Abstract
AIM: To detect the prevalence of small bowel polyps by wireless capsule endoscopy (WCE) in patients with familial adenomatous polyposis (FAP).
METHODS: We examined prospectively 14 patients with FAP to assess the location, size and number of small-intestinal polyps. Patients’ age, sex, years of observation after surgery, type of surgery, duodenal polyps and colorectal cancer at surgery were analyzed.
RESULTS: During WCE, polyps were detected in 9/14 (64.3%) patients. Duodenal adenomatous polyps were found in nine (64.3%) patients, and jejunal and ileal polyps in seven (50%) and eight (57.1%), respectively. The Spigelman stage of duodenal polyposis was associated with the presence of jejunal and ileal polyps. Identification of the ampulla of Vater was not achieved with WCE. Importantly, the findings of WCE had no immediate impact on the further clinical management of FAP patients. No procedure-related complications were observed in the patients.
CONCLUSION: WCE is a promising noninvasive new method for the detection of small-intestinal polyps. Further investigation is required to determine which phenotype of FAP is needed for surveillance with WCE.
Keywords: Wireless capsule endoscopy, Familial adenomatous polyposis, Intestinal polyps, Duodenal neoplasms, Adenoma
INTRODUCTION
Familial adenomatous polyposis (FAP) is a dominant inherited syndrome with an incidence of 1 in 11 000. It is caused by an alteration of the FAP (APC) gene that is located on chromosome 5q21, which causes multiple disorders of the development of the ecto-, endo- and mesoderm. The syndrome is characterized by the presence of adenomatous polyps in the gastrointestinal tract, mainly in the colon, rectum and duodenum, with a demonstrated adenoma-carcinoma sequence[1-3]. The duodenum is characterized by the presence of adenomas in 80% of patients with FAP and the development of periampullary cancer in 4%[4,5]. In patients who have undergone colectomy, periampullary cancer is the main cause of death. Between five and 10% of FAP patients die from upper gastrointestinal cancer, which is frequently periampullary in origin. In an attempt to prevent malignancy, a screening program appears to be mandatory to detect particularly those patients most at risk of developing the disease. Therefore, endoscopic surveillance of the second part of the duodenum with side-viewing endoscopy is advised. Since it was introduced in 2000, wireless capsule endoscopy (WCE) has opened the way for the noninvasive and painless test of the entire small intestine, thereby becoming the gold standard for endoscopic evaluation of the small bowel in several clinical situations, including surveillance of polyposis syndromes. There have been only a few studies that have evaluated the utility of WCE in detecting small-intestinal polyps in patients with FAP[6-12].
The aim of our prospective study was to investigate the diagnostic yield of WCE in being able to detect adenomatous polyps in a Greek FAP cohort,and to establish potential risk factors for small-bowel polyp development for a more targeted surveillance with WCE.
MATERIALS AND METHODS
We performed an open prospective, non-randomized clinical trial from September 2007 to September 2008, which evaluated the use of WCE in FAP patients. The study was conducted in accordance with good clinical practice, as set forth by the Helsinki agreements and their later amendments. The study was approved by our hospital Ethics Committee and informed consent was obtained from all patients.
We included male and female patients, aged 18-70 years, who were referred to our clinic. Patients excluded were those with severe swallowing disorders, implanted cardiac pacemaker or other electronic devices, pregnant women, patients with a clinical suspicion of small-bowel obstruction/pseudo-obstruction, strictures or fistulas, and children under 10 years old[13,14].
The following information was gathered from patients’ records: age, sex, diagnostic [endoscopy, small-bowel radiography, computed tomography (CT)] and surgical procedures (type of colectomy and time of surgery) before WCE. All the procedures were performed on an outpatient basis, in the morning, after an overnight fast. Bowel preparation was performed with 4 L polyethylene glycol solution given 15 h before the procedure. Patients were allowed to drink clear fluids 2 h after capsule ingestion. Furthermore, the patients were able to maintain their normal activities while the capsule was passing through the digestive tract. Patients returned to the hospital 8 h after capsule ingestion. The registration device and the antennae were disconnected from the patient and a questionnaire about symptom occurrence and overall satisfaction with the procedure was completed. On each of the 2 d following the procedure, a telephone call was made to inquire about any symptoms and to confirm that the capsule had been expelled. In view that the major risk from WCE is capsule retention or impaction, all patients were instructed to contact the study staff should they develop any gastrointestinal symptoms during or after WCE.
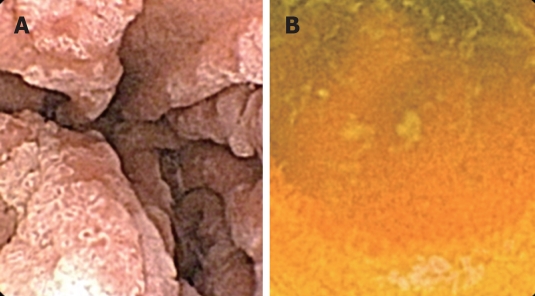
Capsule videorecordings were reviewed by a single experienced endoscopist (Katsinelos P) who previously had performed more than 200 WCE procedures. A polyp was defined as a discrete mass of tissue that protruded into the bowel lumen. The location of small-bowel polyps was approximately estimated as duodenal (Figure 1A), jejunal, or ileal (Figure 1B), according to the timing of polyp appearance after entrance of the capsule to the duodenum, the total small-bowel passage time, and the endoscopic appearance of the small-intestinal mucosa. Entrance to the duodenum is easy to detect because it begins just after the pylorus, which can be identified easily. The location of small-bowel polyps was estimated by analyzing the WCE transit time between pylorus passage and ileocecal valve or pouch ileostoma. The duodenum was designated to be the small bowel that was visualized during the first 15 min after the capsule exited the pylorus, while the jejunum and ileum were designated to be the small bowel that was visualized after < 50% and > 50% of small-bowel transit time, respectively. Moreover, the prominent folds and high narrow villi characterized the jejunum, while fewer folds and shorter villi were observed in the ileum. WCE allows only an approximate estimation of the size of polyps, therefore, based on previous experience[11], we classified polyps as small or large, using an open pylorus orifice (diameter 10 mm) as a reference for polyp size estimation. Small and large polyps were classified with a diameter < 10 mm and > 10 mm, respectively.
Figure 1.
Wireless capsule endoscopy (WCE) view. A: Large and small mushroom-shaped adenomas in the distal duodenum; B: Small ileal polyp.
Following WCE, conventional endoscopy was performed within 2 wk in all patients. Standard duodenoscopy up to the second part of the duodenum was performed with a forward-looking gastroscope and a side-viewing duodenoscope, on an outpatient basis. To reduce motility artifacts, 20 mg butylscopolamine were administered intravenously. Biopsies and polypectomies were performed for staging of duodenal disease according to the Spigelman classification (Table 1)[15].
Table 1.
Spigelman classification of duodenal polyposis (adenomas in FAP)[15]
|
Number of points |
|||
| 1P | 2P | 3P | |
| Number of polyps | 1-4 | 5-20 | > 20 |
| Polyp size (mm) | 1-4 | 5-20 | > 10 |
| Histology | Tubulous | Tubulovillous | Villous |
| Dysplasia | Mild | Moderate | Severe |
| Stage | Spigelman score | ||
| 0 | 0 | ||
| I | 1-4 | ||
| II | 5-6 | ||
| III | 7-8 | ||
| IV | 9-12 | ||
The primary end point of the study was to identify the number and size of small-bowel polyps in each patient, and the secondary end point was the impact of WCE findings on the management of the patients.
RESULTS
Fourteen patients (9 men, median age 34 years, range: 22-56 years) with FAP were recruited. Eight patients had undergone total proctocolectomy with ileal-pouch-anal anastomosis, four had undergone ileorectal anastomosis, and two were examined before colectomy (Table 2).
Table 2.
Clinical characteristics of the 14 patients with FAP studied with WCE
| Patient No. | Sex | Age (yr) | Time of surgery before WCE (yr) | Type of surgery | No. of colon polyps | Colon cancer |
| 1 | M | 54 | 4 | IPAA | > 1000 | No |
| 2 | F | 23 | BS | BS | > 100 | No |
| 3 | M | 53 | 5 | IPAA | > 100 | No |
| 4 | F | 27 | 5 | IPAA | > 100 | No |
| 5 | M | 28 | 4 | IPAA | > 100 | No |
| 6 | M | 22 | BS | BS | > 100 | No |
| 7 | M | 53 | 2 | IRA | > 1000 | No |
| 8 | F | 26 | 3 | IPAA | > 100 | No |
| 9 | M | 56 | 18 | IRA | > 1000 | No |
| 10 | F | 29 | 10 | IPAA | > 100 | No |
| 11 | M | 54 | 4 | IPAA | > 1000 | Yes |
| 12 | M | 36 | 15 | IRA | > 100 | No |
| 13 | F | 41 | 17 | IRA | > 100 | No |
| 14 | M | 32 | 7 | IPAA | > 100 | No |
FAP: Familial adenomatous polyposis; WCE: Wireless capsule endoscopy; IPAA: Ileal pouch-anal anastomosis; IRA: Ileorectal anastomosis; BS: Before surgery.
Endoscopic investigation of the entire length of the small bowel was achieved in all patients. The quality was considered as good except for one case in which food debris in the duodenum, jejunum and ileum made reading the film very difficult; in the last case, the procedure was repeated and the patient was asked to avoid food intake prior to the examination. Mean gastric and small-bowel transit time were 36 (range: 12-58) and 256 (range: 128-360) min, respectively. No abnormal additional findings were identified. Overall, 81 polyps, mainly small (96.3%), were detected by WCE. The presence, size and location of duodenal, jejunal and ileal polyps were related to the Spigelman stage of duodenal polyposis and age of the patient, but not sex (Table 3). None of the five young FAP subjects with Spigelman stage 0 had small-bowel polyps detected. Three large sessile polyps were found in the duodenum in one patient with Spigelman stage IV disease. All other polyps detected were small (Table 3). WCE was inferior diagnostically to standard duodenoscopy and gastroscopy regarding the second part of the duodenum and especially the ampullary region. The capsule technique could not identify the papilla of Vater in any of our patients and four small ampullary adenomas were missed by WCE as compared with duodenoscopy. Endoscopic polypectomy of duodenal adenomas was performed in five patients, and biopsies were taken from the rest of the patients. Histological examination of the specimens confirmed the diagnosis of tubular or tubulovillous adenomas with low-grade dysplasia in one case with large polyps. We detected small ileal white-colored polyps with a normally appearing mucosal surface in two young patients (both Spigelman stage 0), and we classified these lesions as lymphoid hyperplasia, which occurs commonly in the terminal ileum and rectum associated with FAP, especially in young patients[16].
Table 3.
Distribution and size of polyps according to Spigelman stage as assessed by WCE
| Patient No. | Sex | Spigelman stage | Duodenal | Jejunal | Ileal | Rectal stump |
| 1 | M | IV | 13 | 3 | 5 | No |
| 2 | M | 0 | ||||
| 3 | F | II | 3 | 2 | 4 | No |
| 4 | M | I | 3 | 2 | No | |
| 5 | F | I | 2 | 1 | 3 | No |
| 6 | M | 0 | No | |||
| 7 | M | III | 4 | 4 | 3 | Yes |
| 8 | F | 0 | Yes | |||
| 9 | M | III | 7 | 3 | 2 | Yes |
| 10 | M | 0 | No | |||
| 11 | M | II | 4 | 1 | 2 | Yes |
| 12 | M | I | 3 | No | ||
| 13 | F | I | 4 | 1 | 2 | No |
| 14 | M | 0 | No |
All the patients described the procedure as comfortable and were willing to repeat it had it been deemed necessary. Difficulty/inability to swallow the capsule or clinically significant complications, including symptomatic capsule retention and aspiration, did not occur during the procedure. Five patients previously had undergone enteroclysis, and although a comparison of the two methods was not within the scope of this study, as there was a significant time lapse between them, all the patients preferred WCE when they were asked to compare it with enteroclysis. All patients reported no pain or discomfort when contacted 1 wk after the WCE examination.
DISCUSSION
Small-intestinal adenomas can occur in FAP patients, but their prevalence varies, depending of the modality used for their detection[17]. The advent of WCE in 2000 has changed noticeably the diagnosis and management of numerous diseases of the small intestine, including polyps associated with FAP[14]. Our study shows that WCE is able to detect even small polyps in the entire small intestine in subjects with FAP. We found jejunal and ileal polyps to be common. The frequency and number of polyps and the length of small bowel involvement was found to increase with Spigelman classification (Table 3). All polyps were small except for three in the duodenum. These findings were similar to those previously reported by other studies[6-11], although Iaquino et al[12] have found the presence of duodenal adenomas to be the only clinical feature predictive of small-intestinal adenoma, but not associated with Spigelman stage. We cannot define the true sensitivity of WCE for detection of small-bowel adenomas because the lack of visualization of the entire small-bowel mucosa by WCE leads to underestimation of polyp burden. To achieve this, we would have to compare the performance of WCE with that of the criterion standard of surgical enteroscopy. However, given the invasiveness and the high morbidity rate of the latter procedure, such a study would be extremely difficult to perform. The advent of double or single balloon enteroscopy of the small bowel may have opened a new avenue to gain less invasive access, even to polyps located in the distal small bowel. Double balloon enteroscopy appears to be equivalent to an intraoperative enteroscopy for scrutiny of small-intestinal polyps in FAP[17]. The region around the papilla of Vater was not visualized in any of our patients, which calls for the mandatory use of side-view duodenoscopy for staging duodenal disease.
Magnetic resonance enteroclysis combines the advantages of cross-sectional resonance with those of the volume challenge of conventional enteroclysis in the recognition and characterization of small-bowel-wall abnormalities, including initial tumors. There are few promising reports about the role of magnetic resonance enteroclysis and CT enteroclysis in the diagnostic algorithm of small-bowel neoplasms[18]. Whether the use of WCE in combination with these new diagnostic techniques will lead to earlier diagnosis of small-intestinal polyps in FAP patients remains to be elucidated in the future.
We observed no complications from WCE in our study. Other reports of WCE performed in individuals with FAP also have failed to detect any complications[6-12].
Forward and side-viewing endoscopic surveillance for gastric and duodenal/periampullary neoplasia is recommended for all individuals with FAP[19,20]. The frequency of surveillance should be based on the Spigelman classification of duodenal polyposis[19,20]. However, the implication of jejunal and ileal adenomas in FAP is unknown. The risk of cancer distal to the duodenum in FAP has been reported much more rarely than that of duodenal and periampullary carcinoma[21]. The lack of data may rely on the fact that patients with FAP usually are not studied because of the low incidence of non-duodenal small-bowel cancer[21]. Therefore, should a search for small bowel adenomas with WCE be performed in all patients with FAP? Keeping in mind the high cost of WCE, identification of a subset of FAP patients who might be at the highest risk for developing small bowel tumor is desired. The analysis of germline APC gene mutation was not available in our patients, to compare with WCE findings. However, as reported by other investigators, the incidence of small-intestinal adenomas is correlated with mutations found in exon 15[22]. Mutations in this exon traditionally have been associated with a more aggressive phenotype[22,23]. The identification of genotypic factors that predict the phenotype of small-bowel adenomas is important. It has been suggested that WCE should be performed only in patients with exon 15 mutations[12], thereby requiring relative WCE surveillance. This approach may allow for a more cost-effective evaluation of FAP patients. Obviously, the current genotype-phenotype correlation must be confirmed in a larger cohort of FAP patients.
The frequency of WCE surveillance of jejunal and ileal adenomatous polyps in patients with FAP remains unknown. The detection of these small polyps in our study and previous studies had no immediate impact on the clinical management, other than establishing further surveillance intervals in these patients[6-12]. The tendency is for WCE to become the standard imaging modality for small-bowel surveillance, since Spigelman stage III and IV patients have a high burden of small-intestinal adenomas on WCE (Table 3). With the potential exception of the mentioned high risk of FAP patients developing small-bowel cancer, we recommend surveillance every 3-5 years in these patients; despite more data on the prevalence of small-bowel polyps in patients with advanced stage (III or IV) duodenal polyposis being needed to understand the utility of WCE in these groups. The small number of polyps observed in our FAP patients with Spigelman stage 0-II disease (Table 3) is in accordance with other studies[6-8]. We agree with other investigators’[6-10] recommendations that WCE is not useful for routine small-bowel surveillance in these patients. Although management of jejunal and ileal polyps has not as yet been well defined considering the adenomatous nature of polyps in FAP, it seems reasonable to remove these polyps that are easily accessible by endoscopy. Whenever endoscopic polypectomy cannot be performed, although there is not enough evidence to propose surgical resection, surveillance with WCE seems advisable.
In conclusion, WCE is noninvasive, safe and comfortable, and can be performed on an ambulatory basis in FAP patients. It is effective for the detection of small-bowel polyps, but larger studies are needed to define better the impact of WCE on the clinical outcome of FAP patients with small-intestinal polyps, to elaborate which mutant gene carries the highest prevalence of small-intestinal adenomas, and to decide the timing of surveillance and polypectomy treatment by double or single balloon enteroscopy.
COMMENTS
Background
Endoscopic surveillance of the duodenum and periampullary area is recommended in patients with familiar adenomatous polyposis (FAP), because 4% of patients develop cancer. However, the significance of the presence of jejunal and ileal polyps in patients with FAP is unknown.
Research frontiers
FAP is a dominant inherited syndrome characterized by the presence of adenomatous polyps in the gastrointestinal tract, with a demonstrated adenoma-carcinoma sequence. In the present study, the authors investigated the diagnostic utility of wireless capsule endoscopy (WCE) in detecting small intestine polyps in a Greek FAP cohort.
Innovations and breakthroughs
Few studies have evaluated the utility of WCE in detecting small-intestinal polyps and their clinical significance in patients with FAP. The rate of detection of small polyps in our patients was high but had no immediate impact on clinical management, other than establishing further surveillance intervals in these patients.
Applications
This study represents a new role for WCE in the examination of the small intestine in FAP patients and emphasizes the need for a highly targeted surveillance based on Spigleman classification.
Terminology
WCE is a technology that uses a swallowed video capsule to take photographs of the inside of the esophagus, stomach, and small intestine. Since it was introduced in 2000, it has become the gold standard for endoscopic evaluation of the small bowel in several clinical situations, including surveillance of polyposis syndromes.
Peer review
This is an interesting observational study of the role of WCE in screening for small-intestinal polyps in a small cohort of patients with established FAP.
Footnotes
Peer reviewer: John K Marshall, MD, Associate Professor of Medicine, Division of Gastroenterology (4W8), McMaster University Medical Centre, 1200 Main Street West, Hamilton, Ontario L8N 3Z5, Canada
S- Editor Cheng JX L- Editor Kerr C E- Editor Ma WH
References
- 1.Bodmer WF, Bailey CJ, Bodmer J, Bussey HJ, Ellis A, Gorman P, Lucibello FC, Murday VA, Rider SH, Scambler P. Localization of the gene for familial adenomatous polyposis on chromosome 5. Nature. 1987;328:614–616. doi: 10.1038/328614a0. [DOI] [PubMed] [Google Scholar]
- 2.Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB, Smith KJ, Preisinger AC, Hedge P, McKechnie D. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- 3.Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H, Joslyn G, Stevens J, Spirio L, Robertson M. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66:589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- 4.Burke CA, Beck GJ, Church JM, van Stolk RU. The natural history of untreated duodenal and ampullary adenomas in patients with familial adenomatous polyposis followed in an endoscopic surveillance program. Gastrointest Endosc. 1999;49:358–364. doi: 10.1016/s0016-5107(99)70013-1. [DOI] [PubMed] [Google Scholar]
- 5.Bjork J, Akerbrant H, Iselius L, Bergman A, Engwall Y, Wahlstrom J, Martinsson T, Nordling M, Hultcrantz R. Periampullary adenomas and adenocarcinomas in familial adenomatous polyposis: cumulative risks and APC gene mutations. Gastroenterology. 2001;121:1127–1135. doi: 10.1053/gast.2001.28707. [DOI] [PubMed] [Google Scholar]
- 6.Schulmann K, Hollerbach S, Kraus K, Willert J, Vogel T, Moslein G, Pox C, Reiser M, Reinacher-Schick A, Schmiegel W. Feasibility and diagnostic utility of video capsule endoscopy for the detection of small bowel polyps in patients with hereditary polyposis syndromes. Am J Gastroenterol. 2005;100:27–37. doi: 10.1111/j.1572-0241.2005.40102.x. [DOI] [PubMed] [Google Scholar]
- 7.Mata A, Llach J, Castells A, Rovira JM, Pellise M, Gines A, Fernandez-Esparrach G, Andreu M, Bordas JM, Pique JM. A prospective trial comparing wireless capsule endoscopy and barium contrast series for small-bowel surveillance in hereditary GI polyposis syndromes. Gastrointest Endosc. 2005;61:721–725. doi: 10.1016/s0016-5107(05)00289-0. [DOI] [PubMed] [Google Scholar]
- 8.Wong RF, Tuteja AK, Haslem DS, Pappas L, Szabo A, Ogara MM, DiSario JA. Video capsule endoscopy compared with standard endoscopy for the evaluation of small-bowel polyps in persons with familial adenomatous polyposis (with video) Gastrointest Endosc. 2006;64:530–537. doi: 10.1016/j.gie.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 9.Caspari R, von Falkenhausen M, Krautmacher C, Schild H, Heller J, Sauerbruch T. Comparison of capsule endoscopy and magnetic resonance imaging for the detection of polyps of the small intestine in patients with familial adenomatous polyposis or with Peutz-Jeghers’ syndrome. Endoscopy. 2004;36:1054–1059. doi: 10.1055/s-2004-826041. [DOI] [PubMed] [Google Scholar]
- 10.Burke CA, Santisi J, Church J, Levinthal G. The utility of capsule endoscopy small bowel surveillance in patients with polyposis. Am J Gastroenterol. 2005;100:1498–1502. doi: 10.1111/j.1572-0241.2005.41506.x. [DOI] [PubMed] [Google Scholar]
- 11.Barkay O, Moshkowitz M, Fireman Z, Shemesh E, Goldray O, Revivo M, Kessler A, Halpern Z, Orr-Urtreger A, Arber N. Initial experience of videocapsule endoscopy for diagnosing small-bowel tumors in patients with GI polyposis syndromes. Gastrointest Endosc. 2005;62:448–452. doi: 10.1016/s0016-5107(05)01582-8. [DOI] [PubMed] [Google Scholar]
- 12.Iaquinto G, Fornasarig M, Quaia M, Giardullo N, D’Onofrio V, Iaquinto S, Di Bella S, Cannizzaro R. Capsule endoscopy is useful and safe for small-bowel surveillance in familial adenomatous polyposis. Gastrointest Endosc. 2008;67:61–67. doi: 10.1016/j.gie.2007.07.048. [DOI] [PubMed] [Google Scholar]
- 13.Storch I, Barkin JS. Contraindications to capsule endoscopy: do any still exist? Gastrointest Endosc Clin N Am. 2006;16:329–336. doi: 10.1016/j.giec.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 14.Waterman M, Eliakim R. Capsule enteroscopy of the small intestine. Abdom Imaging. 2009;34:452–458. doi: 10.1007/s00261-008-9431-5. [DOI] [PubMed] [Google Scholar]
- 15.Spigelman AD, Williams CB, Talbot IC, Domizio P, Phillips RK. Upper gastrointestinal cancer in patients with familial adenomatous polyposis. Lancet. 1989;2:783–785. doi: 10.1016/s0140-6736(89)90840-4. [DOI] [PubMed] [Google Scholar]
- 16.Dorazio RA, Whelan TJ Jr. Lymphoid hyperplasia of the terminal ileum associated with familial polyposis coli. Ann Surg. 1970;171:300–302. doi: 10.1097/00000658-197002000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsumoto T, Esaki M, Yanaru-Fujisawa R, Moriyama T, Yada S, Nakamura S, Yao T, Iida M. Small-intestinal involvement in familial adenomatous polyposis: evaluation by double-balloon endoscopy and intraoperative enteroscopy. Gastrointest Endosc. 2008;68:911–919. doi: 10.1016/j.gie.2008.02.067. [DOI] [PubMed] [Google Scholar]
- 18.Pennazio M, Rondonotti E, de Franchis R. Capsule endoscopy in neoplastic diseases. World J Gastroenterol. 2008;14:5245–5253. doi: 10.3748/wjg.14.5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burke C. Risk stratification for periampullary carcinoma in patients with familial adenomatous polyposis: does theodore know what to do now? Gastroenterology. 2001;121:1246–1248. doi: 10.1053/gast.2001.29265. [DOI] [PubMed] [Google Scholar]
- 20.Groves CJ, Saunders BP, Spigelman AD, Phillips RK. Duodenal cancer in patients with familial adenomatous polyposis (FAP): results of a 10 year prospective study. Gut. 2002;50:636–641. doi: 10.1136/gut.50.5.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allen BA, Terdiman JP. Hereditary polyposis syndromes and hereditary non-polyposis colorectal cancer. Best Pract Res Clin Gastroenterol. 2003;17:237–258. doi: 10.1016/s1521-6918(02)00149-x. [DOI] [PubMed] [Google Scholar]
- 22.Friedl W, Caspari R, Sengteller M, Uhlhaas S, Lamberti C, Jungck M, Kadmon M, Wolf M, Fahnenstich J, Gebert J, et al. Can APC mutation analysis contribute to therapeutic decisions in familial adenomatous polyposis? Experience from 680 FAP families. Gut. 2001;48:515–521. doi: 10.1136/gut.48.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bertario L, Russo A, Sala P, Varesco L, Giarola M, Mondini P, Pierotti M, Spinelli P, Radice P. Multiple approach to the exploration of genotype-phenotype correlations in familial adenomatous polyposis. J Clin Oncol. 2003;21:1698–1707. doi: 10.1200/JCO.2003.09.118. [DOI] [PubMed] [Google Scholar]