Abstract
AIM: To evaluate whether adding azithromycin to first-line Helicobacter pylori (H pylori) eradication improved eradication and reduced side effects.
METHODS: Eligible articles were identified by searches of electronic databases. We included all randomized trials that compared azithromycin-containing with standard triple-therapy regimens for first-line treatment of H pylori infection. Statistical analysis was performed with Review Manager 5.0.10. Sub-analyses were also performed.
RESULTS: We identified 14 randomized trials (1431 patients). Pooled H pylori eradication rates were 72.01% (95% CI: 58.09%-85.93%) and 69.78% (95% CI: 66.47%-73.09%) for patients with or without azithromycin by intention-to-treat analysis, and the odds ratio (OR) was 1.17 (95% CI: 0.64-2.14). The occurrence of side effects differed significantly and was 15.81% (95% CI: 12.50%-19.12%) and 25.20% (95% CI: 21.44%-28.96%) for treatment with or without azithromycin, respectively, and the summary OR was 0.58 (95% CI: 0.41-0.82). Furthermore, the azithromycin-containing group had a lower occurrence of diarrhea, nausea and taste disturbance.
CONCLUSION: Our review suggests that azithromycin-containing triple-therapy regimens could be equally effective in eradication of H pylori compared with standard first-line triple-therapy regimens.
Keywords: Azithromycin, Helicobacter pylori, Combination drug therapy, Adverse effects
INTRODUCTION
Infection caused by Helicobacter pylori (H pylori), one of the most common pathogens worldwide, causes chronic gastritis and increases the risk of peptic ulcer and gastric cancer. Although some H pylori-positive individuals are asymptomatic, many experience symptoms such as dyspepsia. It is increasingly common to screen patients, even those with mild symptoms, for H pylori infection, and to treat them actively. The first-line treatment for H pylori infection, as recommended by the Maastricht III Consensus Report, is 7-d triple therapy that includes clarithromycin, amoxicillin and a proton-pump inhibitor (PPI)[1]. Even though this triple therapy is effective and its short duration helps maintain patient compliance, a considerable number of patients experience undesirable side effects.
In first-line therapy, eradication rates using combinations of PPI-based triple therapies range from 75% to 98%, with most of them near 80%[2]. This signifies that up to 20% of patients are expected to be treatment failures, a value which could be even higher in areas with a high prevalence of resistant H pylori strains. The recommended second-line therapy is a quadruple regimen composed of tetracycline, metronidazole, bismuth salts and a PPI; however, the efficacy of this regimen is limited by poor compliance, treatment duration, number and dose of the prescribed drugs, and bacterial antibiotic resistance.
Gastroenterologists and microbiologists continue the search for new therapies because of the increasing number of target subjects for H pylori and the physiological and pharmacoeconomic burden of a second course of therapy. Among the new options against H pylori brought to light recently, azithromycin has attracted substantial interest. Azithromycin is a macrolide antibiotic that has been shown to reach high concentrations in gastric tissue after oral administration; furthermore, these high concentrations are maintained for several days, which make it potentially useful in the eradication of H pylori[3]. Clinical trials with triple therapy regimens that contain azithromycin have reported eradication rates of approximately 60%-80%, depending on the regimen and azithromycin dose used[4,5]. However, results from some other available trials utilizing azithromycin have yielded conflicting results. The primary aim of the present meta-analysis was to evaluate whether adding azithromycin to H pylori eradication regimens could improve eradication and reduce side effects.
MATERIALS AND METHODS
Selection of studies
Studies evaluating azithromycin-containing triple therapy for the eradication of H pylori were considered. For the meta-analysis, the selection criteria were as follows: (1) articles that reported comparative randomized controlled trials (RCTs); (2) studies had to include at least two branches of treatment that consisted of (a) triple first-line therapy (one PPI and two antibiotics) and (b) azithromycin-containing triple regimen; (3) study population consisted of subjects who had never been treated for H pylori infection previously; and (4) data for successful eradication and/or side effects were available.
Search strategy for identification of studies
Trials were identified by searching the Cochrane Controlled Trials Register (Issue 2, 2009), PubMed (1966 to May 2009), Embase (1980 to May 2009), Science Citation Index (1945 to May 2009) and the Chinese Biomedical Database (1981 to May 2009). A search strategy was constructed by using a combination of the following words: (Helicobacter pylori OR H pylori) AND (azithromycin). Articles published in any language were included. Reference lists from the trials selected by electronic searching were hand-searched to identify further relevant trials. We also conducted a manual search of abstracts from 1995 to May 2009 from the following congresses: International Workshop of the European Helicobacter Study Group, American Digestive Disease Week (DDW), and United European Gastroenterology Week (UEGW). Abstracts of the articles selected in each of these multiple searches were reviewed and those meeting the inclusion criteria were recorded. References of reviews on H pylori treatment with azithromycin, and from the articles selected for the study, were also examined for articles that met the inclusion criteria. Authors of some identified trials were asked whether they knew of additional studies, including unpublished randomized ones. In case of duplicate reports, or studies obviously reporting results from the same study population, only the latest published results were used.
Data extraction
Standardized data abstraction sheets were prepared. Data were exacted for study quality, dose and duration of azithromycin treatment, anti-H pylori regimens, and the number, sex and age of enrolled subjects, diagnostic methods of testing H pylori infection before enrolling and after completing the study, and scoring systems for assessing side effects. Key outcome data, such as eradication rates, occurrence of diarrhea, nausea, taste disturbance and abdominal pain were abstracted from all included studies. All articles were examined independently for eligibility by two reviewers. Disagreements were resolved by consulting a third reviewer. Quality was assessed using the Jadad score system based on three items, randomization, double blinding and description of withdrawals/dropouts. We considered that they were low quality when scores were < 3.
Data synthesis
Data were entered into the Cochrane Collaboration review manager programme RevMan 5.0.10 (released on May 16, 2008). The outcome measure examined was the OR of improving H pylori eradication rates and reducing side effects with azithromycin compared to without azithromycin-containing triple regimens. Categorical variables were compared with the χ2 test, and P < 0.05 was considered statistically significant. Eradication rates and side effects were analyzed based on a fixed-effects model using the methods of Mantel-Haenszel[6], both by intention-to-treat and per-protocol. Heterogeneity between the studies was assessed by χ2 test. Statistical significance of heterogeneity was set at 0.10. If significant heterogeneity existed, it would have been inappropriate to combine the data for further analysis using a fixed-effects model, while the random model was used for calculations.
Sub-analyses
In the meta-analysis, sub-analyses of H pylori eradication efficacy were planned, depending on: (1) the type of drugs co-prescribed with azithromycin (combination with amoxicillin and a PPI was the most widely prescribed); (2) the duration and dose of azithromycin therapy; (3) age of the subjects involved; and (4) quality of the studies (based on quality score proposed by Jadad, see appropriate section). Finally, we used funnel plot asymmetry to detect any publication bias in the meta-analysis, and Egger’s regression test to measure funnel plot asymmetry.
RESULTS
Description of the studies
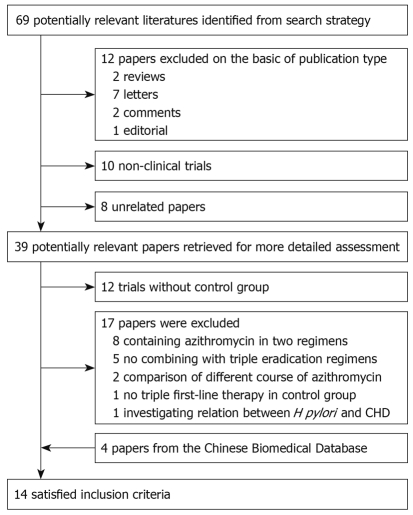
The bibliographical search yielded a total of 69 studies. Of these, 12 articles were excluded owing to publication type, i.e. two reviews, seven letters, two comments, and one editorial. We excluded 18 articles (10 non-clinical trials and eight unrelated articles) after examining the title and abstract, which left 39 potentially relevant articles for more detailed assessment. Of these potential eligible articles, 12 trials without a control group were excluded, and then we excluded another 17 articles, because of no combining with triple eradication regimens[7-11], containing azithromycin in two regimens[12-19], comparison of different treatment course of azithromycin[20,21], no triple first-line therapy in control group[22], and investigating relation between H pylori eradication and coronary heart disease[23]. Furthermore, we identified four additional articles from the Chinese Biomedical Database (1981 to May 2009). Finally, 14 RCTs met the inclusion criteria[24-37]. The flowchart of reviews showed the detailed process of selection (Figure 1). The characteristics of 14 trials included in the meta-analysis are summarized in Table 1, including quality score.
Figure 1.
Flowchart of study selection. H pylori: Helicobacter pylori.
Table 1.
Characteristics of included studies comparing Helicobacter pylori (H pylori) eradication efficacy of azithromycin-containing triple therapy versus standard triple therapy
| Authors | Country | Form | Trial design | Case No. (Az/con) | Patients | Diagnostic methods | Azithromycin regimen | % Eradication (n) | % Adverse effects (n) | Triple therapy | Days of antibiotics | % Eradication (n) | % Adverse effects (n) | Q |
| Lu et al[24], 2007 | China | JA | Single centre RCT | 85 (43/42) | H pylori positive | RUT/ | O (20 mg bid) | ITT 84 (36/43) | 26 (11/43) | O (20 mg bid) | 7 | ITT 69 (29/42) | 19 (8/42) | 3 |
| Chronic active gastritis | RUT (30 d later) | Lev (200 mg bid) | PP 90 (36/40) | A (1 g bid) | PP 72.5 (29/40) | |||||||||
| Az (500 mg o.d.) | C (500 mg bid) | |||||||||||||
| Kang et al[25], 2006 | Korea | JA | Single centre RCT | 78 (17/61) | H pylori positive | Histology + RUT or UBT/ | O (20 mg bid) | ITT 70.6 (12/17) | 11.8 (2/17) | O (20 mg bid) | 7 | ITT 80.3 (49/61) | 41.0 (25/61) | 3 |
| Adults | Histology + RUT | Lev (500 mg o.d.) | PP 70.6 (12/17) | A (1 g bid) | PP 80.3 (49/61) | |||||||||
| or UBT (8 wk later) | Az (500 mg o.d.) | C (500 mg bid) | ||||||||||||
| Iacopini et al[26], 2005 | Italy | JA | Single centre RCT | 164 (83/81) | H pylori positive | Histology + UBT/ | E (20 mg o.d.) | ITT 65 (54/83) | 12 (9/77) | E (20 mg bid) | 7 | ITT 65 (53/81) | 30 (22/70) | 4 |
| Peptic ulcer and | UBT + HpSA | Lev (500 mg o.d.) | PP 70 (54/77) | A (1 g bid) | PP 76 (53/70) | |||||||||
| GERD adults | (8 wk later) | Az (500 mg o.d.) | C (500 mg bid) | |||||||||||
| Zhao et al[27], 2005 | China | JA | Single centre RCT | 98 (49/49) | H pylori positive | Histology + RUT/ | O (20 mg bid) | ITT 73.5 (36/49) | / | O (20 mg bid) | 7 | ITT 30.6 (15/49) | / | 3 |
| Active duodenal ulcer | RUT + Histology | A (1 g bid) | PP 76.6 (36/47) | A (1 g bid) | PP 31.3 (15/48) | |||||||||
| (4 wk later) | Az (1 g o.d.) 3 d | M (500 mg bid) | ||||||||||||
| Chen et al[28], 2004 | China | JA | Single centre RCT | 100 (55/45) | H pylori positive | Histology + UBT/ | O (40 mg o.d.) | ITT 92.7 (51/55) | 5 (3/55) | O (40 mg o.d.) | 7 | ITT 93.3 (42/45) | 17.8 (8/45) | 2 |
| Active duodenal ulcer | UBT (6 wk later) | A (1 g bid) | PP 92.7 (51/55) | A (1 g bid) | PP 93.3 (42/45) | |||||||||
| Az (500 mg o.d.) 3 d | C (500 mg bid) | |||||||||||||
| Chen et al[29], 2002 | China | JA | Single centre RCT | 47 (24/23) | H pylori positive | Histology + RUT/ | L (30 mg o.d.) | ITT 92 (22/24) | 4 (1/24) | O (20 mg bid) | 7 | ITT 91 (21/23) | 9 (2/23) | 3 |
| Chronic gastritis adults | UBT (4 wk later) | M (400 mg bid) 3 d | PP 92 (22/24) | M (400 mg bid) | PP 91 (21/23) | |||||||||
| Az (500 mg o.d.) 3 d | C (500 mg bid) | |||||||||||||
| Ivashkin et al[30], 2002 | Russia | JA | Multicenter RCT | 100 (50/50) | H pylori positive | Histology + RUT/ | O (20 mg bid) | ITT 72 (36/50) | / | O (20 mg bid) | 7 | ITT 30 (15/50) | / | 4 |
| Active duodenal | Histology + RUT (8 wk later) | A (1 g bid) | PP 75 (36/48) | A (1 g bid) | PP 31 (15/49) | |||||||||
| Ulcer adults | Az (1 g o.d.) 3 d | M (500 mg bid) | ||||||||||||
| Laurent et al[31], 2001 | France | JA | Multicenter RCT | 247 (64/78/70) | H pylori positive | Histology + RUT/ | O (20 mg bid) | ITT 37.5 (24/64) | 56.9 (33/58) | O (20 mg bid) | 7 | ITT 71.8/61.4 (56/78) (43/70) | 57.7/66.1 | 4 |
| Non-ulcer dyspepsia | UBT (4-6 wk later) | A (1 g bid) | PP 41.4 (24/58) | C (500 mg bid)/C (250 mg bid) | PP 78.9/69.4 (56/71) (43/62) | (41/71) (41/62) | ||||||||
| Az (500 mg o.d.) day 1 + (250 mg o.d.) days 2-5 | A (1 g bid)/M (500 mg bid) | |||||||||||||
| Vcev et al[32], 2000 | Croatia | JA | Single centre RCT | 110 (55/55) | H pylori positive | Histology + RUT/ | P (40 mg bid) | ITT 71 (39/55) | 14 (7/50) | P (40 mg bid) | 7 | ITT 78 (43/55) | 17 (9/53) | 3 |
| Active duodenal ulcer | Histology + RUT | A (1 g bid) | PP 78 (39/50) | A (1 g bid) | PP 81 (43/53) | |||||||||
| (8 wk later) | Az (500 mg o.d.) 6 d | C (500 mg bid) | ||||||||||||
| Laine et al[33], 1999 | USA | JA | Single centre RCT | 120 (40/40/40) | H pylori positive | Histology or serology + UBT/ | O (80 mg o.d.) | ITT 65 (26/40) | 3 (1/38) | O (80 mg o.d.) | 10 | ITT 35/78 (14/40) (31/40) | 8/15 (3/37) (5/33) | 3 |
| Symptomatic and | UBT (6 wk later) | M (750 mg o.d.) | PP 66 (25/38) | M (750 mg o.d.) | PP 35/79 (13/37) (26/33) | |||||||||
| Asymptomatic adults | Az (500 mg o.d.) 7 d | A (1.5 g o.d.)/C (1 g o.d.) | ||||||||||||
| Trevisani et al[34], 1998 | Italy | JA | Single centre RCT | 160 (80/80) | H pylori positive | RUT + Histology/ | L (30 mg bid) days 1-4 | ITT 73.3 (59/80) | 1.3 (1/73) | O (20 mg o.d.) | 7 | ITT 81.2 (65/80) | 2.6 (2/76) | 4 |
| Symptomatic adults | RUT + Histology (4 wk later) | T (2000 mg o.d.) day 3 | PP 80.8 (59/73) | C (250 mg bid) | PP 85.5 (65/76) | |||||||||
| Az (500 mg o.d.) days 2-4 | T (500 mg bid) | |||||||||||||
| Caselli et al[35], 1997 | Italy | JA | Multicenter RCT | 120 (60/60) | H pylori positive | Histology + RUT/ | L (30 mg o.d.) | ITT 93.3 (56/60) | / | O (20 mg o.d.) | 7 | ITT 86.7 (52/60) | / | 3 |
| Gastritis with or | Histology (7-8 wk later) | M (250 mg bid) 3 d | PP 93.3 (56/60) | C (250 mg bid) | PP 91.2 (52/57) | |||||||||
| without peptic ulcer | Az (500 mg o.d.) 3 d | T (500 mg bid) | ||||||||||||
| Leri et al[36], 1997 | Italy | Ab | Single centre RCT | 123 (41/41/41) | H pylori positive | Histology + RUT | O (20 mg bid) | ITT 68 (28/41) | / | O (20 mg bid) | 14 | ITT 80/97 (33/41) (40/41) | / | 2 |
| M (500 mg bid) 10 d | M (500 mg bid) 10 d | |||||||||||||
| Az (500 mg o.d.) 6 d | A (1 g bid) /C (500 mg t.d.) | |||||||||||||
| Cammarota et al[37], 1996 | Italy | JA | Single centre RCT | 70 (35/35) | H pylori positive | Histology + RUT/ | L (30 mg o.d.) | ITT 57 (20/35) | 18 (6/33) | L (30 mg o.d.) | 7 | ITT 80 (28/35) | 26 (9/34) | 3 |
| Symptomatic adults | Histology + RUT (8 wk later) | A (1 g bid) | PP 61 (20/33) | A (1 g bid) | PP 82 (28/34) | |||||||||
| Az (500 mg o.d.) 3 d | C (250 mg bid) |
Ab: Abstract; JA: Journal article; C: Clarithromycin; A: Amoxicillin; Az: Azithromycin; M: Metronidazole; T: Tinidazole; Lev: Levofloxacin; E: Esomeprazole; P: Pantorazole; O: Omeprazole; L: Lanzoprazole; UBT: 13C-urea breath test; RUT: Rapid urease test; HpSA: H pylori stool antigen; Q: quality score; RCT: Randomized controlled trial; ITT: Intent-to-treat analysis; PP: Per-protocol analysis.
Eradication rates
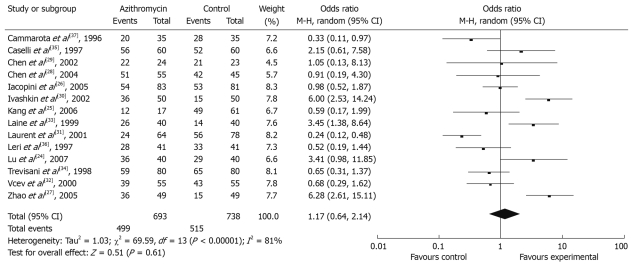
Fourteen studies that described H pylori eradication rates were selected for the meta-analysis. Four of these reported significantly improved eradication rates, and the remaining 10 had similar efficacy for H pylori eradication. Pooled eradication rates were achieved in 499 of 693 patients with azithromycin supplementation (72.01%, 95% CI: 58.09%-85.93%) and in 515 of 738 patients with azithromycin without regimen (69.78%, 95% CI: 66.47%-73.09%) by intention-to-treat analysis, the OR was 1.17 (95% CI: 0.64-2.14) (Figure 2). Overall, per-protocol eradication rates were 75.81% (95% CI: 72.44%-79.18%) and 72.44% (95% CI: 69.05%-75.83%) for azithromycin supplementation and azithromycin without regimen, respectively (OR 1.22, 95% CI: 0.61-2.43).
Figure 2.
Effect of azithromycin-containing triple therapy versus standard triple therapy on eradication rates by intention-to-treat analysis.
Side effects
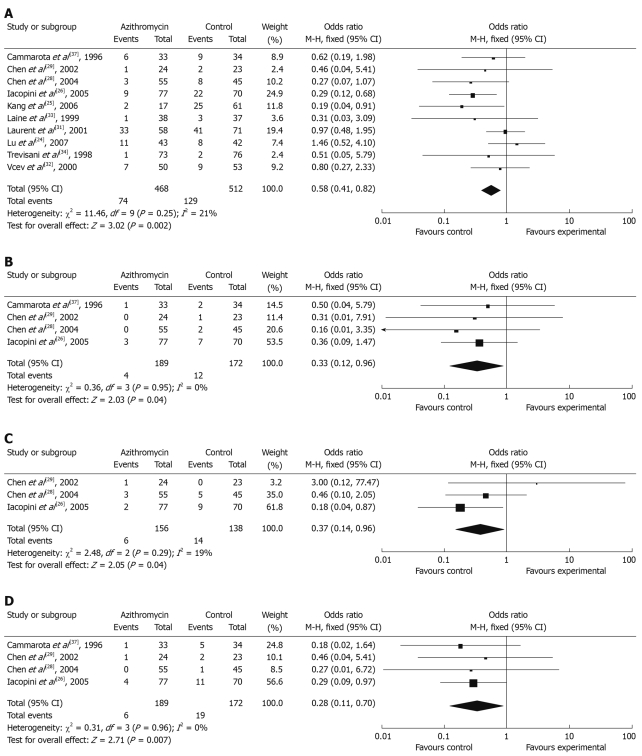
Total side effects were initially performed for meta-analysis. Data for the occurrence of side effects were obtained from 10 RCTs. Five of these studies reported a significant decrease in the occurrence of gastrointestinal side effects. The total number of side effects with azithromycin supplementation differed significantly from azithromycin without regimen: 15.81% (95% CI: 12.50%-19.12%) and 25.20% (95% CI: 21.44%-28.96%), and the summary OR was 0.58 (95% CI: 0.41-0.82) (Figure 3A). Individual symptoms during eradication therapy, such as nausea, diarrhea, abdominal pain, and taste disturbance were also analyzed. Incidence of diarrhea (2.13% vs 6.98%) (Figure 3B), nausea (3.85% vs 10.14%) (Figure 3C) and taste disturbance (3.17% vs 11.05%) (Figure 3D) were lower in the azithromycin supplementation group (OR: 0.33 vs 0.37 vs 0.28, 95% CI: 0.12-0.96 vs 0.14-0.96 vs 0.11-0.70).
Figure 3.
Effect of azithromycin-containing triple therapy versus standard triple therapy on the incidence of total side effects (A), diarrhea (B), nausea (C), and taste disturbance (D).
Sub-analyses
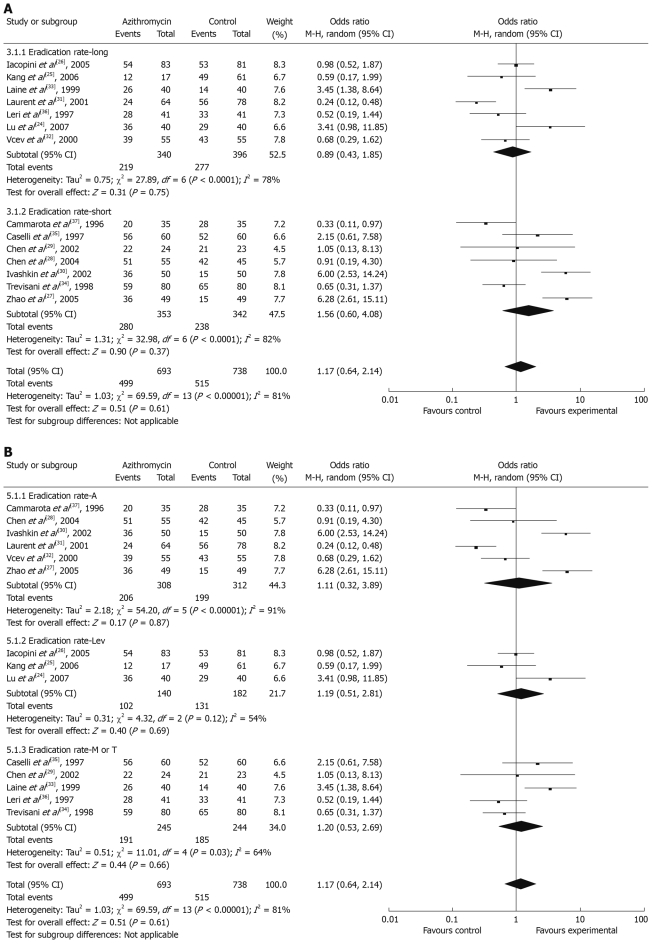
Sub-analyses for the meta-analysis were planned depending on subject age, symptoms before enrollment, course of azithromycin, and choice of antibiotics. We divided all eligible trials into long- and short-course subgroups, Az+A subgroup, Az+Lev subgroup and Az+M/T subgroup. There was no significant difference between the long-course and short-course subgroups; the summary ORs were 0.89 (95% CI: 0.43-1.85) and 1.56 (95% CI: 0.60-4.08), respectively (Figure 4A). For antibiotics sub-analysis, Az+A subgroup, Az+Lev subgroup and Az+M/T subgroup all had no significant difference; the summary ORs were 1.11 (95% CI: 0.32-3.89), 1.19 (95% CI: 0.51-2.81) and 1.20 (95% CI: 0.53-2.69), respectively (Figure 4B).
Figure 4.
Meta-analysis of eradication rates by treatment course (A) and different antibiotics (B).
Publication bias
We found that the funnel plot had a slightly asymmetrical distribution, but Egger’s regression test[38] suggested no significant asymmetry of the funnel plot (P = 0.84), which indicated no evidence of substantial publication bias.
DISCUSSION
For H pylori eradication therapy, clinical trails are undertaken to search for simpler but equally or more effective regimens. The modern macrolides are a focus of attention from that point of view. Azithromycin, a new-generation macrolide, has some special attributes that make it a promising compound in regimens for H pylori eradication. Following the administration of a single oral dose, azithromycin readily accumulates in the human gastric mucosa, subsequently redistributes from mucosal tissue to the mucus layer, and from the mucus to gastric juice. There, it reaches gastric tissue concentrations that persist above the minimal concentration for 90% inhibition (MIC90) for H pylori (0.25 μg/mL) over a 5-d period, thus leading to exposure of the microorganism to consistent amounts of this drug. The high tissue affinity and the absorption of the drug after oral administration are reduced when given during or after a meal. The pharmacological properties of azithromycin make it possible to use shorter courses, therefore, the problem was to define an optimal dose and duration of azithromycin in triple therapy.
Azithromycin is able to reach high gastric concentrations that persist for several days, and therefore, it can be administered at a dose of 500 mg once daily for only 3 d during a 7-d triple eradication regimen. The published trials that have used this antibiotic have yielded conflicting results, and have reported a wide range of eradication rates. Administration with meals markedly reduces azithromycin absorption, therefore, this might account for the low eradication rates observed in some studies[21]. In treatment regimens in which azithromycin was given to fasting patients, the cure rate was in the range 86%-93%[13,37]. Recently, short-term treatments of only 3 d, using a PPI plus azithromycin 500 mg and tinidazole 1000-2000 mg daily, have been found to promote eradication in 81%-88% of cases[23,39]. In contrast with the results reported in early studies that have used azithromycin for 2 wk and in repeated daily doses[40], side effects are scarce if the drug is administered once daily for a few days. In subanalyses, we also found that H pylori eradication rate had no significant difference between the long-course and short course subgroups.
H pylori eradication depends on a number of factors, including patient compliance, side effects, bacterial resistance, poor drug distribution or concentration, geographic differences, and socio-economic conditions. Optimization of H pylori eradication therapy remains an ongoing challenge worldwide. Although a great deal of research has focused on treatment of H pylori since the discovery of its crucial role in gastrointestinal disease, currently up to 25% of patients enrolled in clinical trials are treatment failures, even using the widely accepted and efficacious regimens that have gained inclusion in consensus guidelines[41]. A disappointing cure rate of < 80% after 7-d triple therapy was confirmed in the present study. Guidelines often suggest that an acceptable success rate for a particular therapy against H pylori infection should be > 80% on an intention-to-treat basis. However, clinical trials with azithromycin have displayed considerable variation with respect to the regimens used and the results obtained. Eradication rates varying between 93% and 22% have been reported[20,30,42]. The results of our meta-analysis demonstrated pooled H pylori eradication rates were 72.01% and 69.78% for patients with or without azithromycin by intention-to-treat analysis, respectively, and no significant difference was observed between the two regimens.
H pylori has cross resistance to macrolides; e.g. a strain that is resistant to clarithromycin is resistant to every other macrolide. The level of clarithromycin resistance is unfortunately showing a tendency to increase. The effect of drug synergism is of great value in combination treatment to heal H pylori infection. Lepper et al[43] have demonstrated an in vitro synergistic effect of azithromycin and the PPI lansoprazole. They have speculated that this effect might enhance eradication rates even with macrolide-resistant H pylori strains, because of the unique pharmacological properties of the combination. Azithromycin could provide a potent anti-H pylori effect and could simplify the bulky triple therapy.
Antibiotic-associated gastrointestinal side effects such as diarrhea, nausea, vomiting, bloating and abdominal pain represent a serious drawback of anti-H pylori therapy, although they are mild in most cases, but usually result in non-compliance. The quadruple regimen is associated with a relatively high incidence of side effects. In contrast, azithromycin is generally well tolerated, and most side effects associated with its use are mild to moderate in severity and transient. In our systematic review, we found that the total number of side effects with azithromycin supplementation was significantly lower than with azithromycin without regimen: 15.81% vs 25.20%; the summary OR was 0.58 (95% CI: 0.41-0.82). Moreover, the incidence of diarrhea (2.13% vs 6.98%), nausea (3.85% vs 10.14%) and taste disturbance (3.17% vs 11.05%) were lower in the azithromycin supplementation group. Our results showed that azithromycin had a positive impact on some H pylori therapy-related side effects. Several methodological weaknesses may limit the validity and generalizability of our meta-analysis. For example, there were no studies involving patients from Africa and South America.
In summary, the conclusion of this systematic review and meta-analysis is that, for first-time treatment, azithromycin-containing triple therapy has equal efficacy to that of standard triple eradication therapy. A combination of azithromycin, amoxicillin and a PPI constitutes an encouraging empirical first-line strategy. Furthermore, azithromycin-containing triple therapy showed a lower occurrence of drug-related side effects.
COMMENTS
Background
Colonization with Helicobacter pylori (H pylori) causes a wide range of upper gastrointestinal disorders in humans. Unfortunately, eradication therapy is not always successful, and can even induce several side effects. Azithromycin has some special attributes that make it a promising compound in the regimens for H pylori eradication.
Research frontiers
In first-line therapy, H pylori eradication rates using proton-pump inhibitor (PPI)-based triple therapy are about 80%. This signifies that up to 20% of patients are expected to be treatment failures and it could be even higher in areas with a high prevalence of resistant H pylori strains. In this study, the authors demonstrated that, for first-time treatment, azithromycin-containing triple therapy has equal efficacy to standard triple eradication therapy.
Innovations and breakthroughs
Recent studies have shown that azithromycin is a promising compound in regimens for H pylori eradication. Our meta-analysis demonstrated that azithromycin-containing triple therapy has equal efficacy to standard triple eradication therapy, and has a lower occurrence of side effects. A combination of azithromycin, amoxycillin and a PPI constitutes an encouraging empirical first-line strategy.
Applications
By understanding the effect of azithromycin in H pylori eradication, this study represents a new encouraging strategy for first-time treatment, and it could decrease the physiological and pharmacoeconomic burden of second courses of therapy.
Terminology
Azithromycin is a new-generation macrolide and has some special attributes. It is able to reach high gastric concentrations that persist for several days, and therefore may be administered at a dose of 500 mg once daily for only 3 d during 7-d triple eradication therapy.
Peer review
The authors performed a meta-analysis and demonstrated that azithromycin-containing triple therapy has equal efficacy to standard triple H pylori eradication therapy. This was an original and good study.
Footnotes
Peer reviewers: Siegfried Wagner, Professor, Medical Clinic II, Klinikum Deggendorf, Perlasberger Str. 41, Deggendorf 94469, Germany; Özlem Yilmaz, PhD, Associate Professor of Microbiology, Department of Microbiology and Clinical Microbiology, Dokuz Eylul University, School of Medicine, Inciralti 35340, Izmir, Turkey
S- Editor Tian L L- Editor Kerr C E- Editor Lin YP
References
- 1.Malfertheiner P, Megraud F, O'Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772–781. doi: 10.1136/gut.2006.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perri F, Qasim A, Marras L, O'Morain C. Treatment of Helicobacter pylori infection. Helicobacter. 2003;8 Suppl 1:53–60. doi: 10.1046/j.1523-5378.2003.00162.x. [DOI] [PubMed] [Google Scholar]
- 3.Mégraud F, Darmaillac V, Brügmann D. [Helicobacter pylori and azithromycin] Pathol Biol (Paris) 1995;43:555–560. [PubMed] [Google Scholar]
- 4.Chey WD, Fisher L, Barnett J, Delvalle J, Elta GH, Hasler WL, Nostrant T, Palaniappan J, Scheiman J. Low- versus high-dose azithromycin triple therapy for Helicobacter pylori infection. Aliment Pharmacol Ther. 1998;12:1263–1267. doi: 10.1046/j.1365-2036.1998.00422.x. [DOI] [PubMed] [Google Scholar]
- 5.Dohmen W, Seelis RE. The role of azithromycin in the treatment of Helicobacter pylori infection - a retrospective report. Infection. 1998;26:256–262. doi: 10.1007/BF02962382. [DOI] [PubMed] [Google Scholar]
- 6.Leonard T, Duffy JC. A Bayesian fixed effects analysis of the Mantel-Haenszel model applied to meta-analysis. Stat Med. 2002;21:2295–2312. doi: 10.1002/sim.1048. [DOI] [PubMed] [Google Scholar]
- 7.Mousavi S, Toussy J, Yaghmaie S, Zahmatkesh M. Azithromycin in one week quadruple therapy for H pylori eradication in Iran. World J Gastroenterol. 2006;12:4553–4556. doi: 10.3748/wjg.v12.i28.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Altintaş E, Ulu O, Sezgin O, Aydin O, Camdeviren H. Comparison of ranitidine bismuth citrate, tetracycline and metronidazole with ranitidine bismuth citrate and azithromycin for the eradication of Helicobacter pylori in patients resistant to PPI based triple therapy. Turk J Gastroenterol. 2004;15:90–93. [PubMed] [Google Scholar]
- 9.Sullivan B, Coyle W, Nemec R, Dunteman T. Comparison of azithromycin and clarithromycin in triple therapy regimens for the eradication of Helicobacter pylori. Am J Gastroenterol. 2002;97:2536–2539. doi: 10.1111/j.1572-0241.2002.06036.x. [DOI] [PubMed] [Google Scholar]
- 10.Bujanda L, Muñoz C, Sánchez A, Iriondo C, Cosme A. Ranitidine bismuth citrate plus azithromycin for Helicobacter pylori eradication. J Clin Gastroenterol. 2000;30:337–338. doi: 10.1097/00004836-200004000-00033. [DOI] [PubMed] [Google Scholar]
- 11.Bertoni G, Sassatelli R, Nigrisoli E, Tansini P, Bianchi G, Della Casa G, Bagni A, Bedogni G. Triple therapy with azithromycin, omeprazole, and amoxicillin is highly effective in the eradication of Helicobacter pylori: a controlled trial versus omeprazole plus amoxicillin. Am J Gastroenterol. 1996;91:258–263. [PubMed] [Google Scholar]
- 12.Tindberg Y, Casswall TH, Blennow M, Bengtsson C, Granström M, Sörberg M. Helicobacter pylori eradication in children and adolescents by a once daily 6-day treatment with or without a proton pump inhibitor in a double-blind randomized trial. Aliment Pharmacol Ther. 2004;20:295–302. doi: 10.1111/j.1365-2036.2004.02077.x. [DOI] [PubMed] [Google Scholar]
- 13.Anagnostopoulos GK, Kostopoulos P, Margantinis G, Tsiakos S, Arvanitidis D. Omeprazole plus azithromycin and either amoxicillin or tinidazole for eradication of Helicobacter pylori infection. J Clin Gastroenterol. 2003;36:325–328. doi: 10.1097/00004836-200304000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Flores S, Opazo H, Valderrama D, Aguilera R, Marchese A, Valderrama S. [Triple therapy of short-term with azithromycin, amoxycillin and omeprazole for the eradication of Helicobacter pylori] Rev Med Chil. 2000;128:509–512. [PubMed] [Google Scholar]
- 15.Vcev A, Stimac D, Vceva A, Takac B, Pezerovíc D, Ivandíc A. High dose omeprazole plus amoxicillin and azithromycin in eradication of Helicobacter pylori in duodenal ulcers. Helicobacter. 1999;4:54–57. doi: 10.1046/j.1523-5378.1999.09041.x. [DOI] [PubMed] [Google Scholar]
- 16.Cammarota G, Papa A, Cianci R, Cannizzaro O, Armuzzi A, Gasbarrini A, Addolorato G, Gasbarrini GB. Three-day antibiotic therapy with azithromycin and tinidazole plus lansoprazole or pantoprazole to cure Helicobacter pylori infection: a pilot study. Eur J Gastroenterol Hepatol. 1999;11:247–250. doi: 10.1097/00042737-199903000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Vcev A, Vceva A, Takac B, Dmitrović B, Stimac D, Stimac T, Kovac D, Pezerović D, Blazanović A, Ivandić A, et al. Omeprazole, azithromycin and amoxicillin or amoxicillin plus clavulanic acid in eradication of Helicobacter pylori in duodenal ulcer disease. Acta Med Croatica. 1998;52:209–214. [PubMed] [Google Scholar]
- 18.Vcev A, Vceva A, Stimac D, Takac B, Dmitrović B, Kovac D. Omeprazole, azithromycin and either amoxycillin or metronidazole in eradication of Helicobacter pylori in duodenal ulcer patients. Aliment Pharmacol Ther. 1998;12:453–456. doi: 10.1046/j.1365-2036.1998.00332.x. [DOI] [PubMed] [Google Scholar]
- 19.Vcev A, Vceva A, Ivandić A, Mihaljević S, Micunović N, Dmitrović B, Vuković D, Takac B, Gardasanić J, Horonitz M, et al. [Omeprazole and azithromycin with and without metronidazole in the eradication of Helicobacter pylori in duodenal ulcer disease] Lijec Vjesn. 1997;119:210–213. [PubMed] [Google Scholar]
- 20.Chahine C, Moukhachen O, Chedid M, Araj GF, Sharara AI. Ultrashort regimen of lansoprazole-amoxicillin-azithromycin for eradicating Helicobacter pylori. Am J Health Syst Pharm. 2001;58:1819–1823. doi: 10.1093/ajhp/58.19.1819. [DOI] [PubMed] [Google Scholar]
- 21.Calabrese C, Di Febo G, Areni A, Scialpi C, Biasco G, Miglioli M. Pantoprazole, azithromycin and tinidazole: short duration triple therapy for eradication of Helicobacter pylori infection. Aliment Pharmacol Ther. 2000;14:1613–1617. doi: 10.1046/j.1365-2036.2000.00879.x. [DOI] [PubMed] [Google Scholar]
- 22.Frota LC, da Cunha Mdo P, Luz CR, de Araujo-Filho AH, Frota LA, Braga LL. Helicobacter pylori eradication using tetracycline and furazolidone versus amoxicillin and azithromycin in lansoprazole based triple therapy: an open randomized clinical trial. Arq Gastroenterol. 2005;42:111–115. doi: 10.1590/s0004-28032005000200009. [DOI] [PubMed] [Google Scholar]
- 23.Stone AF, Mendall MA, Kaski JC, Edger TM, Risley P, Poloniecki J, Camm AJ, Northfield TC. Effect of treatment for Chlamydia pneumoniae and Helicobacter pylori on markers of inflammation and cardiac events in patients with acute coronary syndromes: South Thames Trial of Antibiotics in Myocardial Infarction and Unstable Angina (STAMINA) Circulation. 2002;106:1219–1223. doi: 10.1161/01.cir.0000027820.66786.cf. [DOI] [PubMed] [Google Scholar]
- 24.Lu XJ, Zhao TY, Jia HY. Analysis of the effect on eradication treatment of Helicobacter pylori in 85 patients. Zhongguo Yiyao Daobao. 2007;27:35–36. [Google Scholar]
- 25.Kang MS, Park DI, Yun JW, Oh SY, Yoo TW, Park JH, Kim HJ, Cho YK, Sohn CI, Jeon WK, et al. [Levofloxacin-azithromycin combined triple therapy for Helicobacter pylori eradication] Korean J Gastroenterol. 2006;47:30–36. [PubMed] [Google Scholar]
- 26.Iacopini F, Crispino P, Paoluzi OA, Consolazio A, Pica R, Rivera M, Palladini D, Nardi F, Paoluzi P. One-week once-daily triple therapy with esomeprazole, levofloxacin and azithromycin compared to a standard therapy for Helicobacter pylori eradication. Dig Liver Dis. 2005;37:571–576. doi: 10.1016/j.dld.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Zhao Y, Hang YQ. Effect of PPI-triple therapy in eradication treatment of Helicobacter pylori. Zhongguo Shiyong Xiangcun Yisheng Zazhi. 2005;12:30–31. [Google Scholar]
- 28.Chen H, Li BT, Zhou HM. Effect of azithromycin, omeprazole and amoxicillin triple regimen in eradication treatment of Helicobacter pylori. Zhongguo Quanke Yixue. 2004;24:1858–1859. [Google Scholar]
- 29.Chen ZQ, Zhang J, Kong CM. Effect therapy cost of short-term low-d ose therapy with azithromycin, metronidazole and lansoprazole for eradication of Helicoba cter pylori. Zhongguo Xinyao Yu Linchuang Zazhi. 2002;21:687–689. [Google Scholar]
- 30.Ivashkin VT, Lapina TL, Bondarenko OY, Sklanskaya OA, Grigoriev PY, Vasiliev YV, Yakovenko EP, Gulyaev PV, Fedchenko VI. Azithromycin in a triple therapy for H.pylori eradication in active duodenal ulcer. World J Gastroenterol. 2002;8:879–882. doi: 10.3748/wjg.v8.i5.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laurent J, Mégraud F, Fléjou JF, Caekaert A, Barthélemy P. A randomized comparison of four omeprazole-based triple therapy regimens for the eradication of Helicobacter pylori in patients with non-ulcer dyspepsia. Aliment Pharmacol Ther. 2001;15:1787–1793. doi: 10.1046/j.1365-2036.2001.01104.x. [DOI] [PubMed] [Google Scholar]
- 32.Vcev A, Stimac D, Ivandić A, Vceva A, Takac B, Pezerović D. Pantoprazole, amoxycillin and either azithromycin or clarithromycin for eradication of Helicobacter pylori in duodenal ulcer. Aliment Pharmacol Ther. 2000;14:69–72. doi: 10.1046/j.1365-2036.2000.00662.x. [DOI] [PubMed] [Google Scholar]
- 33.Laine L, Estrada R, Trujillo M, Cheybani K, Yeramian P, Smith S, Neil G. Once-daily therapy for H. pylori infection: a randomized comparison of four regimens. Am J Gastroenterol. 1999;94:962–966. doi: 10.1111/j.1572-0241.1999.995_r.x. [DOI] [PubMed] [Google Scholar]
- 34.Trevisani L, Sartori S, Caselli M, Ruina M, Verdianelli G, Abbasciano V. A four-day low dose triple therapy regimen for the treatment of Helicobacter pylori infection. Am J Gastroenterol. 1998;93:390–393. doi: 10.1111/j.1572-0241.1998.00390.x. [DOI] [PubMed] [Google Scholar]
- 35.Caselli M, Trevisani L, Tursi A, Sartori S, Ruina M, Luzzi I, Gaudenzi P, Alvisi V, Gasbarrini G. Short-term low-dose triple therapy with azithromycin, metronidazole and lansoprazole appears highly effective for the eradication of Helicobacter pylori. Eur J Gastroenterol Hepatol. 1997;9:45–48. doi: 10.1097/00042737-199701000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Leri O, Perinelli P, Mastropasqua M, Tubili S, Losi T. [Effects of the triple therapy in the eradication of Helicobacter pylori: comparison of 3 different antibiotics] Clin Ter. 1997;148:617–622. [PubMed] [Google Scholar]
- 37.Cammarota G, Tursi A, Papa A, Montalto M, Veneto G, Cuoco L, Fedeli G, Gasbarrini G. Helicobacter pylori eradication using one-week low-dose lansoprazole plus amoxycillin and either clarithromycin or azithromycin. Aliment Pharmacol Ther. 1996;10:997–1000. doi: 10.1046/j.1365-2036.1996.d01-533.x. [DOI] [PubMed] [Google Scholar]
- 38.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trevisani L, Sartori S, Galvani F, Ruina M, Caselli M, Verdianelli G, Abbasciano V. Evaluation of a new ultrashort triple therapy for Helicobacter pylori disease. Aliment Pharmacol Ther. 1998;12:1269–1272. doi: 10.1046/j.1365-2036.1998.00430.x. [DOI] [PubMed] [Google Scholar]
- 40.al-Assi MT, Genta RM, Karttunen TJ, Cole RA, Graham DY. Azithromycin triple therapy for Helicobacter pylori infection: azithromycin, tetracycline, and bismuth. Am J Gastroenterol. 1995;90:403–405. [PubMed] [Google Scholar]
- 41.Altintas E, Sezgin O, Ulu O, Aydin O, Camdeviren H. Maastricht II treatment scheme and efficacy of different proton pump inhibitors in eradicating Helicobacter pylori. World J Gastroenterol. 2004;10:1656–1658. doi: 10.3748/wjg.v10.i11.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu WZ, Xiao SD, Shi Y, Wu SM, Zhang DZ, Xu WW, Tytgat GN. Furazolidone-containing short-term triple therapies are effective in the treatment of Helicobacter pylori infection. Aliment Pharmacol Ther. 1999;13:317–322. doi: 10.1046/j.1365-2036.1999.00492.x. [DOI] [PubMed] [Google Scholar]
- 43.Lepper PM, Moricke A, Glasbrenner B, Trautman M. Demonstration of in-vitro synergism between proton-pump inhibitors and macrolides against Helicobacter pylori (Abstr) Gut. 2000;47 Suppl I:A110. [Google Scholar]