Abstract
AIM: To investigate the possible correlation between osteoglycin expression and gelatinase activity of mouse hepatocarcinoma Hca-F cells.
METHODS: A eukaryotic expression plasmid pIRESpuro3 osteoglycin(+) was constructed and transfected into Hca-F cells to investigate the possible correlation between osteoglycin expression and gelatinase activity of Hca-F cells cultured with extract of lymph node, liver, spleen or in DMEM medium. The activity of gelatinases was examined through zymographic analysis.
RESULTS: High expression of osteoglycin attenuated the gelatinase activity of Hca-F cells cultured with extract of lymph node, and at the same time, decreased the metastatic potential of Hca-F cells to peripheral lymph nodes in vivo.
CONCLUSION: High expression of osteoglycin decreases the gelatinase activity of Hca-F cells cultured with extract of lymph node; regulation of gelatinase activity might be one of mechanisms that osteoglycin contributes to lymphatic metastasis suppression.
Keywords: Osteoglycin, Transfection, Hepatocellular carcinoma, Neoplasm metastasis, Genes, Gelatinases
INTRODUCTION
Most cancer lesions metastasize through the lymphatic system and the status of regional lymph nodes is the most important indicator of a patient’s prognosis[1]. But the molecular mechanism of lymphatic metastasis remains unclear. Hca-P and Hca-F are synogenetic mouse hepatocarcinoma cell lines, when inoculated subcutaneously in 615-mice, they metastasized only to the lymph nodes but not to other organs, Hca-P cells illustrated a low metastatic potential (lymphatic metastasis rate < 30%), while Hca-F cells showed a high metastatic potential (lymphatic metastasis rate > 80%)[2,3]. In our previous study, we found that osteoglycin was highly expressed in Hca-P cells and lowly expressed in Hca-F cells with suppressively subtracted hybridization (SSH) technique. Osteoglycin (OGN) is a member of proteoglycans (PGs) called small leucine-rich proteoglycans (SLRPs) residing in the extracellular matrix of connective tissues which are involved in matrix assembly, cellular growth and migration[4]. There are few reports about the relationship between osteoglycin and tumor metastasis. We subsequently transfected osteoglycin into Hca-F cells and found that high expression of osteoglycin inhibited the metastatic behavior of Hca-F cells[5]. However, the mechanism of osteoglycin regulating metastasis is elusive.
Gelatinases/type IV collagenases belong to matrix metalloproteinase (MMP) family, including gelatinase A (also known as MMP2, 72 kDa) and gelatinase B (also known as MMP9, 92 kDa), they are secreted in a proenzyme form and activated extracellularly[6]. Gelatinases mainly degrade collagen IV and a number of other ECM proteins, such as Col I, V, VII, IX, fibronectin, laminin, elastin and vitronectin[7]. As the most frequently studied MMPs in tumor research, gelatinases are suggested to play critical roles in tumor invasion and metastasis[8].
In this study, we resorted to gene transfection technique to explore the possible correlation between osteoglycin expression and gelatinase activity of murine hepatocarcinoma Hca-F cells with a high metastatic potential. We found that high expression of osteoglycin decreased the gelatinase activity of Hca-F cells cultured with extract of lymph node, and at the same time, decreased the metastatic potential of Hca-F cells to peripheral lymph nodes in vivo; regulation of gelatinase activity might be one of mechanisms that osteoglycin contributes to lymphatic metastasis suppression.
MATERIALS AND METHODS
Cell culture and animals
Mouse hepatocarcinoma Hca-P cells and Hca-F cells (established by Department of Pathology, Dalian Medical University) were cultured in DMEM (Invitrogen) supplemented with antibiotics (1 × penicillin/streptomycin 100 U/mL, Invitrogen), 10% FBS (Invitrogen) and cultured in a humidified incubator at 37°C with 50 mL/L CO2; inbred 615-mice (male, 8 wk old) were provided by Animal Facility of Dalian Medical University.
Construction of targeting vector
The osteoglycin coding sequence was amplified by polymerase chain reaction (PCR). Briefly, total RNA from 1 × 107 Hca-F cells was isolated with Trizol (Invitrogen). A High Fidelity PrimeScript RT-PCR kit (TaKaRa) was used to synthesize the cDNA according to the manufacturer’s protocol. PCR was carried out with primer sets P1, 5'-GAATTCATGGAGACTGTGCACTCTA-3' (forward), and P2, 5'-GCGGCCGCTTAGAAGTATGACCCTA-3' (reverse), containing EcoRI and NotI sites, respectively (underlined). Using obtained cDNA as a template, PCR was carried out under the following conditions: 30 cycles of denaturation for 10 s at 98°C, annealing for 15 s at 55°C, and extension for 60 s at 72°C. After digestion by EcoRI and NotI enzymes, the PCR product was cloned into pIRESpuro3 vector digested by the same enzymes and designated as pIRESpuro osteoglycin(+). Sequence and orientation were confirmed by DNA sequencing using a BigDye Terminator V3.1 cycle sequencing kit (Applied Biosystems).
Cell transfection and screening
Hca-F cells incubated in antibiotic-free medium with 10% FBS (Invitrogen) were transferred to a 6-well culture plate and incubated at 37°C, CO2 incubator to obtain 60%-80% confluence, and then were stably transfected with pIRESpuro3 and pIRESpuro3 osteoglycin(+) using TransIT-LT1 Transfection Reagent (TaKaRa) according to the protocol provided by the manufacturer. Two µg plasmid DNA was added to each transfection. The transfected Hca-F cells were selected by puromycin (Clontech) for 2 wk and maintained in medium containing 0.5 mg/L puromycin.
RT-PCR analysis
For RT-PCR analysis of osteoglycin mRNA levels, total RNA was isolated from cells using Trizol (Invitrogen) and cDNA was synthesized with High Fidelity PrimeScriptTM RT-PCR Kit (TaKaRa) according to the manufacturer’s instruction. The sequences of the primers were as follows: F1: 5'-TTCTCCTGCTACTCTTCGTG-3' and R1: 5'-AAGCAGACACACAACAGGCA-3' for osteoglycin; and F1: 5'-CGGGACCTGACAGACTACCT-3' and R1: 5'-AGCACTGTGTTGGCATAGAG-3' for β-actin, respectively. PCR analysis was performed under the following conditions: 30 cycles of denaturation for 10 s at 98°C, annealing for 15 s at 55°C, and extension for 30 s at 72°C. The amplified products were analyzed by agarose gel electrophoresis using 1.6% gel, followed by ethidium bromide staining. The bands were analyzed with LabWorks (UVP GDS-800 Version 4.0).
Western blotting analysis
Western blotting analysis was carried out to evaluate osteoglycin protein levels. Cellular protein was extracted with lysis buffer [20 mmol/L Tris (pH 7.5), 150 mmol/L NaCl, 1 mmol/L MgCl2, 2 mmol/L EGTA, 10% glycerol, 0.15% sodium dodecylsulfate, 1% deoxycholate, 1% Triton X-100, and 1% anti-protease cocktail (Sigma)]. The extracted proteins were subjected to 10% sodiumdodecylsulfate-polyacrylamide gel electrophoresis, blotted onto polyvinylidene difluoride membranes (Invitrogen), then probed with goat anti-mouse osteoglycin polyclonal antibody and β-actin monoclonal antibody (Santa Cruz) followed by secondary antibody conjugated to horseradish peroxidase (Santa Cruz) and detected by enhanced chemiluminescence (Amersham Biosciences). The bands were analyzed with LabWorks (UVP GDS-800 Version 4.0).
In vivo tumor metastasis assay
Ninety inbred 615-mice were randomly divided into 3 groups. Hca-F cells (F), Hca-F cells transfected with pIRESpuro3 (F0), or Hca-F cells transfected with pIRESpuro3 osteoglycin(+) [F(+)] were inoculated subcutaneously at 2 × 106 tumor cells of approximately 0.05 mL cell suspension into the left foot of each mouse in each group. They were terminated on the 28th day after inoculation, the implanted tumor and their axillary lymph nodes, inguinal lymph nodes, and popliteal lymph nodes were hematoxylin eosin (HE) stained and examined under microscope. The mouse which had at least one metastatic axillary lymph node or one metastatic inguinal lymph node or one metastatic popliteal lymph node was considered as a metastatic mouse. The lymph node metastatic rate of tumor-burden mice = metastatic mice/total mice.
The lymph node metastatic rates of F, F0 and F(+) cells burden mice were calculated. The number of positive lymph nodes per mouse was also evaluated.
Zymographic analysis
The F, Hca-P (P), F0 and F(+) cells were put into different wells at 5 × 105, and then added 50 mg extract of lymph node, liver or spleen respectively. The Dulbecco’s Modified Eagle Media (DMEM) was placed into each well up to 1 mL. DMEM medium containing only F, P, F0 or F(+) cells, and DMEM medium added only extracts of lymph node, liver or spleen served as controls. These cells were cultured at 37°C for 24 h. The supernatant of cultured cells was collected by centrifugation at 3000 × g. Gelatinases contained in supernatants of each cell with or without extracts of lymph node, liver or spleen were detected through zymographic analysis according to the method described by Fridman[9]. The bands were analyzed with LabWorks (UVP GDS-800 Version 4.0).
Statistical analysis
Data were presented as means ± SD and analyzed by the Student’s t test, analysis of variance and χ2 test using SPSS 11.5. P < 0.05 was considered statistically significant.
RESULTS
Osteoglycin expression at mRNA and protein level
The relative mRNA and protein levels of osteoglycin were determined by RT-PCR and Western blotting analysis, respectively. Compared with F and F0 cells, F(+) cells showed significantly higher expression of osteoglycin at both mRNA and protein levels; however, no significant difference of osteoglycin expression was found between F0 and F cells. Transfection of osteoglycin into Hca-F cells resulted in high expression of osteoglycin at both mRNA and protein levels. Osteoglycin was highly expression at both mRNA and protein levels in P cells (Figure 1).
Figure 1.
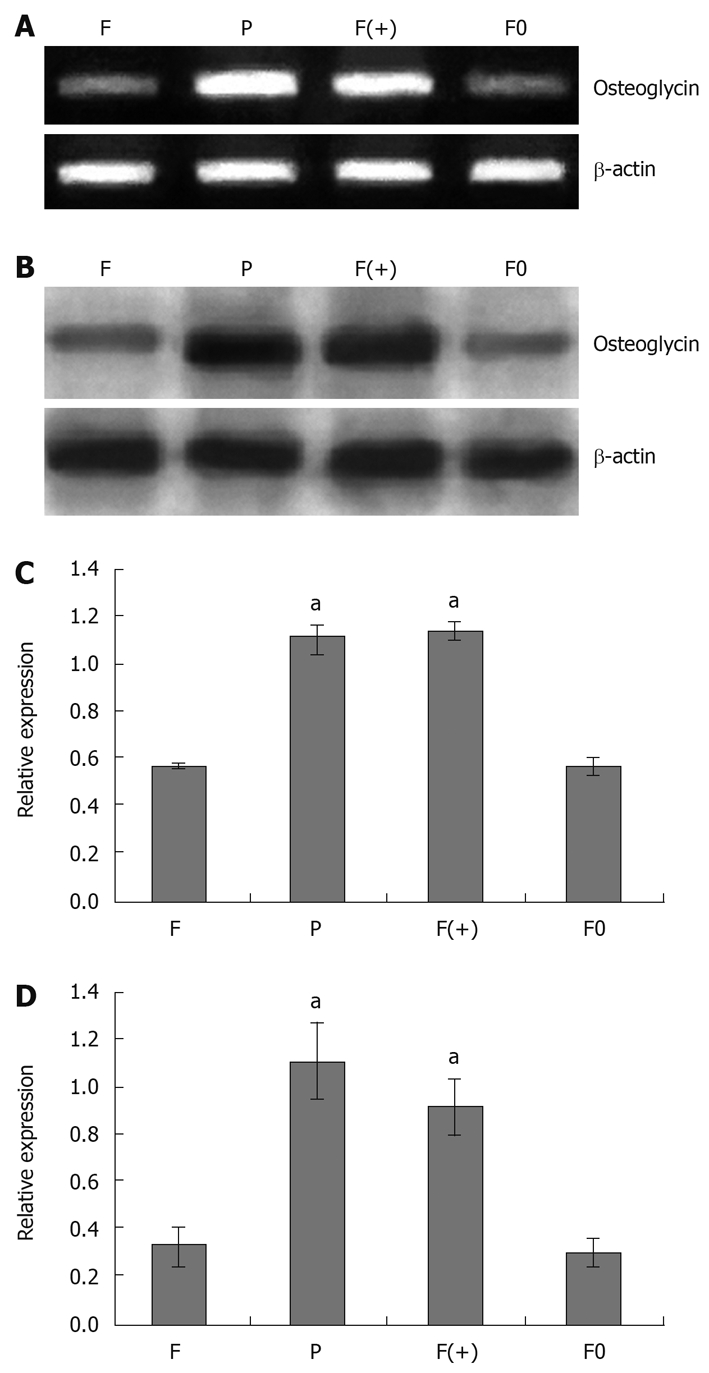
Analysis of osteoglycin expression. RT-PCR analysis (A) and Western blot analysis (B) of osteoglycin expression in mouse hepatocarcinoma cells; relative signal intensities of osteoglycin mRNA (C) and protein (D) levels were normal as against those of β-actin by LabWorks (UVP GDS-800 Version 4.0) analysis (compared with F cells, aP < 0.05). F: Hca-F cells; P: Hca-P cells; F(+): Hca-F cells transfected with pIRESpuro3 osteoglycin(+); F0: Hca-F cells transfected with pIRESpuro3. β-actin was used as an internal control.
In vivo tumor metastasis assay
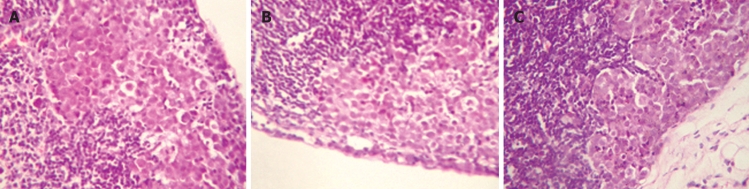
F, F0 and F(+) cells were injected subcutaneously into the left foot of 615-mice. The implanted tumors were palpable on the 7th day after inoculation. On the 28th day after inoculation, 53.3% (16/30) F(+) cells burden mice developed lymphatic metastasis, while 80% (24/30, P < 0.05) F cells burden mice and 83.3% (25/30, P < 0.05) F0 cells burden mice developed lymphatic metastasis. Hca-F cells with transfected osteoglycin showed significant decrease in metastasis potential to lymph node (Figure 2). The result supported the fact that osteoglycin acted as a tumor lymphatic metastasis suppressed gene.
Figure 2.
Metastatic lymph nodes of tumor-burden mice inoculated with Hca-F cells (A), Hca-F cells transfected with pIRESpuro3 (B), or Hca-F cells transfected with pIRESpuro3 osteoglycin(+) (C). Lymph nodes of tumor-burden mice were HE stained and examined under microscope.
No significant difference was found in the number of positive lymph nodes per mouse in F(+), F and F0 cells burden mice.
Zymographic analysis
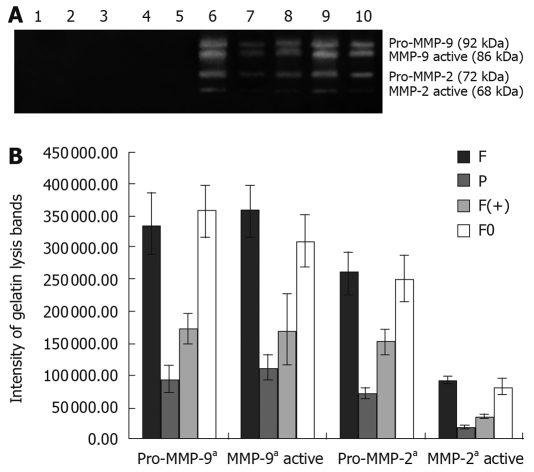
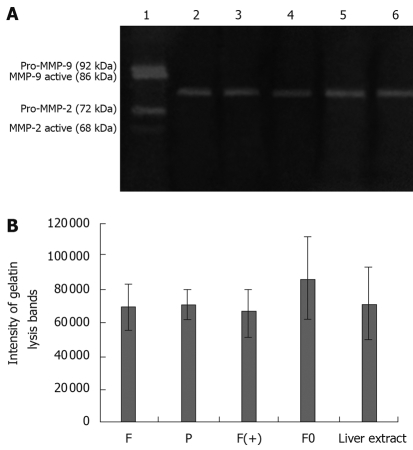
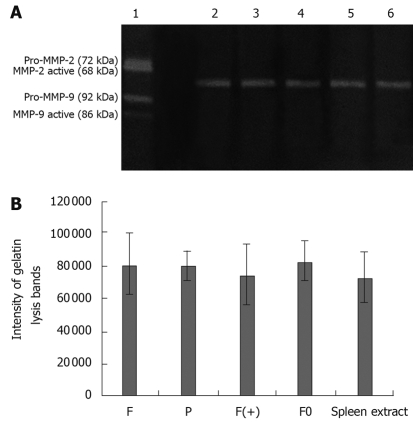
When cultured in DMEM, no cell produced any gelatinase (no gelatinase was detected in the supernatant of each cell). However, when cultured with extract of lymph node, all cells produced gelatinases (Pro-MMP-9, MMP-9 active, Pro-MMP-2 and MMP-2 active were detected in the supernatant of each cell). The quantity of gelatinases produced by tumor cells were closely associated with the metastatic potential of each tumor cell (quantity of MMP2 and MMP9 detected in the supernatant of F and F0 cells were much higher than those detected in F(+) and P cells (P < 0.05). High expression of osteoglycin via transfecion of osteoglycin attenuated the secretion of gelatinases in Hca-F cells cultured with extract of lymph node (quantities of MMP2 and MMP9 detected in the supernatant of F(+) cells were much lower than those detected in F and F0 cells (P < 0.05). The extract of lymph node did not contain any gelatinase (Figure 3). Gelatin lysis bands were found in the zymograms of the supernatant of all cells cultured with extract of liver, and the same gelatin lysis bands were found in the zymograms of the extract of liver, and their intensities were almost the same (Figure 4); gelatin lysis bands were also found in the zymograms of the supernatant of all cells cultured with extract of spleen, and in the zymograms of the extract of spleen, with similar intensities (Figure 5). Therefore, we think that all cells in the liver and spleen did not produce any gelatinases.
Figure 3.
Zymographic analysis of MMPs activity of tumor cells in DMEM with or without lymph node extract (A); the intensity of gelatin lysis bands obtained by scanning densitometry (LabWorks UVP GDS-800 Version 4.0, multiple comparisons, aP < 0.05) (B). 1. L; 2. F; 3. P; 4. F(+); 5. F0; 6. F; 7. P; 8. F(+); 9. F0. 10. Type IV collagenases. 1. L: lymph node extract; 2-5: cells in DMEM. 6-10: cells cultured with lymph node extract. F: Hca-F cells; P: Hca-P cells; F(+): Hca-F cells transfected with pIRESpuro3 osteoglycin(+); F0: Hca-F cells transfected with pIRESpuro3.
Figure 4.
Zymographic analysis of MMPs activity of tumor cells in liver extract (A); the intensity of gelatin lysis bands obtained by scanning densitometry (LabWorks UVP GDS-800 Version 4.0) (B). 1. Type IV collagenases; 2. F; 3. P; 4. F(+); 5. F0; 6. Liver extract. F: Hca-F cells; P: Hca-P cells; F(+): Hca-F cells transfected with pIRESpuro3 osteoglycin(+); F0: Hca-F cells transfected with pIRESpuro3.
Figure 5.
Zymographic analysis of MMPs activity of tumor cells in spleen extract (A); the intensity of gelatin lysis bands obtained by scanning densitometry (LabWorks UVP GDS-800 Version 4.0) (B). 1. Type IV collagenases; 2. F; 3. P; 4. F(+); 5. F0; 6. spleen extract. F: Hca-F cells; P: Hca-P cells; F(+): Hca-F cells transfected with pIRESpuro3 osteoglycin(+); F0: Hca-F cells transfected with pIRESpuro3.
DISCUSSION
The metastatic potential of tumor cells is believed to be regulated by interactions between the tumor cells and their extracellular environment (extracellular matrix)[10,11]. Being a matrix molecule, osteoglycin participates in the organization and regulation of the extracellular matrix and might influence the tumor metastasis, as exemplified by studies in vivo that osteoglycin played a role in collagen fibrillogenesis[12,13], a process essential in metastasis[11,12]. In addition to its extracellular matrix functions, osteoglycin, like other members of SLRPs, also plays a role in regulation of cell biological behavior[4]. As illustrated in the literature, the expression of mimecan was high at mRNA level in corneal keratocytes cultured in low-serum or serum-free media, but was attenuated if these cells were cultured in media containing serum[14]. Osteoglycin mRNA was absent or at a low level in the majority of cancer cell lines and tumors[15]. Bioactive such as p53, basic fibroblast growth factor, interferon-γ and bone morphogenetic protein-1/tolloid-related metalloproteinases interacted with osteoglycin[16-20]. In the earlier studies, we found that osteoglycin was highly expressed in Hca-P cells and lowly expressed in Hca-F cells, and that osteoglycin acted as a tumor lymphatic metastasis suppressed gene[5]. However, no data identified intrinsic mechanism for osteoglycin regulation of tumor lymphatic metastasis.
Hca-P and Hca-F are syngenetic mouse hepatocarcinoma cell lines presenting a specific potential of lymphatic metastasis with a significant difference in their potential of metastasis[2,3], which provide good experimental models for lymph node metastasis.
Cell adhesion to extracellular matrices is a determinant for cell migration and invasion[21,22]. Osteoglycin, being a matrix molecule, as we once assumed, would probably affect adhesive capacity of tumor cells, whereby influencing tumor migration and invasion. However, our previous work showed that adhesion was not responsible for the contribution of osteoglycin to lymphatic metastasis inhibition[5]. As the main mediators of extracellular matrix degradation, gelatinases play an important role in tumor metastasis as demonstrated in gastrointestinal cancer[23,24], breast cancer[25], hepatocarcinoma[26], etc. Inhibition of the gelatinase activity can reduce the metastatic potential of cancer cells[27]. In the present study, high expression of osteoglycin via osteoglycin transfection attenuated the secretion of gelatinases (Pro-MMP-9, MMP-9 active, Pro-MMP-2 and MMP-2 active) in Hca-F cells cultured with extract of lymph node, and at the same time, decreased the metastatic potential of Hca-F cells to peripheral lymph nodes in vivo, which suggested that regulation of gelatinase activity might be one of mechanisms that osteoglycin contributes to lymphatic metastasis suppression. Moreover, osteoglycin expression only influenced gelatinase activity of Hca-F cells cultured with extract of lymph node, but failed to influence gelatinase activity of Hca-F cell cultured with extracts of liver and spleen or in DMEM medium, demonstrating a lymph node environment-selective metastasis suppression, which further supported the fact that osteoglycin acted as lymphatic metastasis suppression gene. The mechanism of osteoglycin impact on gelatinases is unclear. Some of the SLRPs members bind and modulate TGF-β and cytokines such as TNF-α[28,29] and play roles in EGFR activation pathway and the NF-κB signal transduction system as well[30,31]. And these bioactives (TGF-β, TNF-α, EGF and NF-κB) are also the regulators of gelatinase activity[6,32], which implicates that SLRPs might involve in the regulation of gelatinase activity. Further studies are needed to clarify the interaction between gelatinases and osteoglycin.
COMMENTS
Background
Lymphatic metastasis is responsible for the early stage of tumor metastasis and acts as the most important indicator of a patient’s prognosis. But the molecular mechanism of lymphatic metastasis remains poorly understood.
Research frontiers
The metastatic potential of tumor cells is believed to be regulated by interactions between the tumor cells and their extracellular environment (extracellular matrix). Matrix molecules play important roles in tumor metastasis. Osteoglycin, as one of matrix molecules, is suggested to play a part in matrix assembly, cell growth and migration. However, there has been no report on osteoglycin and tumor metastasis.
Innovations and breakthroughs
The authors first report that osteoglycin acted as a tumor lymphatic metastasis suppression gene, and regulation of gelatinase activity might be one of mechanisms that osteoglycin contributes to lymphatic metastasis suppression.
Applications
This study may help for therapeutic intervention in tumor metastasis.
Terminology
Osteoglycin belongs to a small leucine-rich proteoglycan (SLRP) gene family, as one of the matrix molecules, it is reported to participate in the organization and regulation of the extracellular matrix. In addition to its extracellular matrix functions, osteoglycin, like other members of SLRPs, also plays a role in regulation of cell biological behavior, cell growth and migration, etc.
Peer review
In this study, it was observed that osteoglycin upregulation, induced by its transfection into mouse hepatocarcinoma Hca-F cells, results in a decrease in gelatinase activity and metastatic potential of Hca-F cells. The effect of osteoglycin transfection on gelatinase activity has been convincingly demonstrated.
Footnotes
Supported by National Natural Science Foundation of China, No. 30500586
Peer reviewer: Francesco Feo, Professor, Department of Biomedical Sciences, Section of Experimental Pathology and Oncology, University of Sassari, Via P, Manzella 4, 07100 Sassari, Italy
S- Editor Wang JL L- Editor Ma JY E- Editor Ma WH
References
- 1.Das S, Skobe M. Lymphatic vessel activation in cancer. Ann N Y Acad Sci. 2008;1131:235–241. doi: 10.1196/annals.1413.021. [DOI] [PubMed] [Google Scholar]
- 2.Chu H, Zhou H, Liu Y, Liu X, Hu Y, Zhang J. Functional expression of CXC chemokine recepter-4 mediates the secretion of matrix metalloproteinases from mouse hepatocarcinoma cell lines with different lymphatic metastasis ability. Int J Biochem Cell Biol. 2007;39:197–205. doi: 10.1016/j.biocel.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Zhou H, Jia L, Wang S, Wang H, Chu H, Hu Y, Cao J, Zhang J. Divergent expression and roles for caveolin-1 in mouse hepatocarcinoma cell lines with varying invasive ability. Biochem Biophys Res Commun. 2006;345:486–494. doi: 10.1016/j.bbrc.2006.03.246. [DOI] [PubMed] [Google Scholar]
- 4.Williamson RE, Darrow KN, Giersch AB, Resendes BL, Huang M, Conrad GW, Chen ZY, Liberman MC, Morton CC, Tasheva ES. Expression studies of osteoglycin/mimecan (OGN) in the cochlea and auditory phenotype of Ogn-deficient mice. Hear Res. 2008;237:57–65. doi: 10.1016/j.heares.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cui X, Song B, Hou L, Wei Z, Tang J. High expression of osteoglycin decreases the metastatic capability of mouse hepatocarcinoma Hca-F cells to lymph nodes. Acta Biochim Biophys Sin (Shanghai) 2008;40:349–355. doi: 10.1111/j.1745-7270.2008.00392.x. [DOI] [PubMed] [Google Scholar]
- 6.Ries C, Kolb H, Petrides PE. Regulation of 92-kD gelatinase release in HL-60 leukemia cells: tumor necrosis factor-alpha as an autocrine stimulus for basal- and phorbol ester-induced secretion. Blood. 1994;83:3638–3646. [PubMed] [Google Scholar]
- 7.Cohen M, Meisser A, Bischof P. Metalloproteinases and human placental invasiveness. Placenta. 2006;27:783–793. doi: 10.1016/j.placenta.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 9.Fridman R, Sweeney TM, Zain M, Martin GR, Kleinman HK. Malignant transformation of NIH-3T3 cells after subcutaneous co-injection with a reconstituted basement membrane (matrigel) Int J Cancer. 1992;51:740–744. doi: 10.1002/ijc.2910510513. [DOI] [PubMed] [Google Scholar]
- 10.Lunt SJ, Chaudary N, Hill RP. The tumor microenvironment and metastatic disease. Clin Exp Metastasis. 2009;26:19–34. doi: 10.1007/s10585-008-9182-2. [DOI] [PubMed] [Google Scholar]
- 11.Pollard JW. Macrophages define the invasive microenvironment in breast cancer. J Leukoc Biol. 2008;84:623–630. doi: 10.1189/jlb.1107762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ameye L, Young MF. Mice deficient in small leucine-rich proteoglycans: novel in vivo models for osteoporosis, osteoarthritis, Ehlers-Danlos syndrome, muscular dystrophy, and corneal diseases. Glycobiology. 2002;12:107R–116R. doi: 10.1093/glycob/cwf065. [DOI] [PubMed] [Google Scholar]
- 13.Tasheva ES, Koester A, Paulsen AQ, Garrett AS, Boyle DL, Davidson HJ, Song M, Fox N, Conrad GW. Mimecan/osteoglycin-deficient mice have collagen fibril abnormalities. Mol Vis. 2002;8:407–415. [PubMed] [Google Scholar]
- 14.Plaas AH, West LA, Thonar EJ, Karcioglu ZA, Smith CJ, Klintworth GK, Hascall VC. Altered fine structures of corneal and skeletal keratan sulfate and chondroitin/dermatan sulfate in macular corneal dystrophy. J Biol Chem. 2001;276:39788–39796. doi: 10.1074/jbc.M103227200. [DOI] [PubMed] [Google Scholar]
- 15.Tasheva ES, Maki CG, Conrad AH, Conrad GW. Transcriptional activation of bovine mimecan by p53 through an intronic DNA-binding site. Biochim Biophys Acta. 2001;1517:333–338. doi: 10.1016/s0167-4781(00)00288-8. [DOI] [PubMed] [Google Scholar]
- 16.Shanahan CM, Cary NR, Osbourn JK, Weissberg PL. Identification of osteoglycin as a component of the vascular matrix. Differential expression by vascular smooth muscle cells during neointima formation and in atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 1997;17:2437–2447. doi: 10.1161/01.atv.17.11.2437. [DOI] [PubMed] [Google Scholar]
- 17.Tasheva ES, Funderburgh ML, McReynolds J, Funderburgh JL, Conrad GW. The bovine mimecan gene. Molecular cloning and characterization of two major RNA transcripts generated by alternative use of two splice acceptor sites in the third exon. J Biol Chem. 1999;274:18693–18701. doi: 10.1074/jbc.274.26.18693. [DOI] [PubMed] [Google Scholar]
- 18.Tasheva ES. Analysis of the promoter region of human mimecan gene. Biochim Biophys Acta. 2002;1575:123–129. doi: 10.1016/s0167-4781(02)00245-2. [DOI] [PubMed] [Google Scholar]
- 19.Tasheva ES, Conrad GW. Interferon-gamma regulation of the human mimecan promoter. Mol Vis. 2003;9:277–287. [PubMed] [Google Scholar]
- 20.Ge G, Seo NS, Liang X, Hopkins DR, Hook M, Greenspan DS. Bone morphogenetic protein-1/tolloid-related metalloproteinases process osteoglycin and enhance its ability to regulate collagen fibrillogenesis. J Biol Chem. 2004;279:41626–41633. doi: 10.1074/jbc.M406630200. [DOI] [PubMed] [Google Scholar]
- 21.Gravdal K, Halvorsen OJ, Haukaas SA, Akslen LA. A switch from E-cadherin to N-cadherin expression indicates epithelial to mesenchymal transition and is of strong and independent importance for the progress of prostate cancer. Clin Cancer Res. 2007;13:7003–7011. doi: 10.1158/1078-0432.CCR-07-1263. [DOI] [PubMed] [Google Scholar]
- 22.Lyons AJ, Jones J. Cell adhesion molecules, the extracellular matrix and oral squamous carcinoma. Int J Oral Maxillofac Surg. 2007;36:671–679. doi: 10.1016/j.ijom.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Alakus H, Grass G, Hennecken JK, Bollschweiler E, Schulte C, Drebber U, Baldus SE, Metzger R, Holscher AH, Monig SP. Clinicopathological significance of MMP-2 and its specific inhibitor TIMP-2 in gastric cancer. Histol Histopathol. 2008;23:917–923. doi: 10.14670/HH-23.917. [DOI] [PubMed] [Google Scholar]
- 24.Herszenyi L, Sipos F, Galamb O, Solymosi N, Hritz I, Miheller P, Berczi L, Molnar B, Tulassay Z. Matrix metalloproteinase-9 expression in the normal mucosa-adenoma-dysplasia-adenocarcinoma sequence of the colon. Pathol Oncol Res. 2008;14:31–37. doi: 10.1007/s12253-008-9004-5. [DOI] [PubMed] [Google Scholar]
- 25.Zhao T, Xia WH, Zheng MQ, Lu CQ, Han X, Sun YJ. Surgical excision promotes tumor growth and metastasis by promoting expression of MMP-9 and VEGF in a breast cancer model. Exp Oncol. 2008;30:60–64. [PubMed] [Google Scholar]
- 26.Matsunaga Y, Koda M, Murawaki Y. Expression of matrix metalloproiteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) in hepatocellular carcinoma tissue, compared with the surrounding non-tumor tissue. Res Commun Mol Pathol Pharmacol. 2004;115-116:143–150. [PubMed] [Google Scholar]
- 27.Tu G, Xu W, Huang H, Li S. Progress in the development of matrix metalloproteinase inhibitors. Curr Med Chem. 2008;15:1388–1395. doi: 10.2174/092986708784567680. [DOI] [PubMed] [Google Scholar]
- 28.Macri L, Silverstein D, Clark RA. Growth factor binding to the pericellular matrix and its importance in tissue engineering. Adv Drug Deliv Rev. 2007;59:1366–1381. doi: 10.1016/j.addr.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 29.Tufvesson E, Westergren-Thorsson G. Tumour necrosis factor-alpha interacts with biglycan and decorin. FEBS Lett. 2002;530:124–128. doi: 10.1016/s0014-5793(02)03439-7. [DOI] [PubMed] [Google Scholar]
- 30.Zafiropoulos A, Tzanakakis GN. Decorin-mediated effects in cancer cell biology. Connect Tissue Res. 2008;49:244–248. doi: 10.1080/03008200802147746. [DOI] [PubMed] [Google Scholar]
- 31.Kresse H, Schonherr E. Proteoglycans of the extracellular matrix and growth control. J Cell Physiol. 2001;189:266–274. doi: 10.1002/jcp.10030. [DOI] [PubMed] [Google Scholar]
- 32.Cho HJ, Lee TS, Park JB, Park KK, Choe JY, Sin DI, Park YY, Moon YS, Lee KG, Yeo JH, et al. Disulfiram suppresses invasive ability of osteosarcoma cells via the inhibition of MMP-2 and MMP-9 expression. J Biochem Mol Biol. 2007;40:1069–1076. doi: 10.5483/bmbrep.2007.40.6.1069. [DOI] [PubMed] [Google Scholar]