Abstract
AIM: To analyze the performance value of high risk factors in population-based colorectal cancer (CRC) screening in China.
METHODS: We compared the performance value of the immunochemical fecal occult blood test (iFOBT) and other high risk factors questionnaire in a population sample of 13 214 community residents who completed both the iFOBT and questionnaire investigation. Patients with either a positive iFOBT and/or questionnaire were regarded as a high risk population and those eligible were asked to undergo colonoscopy.
RESULTS: The iFOBT had the highest positive predictive value and negative predictive value in screening for advanced neoplasia. The iFOBT had the highest sensitivity, lowest number of extra false positive results associated with the detection of one extra abnormality for screening advanced neoplasias and adenomas. A history of chronic cholecystitis or cholecystectomy, chronic appendicitis or appendectomy, and chronic diarrhea also had a higher sensitivity than a history of adenomatous polyps in screening for advanced neoplasias and adenomas. The sensitivity of a history of chronic cholecystitis or cholecystectomy was highest among the 10 high risk factors in screening for non-adenomatous polyps. A history of chronic appendicitis or appendectomy, chronic constipation, chronic diarrhea, mucous and bloody stool, CRC in first degree relatives, malignant tumor and a positive iFOBT also had higher sensitivities than a history of adenomas polyps in screening for non-adenomatous polyps. Except for a history of malignant tumor in screening for non-adenomatous polyps, the gain in sensitivity was associated with an increase in extra false positive results associated with the detection of one extra abnormality.
CONCLUSION: The iFOBT may be the best marker for screening for advanced neoplasias and adenomas. Some unique high risk factors may play an important role in CRC screening in China.
Keywords: Colorectal cancer, Cancer screening, Feces, Occult blood, Risk factors, Predictive value of tests
INTRODUCTION
The incidence of colorectal cancer (CRC) is increasing rapidly, and there is a similar incidence in some Asian populations to that in Western countries because of a more “Westernized” lifestyle and dietary habits[1]. A relatively long time for malignant transformation, together with improved survival associated with early detection of cancer, makes CRC an ideal target for screening. In source-limited Asian countries, the fecal occult blood test (FOBT) is the first choice for CRC screening because of its better population impact[1]. However, bleeding from cancers and precancerous polyps may be intermittent and most small colorectal neoplasias do not tend to bleed[2]. Therefore, the immunochemical FOBT (iFOBT) alone inevitably misses some important lesions that do not bleed, or bleed intermittently. The iFOBT and a high risk factors questionnaire approach as primary screening followed by full colonoscopy examination as follow-up screening, has been recommended by the Department of Disease Control, the Ministry of Health of China as the protocol for population-based CRC screening in China[3]. However, the performance value of the iFOBT and a high risk factors questionnaire is still unknown in CRC screening practice in China. According to the definition of high risk factors by American Cancer Society[4], individuals at higher risk for CRC include “individuals with a history of adenomatous polyps (HAP)”. Therefore we used the data available in CRC screening practice in China to examine the performance value of each high risk factor using an acknowledged high risk factor - HAP - as a reference in CRC screening practice in China.
MATERIALS AND METHODS
CRC screening protocol in China
The CRC screening protocol of China has been published in a recent study[5]. Subjects (age should be defined as ≥ 40 years and ≤ 74 years) who have one or more of the following items are considered to be at high risk of CRC and should undergo colonoscopy: (1) Positive results from the iFOBT; (2) First-degree relatives with CRC; (3) A personal history of cancers or intestinal polyps; (4) 2 or more of the following items: (a) chronic diarrhea; (b) chronic constipation; (c) mucous and bloody stool; (d) history of appendicitis or appendectomy; (e) history of chronic cholecystitis or cholecystectomy; (f) history of psychiatric trauma (e.g. divorce, death of relatives).
Study population
From July 2006 to December 2008, a screening program was implemented following the CRC screening protocol recommended by the Ministry of Health of China, for individuals aged 40-74 years in Xiacheng District, Hangzhou City, China. Among 33 778 targeted residents, 16 918 declined, 3646 participated only in the questionnaire investigation, and 13 214 (39.1%) undertook both the iFOBT and questionnaire investigation.
Study design
The 33 778 subjects, aged 40-74 years, who lived in Xiacheng District were enrolled as the target population for our CRC screening practice. Therefore the targeted population can be classified into average, intermediate or high risk individuals. The targeted population was contacted by Chronic Disease Control (CDC) staff to explain the aim of the study, with an invitation to undergo both tests.
The aim of primary screening was to determine the high risk population among the targeted population by the iFOBT and questionnaire approach. Therefore the primary screening test kits included an iFOBT kit (Acon Biotech Co. Ltd., Hangzhou, China), a detailed instruction sheet, a consent form, and a questionnaire containing high risk items. The iFOBT kit used is a qualitative method, with a hemoglobin detection threshold of 200 ng/mL. Participants were asked to prepare a fecal sample from 3 areas of a stool specimen. No specific dietary restriction was stipulated.
The study was approved by the local ethics committee and all participants gave written informed consent.
Identification of high risk subjects
All participants learned how to use the iFOBT kit and how to fill in the questionnaire sheet under guidance of CDC staff. Feces samples were processed and results were obtained at the central laboratory of the local CDC. Processing and evaluation were not automated but were performed by trained staff and under strict quality control (double reading, control of frequency of positive tests, reproducibility). Scrutineers of the iFOBT were blinded to the subject’s medical records. The screening procedure was considered positive when at least one of the tests was positive. All positive cases resulting from the primary screening were regarded as high risk subjects and those eligible were invited to the follow-up colonoscopy examinations. The CDC staff and primary care managers were responsible for inviting eligible high risk subjects for further colonoscopy examination.
Colonoscopy examination
Colonoscopy examination was performed by gastroenterologists in endoscopy units of local hospitals and all participants gave written informed consent. The gastroenterologists recorded data using a standard form, including the quality of bowel preparation, the completeness of the colonoscopy, the number, size, and localization of any detected lesions, and the occurrence of complications. All polyps detected during the colonoscopy were immediately removed and/or biopsied for histologic diagnosis by pathologists. Those who were suspected of having CRC or had polyps that could not be removed endoscopically were referred for surgery. If a colonoscopy examination failed because of inadequate bowel preparation, inaccessibility of the cecum, or lack of satisfactory colonoscopy results, a subsequent colonoscopy would be performed within 1 mo.
Pathologic examination
In subjects with more than one polyp, the most advanced pathological lesions or the largest lesion was included in the analysis. An advanced neoplasia was comprised of advanced adenomas (an adenoma measuring 10 mm or more in size, adenomas with high grade dysplasia, or an adenomas with villous component ≥ 25%) and invasive cancer[6,7]. Non-adenomatous polyps included juvenile polyps, inflammatory polyps and hyperplastic polyps. Invasive cancer was defined as invasion by malignant cells through the muscularis mucosae. Intramucosal carcinoma and carcinoma in situ were categorized as high grade dysplasia. Pathologic slides of positive lesions were re-examined and diagnosed by consensus by pathologists.
Statistical analysis
The population of participants in the primary screening comprised all patients who had given written consent (n = 16 860). Subjects who accepted only questionnaire investigation (n = 3646) in the primary screening were excluded from the study. Subjects with at least one test positive (n = 1937 were regarded as positive. A colonoscopy examination was not conducted in 382 subjects because of death, health problems, moving or other reasons. A further 695 subjects rejected a colonoscopy examination. Figure 1 provides a flow diagram of the study.
Figure 1.
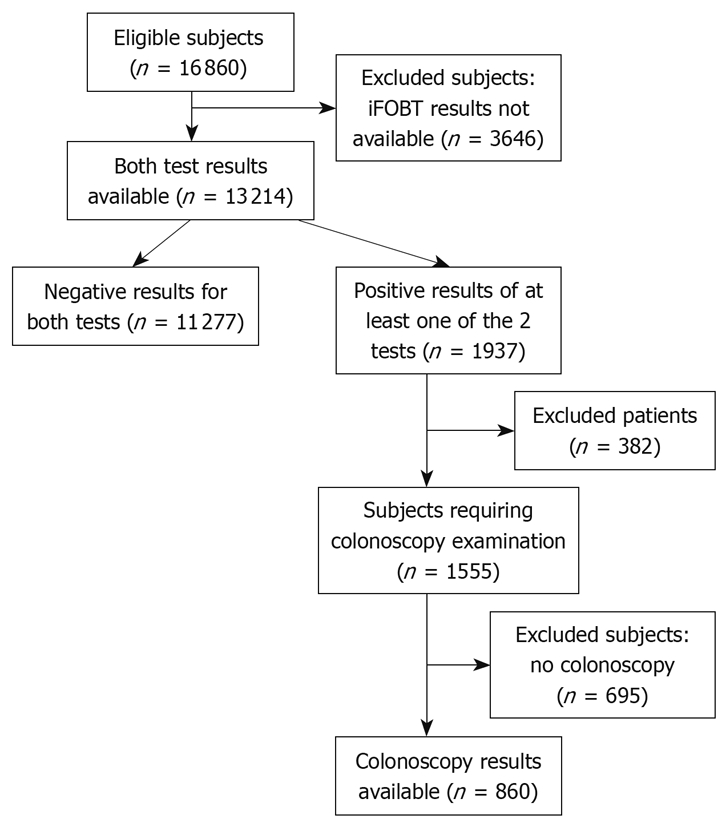
Flow diagram of the study. iFOBT: Immunochemical fecal occult blood test.
As the confirmatory procedure (colonoscopy examination) was restricted to subjects classified as positive in at least one of 2 tests (iFOBT and questionnaire examination) positive, the sensitivity of each high risk item could not be directly estimated. According to the theory originally suggested by Schatzkin et al[8], we therefore compared the relative sensitivity (RSN) by calculating the ratio using HAP as reference. For example, if the number of true positive subjects for one high risk factor is denoted by m and the number of true positive subjects for HAP by n, RSN is calculated as m/n. Confidence intervals (95%) were calculated according to the formulae suggested by Cheng et al[9]. Using the theory recommended by Chock, the number of extra false positives associated with the detection of one extra true positive was denoted FP:TP, which was calculated as the ratio between the difference in the number of false positive subjects with one high risk factor versus HAP and the difference in the number of true positive subjects with one high risk factor versus HAP[10].
RESULTS
Colonoscopic results of the iFOBT and questionnaire
A total of 21 CRC (2.4%) cases, 48 (5.6%) subjects with advanced adenomas, 147 (17%) subjects with adenomas, and 54 (6.3%) subjects with non-adenomatous polyps were detected in 860 colonoscopies. Table 1 shows colonoscopic results of the iFOBT and questionnaire. The iFOBT alone diagnosed 13 cases of cancer, 22 cases of advanced adenomas, 44 cases of adenomas, and 9 cases of non-adenomatous polyps while the questionnaire alone found 4 cases of CRC, 21 cases of advanced adenomas, 91 cases of adenomas, and 43 cases with non-adenomatous polyps. Four cases of CRC, 5 of advanced adenomas, 12 of adenomas, and 2 of non-adenomatous polyps were found in both positives. Table 2 shows the results of colonoscopy according to each high risk item. One perforation was recorded after colonoscopy (0.1%).
Table 1.
Colonoscopy results of iFOBT and questionnaire n (%)
| Colorectal cancer | Advanced adenomas | Adenoma | Non-adenomatous polyps | |
| iFOBT positive only | 13 (61.9) | 22 (45.8) | 44 (29.9) | 9 (16.7) |
| Both positive1 | 4 (19) | 5 (10.4) | 12 (8.2) | 2 (3.6) |
| Only questionnaire positive | 4 (19) | 21 (43.8) | 91 (61.9) | 43 (79.7) |
| Total | 21 (100) | 48 (100) | 147 (100) | 54 (100) |
Both iFOBT and questionnaire positive. iFOBT: Immunochemical fecal occult blood test.
Table 2.
Colonoscopy results of high risk questionnaire items
| Cancer | Advanced adenomas | Adenomas | Non-adenomatous polyps | Normal results | |
| iFOBT | 17 | 27 | 56 | 11 | 128 |
| History of malignant tumor | 1 | 3 | 9 | 7 | 41 |
| Colorectal cancer (CRC) in first degree relatives | 2 | 7 | 29 | 11 | 81 |
| History of adenomatous polyps (HAP) | 0 | 9 | 25 | 6 | 48 |
| History of mucous and bloody stool | 5 | 4 | 15 | 10 | 101 |
| History of chronic diarrhea | 2 | 10 | 29 | 10 | 120 |
| History of chronic constipation | 0 | 8 | 24 | 13 | 102 |
| History of chronic appendicitis or appendectomy | 3 | 9 | 33 | 14 | 122 |
| History of chronic cholecystitis or cholecystectomy | 3 | 14 | 50 | 19 | 169 |
| History of psychiatric trauma | 4 | 5 | 18 | 2 | 73 |
The characteristics of the study population
Table 3 shows the characteristics of the study population. Of 13 214 subjects who completed both the iFOBT and questionnaire investigation, 1937 had at least one positive test. The positive rate of the questionnaire investigation was markedly higher than that of the iFOBT (11.7% vs 3.6%). A colonoscopy examination was not conducted in 382 subjects (19.7%) because of death, health problems, or other reasons. A total of 860 (55.3%) subjects underwent colonoscopy. In subjects undergoing endoscopic examination, 60.3% were iFOBT positive only, 53.2% were questionnaire positive only, 64.9% were positive for both the iFOBT and questionnaire.
Table 3.
Characteristics of the study population n (%)
| Subjects with 2 analyzable tests (n = 13 214) | |
| Sex | |
| Male | 5391 (40.8) |
| Female | 7823 (59.2) |
| Age (yr) | |
| 40-49 | 2711 (20.5) |
| 50-59 | 4704 (35.6) |
| 60-69 | 3683 (27.9) |
| 70-74 | 2116 (16.0) |
| Positive items | |
| iFOBT | 481 (3.6) |
| History of malignant tumor | 172 (1.3) |
| CRC in first degree relatives | 367 (2.8) |
| HAP | 158 (1.2) |
| History of mucous and bloody stool | 430 (3.3) |
| History of chronic diarrhea | 709 (5.4) |
| History of chronic constipation | 902 (6.8) |
| History of chronic appendicitis or appendectomy | 1126 (8.5) |
| History of chronic cholecystitis or cholecystectomy | 1538 (11.6) |
| History of psychiatric trauma | 655 (5) |
Performance of high risk factors in screening for advanced neoplasia
Using HAP as the reference, the sensitivity of iFOBT was highest among all high risk factors. The sensitivities of history of chronic cholecystitis or cholecystectomy, chronic appendicitis or appendectomy, history of chronic diarrhea were also higher than that of HAP. The positive predictive value (PPV) and negative predictive value (NPV) of iFOBT were highest among all high risk factors for advanced neoplasias, but the gain in sensitivity was accompanied by an increase in FP:TP. The iFOBT had the lowest FP:TP ratio (Table 4).
Table 4.
Comparison of the performance of high risk factors in screening advanced neoplasias, adenomas, non-adenomatous polyps using HAP as reference
|
Advanced neoplasias |
Adenomas |
Non-adenomatous polyps |
||||||||||
| PPV (%) | NPV (%) | RSN | FP:TP | PPV (%) | NPV (%) | RSN | FP:TP | PPV (%) | NPV (%) | RSN | FP:TP | |
| iFOBT | 17.70 | 95.70 | 4.9 (2.42-9.87) | 2.29 (1.36-3.83) | 22.50 | 85.10 | 2.24 (1.4-3.52) | 2.58 (1.37-4.87) | 4.40 | 93 | 1.8 (0.71-4.58) | 16 (3.39-75.58) |
| HMT | 6.10 | 91.70 | 0.4 (0.17-1.19) | 13.60 | 82.60 | 0.36 (0.18-0.7) | 10.60 | 94 | 1.17 (0.39-3.48) | |||
| FDR | 5.70 | 91.30 | 1 (0.4-2.519) | 18.50 | 83.20 | 1.16 (0.69-1.96) | 8.25 (0.44-154.47) | 7 | 93.90 | 1.8 (0.67-4.88) | 6.6 (1.16-37.6) | |
| HAP | 9.40 | 92 | 1 | 26 | 84 | 1 | 6.30 | 93.70 | 1 | |||
| MBS | 5.70 | 91.30 | 1 (0.42-2.39) | 9.50 | 81.20 | 0.6 (0.33-1.08) | 6.30 | 93.70 | 1.7 (0.66-4.38) | 13.25 (2.02-86.77) | ||
| HCD | 5.40 | 91 | 1.2 (0.54-2.63) | 24 (1.87-307.35) | 13.90 | 82 | 1.16 (0.72-1.88) | 18 (1.1-293.1) | 4.90 | 93.30 | 1.7 (0.71-4.08) | 18 (3.2-101.33) |
| HCC | 4.80 | 91 | 0.9 (0.35-2.33) | 14.30 | 82.20 | 0.96 (0.56-1.65) | 7.70 | 94.10 | 2.2 (0.88-5.49) | 7.7 (2.26-26.21) | ||
| CAA | 5.50 | 91 | 1.3 (0.59-2.83) | 24.6 (1.63-370.18) | 15.10 | 82.20 | 1.32 (0.8-2.16) | 9.25 (1.52-55.7) | 6.40 | 93.80 | 2.3 (0.88-5.99) | 9.25 (2.96-28.79) |
| CCC | 5.80 | 87.40 | 1.9 (0.87-4.12) | 15.13 (4.47-51.23) | 16.90 | 82.80 | 2 (1.26-3.16) | 4.84 (2.43-9.65) | 6.40 | 93.80 | 3.2 (1.38-7.42) | 9.3 (4.5-19.22) |
| HPT | 7.60 | 91.80 | 1 (0.42-2.39) | 15.30 | 82.60 | 0.72 (0.43-1.2) | 1.70 | 93 | 0.33 (0.07-1.63) | |||
HMT: History of malignant tumor; FDR: History of CRC in first degree relatives; MBS: History of mucous and bloody stool; HCD: History of chronic diarrhea; HCC: History of chronic constipation; CAA: History of chronic appendicitis or appendectomy; CCC: History of chronic cholecystitis or cholecystectomy; HPT: History of psychiatric trauma; PPV: Positive predictive value; NPV: Negative predictive value; RSN: Relative sensitivity; FP:TP ratio: The ratio between the difference in the number of false positive subjects with one high risk factor vs HAP and the difference in the number of true positive subjects with one high risk factor vs HAP. Values for RSN and FP:TP are mean (95% CI). RSN > 1: Sensitivity of the high risk factor is greater than that of HAP.
Performance comparison among high risk factors in screening for adenomas
Using HAP as standard, the sensitivity of iFOBT was also highest among 10 high risk factors. Higher sensitivities were also found in history of chronic appendicitis or appendectomy, chronic diarrhea, CRC in first degree relatives, and chronic cholecystitis or cholecystectomy. The PPV of the iFOBT was 22.5%, just behind that of HAP (26%). The NPV of iFOBT was highest among all high risk factors. The gain in sensitivity was also accompanied by an increase in FP:TP ratio (Table 4).
Performance comparison of high risk factors in screening for non-adenomatous polyps
Using HAP as standard, a history of chronic cholecystitis or cholecystectomy was the most sensitive marker in screening for non-adenomatous polyps. The sensitivities of other high risk factors except history of psychiatric trauma were also higher than that of HAP. The PPV of history of malignant tumor (10.6%) was highest among all high risk factors in screening for non-adenomatous polyps. Except for history of malignant tumor, the gain in sensitivity was accompanied by increase in the FP:TP ratio (Table 4).
DISCUSSION
Colonoscopy is often regarded as the “gold standard” for detection of CRC[11,12]. Direct colonoscopy screening is the most accurate test for CRC. However because of its potential harm, acceptability[13], availability, and expense[14], the use of colonoscopy as a one-step screening method for the whole targeted population is unpractical in China. The use of noninvasive screening tests in primary screening, such as iFOBT and questionnaire investigation, have been adopted as the large scale population screening program in China[15]. The iFOBT and questionnaire investigation focused on different aspects. The iFOBT can detect bleeding lesions and the questionnaire can find lesions which do not bleed or bleed intermittently. Thus they may have different performance in screening colorectal abnormalities. To the best of our knowledge, the current study is the first analysis comparing the performance value of high risk factors in mass CRC screening in China. Because confirmatory examination was limited to subjects who had at least one positive test, studies calculated the RSN and relative false-positive rate in comparing the 2 screening methods[16,17].
Colorectal adenomatous polyps are recognized as precancerous lesions and are responsible for most cases of CRC[18]. Thus far, the important indicator for transition from adenomas to cancer has been the pathologic characteristics of the advanced adenomas. Thus it is important to find advanced adenomas and block the adenoma-carcinoma sequence in CRC screening. The iFOBT had the highest PPV, NPV and RSN, and the lowest FP:TP ratio in screening for advanced neoplasias, indicating that the iFOBT may be superior to other factors in screening for advanced neoplasias. Though some studies in Asian countries have shown that iFOBT is effective in CRC screening[19,20], iFOBT alone may not be enough in CRC screening, because iFOBT inevitably misses some important lesions which do not bleed or bleed intermittently. A history of chronic cholecystitis or cholecystectomy, chronic appendicitis or appendectomy, and chronic diarrhea also had higher sensitivity than HAP, indicating that these unique Chinese high risk factors can detect a larger number of advanced neoplasias. Some studies have found an increase in the risk of CRC following cholecystectomy for gallstones[21-27]. Cholecystectomy also influences the adenoma to cancer transition, ultimately predisposing to the development of CRC[28]. A study from France supported the hypothesis that the appendix, as a lymphoid organ, plays a protective role in colon carcinogenesis[29]. These unique Chinese high risk factors for CRC may play an important role in screening for advanced neoplasias because of their higher sensitivity, which contributed to detection of a greater number of advanced neoplasias.
It would be unsafe to ignore adenomas < 10 mm because 30% of cancer is derived from 6-9 mm adenomas[30]. The questionnaire detected a greater number of adenomas than the iFOBT in our screening study. We also found that the iFOBT still had the highest sensitivity among all high risk factors, followed by history of chronic cholecystitis or cholecystectomy, chronic appendicitis or appendectomy, and chronic diarrhea. The iFOBT may also be superior to other factors in screening for adenomas because of high PPV and NPV, high RSN and low FP:TP ratio. Higher sensitivities indicated the important performance value of these unique Chinese high risk factors.
A history of chronic cholecystitis or cholecystectomy was the most sensitive marker in screening for non-adenomatous polyps, followed by history of chronic appendicitis or appendectomy, and chronic constipation. Though subjects with non-adenomatous polyps were not regarded as having increased risk of CRC, these polyps do not require surveillance colonoscopy, they may serve as a precursor to CRC in subjects with specific genetic and other molecular characteristics[31-34]. Thus it would be unsafe to ignore these polyps.
The study had several drawbacks. Firstly, to evaluate screening test performances among the general population, the ideal is to obtain sensitivity and specificity for all individuals. Because only eligible high risk subjects were invited and only about 55% of population accepted the colonoscopy examination, these results may not be completely representative of the general population. Secondly, although all the study population accepted both the iFOBT and questionnaire investigation, the colonoscopy uptake rate of the iFOBT positive only was higher than that of the questionnaire positive only. This would slightly overestimate the RSN of the iFOBT.
HAP, an acknowledged high risk factor was used as the reference to calculate the relative ratio in this study. Therefore the other variables being compared may be underestimated. Even so, the iFOBT and some unique Chinese high risk factors - history of chronic cholecystitis or cholecystectomy, chronic appendicitis or appendectomy, and history of chronic diarrhea - still play an important role because of the higher sensitivities than that of HAP. The iFOBT may be superior to other factors in screening for advanced neoplasias and adenomas.
COMMENTS
Background
The immunochemical fecal occult blood test (iFOBT) and high risk factors questionnaire approach as primary screening followed by full colonoscopy examination as follow-up screening, has been recommended as the colorectal cancer (CRC) screening guideline for population-based CRC screening in China. The performance value of the iFOBT and the high risk factors questionnaire is still unknown in CRC screening practice in China.
Research frontiers
The limitation of the iFOBT is its low sensitivity for CRC. High risk factors for CRC among a Chinese natural population have been identified through a meta-analysis. The major advantage of the high risk factors questionnaire investigation is that it can detect lesions that do not bleed or bleed intermittently.
Innovations and breakthroughs
This is believed to be the first study comparing the performance value of high risk factors in mass CRC screening in China. In this study, because participants with at least one positive factor were asked to undergo colonoscopy, the sensitivity of each high risk item could not be directly estimated. The authors therefore compared sensitivities by calculating the relative sensitivity using HAP (history of adenomatous polyps, an acknowledged high risk factor) as a reference.
Applications
The study suggests that the iFOBT may be the best marker for screening advanced neoplasias and adenomas. Some unique Chinese high risk factors (history of chronic cholecystitis or cholecystectomy, chronic appendicitis or appendectomy, and history of chronic diarrhea) may play an important role in CRC screening in China because of higher sensitivities than that of HAP.
Peer review
The authors have done much work in this study. The study is worthwhile and well performed.
Acknowledgments
We thank all the gastroenterologists in the local hospitals and all staff at Xiacheng Disease Control Center (CDC) for their assistance and participation.
Footnotes
Supported by 11th 5-Year Key Programs for Science and Technology Development of China, No. 2006BAI02A08
Peer reviewer: Dr. Weidong Tong, Department of General Surgery, Veterans Affairs Medical Center, Medical College of Wisconsin, Milwaukee, WI 53295, United States
S- Editor Wang YR L- Editor Cant MR E- Editor Lin YP
References
- 1.Sung JJ, Lau JY, Young GP, Sano Y, Chiu HM, Byeon JS, Yeoh KG, Goh KL, Sollano J, Rerknimitr R, et al. Asia Pacific consensus recommendations for colorectal cancer screening. Gut. 2008;57:1166–1176. doi: 10.1136/gut.2007.146316. [DOI] [PubMed] [Google Scholar]
- 2.Smith RA, von Eschenbach AC, Wender R, Levin B, Byers T, Rothenberger D, Brooks D, Creasman W, Cohen C, Runowicz C, et al. American Cancer Society guidelines for the early detection of cancer: update of early detection guidelines for prostate, colorectal, and endometrial cancers. Also: update 2001--testing for early lung cancer detection. CA Cancer J Clin. 2001;51:38–75; quiz 77-80. doi: 10.3322/canjclin.51.1.38. [DOI] [PubMed] [Google Scholar]
- 3.Dong ZW. Guidelines of cancer screening, early detection and early treatment of China. 1st ed. Peiking: Peking University Medical Press; 2005. pp. 34–46. [Google Scholar]
- 4.Smith RA, Cokkinides V, Eyre HJ. Cancer screening in the United States, 2007: a review of current guidelines, practices, and prospects. CA Cancer J Clin. 2007;57:90–104. doi: 10.3322/canjclin.57.2.90. [DOI] [PubMed] [Google Scholar]
- 5.Meng W, Bi XW, Bai XY, Pan HF, Cai SR, Zhao Q, Zhang SZ. Barrier-focused intervention to increase colonoscopy attendance among nonadherent high-risk populations. World J Gastroenterol. 2009;15:3920–3925. doi: 10.3748/wjg.15.3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imperiale TF, Wagner DR, Lin CY, Larkin GN, Rogge JD, Ransohoff DF. Risk of advanced proximal neoplasms in asymptomatic adults according to the distal colorectal findings. N Engl J Med. 2000;343:169–174. doi: 10.1056/NEJM200007203430302. [DOI] [PubMed] [Google Scholar]
- 7.Terhaar Sive Droste JS, Craanen ME, van der Hulst RW, Bartelsman JF, Bezemer DP, Cappendijk KR, Meijer GA, Morsink LM, Snel P, Tuynman HA, et al. Colonoscopic yield of colorectal neoplasia in daily clinical practice. World J Gastroenterol. 2009;15:1085–1092. doi: 10.3748/wjg.15.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schatzkin A, Connor RJ, Taylor PR, Bunnag B. Comparing new and old screening tests when a reference procedure cannot be performed on all screenees. Example of automated cytometry for early detection of cervical cancer. Am J Epidemiol. 1987;125:672–678. doi: 10.1093/oxfordjournals.aje.a114580. [DOI] [PubMed] [Google Scholar]
- 9.Cheng H, Macaluso M. Comparison of the accuracy of two tests with a confirmatory procedure limited to positive results. Epidemiology. 1997;8:104–106. doi: 10.1097/00001648-199701000-00017. [DOI] [PubMed] [Google Scholar]
- 10.Chock C, Irwig L, Berry G, Glasziou P. Comparing dichotomous screening tests when individuals negative on both tests are not verified. J Clin Epidemiol. 1997;50:1211–1217. doi: 10.1016/s0895-4356(97)00122-4. [DOI] [PubMed] [Google Scholar]
- 11.Lowenfels AB. Fecal occult blood testing as a screening procedure for colorectal cancer. Ann Oncol. 2002;13:40–43. doi: 10.1093/annonc/mdf074. [DOI] [PubMed] [Google Scholar]
- 12.Gluecker T, Dorta G, Keller W, Jornod P, Meuli R, Schnyder P. Performance of multidetector computed tomography colonography compared with conventional colonoscopy. Gut. 2002;51:207–211. doi: 10.1136/gut.51.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Segnan N, Senore C, Andreoni B, Azzoni A, Bisanti L, Cardelli A, Castiglione G, Crosta C, Ederle A, Fantin A, et al. Comparing attendance and detection rate of colonoscopy with sigmoidoscopy and FIT for colorectal cancer screening. Gastroenterology. 2007;132:2304–2312. doi: 10.1053/j.gastro.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 14.Detsky AS. Screening for colon cancer--can we afford colonoscopy? N Engl J Med. 2001;345:607–608. doi: 10.1056/NEJM200108233450809. [DOI] [PubMed] [Google Scholar]
- 15.Zheng S, Chen K, Liu X, Ma X, Yu H, Chen K, Yao K, Zhou L, Wang L, Qiu P, et al. Cluster randomization trial of sequence mass screening for colorectal cancer. Dis Colon Rectum. 2003;46:51–58. doi: 10.1007/s10350-004-6496-2. [DOI] [PubMed] [Google Scholar]
- 16.Guittet L, Bouvier V, Mariotte N, Vallee JP, Arsène D, Boutreux S, Tichet J, Launoy G. Comparison of a guaiac based and an immunochemical faecal occult blood test in screening for colorectal cancer in a general average risk population. Gut. 2007;56:210–214. doi: 10.1136/gut.2006.101428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng H, Macaluso M, Vermund SH, Hook EW 3rd. Relative accuracy of nucleic acid amplification tests and culture in detecting Chlamydia in asymptomatic men. J Clin Microbiol. 2001;39:3927–3937. doi: 10.1128/JCM.39.11.3927-3937.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 19.Lee KJ, Inoue M, Otani T, Iwasaki M, Sasazuki S, Tsugane S. Colorectal cancer screening using fecal occult blood test and subsequent risk of colorectal cancer: a prospective cohort study in Japan. Cancer Detect Prev. 2007;31:3–11. doi: 10.1016/j.cdp.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Nakama H, Kamijo N, Abdul Fattah AS, Zhang B. Validity of immunological faecal occult blood screening for colorectal cancer: a follow up study. J Med Screen. 1996;3:63–65. doi: 10.1177/096914139600300203. [DOI] [PubMed] [Google Scholar]
- 21.Reid FD, Mercer PM, harrison M, Bates T. Cholecystectomy as a risk factor for colorectal cancer: a meta-analysis. Scand J Gastroenterol. 1996;31:160–169. doi: 10.3109/00365529609031981. [DOI] [PubMed] [Google Scholar]
- 22.Giovannucci E, Colditz GA, Stampfer MJ. A meta-analysis of cholecystectomy and risk of colorectal cancer. Gastroenterology. 1993;105:130–141. doi: 10.1016/0016-5085(93)90018-8. [DOI] [PubMed] [Google Scholar]
- 23.Lagergren J, Ye W, Ekbom A. Intestinal cancer after cholecystectomy: is bile involved in carcinogenesis? Gastroenterology. 2001;121:542–547. doi: 10.1053/gast.2001.27083. [DOI] [PubMed] [Google Scholar]
- 24.Neagoe A, Molnar AM, Acalovschi M, Seicean A, Serban A. Risk factors for colorectal cancer: an epidemiologic descriptive study of a series of 333 patients. Rom J Gastroenterol. 2004;13:187–193. [PubMed] [Google Scholar]
- 25.Schernhammer ES, Leitzmann MF, Michaud DS, Speizer FE, Giovannucci E, Colditz GA, Fuchs CS. Cholecystectomy and the risk for developing colorectal cancer and distal colorectal adenomas. Br J Cancer. 2003;88:79–83. doi: 10.1038/sj.bjc.6600661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shao T, Yang YX. Cholecystectomy and the risk of colorectal cancer. Am J Gastroenterol. 2005;100:1813–1820. doi: 10.1111/j.1572-0241.2005.41610.x. [DOI] [PubMed] [Google Scholar]
- 27.Lowenfels AB. Gallstones and the risk of cancer. Gut. 1980;21:1090–1092. doi: 10.1136/gut.21.12.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Llamas KJ, Torlach LG, Ward M, Bain C. Cholecystectomy and adenomatous polyps of the large bowel. Gut. 1986;27:1181–1185. doi: 10.1136/gut.27.10.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grobost O, Boutron MC, Arveux P, Bedenne L, Chatrenet P, Faivre J. [Appendectomy, cholecystectomy, cholelithiasis and colorectal cancer. A retrospective case control study at the Côte-d'Or] Gastroenterol Clin Biol. 1991;15:594–599. [PubMed] [Google Scholar]
- 30.Loeve F, Brown ML, Boer R, van Ballegooijen M, van Oortmarssen GJ, Habbema JD. Endoscopic colorectal cancer screening: a cost-saving analysis. J Natl Cancer Inst. 2000;92:557–563. doi: 10.1093/jnci/92.7.557. [DOI] [PubMed] [Google Scholar]
- 31.Jass JR. Hyperplastic polyps and colorectal cancer: is there a link? Clin Gastroenterol Hepatol. 2004;2:1–8. doi: 10.1016/s1542-3565(03)00284-2. [DOI] [PubMed] [Google Scholar]
- 32.Brosens LA, van Hattem A, Hylind LM, Iacobuzio-Donahue C, Romans KE, Axilbund J, Cruz-Correa M, Tersmette AC, Offerhaus GJ, Giardiello FM. Risk of colorectal cancer in juvenile polyposis. Gut. 2007;56:965–967. doi: 10.1136/gut.2006.116913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dunlop MG. Guidance on gastrointestinal surveillance for hereditary non-polyposis colorectal cancer, familial adenomatous polypolis, juvenile polyposis, and Peutz-Jeghers syndrome. Gut. 2002;51 Suppl 5:V21–V27. doi: 10.1136/gut.51.suppl_5.v21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franzin G, Zamboni G, Dina R, Scarpa A, Fratton A. Juvenile and inflammatory polyps of the colon--a histological and histochemical study. Histopathology. 1983;7:719–728. doi: 10.1111/j.1365-2559.1983.tb02284.x. [DOI] [PubMed] [Google Scholar]


