Abstract
AIM: To test the hypothesis that pain and affect rather than impaired emptying determine symptom severity in patients with gastroparesis.
METHODS: Adult patients with documented gastroparesis were enrolled prospectively in a single center and asked to complete the Gastroparesis Cardinal Symptom Index (GCSI), Hospital Anxiety and Depression Scale (HADS), the Short Form 12 (SF-12) as quality of life index, rate pain severity and answer 10 open-ended questions.
RESULTS: A total of 55 patients (44 women) participated. Idiopathic (n = 29) or diabetic (n = 11) gastroparesis and connective tissue disease (n = 8) were the most common underlying causes. Antiemetics (n = 30) and prokinetics (n = 32) were most often prescribed. Seventeen patients used opioids on a daily basis. Nausea and/or vomiting (n = 28), pain (n = 24) and bloating (n = 14) were most commonly listed as dominant symptoms. Patients subjectively attributed symptom improvement to nutritional and dietary therapy (n = 11), prokinetics (n = 11), antiemetics (n = 10) or analgesic agents (n = 3). In univariate analyses, the physical subscore of the SF-12 and HADS, but not gastric emptying delay or symptom duration significantly correlated with disease severity as measured by the GCSI. In multivariate analyses, the combination of vomiting, bloating and depression best predicted the overall impact on quality of life.
CONCLUSION: The study confirms the importance of pain and affect in gastroparesis, which requires novel approaches to improve more effectively the quality of life in patients with this disorder.
Keywords: Pain, Depression, Gastroparesis, Quality of life
INTRODUCTION
Gastroparesis is an increasingly diagnosed functional disorder, characterized by delayed emptying of gastric contents. Considering the defining abnormality, prokinetics continue to play a primary role in managing this disorder[1-3]. However, cross-sectional and longitudinal studies have demonstrated a poor correlation between the documented delay in gastric emptying and symptom severity[4-6]. Moreover, motilin agonists do not improve symptoms even though they significantly accelerate gastric emptying[5,7-10]. Thus, other mechanisms must contribute to the clinical manifestation of gastroparesis, such as impaired gastric accommodation, altered distribution of ingested material within the stomach, and peripheral and/or central hyperalgesia[1-4]. The relative importance of any of these potential mechanisms remains unclear. More importantly, there is no evidence that medication that targets these abnormalities is indeed beneficial when used clinically. As we typically cannot change the underlying processes that lead to gastroparesis, treatment remains largely symptomatic with the primary goal of improving the overall quality of life of patients suffering from this, at times, disabling disorder.
We performed a cross-sectional study in patients with confirmed gastroparesis to define better the relative importance of key symptoms of gastroparesis, their impact on the patients’ functional status, and the potential differences between patient subgroups defined by the underlying etiology. The main hypotheses were: that pain significantly contributes to the impaired quality of life in patients with gastroparesis, and that affect plays an important role in determining symptom severity in patients with gastroparesis.
MATERIALS AND METHODS
Patient recruitment
Adult patients with confirmed gastroparesis seen by one investigator (Bielefeldt K) in the Digestive Disorders Clinic of the University of Pittsburgh Medical Center were invited to participate. Patients with gastroparesis had to be symptomatic for at least 6 wk and have objective evidence of impaired gastric function with delayed gastric emptying by scintigraphy, or documentation of retained food within the stomach after a 12-h fasting period obtained by endoscopy or contrast study. Structural abnormalities, such as gastric outlet obstruction or mechanical obstruction in the more distal gastrointestinal tract had to be excluded. The protocol was approved by the Institutional Review Board of the University of Pittsburgh (PRO8020059).
Surveys
After obtaining informed consent, all patients were asked to provide basic demographic information, complete the Gastroparesis Cardinal Symptom Index (GCSI), the Hospital Anxiety and Depression Scale (HADS), and the Medical Outcomes Study Short Form 12 (SF-12).
The GCSI is a self-administered assessment tool developed for patients with gastroparesis. The original survey comprises three subscales: postprandial fullness/early satiation (4 items); nausea/vomiting (3 items), and bloating (2 items). Considering the potential importance of pain, pain rating was added to the scale and scored similarly to the other items. Internal consistency and test-retest reliability were 0.84 and 0.76, respectively[11].
The HADS is a 14-item self-report questionnaire that asks patients to rate symptoms using statements that are converted into a numeric score between 0 and 3. The total score of can range between 0 and 21 for anxiety and depression. Extensive studies support an internal consistency exceeding 0.8. When used in different populations, scores of > 8 have a sensitivity around 80% for depressive or anxiety disorders[12].
The SF-12 is a self-report measure of perceived health, which consists of 12 questions. Two summary scores are generated: a physical health factor score (PHS) and a mental health factor score (MHS). The scores have been constructed with the population norm for each score being 50. Compared with the more extensive Short Form 36 (SF-36), the measure has been shown to be valid with correlations of 0.905 for the SF-36 Physical Component Summary and 0.938 for the SF-36 Mental Component Summary[13].
Open ended questions
Patients were also asked a series of open-ended questions (Table 1) that focused on the impact of gastroparesis on professional and private spheres of their lives. These questions included verbal assessments of the most significant symptoms, perceived treatment effects, and concerns related to the disease.
Table 1.
Open-ended questions
| How does the stomach problem affect your life? |
| How has gastroparesis affected your relationships with family and friends? |
| How has gastroparesis affected your ability to work and your professional life? |
| What do you worry about when you think about your stomach problem? |
| What symptom or problem related to your gastroparesis disturbs you the most? |
| What helped you the most in the treatment of your stomach problem? |
| What do you know and understand about your stomach problem? |
| Patients are often left with questions about their condition. What do you want to know about your disease? |
| What were your experiences as a patient with gastroparesis when you met physicians, nurses and other healthcare providers? |
| What can we do to better serve patients with your condition? |
Data abstraction
Clinical data about nature and duration of symptoms, severity, character, location of pain, weight changes, documented delay in gastric emptying, prior and ongoing treatment were abstracted after the clinical encounter, de-identified and linked to the survey data.
Statistical analysis
Responses to open-ended questions were analyzed by two independent coders who were aware of the underlying diagnosis of gastroparesis, but were blinded to patient identity, survey data as well as duration and etiology of the disorder. The qualitative analysis focused on frequency rather than expressions of severity of factors. A codebook was generated to record responses. Statements supporting the code were highlighted and added to a master file that contained all qualitative data.
Dichotomous variables were analyzed using χ2 statistics. Continuous and categorical variables are given as mean ± SE. Group comparisons were performed using analysis of variance. The GCSI summary score and quality of life as measured by the SF-12 were defined as the main endpoints. In an initial univariate analysis, correlations between the main endpoints and between different measures and the main endpoints were analyzed using Spearman correlation. Only variables with P < 0.1 were entered into the multilinear regression model.
RESULTS
Patients
Between June 2008 and February 2009, a total of 55 patients (mean age: 42.4 ± 1.9 years; 81% women) agreed to participate (Table 2). Gastric emptying studies had documented delayed emptying in all but five patients, who could not tolerate or complete studies because of emesis. In these individuals, retained food (n = 4) or a bezoar (n = 1) had been demonstrated endoscopically prior to enrollment. Thus, all patients met the entry criterion of objectively documented impairment in gastric emptying. However, studies were not performed in a single center with differences in test meals, test duration and data reporting. In 26 patients, the half-emptying time for solid-phase gastric emptying had been calculated and given. One additional patient only underwent a gastric emptying study for liquids that showed a significant delay. In five patients, only gastric retention at 90 or 120 min was quantified and compared with institutional norms. In the remaining patients, only a qualitative comment described delayed gastric emptying without further quantification by radiologists in outside institutions. The mean half-emptying time for the available studies was 319 ± 50 min.
Table 2.
Baseline patient characteristics n (%)
| Variable | All patients | Idiopathic gastroparesis | Diabetic gastroparesis | Connective tissue disease |
| Women | 55 (80) | 29 (90) | 11 (55) | 8 (100) |
| Age (yr) | 42.4 ± 1.9 | 38.1 ± 2.8b | 46.0 ± 2.9 | 57 ± 2.2 |
| Education | ||||
| High school | 38 | 20 | 8 | 5 |
| Bachelor | 9 | 5 | 1 | 1 |
| Graduate | 8 | 4 | 2 | 2 |
| Employed | 15 (27) | 11 (38) | 3 (27) | 0 |
| Annual household income ($) | ||||
| < 20 000 | 20 | 10 | 7 | 0 |
| 20 000-39 000 | 13 | 8 | 2 | 2 |
| 40 000-59 000 | 8 | 5 | 0 | 2 |
| 60 000-79 000 | 7 | 3 | 0 | 2 |
| > 80 000 | 7 | 3 | 2 | 2 |
| Symptom duration (mo) | 32 ± 4 | 24.2 ± 4.2 | 44 ± 15 | 25.8 ± 6.7 |
| Weight loss (pounds) | 3.7 ± 1.6 | 4.5 ± 2.6 | 2.9 ± 2.7 | 7.1 ± 3.6 |
P < 0.01 vs patients with gastroparesis due to connective tissue disease.
The most common causes of gastroparesis were diabetes mellitus (n = 11), connective tissue disease (n = 8), abdominal surgery or trauma (n = 4), osteogenesis imperfecta (n = 1), mitochondrial myopathy (n = 1) and Marfan syndrome (n = 1). In the remaining 29 patients, no underlying cause could be identified. Four of the patients with idiopathic gastroparesis recalled an acute illness prior to the onset of the disease, which suggested a post-infectious form of gastroparesis. Patients with idiopathic gastroparesis were significantly younger than those in the other two major groups (Table 2). Gastroparesis caused by connective tissue disease showed an expected female predominance, considering the preferential manifestation of systemic sclerosis in women[14]. Similarly, 90% of patients with idiopathic gastroparesis were women, while nearly half of the patients with diabetic gastroparesis were men (P < 0.05).
Symptoms of gastroparesis had been present for 32 ± 4 mo. Ten patients reported a significant weight loss that exceeded 5% of their body weight within the preceding 6 mo. The average weight loss for the entire group was 1.7 ± 0.7 kg. All but four patients (93%) complained about nausea. A sense of bloating, fullness and/or early satiation was present in 47 (87%) patients. At least mild pain was mentioned by 44 (81%) patients. The pain was primarily postprandial in 11 patients, with the remaining 34 participants complaining about constant discomfort. The pain was typically located in the epigastrium and described as pressure (n = 18), sharp (n = 12) or burning sensation (n = 7). Three patients mentioned generalized abdominal pain. In one patient, the pain radiated to the right upper quadrant and in another to the chest.
Seven patients (6 idiopathic gastroparesis; 1 diabetic gastroparesis) reported undergoing a cholecystectomy for biliary dyskinesia after symptom onset, which led to transient improvement in five patients. However, symptoms recurred within 3 mo in all but one of these patients, who continued to do well for 4 years before experiencing recurrent problems. Within the month prior to enrollment, 32 (60%) patients were using prokinetic agents, most commonly metoclopramide (Table 3). Ten of these patients experienced at least moderate side effects with extrapyramidal motor disorders (n = 3) worsening fatigue (n = 5) or depression (n = 1), which led to discontinuation of the agent. One patient was switched to domperidone. Three patients with systemic sclerosis and gastroparesis complicated by chronic intestinal pseudo-obstruction were using octreotide (n = 2) or pyridostigmine (n = 1). A total of 30 (55%) patients required daily antiemetic medication, most commonly phenothiazines and/or ondansetron. Eighteen patients (33%) received chronic antidepressant medication; mostly serotonin reuptake inhibitors. In four patients, tricyclic antidepressants or duloxetine were given to improve pain control. Regular use of benzodiazepines was reported by 16 (29%) patients. A total of 17 patients (31%) received chronic opioids for pain management. In two patients, narcotics were given for painful diabetic neuropathy. One patient had sickle cell anemia with severe bone pain. An additional patient with severe joint and muscle pain caused by systemic sclerosis and polymyositis used a fentanyl patch, which left 13 (24%) patients who were taking opioids daily for control of their abdominal pain. As opioids can impair gastric emptying, assessment of gastric function was performed after transient discontinuation of narcotics in all but the two patients who suffered from painful diabetic neuropathy. A total of 11 (20%) patients received nutritional support via enteral (n = 8) or parenteral (n = 3) nutrition.
Table 3.
Treatment of gastroparesis
| Variable | All patients | Idiopathic gastroparesis | Diabetic gastroparesis | Connective tissue disease |
| Prokinetics | ||||
| Metoclopramide | 25 | 11 | 7 | 4 |
| Erythromycin | 7 | 4 | 2 | 1 |
| Other | 4 | 1 | 3 | |
| Antiemetics | ||||
| Phenothiazine | 21 | 10 | 5 | 2 |
| Ondansetron | 20 | 12 | 4 | 1 |
| Scopolamine | 6 | 3 | 2 | |
| Meclizine | 2 | 1 | 1 | |
| Dronabinol | 5 | 3 | ||
| Antidepressives | ||||
| TCA | 3 | 1 | 1 | |
| SSRI | 15 | 4 | 5 | 4 |
| Benzodiazepines | 16 | 10 | 3 | 1 |
| Opioids (daily) | 17 | 7 | 5 | 1 |
| Nutritional support | ||||
| Jejunostomy | 8 | 1 | 4 | |
| TPN | 3 | 2 | 1 | |
TCA: Tricyclic antidepressant; SSRI: Selective serotonin reuptake inhibitor; TPN: Total parenteral nutrition.
Survey data
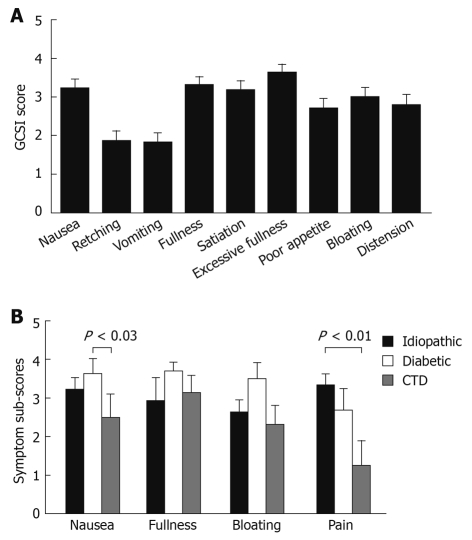
As shown in Table 4, the GCSI summary score demonstrated a moderate symptom severity for the entire group. Group comparisons only showed a trend for a lower overall score in patients with connective tissue disorders as the underlying etiology (P = 0.075). Figure 1A summarizes the individual symptom scores for the entire group. When asked to rate abdominal pain using the GCSI coding scale, the mean pain severity was 2.97 ± 0.24, which was similar to the subjectively rated severity of other symptoms. The pain rating correlated with the pain scale of the SF-12 that assessed the impact of pain over the preceding month (R2 = 0.22, P < 0.01), and the routinely assessed patient pain rating that addressed pain presence on the day of the clinic visit (R2 = 0.36, P < 0.001). A group comparison of component scores revealed more significant nausea for patients with diabetic gastroparesis and higher pain scores for patients with idiopathic gastroparesis (Figure 1B).
Table 4.
Survey data
| Variable | All patients | Idiopathic gastroparesis | Diabetic gastroparesis | Connective tissue disease |
| GCSI | 25.7 ± 1.4 | 24.6 ± 2.0 | 30.7 ± 2.3 | 20.8 ± 2.5 |
| HADS | ||||
| Anxiety | 8.3 ± 0.6 | 8.6 ± 0.9 | 9.3 ± 1.1 | 6.6 ± 1.7 |
| Depression | 7.7 ± 0.7 | 7.1 ± 1.1 | 9.9 ± 1.2 | 6.3 ± 1.5 |
| SF-12 | ||||
| PHS | 31.5 ± 1.4 | 33.3 ± 2.0 | 28.7 ± 2.8 | 31.7 ± 3.6 |
| MHS | 41.7 ± 1.6 | 41.4 ± 2.1 | 37.7 ± 3.5 | 47.7 ± 4.1 |
GCSI: Gastroparesis Cardinal Symptom Index; HADS: Hospital Anxiety and Depression Scale; SF-12: Short Form 12; PHS: Physical health factor score; MHS: Mental health factor score.
Figure 1.
Symptom severity scores in patients with gastroparesis. The individual GCSI scores are shown for the entire group (A). Component sub-scores for the GCSI and pain ratings are summarized for the three major patient groups, to allow comparison between the different etiologies of gastroparesis (B). Significant differences were seen for nausea (diabetes vs connective tissue disease) and pain (idiopathic vs connective tissue disease). CTD: Connective tissue disease; GCSI: Gastroparesis Cardinal Symptom Index.
Patients in all groups had moderately elevated scores for both anxiety and depression, without significant differences between the groups. Using the proposed cutoff score of > 8[12], 40 participants (74%) met screening criteria for anxiety or depression. In 16 patients (29%), anxiety and depression were both above the proposed threshold for clinically relevant affective spectrum disorders.
Compared to a population norm of 50, the mental and the physical component of the SF-12 showed a significant impairment of health-related functional status. There were no significant differences between the groups.
The GCSI summary score showed a significant inverse correlation with the physical but not mental score of the SF-12. It was correlated positively with depression scores, but not HADS anxiety scores, age, symptom duration or degree of gastric emptying delay (Table 5). In order to identify the relative contribution of symptoms, we performed univariate analyses, correlating the physical health scale of the SF-12 with GCSI subscores and pain ratings. Based on these results, we performed multiple linear regressions to identify the most important predictors of poor health function. A combination of GCSI subscores for nausea and bloating and the HADS score for depression best predicted the PHS subscore with an adjusted R2 of 0.44.
Table 5.
Univariate correlation analysis
| Variable | Spearman coefficient | P |
| GCSI | ||
| SF-12 PHS | -0.56 | 0.0001 |
| SF-12 MHS | -0.212 | 0.12 |
| HADS-anxiety | 0.17 | 0.22 |
| HADS-depression | 0.33 | 0.02 |
| T-1/2 | 0.05 | 0.82 |
| Symptom duration | -0.08 | 0.56 |
| Age | -0.08 | 0.55 |
| PHS | ||
| GCSI-sub score nausea | -0.52 | 0.0001 |
| GCSI-sub score fullness | -0.4 | 0.003 |
| GCSI-sub score bloating | -0.4 | 0.003 |
| GCSI-sub score pain | -0.35 | 0.01 |
| HADS-depression | -0.44 | 0.001 |
Qualitative data
When asked to describe the impact of gastroparesis on their lives, most patients focused on the effects of interactions with family, friends or colleagues (n = 37). Three main topics emerged in the more detailed analyses of responses. First, eating out, dinners or other social functions were seen as causing difficulties because of the limited ability to tolerate food (n = 26). One patient explained these problems: “[With] every- and anything that goes on socially there is food. When you can’t eat, it is really hard to cope with. People feel funny asking you out to dinner or invite you to something where there will be food.” The second problem was related to fatigue, which made it difficult for patients to meet expectations (n = 14) as exemplified by statements such as “I push myself to get out of bed every morning” or “I can barely make it through the day”. The third major topic revolved around the frustration and emotional impact of the disease as patients experienced difficulties interacting with others (n = 16). One patient summarized her experience: “…enjoying life has become difficult because my mind is always on my stomach”.
Participants cited a significant strain on their relationships with partners and/or family members (n = 13). Such problems could be a simple consequence of limitations imposed by their disease, as this woman explained: “…having to sleep sitting up doesn’t contribute to closeness with your partner”. A young mother with idiopathic gastroparesis reported that her disease “…has prevented me from caring for my children and participating in their activities”. In other cases, it was more anxieties and concerns in response to the physical evidence of illness, such as frequent vomiting: “…children - always frightened I will never get well or I will die”. Some patients reported a gradual withdrawal of others in the face of ongoing illness: “My friends don’t really call anymore because they know I am always feeling sick. … I also broke off a wedding engagement with someone I was with for 9 years because he couldn’t stand how sick I was and felt I was holding him back”.
When asked about the impact of gastroparesis on their professional activity, three patients described themselves as retired and seven responded that they were on disability (in 2 cases, for reasons other than gastroparesis). An additional eight patients mentioned that they felt forced to quit jobs or school because of their disease. One patient who had successfully completed college education explained his difficulties: “…nausea is consuming… [making it] difficult to concentrate on anything else…” Another participant described the impact as being “…unable to work… therefore, I feel worthless. I feel like my stomach problems wasted everything I achieved”.
Patients were also asked to explain their most bothersome symptoms and their concerns related to gastroparesis. Nausea or vomiting were listed by 28 participants, followed by pain (n = 24), bloating (n = 14) and emotional difficulties (n = 6). Nearly half (n = 26) of the patients mentioned the fear of an unrelenting chronic disease as their main concern, as shown by the following quote: “I worry that I’ll never feel like a normal person”. These concerns expressed themselves in some instances through anxiety that a more serious, perhaps even lethal disease caused the problems. Despite prior and extensive investigations and explanations, five patients were afraid that they had cancer.
When describing the perceived impact of treatment they had received for gastroparesis, patients most commonly listed the benefits of dietary and nutritional therapy (n = 11), prokinetics (n = 11) and antiemetics (n = 10). However, 10 patients mentioned that no treatment had led to any improvement. Others reported benefits of acid suppression (n = 5), relaxation techniques (n = 3) and analgesics (n = 3).
Patients also described their experiences with healthcare providers, with 15 (27%) expressing frustration or dissatisfaction. They primarily focused on a perceived lack of knowledge or understanding, which may have contributed to a perceived diagnostic delay and limited symptomatic improvement. One patient summarized her experiences: “I’ve had horrifying experiences from the medical community. Most physicians just say what you can [eat] or drink [like] Ensure”.
DISCUSSION
Our results provide a cross-sectional picture of gastroparesis and its impact on patients seen in a tertiary referral center. Consistent with prior studies, most patients were women in their thirties and early forties, with the majority suffering from idiopathic forms of gastroparesis[11,15-18]. Overall disease severity as judged by the GCSI score, other symptom severity scores or the physical and mental component of the SF-12 were comparable to those reported in previously published studies[6,11,15,16]. Similarly, the frequency of different symptoms, such as nausea, vomiting, early satiation and pain, were consistent with prior reports[6,16,18-21]. About 10% of patients with idiopathic gastroparesis recalled an acute symptom onset after a flu-like illness, which suggested a post-infectious form of the disease. These results are slightly lower than previously reported[15,22]. We also did not see differences in the apparent clinical course based on patient recall, as all individuals with post-infectious forms of gastroparesis had symptoms for more than 1 year and complained about ongoing and at least moderately severe symptoms. However, the cross-sectional design and relatively small sample size do not allow definitive conclusions to be drawn. While the symptom pattern of patients with diabetic and idiopathic gastroparesis did not differ, patients with systemic sclerosis complained less about pain compared to the other groups, which may be important in management decisions[18].
Impact of gastroparesis
When asked to rate their overall health, more than two thirds used descriptors of fair or poor, which demonstrated the significant impact of gastroparesis, which is also shown by the low score in the physical functioning domain of the SF-12. We included a qualitative analysis of open-ended questions to capture important details that led to impairment of quality of life. Most of the comments focused on social interactions with family, friends or colleagues rather than symptoms. A striking finding was the high number of patients who reported significant problems in their professional activities that may have contributed to unemployment or disability. Despite a mean age below 45 years, only about one quarter of study participants was fully employed. The number of individuals not working and/or on disability benefit was higher than reported for inflammatory bowel disease[23,24]. Consistent with the impact of the disease on professional lives, more than half of the patients reported household incomes well below the national average, which also has been observed in other disorders associated with chronic pain[25]. Chronic illness certainly increases work absenteeism and may even result in permanent disability[26-29]. Our findings suggest a disproportionate impact in patients with gastroparesis, which corresponded with patient statements about their own disease experience and functional health status. Additional studies with larger patients groups and longitudinal design are needed to better define the effect of impaired gastric function on professional productivity.
Not surprisingly, our results also highlighted the importance of food intake. Dietary management and nutritional therapy primarily and appropriately target the caloric and nutritional value of ingested materials, which relies on changes in meal volume and frequency, food consistency, and in more severe cases, even enteral or parenteral alimentation[2,30]. Although successful in preventing the development of nutritional deficiencies, these measures do not address the hedonic and social aspects related to eating. Aversive reactions to the previously pleasant experience of food intake, the inability to fully participate in daily routines, such as dinners, fully meet professional obligations associated with eating or drinking, or enjoy dining out with friends reminded patients on a daily basis how the illness had changed their lives. The answers often hinted at resentments, when healthcare providers did not appreciate this fact and briefly told them that simple dietary adjustments would solve their problems, without acknowledging the tremendous impact on quality of life.
Gastroparesis and affect
The high scores for anxiety and depression and patient statements certainly demonstrated the association between mood and impaired gastric function. Our design did not allow us to determine whether and how these factors are causally related. Interestingly, in the multivariate model, depression but not anxiety significantly contributed to the overall impairment in health status. Only one study has described the prevalence of depression in a large group of patients with gastroparesis, with nearly one quarter carrying the diagnosis of depressive illness[15]. This figure corresponds with the reported chronic use of antidepressant medications in our patient group. Our findings also fall in line with previous results obtained in patients with functional dyspepsia, in whom depression significantly correlated with the physical domain of quality of life[31,32]. Recent evidence suggests that depression is the key determinant of symptom severity in functional dyspepsia, which is primarily mediated through somatization[33]. Considering the overlap between functional dyspepsia and gastroparesis, additional studies will be needed to better define the role of psychological mechanisms in disease severity and manifestations.
Pain and gastroparesis
The recent consensus statement of the American Gastroenterological Association comments on pain in gastroparesis as being relatively common, but typically not the primary symptom or concern[3]. Our findings, especially the patient comments, argue against this statement, when one considers the high prevalence of pain and subjective pain ratings, which has been emphasized previously by Hoogerwerf et al[18]. The relevance of pain was also reflected in the treatments received by patients. Despite concerns about the use of opioids in patients with gastroparesis, about 30% of the patients regularly used narcotics. Only one study has mentioned specifically narcotic use in patients with gastroparesis, and has reported an even higher number of close to 50%, which may have been caused partly by selection bias, because all patients underwent implantation of a gastric electrical stimulator[16]. The interaction between narcotic use and impaired gastric emptying is admittedly complex, as opioids affect gastrointestinal motility. However, virtually all assessments of gastric function were performed when patients did not receive narcotics. It is thus unlikely that the documented delay in gastric emptying seen in our patients was solely a consequence of medication.
Beyond its prevalence, pain remains a significant challenge in managing gastroparesis. The conventional approaches with dietary and/or prokinetic therapy do not provide significant benefit[18]. Tricyclic antidepressants (TCAs), selective serotonin reuptake inhibitors (SSRIs) or serotonin/norepinephrine reuptake inhibitors have been used in functional dyspepsia and other visceral pain syndromes[34-36]. Although they may acutely lower pain thresholds in healthy volunteers[37-39], efficacy in patients has not been demonstrated consistently[32,40,41]. Gabapentin or pregabalin similarly may decrease sensory thresholds acutely[42], but have not been shown to possess true analgesic properties in patients[43]. Considering the negative impact of opioids on gastric emptying and their addictive potential, kappa opioids agonists have been tried in gastroparesis or functional dyspepsia, but were not superior to placebo[44,45]. In view of recent advances in our understanding of visceral sensory mechanisms, we will have to see whether other potential targets for pharmacological interventions, such as purinergic receptors, TRPV1 or TRPA1, will provide greater benefit for these patients[46,47].
Gastroparesis and gastric emptying
Although gastroparesis is defined by delayed emptying, our study did not show a correlation between symptom severity and half-emptying time as an objective and quantitative measure of this impairment. Only minor differences emerged in comparisons between symptomatic individuals with (gastroparesis) and without (functional dyspepsia) delayed gastric emptying, which did not allow differentiation between the groups based on symptoms only[6,48]. In addition, potent prokinetics have a limited or no impact on symptoms, which further argues against a primary role of transit delay as a determinant of disease severity[8,9]. Consistent with this conclusion, only a small fraction of patients reported subjectively perceived benefit when taking prokinetics. This relatively low response rate contrasts starkly with the relatively frequent adverse effects. More than one third of the patients who received metoclopramide had to discontinue the medication because of side effects; a rate that is in line with previous reports[1,2]. These results are likely caused by the complex pathophysiology of gastroparesis, which includes impaired accommodation, delayed emptying, hypersensitivity and (preexisting or secondarily evolving) affective spectrum disorders, all of which contribute to the clinical manifestation of the disorder[4,30].
Study limitations
As is true for most clinical studies, our investigation was conducted in a tertiary referral center, which biases and skews findings as a result of the likely higher proportion of difficult-to-treat patients. However, patient age, sex distribution, the high proportion of patients with idiopathic gastroparesis and self-reported symptom severity were comparable with prior studies that used similar scoring systems[4,15,16,49]. The relatively high number of patients with systemic sclerosis and the slightly smaller group of diabetic patients likely reflects institutional idiosyncrasies. A cross-sectional study design certainly comes with limitations. It enabled us to identify association between symptom severity and its potential determinants, from underlying etiology to treatment. Although we noticed an important impact of depressive symptoms, longitudinal studies are needed to better define this relationship and correlate time- and/or treatment-dependent changes in the various indices. Moreover, our data cannot identify psychological or physiological mechanisms that mediate the interactions between affect and symptoms, which will require more detailed investigations. Similarly, our study was not designed to measure systematically the effects of different treatments in patients with gastroparesis. More than half of the patients were enrolled during their initial encounter, with data thus reflecting the approaches of many referring physicians. We also used previously obtained data on gastric emptying, which provided less standardized but still objective evidence of impaired gastric function in all patients. Although appropriate for reducing increasing healthcare costs and typical for clinical practice[11], it limited our ability to correlate fully this disease-defining variable with symptom severity.
Taken together, our data raise questions about some of the key premises that we as clinicians use when approaching patients with gastroparesis. Delayed gastric emptying still remains the defining and only routinely available diagnostic tool to identify this disorder. However, it may detract from the much more complex pathophysiology and may even misguide our treatment. As we cannot cure the illness, our treatment has to focus on improving the overall quality of life. A paradigm shift may be needed to take into account the important influence of under-recognized and under-treated problems, such as pain or affect. Pain assessment should be included in the GCSI. Gastric electrical stimulation was initially thought to alter gastric motility, with investigators now increasingly speculating on its effect on visceral sensation and affect[20,50,51]. Some data are promising, especially in diabetic patients[16,20,50,52]. However, the high cost, frequent need of reoperation, and the limited benefit in patients with significant pain and/or idiopathic gastroparesis clearly force us to look for alternatives. Our results may be seen as initial, yet circumstantial evidence that supports psychologically based interventions, which have been quite successful in functional bowel disease and have shown promise in a small case series[53].
COMMENTS
Background
Gastroparesis is a chronic disorder that is characterized by significant dyspeptic symptoms and delayed gastric emptying. Despite the defining abnormality in gastric motor function, prokinetics typically have limited efficacy, which leaves patients with persistent symptoms and poor quality of life.
Research frontiers
Anxiety and depression play an important role in the clinical manifestation of many functional disorders, which range from irritable bowel syndrome to fibromyalgia. We prospectively enrolled patients with gastroparesis to better understand the relative importance of different gastrointestinal symptoms and associated or coexisting emotional factors on their quality of life.
Innovations and breakthroughs
Our data highlight that depression plays a significant role in the overall impact of gastroparesis on quality of life. The findings also emphasize the importance of pain as a symptom of gastroparesis, which is under-appreciated and under-treated.
Applications
The results stress the need to shift treatment strategies for gastroparesis away from approaches that simply try to accelerate gastric emptying. In our clinical assessment, we need to assess the different symptoms including pain, and design a therapy that takes into account the primary problems the individual patients experience. Because affect, especially depression, significantly impairs quality of life, diagnostic approaches and treatment should address emotional factors. In future research, we will have to define better the interrelationship between altered gastric function and emotion to understand underlying mechanisms. Interactions will likely be reciprocal, which raises the question of how much psychologically or psychiatrically oriented treatment may be beneficial in patients with gastroparesis.
Terminology
Gastroparesis is a chronic disorder that is characterized by symptoms of nausea, vomiting, fullness and bloating after meals, and a delay in gastric emptying, which is typically determined using standardized scintigraphic tests.
Peer review
The article fits into the increasing evidence that structural and especially functional disorders of the gastrointestinal tract should be seen as biopsychocial phenomena that require more holistic diagnostic and therapeutic approaches.
Footnotes
Peer reviewer: Volker F Eckardt, MD, Professor, Chief, Department of Gastroenterology, Deutsche Klinik für Diagnostik, Aukammallee 33, 65191 Wiesbaden, Germany
S- Editor Tian L L- Editor Kerr C E- Editor Lin YP
References
- 1.Reddymasu SC, McCallum RW. Pharmacotherapy of gastroparesis. Expert Opin Pharmacother. 2009;10:469–484. doi: 10.1517/14656560902722505. [DOI] [PubMed] [Google Scholar]
- 2.Hasler WL. Gastroparesis--current concepts and considerations. Medscape J Med. 2008;10:16. [PMC free article] [PubMed] [Google Scholar]
- 3.Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1592–1622. doi: 10.1053/j.gastro.2004.09.055. [DOI] [PubMed] [Google Scholar]
- 4.Karamanolis G, Caenepeel P, Arts J, Tack J. Determinants of symptom pattern in idiopathic severely delayed gastric emptying: gastric emptying rate or proximal stomach dysfunction? Gut. 2007;56:29–36. doi: 10.1136/gut.2005.089508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arts J, Caenepeel P, Verbeke K, Tack J. Influence of erythromycin on gastric emptying and meal related symptoms in functional dyspepsia with delayed gastric emptying. Gut. 2005;54:455–460. doi: 10.1136/gut.2003.035279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cassilly DW, Wang YR, Friedenberg FK, Nelson DB, Maurer AH, Parkman HP. Symptoms of gastroparesis: use of the gastroparesis cardinal symptom index in symptomatic patients referred for gastric emptying scintigraphy. Digestion. 2008;78:144–151. doi: 10.1159/000175836. [DOI] [PubMed] [Google Scholar]
- 7.Dhir R, Richter JE. Erythromycin in the short- and long-term control of dyspepsia symptoms in patients with gastroparesis. J Clin Gastroenterol. 2004;38:237–242. doi: 10.1097/00004836-200403000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Talley NJ, Verlinden M, Geenen DJ, Hogan RB, Riff D, McCallum RW, Mack RJ. Effects of a motilin receptor agonist (ABT-229) on upper gastrointestinal symptoms in type 1 diabetes mellitus: a randomised, double blind, placebo controlled trial. Gut. 2001;49:395–401. doi: 10.1136/gut.49.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCallum RW, Cynshi O. Clinical trial: effect of mitemcinal (a motilin agonist) on gastric emptying in patients with gastroparesis - a randomized, multicentre, placebo-controlled study. Aliment Pharmacol Ther. 2007;26:1121–1130. doi: 10.1111/j.1365-2036.2007.03461.x. [DOI] [PubMed] [Google Scholar]
- 10.McCallum RW, Cynshi O. Efficacy of mitemcinal, a motilin agonist, on gastrointestinal symptoms in patients with symptoms suggesting diabetic gastropathy: a randomized, multi-center, placebo-controlled trial. Aliment Pharmacol Ther. 2007;26:107–116. doi: 10.1111/j.1365-2036.2007.03346.x. [DOI] [PubMed] [Google Scholar]
- 11.Revicki DA, Rentz AM, Dubois D, Kahrilas P, Stanghellini V, Talley NJ, Tack J. Development and validation of a patient-assessed gastroparesis symptom severity measure: the Gastroparesis Cardinal Symptom Index. Aliment Pharmacol Ther. 2003;18:141–150. doi: 10.1046/j.1365-2036.2003.01612.x. [DOI] [PubMed] [Google Scholar]
- 12.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 13.Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Domsic R, Fasanella K, Bielefeldt K. Gastrointestinal manifestations of systemic sclerosis. Dig Dis Sci. 2008;53:1163–1174. doi: 10.1007/s10620-007-0018-8. [DOI] [PubMed] [Google Scholar]
- 15.Soykan I, Sivri B, Sarosiek I, Kiernan B, McCallum RW. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig Dis Sci. 1998;43:2398–2404. doi: 10.1023/a:1026665728213. [DOI] [PubMed] [Google Scholar]
- 16.Maranki JL, Lytes V, Meilahn JE, Harbison S, Friedenberg FK, Fisher RS, Parkman HP. Predictive factors for clinical improvement with Enterra gastric electric stimulation treatment for refractory gastroparesis. Dig Dis Sci. 2008;53:2072–2078. doi: 10.1007/s10620-007-0124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedenberg FK, Palit A, Parkman HP, Hanlon A, Nelson DB. Botulinum toxin A for the treatment of delayed gastric emptying. Am J Gastroenterol. 2008;103:416–423. doi: 10.1111/j.1572-0241.2007.01676.x. [DOI] [PubMed] [Google Scholar]
- 18.Hoogerwerf WA, Pasricha PJ, Kalloo AN, Schuster MM. Pain: the overlooked symptom in gastroparesis. Am J Gastroenterol. 1999;94:1029–1033. doi: 10.1111/j.1572-0241.1999.01008.x. [DOI] [PubMed] [Google Scholar]
- 19.Silvers D, Kipnes M, Broadstone V, Patterson D, Quigley EM, McCallum R, Leidy NK, Farup C, Liu Y, Joslyn A. Domperidone in the management of symptoms of diabetic gastroparesis: efficacy, tolerability, and quality-of-life outcomes in a multicenter controlled trial. DOM-USA-5 Study Group. Clin Ther. 1998;20:438–453. doi: 10.1016/s0149-2918(98)80054-4. [DOI] [PubMed] [Google Scholar]
- 20.Lin Z, Hou Q, Sarosiek I, Forster J, McCallum RW. Association between changes in symptoms and gastric emptying in gastroparetic patients treated with gastric electrical stimulation. Neurogastroenterol Motil. 2008;20:464–470. doi: 10.1111/j.1365-2982.2007.01054.x. [DOI] [PubMed] [Google Scholar]
- 21.Soykan I, Sarosiek I, McCallum RW. The effect of chronic oral domperidone therapy on gastrointestinal symptoms, gastric emptying, and quality of life in patients with gastroparesis. Am J Gastroenterol. 1997;92:976–980. [PubMed] [Google Scholar]
- 22.Bityutskiy LP, Soykan I, McCallum RW. Viral gastroparesis: a subgroup of idiopathic gastroparesis--clinical characteristics and long-term outcomes. Am J Gastroenterol. 1997;92:1501–1504. [PubMed] [Google Scholar]
- 23.Ananthakrishnan AN, Weber LR, Knox JF, Skaros S, Emmons J, Lundeen S, Issa M, Otterson MF, Binion DG. Permanent work disability in Crohn's disease. Am J Gastroenterol. 2008;103:154–161. doi: 10.1111/j.1572-0241.2007.01561.x. [DOI] [PubMed] [Google Scholar]
- 24.Reinisch W, Sandborn WJ, Bala M, Yan S, Feagan BG, Rutgeerts P, Radford-Smith G, Xu S, Eisenberg D, Olson A, et al. Response and remission are associated with improved quality of life, employment and disability status, hours worked, and productivity of patients with ulcerative colitis. Inflamm Bowel Dis. 2007;13:1135–1140. doi: 10.1002/ibd.20165. [DOI] [PubMed] [Google Scholar]
- 25.Bingefors K, Isacson D. Epidemiology, co-morbidity, and impact on health-related quality of life of self-reported headache and musculoskeletal pain--a gender perspective. Eur J Pain. 2004;8:435–450. doi: 10.1016/j.ejpain.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Cash B, Sullivan S, Barghout V. Total costs of IBS: employer and managed care perspective. Am J Manag Care. 2005;11:S7–S16. [PubMed] [Google Scholar]
- 27.Dean BB, Aguilar D, Barghout V, Kahler KH, Frech F, Groves D, Ofman JJ. Impairment in work productivity and health-related quality of life in patients with IBS. Am J Manag Care. 2005;11:S17–S26. [PubMed] [Google Scholar]
- 28.Leong SA, Barghout V, Birnbaum HG, Thibeault CE, Ben-Hamadi R, Frech F, Ofman JJ. The economic consequences of irritable bowel syndrome: a US employer perspective. Arch Intern Med. 2003;163:929–935. doi: 10.1001/archinte.163.8.929. [DOI] [PubMed] [Google Scholar]
- 29.Kivimäki M, Ferrie JE, Hagberg J, Head J, Westerlund H, Vahtera J, Alexanderson K. Diagnosis-specific sick leave as a risk marker for disability pension in a Swedish population. J Epidemiol Community Health. 2007;61:915–920. doi: 10.1136/jech.2006.055426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abell TL, Bernstein RK, Cutts T, Farrugia G, Forster J, Hasler WL, McCallum RW, Olden KW, Parkman HP, Parrish CR, et al. Treatment of gastroparesis: a multidisciplinary clinical review. Neurogastroenterol Motil. 2006;18:263–283. doi: 10.1111/j.1365-2982.2006.00760.x. [DOI] [PubMed] [Google Scholar]
- 31.Haag S, Senf W, Häuser W, Tagay S, Grandt D, Heuft G, Gerken G, Talley NJ, Holtmann G. Impairment of health-related quality of life in functional dyspepsia and chronic liver disease: the influence of depression and anxiety. Aliment Pharmacol Ther. 2008;27:561–571. doi: 10.1111/j.1365-2036.2008.03619.x. [DOI] [PubMed] [Google Scholar]
- 32.van Kerkhoven LA, Laheij RJ, Aparicio N, De Boer WA, Van den Hazel S, Tan AC, Witteman BJ, Jansen JB. Effect of the antidepressant venlafaxine in functional dyspepsia: a randomized, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol. 2008;6:746–752; quiz 718. doi: 10.1016/j.cgh.2008.02.051. [DOI] [PubMed] [Google Scholar]
- 33.Van Oudenhove L, Vandenberghe J, Geeraerts B, Vos R, Persoons P, Fischler B, Demyttenaere K, Tack J. Determinants of symptoms in functional dyspepsia: gastric sensorimotor function, psychosocial factors or somatisation? Gut. 2008;57:1666–1673. doi: 10.1136/gut.2008.158162. [DOI] [PubMed] [Google Scholar]
- 34.Drossman DA, Toner BB, Whitehead WE, Diamant NE, Dalton CB, Duncan S, Emmott S, Proffitt V, Akman D, Frusciante K, et al. Cognitive-behavioral therapy versus education and desipramine versus placebo for moderate to severe functional bowel disorders. Gastroenterology. 2003;125:19–31. doi: 10.1016/s0016-5085(03)00669-3. [DOI] [PubMed] [Google Scholar]
- 35.Tack J, Broekaert D, Fischler B, Van Oudenhove L, Gevers AM, Janssens J. A controlled crossover study of the selective serotonin reuptake inhibitor citalopram in irritable bowel syndrome. Gut. 2006;55:1095–1103. doi: 10.1136/gut.2005.077503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tack J, Sarnelli G. Serotonergic modulation of visceral sensation: upper gastrointestinal tract. Gut. 2002;51 Suppl 1:i77–i80. doi: 10.1136/gut.51.suppl_1.i77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tack J, Broekaert D, Coulie B, Fischler B, Janssens J. Influence of the selective serotonin re-uptake inhibitor, paroxetine, on gastric sensorimotor function in humans. Aliment Pharmacol Ther. 2003;17:603–608. doi: 10.1046/j.1365-2036.2003.01469.x. [DOI] [PubMed] [Google Scholar]
- 38.Gorelick AB, Koshy SS, Hooper FG, Bennett TC, Chey WD, Hasler WL. Differential effects of amitriptyline on perception of somatic and visceral stimulation in healthy humans. Am J Physiol. 1998;275:G460–G466. doi: 10.1152/ajpgi.1998.275.3.G460. [DOI] [PubMed] [Google Scholar]
- 39.Chial HJ, Camilleri M, Ferber I, Delgado-Aros S, Burton D, McKinzie S, Zinsmeister AR. Effects of venlafaxine, buspirone, and placebo on colonic sensorimotor functions in healthy humans. Clin Gastroenterol Hepatol. 2003;1:211–218. doi: 10.1053/jcgh.2003.50031. [DOI] [PubMed] [Google Scholar]
- 40.Quartero AO, Meineche-Schmidt V, Muris J, Rubin G, de Wit N. Bulking agents, antispasmodic and antidepressant medication for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2005:CD003460. doi: 10.1002/14651858.CD003460.pub2. [DOI] [PubMed] [Google Scholar]
- 41.Jackson JL, O'Malley PG, Tomkins G, Balden E, Santoro J, Kroenke K. Treatment of functional gastrointestinal disorders with antidepressant medications: a meta-analysis. Am J Med. 2000;108:65–72. doi: 10.1016/s0002-9343(99)00299-5. [DOI] [PubMed] [Google Scholar]
- 42.Lee KJ, Kim JH, Cho SW. Gabapentin reduces rectal mechanosensitivity and increases rectal compliance in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2005;22:981–988. doi: 10.1111/j.1365-2036.2005.02685.x. [DOI] [PubMed] [Google Scholar]
- 43.Houghton LA, Fell C, Whorwell PJ, Jones I, Sudworth DP, Gale JD. Effect of a second-generation alpha2delta ligand (pregabalin) on visceral sensation in hypersensitive patients with irritable bowel syndrome. Gut. 2007;56:1218–1225. doi: 10.1136/gut.2006.110858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones KL, Wishart JM, Berry MK, Abitbol JL, Horowitz M. Effects of fedotozine on gastric emptying and upper gastrointestinal symptoms in diabetic gastroparesis. Aliment Pharmacol Ther. 2000;14:937–943. doi: 10.1046/j.1365-2036.2000.00790.x. [DOI] [PubMed] [Google Scholar]
- 45.Talley NJ, Choung RS, Camilleri M, Dierkhising RA, Zinsmeister AR. Asimadoline, a kappa-opioid agonist, and satiation in functional dyspepsia. Aliment Pharmacol Ther. 2008;27:1122–1131. doi: 10.1111/j.1365-2036.2008.03676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bielefeldt K, Christianson JA, Davis BM. Basic and clinical aspects of visceral sensation: transmission in the CNS. Neurogastroenterol Motil. 2005;17:488–499. doi: 10.1111/j.1365-2982.2005.00671.x. [DOI] [PubMed] [Google Scholar]
- 47.Bielefeldt K, Davis BM. Differential effects of ASIC3 and TRPV1 deletion on gastroesophageal sensation in mice. Am J Physiol Gastrointest Liver Physiol. 2008;294:G130–G138. doi: 10.1152/ajpgi.00388.2007. [DOI] [PubMed] [Google Scholar]
- 48.Talley NJ, Locke GR 3rd, Lahr BD, Zinsmeister AR, Tougas G, Ligozio G, Rojavin MA, Tack J. Functional dyspepsia, delayed gastric emptying, and impaired quality of life. Gut. 2006;55:933–939. doi: 10.1136/gut.2005.078634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Revicki DA, Rentz AM, Tack J, Stanghellini V, Talley NJ, Kahrilas P, De La Loge C, Trudeau E, Dubois D. Responsiveness and interpretation of a symptom severity index specific to upper gastrointestinal disorders. Clin Gastroenterol Hepatol. 2004;2:769–777. doi: 10.1016/s1542-3565(04)00348-9. [DOI] [PubMed] [Google Scholar]
- 50.Abell T, McCallum R, Hocking M, Koch K, Abrahamsson H, Leblanc I, Lindberg G, Konturek J, Nowak T, Quigley EM, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125:421–428. doi: 10.1016/s0016-5085(03)00878-3. [DOI] [PubMed] [Google Scholar]
- 51.Jones MP. Is gastric electrical stimulation an effective therapy for patients with drug-refractory gastroparesis? Nat Clin Pract Gastroenterol Hepatol. 2008;5:368–370. doi: 10.1038/ncpgasthep1157. [DOI] [PubMed] [Google Scholar]
- 52.Lin Z, Forster J, Sarosiek I, McCallum RW. Effect of high-frequency gastric electrical stimulation on gastric myoelectric activity in gastroparetic patients. Neurogastroenterol Motil. 2004;16:205–212. doi: 10.1111/j.1365-2982.2004.00503.x. [DOI] [PubMed] [Google Scholar]
- 53.Rashed H, Cutts T, Abell T, Cowings P, Toscano W, El-Gammal A, Adl D. Predictors of response to a behavioral treatment in patients with chronic gastric motility disorders. Dig Dis Sci. 2002;47:1020–1026. doi: 10.1023/a:1015029805498. [DOI] [PubMed] [Google Scholar]