis, require early diagnosis and intensive treatment. As already discussed, treatment options are limited and dietary weight loss is recommended. Although diets are often difficult to adhere to, they also have an enormous preventive value. In fact, a management program (Figure 2) that incorporates and encourages an adequate diet and age-appropriate physical activities may not only promote a healthy lifestyle, but also prevent the development of NAFLD/NASH.
Figure 2.
Management flowchart for NAFLD/NASH treatment and prevention. Elf: Enhanced liver fibrosis; BMI-WC: Body mass index-waist circumference; LFTs: Liver function tests; MS: Metabolic syndrome; US: Ultrasonography.
NAFLD-preventive effects of diet and exercise
Weight loss has been, until now, the only proven therapy for pediatric NAFLD. Thus, diet and exercise can be considered the first-line of defense for preventing the onset of NAFLD and progression to NASH in children at high risk. As demonstrated by several recent studies, the high-risk category comprises not only overweight/obese subjects, but also children with insulin resistance, metabolic syndrome, type 2 diabetes, and those with low birth weight[2,60,61].
However, recommendations for lifestyle modifications should be chosen according to the patients’ general health because very rapid weight loss might expose patients to severe metabolic consequences and increased mortality risk.
We recommend a hypocaloric diet of 25-30 cal/kg per day in case of overweight/obese subjects, and isocaloric diet (40-45 cal/kg per day) for normal-body-weight children. The amount of calories prescribed also takes into account physical activity and daily routines. Diet composition consists of low gastrointestinal carbohydrate (50%-60%), fat (23%-30%), and protein (15%-20%); a fat composition of two-thirds unsaturated and one-third saturated; and a ω6/ω3 ratio of approximately 4:1, in accordance with the Italian Recommended Dietary Allowances. Diet is tailored to individual preferences to improve compliance, which may be particularly poor in children and adolescents. A moderate daily exercise program, consisting of 45 min/d of aerobic physical activity, is also recommended. At each visit, subjects or their parents fill out a 3-d dietary and physical activity recall to evaluate adherence to lifestyle recommendations. A multidisciplinary team including dieticians, hepatologists, endocrinologists, psychologists, and cardiologists evaluates and closely follows up the patients. Participants and their parents are instructed on how to exercise, and maintain adherence to the exercise program, by a skilled exercise physiologist as part of our multidisciplinary program. Every 6 mo after treatment, children with NAFLD undergo ultrasonography, laboratory analyses, dietician evaluation and psychological tests. In Table 1, we report our success and failure rates after therapeutic intervention in children with biopsy-proven NAFLD.
Table 1.
Success and failure rates after therapeutic intervention in children with liver-biopsy-proven NAFLD n (%)
| Lifestyle intervention | Vitamin E | Metformin | |
| n | 29 | 25 | 28 |
| Complete success (all endpoints) | 11 (38) | 9 (36) | 2 (7) |
| Partial success | |||
| Serum ALT normalization | 23 (79) | 13 (52) | 23 (75) |
| HOMA-IR ≤ 1 restoration | 11 (38) | 9 (36) | 2 (7) |
| NAS amelioration | 19 (65) | 17 (68) | 16 (57) |
| Complete failure | 4 (14) | 8 (32) | 2 (7) |
NAFLD: Nonalcoholic fatty liver disease; HOMA-IR: Homeostatic model assessment of insulin resistance; NAS: NAFLD activity score; ALT: Alanine transaminase.
Thus, recommendations for lifestyle intervention in children at high risk and in subjects with fatty liver should follow a program based on an integrated care model that encourages patients, as well as family members, to adopt diet and exercise goals to prevent NAFLD/NASH development.
Integrated care model
NAFLD patients require a multidisciplinary approach that involves health professionals with different areas of expertise. This is even more crucial for pediatric patients, whose care also involves their families and other care providers, such as school personnel. In this respect, it is necessary to identify clearly a case manager with a strong leadership role, who can coordinate case management. Case management is defined as the process of planning, co-ordinating, managing and reviewing the care provided in order to ensure that it responds to the appraisal needs. The challenge of this model is for different professionals to work transversally and concurrently, placing the patient at the core of the system. An effective case management system should therefore be based on different steps[62].
The first step is the strategic planning and preparation of services, which includes different activities, with the integration of skills in a team of professionals who identify and overcome individual and organizational barriers, to guarantee timely access to health care. Coordination, monitoring and evaluation of the results gained by the effort of these integrated teams should be conducted on a regular basis, because they are paramount to achieving proper implementation.
The second step is the management of information. In fact, in order to work in an integrated manner, it is essential to achieve good communication among all the professional specialties involved.
The third step of this model stems from the flow of information between the various professionals and the patient. In this regard, there must be an educational function towards the patients and their relatives, in order that they may also become actively involved in the decisions and in the implementation of required treatments.
At the Bambino Gesù Hospital, this integrated care model is becoming the health-care model of choice. Beginning in 2003, the Liver Unit implemented the multidisciplinary outpatient clinic for the diagnosis and monitoring of patients affected by NAFLD/NASH. The hepatologist acts as case manager, establishes the individual patient’s care program, and coordinates clinical activities with the goal of ensuring that all of them are conducted on the day of the outpatient visit.
In addition to the hepatologist, the multidisciplinary team includes: the endocrinologist, because these patients frequently present with metabolic syndrome, with a predisposition to hyperinsulinism, to glucose intolerance and to type 2 diabetes; the cardiologist, who takes care of cardiovascular issues, ranging from arterial hypertension to increased risk of cardiovascular diseases; the radiologist, for the monitoring of hepatic lesions; the dietician for following appropriate dietary changes and increased physical activity prescribed on an individual basis; and the psychologist to consider psychological attitudes which may have preceded, and perpetuate an unhealthy diet and sedentary life style.
As defined in the program, the results of health care and clinical outcomes are evaluated by the team, and are the object of collegial discussion in order to jointly identify obstacles and the most appropriate way to overcome them.
This organisational model, in which the case manager has a central role, guarantees the quality and efficiency of the multi-specialist care process, leading to a favorable cost/benefit ratio, both at the individual and societal level. In our hospital, this model has worked reasonably well. In fact, it has allowed us to improve considerably patient compliance and medication adherence, reaching values close to 80%[43].
CONCLUSION
NAFLD/NASH has become the leading cause of pediatric liver disease in westernized countries. Several trials are ongoing to establish pharmacological treatment of pediatric disease, but health and nutrition strategies, such as better exercise habits and comprehensive approach to weight management in the school and home surroundings, can reduce the public health impact of pediatric NAFLD. We believe that our integrated care model not only provides an efficient and effective model for the care management of NAFLD patients, who need the intervention of health-care providers with different background and expertise, but also offers a good starting point for a national program of nutritional education and exercise for preventing the disease in children at high risk (i.e. overweight/obese children).
Footnotes
Peer reviewers: Philip Rosenthal, MD, Professor of Pediatrics & Surgery, UCSF, 500 Parnassus Avenue, Box 0136, MU 4-East, San Francisco, CA 94143, United States; Nahum Méndez-Sánchez, MD, PhD, Department of Biomedical Research, Gastroenterology & Liver Unit, Medica Sur Clinic & Foundation, Puente de Piedra 150, Col. Toriello Guerra, Tlalpan 14050, México City, México
S- Editor Tian L L- Editor Kerr C E- Editor Tian L
References
- 1.Barshop NJ, Sirlin CB, Schwimmer JB, Lavine JE. Review article: epidemiology, pathogenesis and potential treatments of paediatric non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2008;28:13–24. doi: 10.1111/j.1365-2036.2008.03703.x. [DOI] [PubMed] [Google Scholar]
- 2.Alisi A, Manco M, Panera N, Nobili V. Association between type two diabetes and non-alcoholic fatty liver disease in youth. Ann Hepatol. 2009;8 Suppl 1:S44–S50. [PubMed] [Google Scholar]
- 3.Alisi A, Manco M, Vania A, Nobili V. Pediatric nonalcoholic fatty liver disease in 2009. J Pediatr. 2009;155:469–474. doi: 10.1016/j.jpeds.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 4.Patton HM, Sirlin C, Behling C, Middleton M, Schwimmer JB, Lavine JE. Pediatric nonalcoholic fatty liver disease: a critical appraisal of current data and implications for future research. J Pediatr Gastroenterol Nutr. 2006;43:413–427. doi: 10.1097/01.mpg.0000239995.58388.56. [DOI] [PubMed] [Google Scholar]
- 5.Papandreou D, Rousso I, Mavromichalis I. Update on non-alcoholic fatty liver disease in children. Clin Nutr. 2007;26:409–415. doi: 10.1016/j.clnu.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Dunn W, Schwimmer JB. The obesity epidemic and nonalcoholic fatty liver disease in children. Curr Gastroenterol Rep. 2008;10:67–72. doi: 10.1007/s11894-008-0011-1. [DOI] [PubMed] [Google Scholar]
- 7.Kinugasa A, Tsunamoto K, Furukawa N, Sawada T, Kusunoki T, Shimada N. Fatty liver and its fibrous changes found in simple obesity of children. J Pediatr Gastroenterol Nutr. 1984;3:408–414. doi: 10.1097/00005176-198406000-00018. [DOI] [PubMed] [Google Scholar]
- 8.Kawasaki T, Hashimoto N, Kikuchi T, Takahashi H, Uchiyama M. The relationship between fatty liver and hyperinsulinemia in obese Japanese children. J Pediatr Gastroenterol Nutr. 1997;24:317–321. doi: 10.1097/00005176-199703000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Tazawa Y, Noguchi H, Nishinomiya F, Takada G. Serum alanine aminotransferases activity in obese children. Acta Paediatr. 1997;86:238–241. doi: 10.1111/j.1651-2227.1997.tb08881.x. [DOI] [PubMed] [Google Scholar]
- 10.Zou CC, Liang L, Hong F, Fu JF, Zhao ZY. Serum adiponectin, resistin levels and non-alcoholic fatty liver disease in obese children. Endocr J. 2005;52:519–524. doi: 10.1507/endocrj.52.519. [DOI] [PubMed] [Google Scholar]
- 11.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 12.Manco M, Bottazzo G, DeVito R, Marcellini M, Mingrone G, Nobili V. Nonalcoholic fatty liver disease in children. J Am Coll Nutr. 2008;27:667–676. doi: 10.1080/07315724.2008.10719744. [DOI] [PubMed] [Google Scholar]
- 13.Patton HM, Lavine JE, Van Natta ML, Schwimmer JB, Kleiner D, Molleston J. Clinical correlates of histopathology in pediatric nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1961.e2–1971.e2. doi: 10.1053/j.gastro.2008.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nobili V, Reale A, Alisi A, Morino G, Trenta I, Pisani M, Marcellini M, Raucci U. Elevated serum ALT in children presenting to the emergency unit: Relationship with NAFLD. Dig Liver Dis. 2009;41:749–752. doi: 10.1016/j.dld.2009.02.048. [DOI] [PubMed] [Google Scholar]
- 15.Manco M, Marcellini M, Devito R, Comparcola D, Sartorelli MR, Nobili V. Metabolic syndrome and liver histology in paediatric non-alcoholic steatohepatitis. Int J Obes (Lond) 2008;32:381–387. doi: 10.1038/sj.ijo.0803711. [DOI] [PubMed] [Google Scholar]
- 16.Ong JP, Younossi ZM. Epidemiology and natural history of NAFLD and NASH. Clin Liver Dis. 2007;11:1–16, vii. doi: 10.1016/j.cld.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Marra F, Gastaldelli A, Svegliati Baroni G, Tell G, Tiribelli C. Molecular basis and mechanisms of progression of non-alcoholic steatohepatitis. Trends Mol Med. 2008;14:72–81. doi: 10.1016/j.molmed.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 18.de Alwis NM, Day CP. Non-alcoholic fatty liver disease: the mist gradually clears. J Hepatol. 2008;48 Suppl 1:S104–S112. doi: 10.1016/j.jhep.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Pardee PE, Lavine JE, Schwimmer JB. Diagnosis and treatment of pediatric nonalcoholic steatohepatitis and the implications for bariatric surgery. Semin Pediatr Surg. 2009;18:144–151. doi: 10.1053/j.sempedsurg.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Socha P, Horvath A, Vajro P, Dziechciarz P, Dhawan A, Szajewska H. Pharmacological interventions for nonalcoholic fatty liver disease in adults and in children: a systematic review. J Pediatr Gastroenterol Nutr. 2009;48:587–596. doi: 10.1097/MPG.0b013e31818e04d1. [DOI] [PubMed] [Google Scholar]
- 21.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–1219. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 22.Bugianesi E. Non-alcoholic steatohepatitis and cancer. Clin Liver Dis. 2007;11:191–207, x-xi. doi: 10.1016/j.cld.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Day CP. Genes or environment to determine alcoholic liver disease and non-alcoholic fatty liver disease. Liver Int. 2006;26:1021–1028. doi: 10.1111/j.1478-3231.2006.01323.x. [DOI] [PubMed] [Google Scholar]
- 24.Alisi A, Nobili V. Molecular genetics of NASH: the role of polymorphisms. J Hepatol. 2007;47:868–869; author reply 870-871. doi: 10.1016/j.jhep.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Tessari P, Coracina A, Cosma A, Tiengo A. Hepatic lipid metabolism and non-alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2009;19:291–302. doi: 10.1016/j.numecd.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 26.Petta S, Muratore C, Craxì A. Non-alcoholic fatty liver disease pathogenesis: the present and the future. Dig Liver Dis. 2009;41:615–625. doi: 10.1016/j.dld.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tilg H, Moschen AR. Insulin resistance, inflammation, and non-alcoholic fatty liver disease. Trends Endocrinol Metab. 2008;19:371–379. doi: 10.1016/j.tem.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Loria P, Carulli L, Bertolotti M, Lonardo A. Endocrine and liver interaction: the role of endocrine pathways in NASH. Nat Rev Gastroenterol Hepatol. 2009;6:236–247. doi: 10.1038/nrgastro.2009.33. [DOI] [PubMed] [Google Scholar]
- 30.Atzori L, Poli G, Perra A. Hepatic stellate cell: a star cell in the liver. Int J Biochem Cell Biol. 2009;41:1639–1642. doi: 10.1016/j.biocel.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Begriche K, Igoudjil A, Pessayre D, Fromenty B. Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion. 2006;6:1–28. doi: 10.1016/j.mito.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Lerret SM, Skelton JA. Pediatric nonalcoholic fatty liver disease. Gastroenterol Nurs. 2008;31:115–119. doi: 10.1097/01.SGA.0000316530.31366.6e. [DOI] [PubMed] [Google Scholar]
- 33.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Clore JN. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 34.Feldstein AE, Werneburg NW, Canbay A, Guicciardi ME, Bronk SF, Rydzewski R, Burgart LJ, Gores GJ. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology. 2004;40:185–194. doi: 10.1002/hep.20283. [DOI] [PubMed] [Google Scholar]
- 35.Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology. 2004;40:46–54. doi: 10.1002/hep.20280. [DOI] [PubMed] [Google Scholar]
- 36.Balsano C, Alisi A, Nobili V. Liver fibrosis and therapeutic strategies: the goal for improving metabolism. Curr Drug Targets. 2009;10:505–512. doi: 10.2174/138945009788488459. [DOI] [PubMed] [Google Scholar]
- 37.Loomba R, Sirlin CB, Schwimmer JB, Lavine JE. Advances in pediatric nonalcoholic fatty liver disease. Hepatology. 2009;50:1282–1293. doi: 10.1002/hep.23119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edmison JM, Kalhan SC, McCullough AJ. Obesity, hepatic metabolism and disease. Nestle Nutr Workshop Ser Pediatr Program. 2009;63:163–172; discussion 172-176, 259-268. doi: 10.1159/000209980. [DOI] [PubMed] [Google Scholar]
- 39.Reinehr T, Schmidt C, Toschke AM, Andler W. Lifestyle intervention in obese children with non-alcoholic fatty liver disease: 2-year follow-up study. Arch Dis Child. 2009;94:437–442. doi: 10.1136/adc.2008.143594. [DOI] [PubMed] [Google Scholar]
- 40.Vos MB, McClain CJ. Nutrition and nonalcoholic fatty liver disease in children. Curr Gastroenterol Rep. 2008;10:308–315. doi: 10.1007/s11894-008-0061-4. [DOI] [PubMed] [Google Scholar]
- 41.Wang CL, Liang L, Fu JF, Zou CC, Hong F, Xue JZ, Lu JR, Wu XM. Effect of lifestyle intervention on non-alcoholic fatty liver disease in Chinese obese children. World J Gastroenterol. 2008;14:1598–1602. doi: 10.3748/wjg.14.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nobili V, Manco M, Ciampalini P, Alisi A, Devito R, Bugianesi E, Marcellini M, Marchesini G. Metformin use in children with nonalcoholic fatty liver disease: an open-label, 24-month, observational pilot study. Clin Ther. 2008;30:1168–1176. doi: 10.1016/j.clinthera.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 43.Nobili V, Manco M, Devito R, Di Ciommo V, Comparcola D, Sartorelli MR, Piemonte F, Marcellini M, Angulo P. Lifestyle intervention and antioxidant therapy in children with nonalcoholic fatty liver disease: a randomized, controlled trial. Hepatology. 2008;48:119–128. doi: 10.1002/hep.22336. [DOI] [PubMed] [Google Scholar]
- 44.Kim HK, Park JY, Lee KU, Lee GE, Jeon SH, Kim JH, Kim CH. Effect of body weight and lifestyle changes on long-term course of nonalcoholic fatty liver disease in Koreans. Am J Med Sci. 2009;337:98–102. doi: 10.1097/MAJ.0b013e3181812879. [DOI] [PubMed] [Google Scholar]
- 45.Uslan I, Acarturk G, Karaca E, Albayrak R, Yuksel S, Colbay M, Karaman O, Akcan Y. The effects of weight loss on normal transaminase levels in obese patients. Am J Med Sci. 2007;334:327–330. doi: 10.1097/MAJ.0b013e3181557702. [DOI] [PubMed] [Google Scholar]
- 46.Huang MA, Greenson JK, Chao C, Anderson L, Peterman D, Jacobson J, Emick D, Lok AS, Conjeevaram HS. One-year intense nutritional counseling results in histological improvement in patients with non-alcoholic steatohepatitis: a pilot study. Am J Gastroenterol. 2005;100:1072–1081. doi: 10.1111/j.1572-0241.2005.41334.x. [DOI] [PubMed] [Google Scholar]
- 47.Roberts EA, Yap J. Nonalcoholic Fatty Liver Disease (NAFLD): Approach in the Adolescent Patient. Curr Treat Options Gastroenterol. 2006;9:423–431. doi: 10.1007/BF02738532. [DOI] [PubMed] [Google Scholar]
- 48.Roberts EA. Non-alcoholic fatty liver disease (NAFLD) in children. Front Biosci. 2005;10:2306–2318. doi: 10.2741/1699. [DOI] [PubMed] [Google Scholar]
- 49.Dufour JF, Oneta CM, Gonvers JJ, Bihl F, Cerny A, Cereda JM, Zala JF, Helbling B, Steuerwald M, Zimmermann A. Randomized placebo-controlled trial of ursodeoxycholic acid with vitamin e in nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2006;4:1537–1543. doi: 10.1016/j.cgh.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 50.Lavine JE. Vitamin E treatment of nonalcoholic steatohepatitis in children: a pilot study. J Pediatr. 2000;136:734–738. [PubMed] [Google Scholar]
- 51.Zuhri-Yafi MI, Brosnan PG, Hardin DS. Treatment of type 2 diabetes mellitus in children and adolescents. J Pediatr Endocrinol Metab. 2002;15 Suppl 1:541–546. [PubMed] [Google Scholar]
- 52.Schwimmer JB, Middleton MS, Deutsch R, Lavine JE. A phase 2 clinical trial of metformin as a treatment for non-diabetic paediatric non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2005;21:871–879. doi: 10.1111/j.1365-2036.2005.02420.x. [DOI] [PubMed] [Google Scholar]
- 53.Miyazaki Y, Mahankali A, Matsuda M, Mahankali S, Hardies J, Cusi K, Mandarino LJ, DeFronzo RA. Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2002;87:2784–2791. doi: 10.1210/jcem.87.6.8567. [DOI] [PubMed] [Google Scholar]
- 54.Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Oliver D, Bacon BR. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology. 2003;38:1008–1017. doi: 10.1053/jhep.2003.50420. [DOI] [PubMed] [Google Scholar]
- 55.Juurinen L, Kotronen A, Graner M, Yki-Jarvinen H. Rosiglitazone reduces liver fat and insulin requirements and improves hepatic insulin sensitivity and glycemic control in patients with type 2 diabetes requiring high insulin doses. J Clin Endocrinol Metab. 2008;93:118–124. doi: 10.1210/jc.2007-1825. [DOI] [PubMed] [Google Scholar]
- 56.Lindor KD, Kowdley KV, Heathcote EJ, Harrison ME, Jorgensen R, Angulo P, Lymp JF, Burgart L, Colin P. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004;39:770–778. doi: 10.1002/hep.20092. [DOI] [PubMed] [Google Scholar]
- 57.Vajro P, Franzese A, Valerio G, Iannucci MP, Aragione N. Lack of efficacy of ursodeoxycholic acid for the treatment of liver abnormalities in obese children. J Pediatr. 2000;136:739–743. [PubMed] [Google Scholar]
- 58.Balmer ML, Siegrist K, Zimmermann A, Dufour JF. Effects of ursodeoxycholic acid in combination with vitamin E on adipokines and apoptosis in patients with nonalcoholic steatohepatitis. Liver Int. 2009;29:1184–1188. doi: 10.1111/j.1478-3231.2009.02037.x. [DOI] [PubMed] [Google Scholar]
- 59.Dufour JF, Oneta CM, Gonvers JJ, Bihl F, Cerny A, Cereda JM, Zala JF, Helbling B, Steuerwald M, Zimmermann A. Randomized placebo-controlled trial of ursodeoxycholic acid with vitamin e in nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2006;4:1537–1543. doi: 10.1016/j.cgh.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 60.Bugianesi E, McCullough AJ, Marchesini G. Insulin resistance: a metabolic pathway to chronic liver disease. Hepatology. 2005;42:987–1000. doi: 10.1002/hep.20920. [DOI] [PubMed] [Google Scholar]
- 61.Nobili V, Alisi A, Panera N, Agostoni C. Low birth weight and catch-up-growth associated with metabolic syndrome: a ten year systematic review. Pediatr Endocrinol Rev. 2008;6:241–247. [PubMed] [Google Scholar]
- 62.Nobili V, Manco M, Raponi M, Marcellini M. Case management in children affected by non-alcoholic fatty liver disease. J Paediatr Child Health. 2007;43:414. doi: 10.1111/j.1440-1754.2007.01092.x. [DOI] [PubMed] [Google Scholar]