Abstract
We report a case of a 56-year-old woman with intrahepatic biliary cystadenoma (IBC) accompanying a tumor embolus in the extrahepatic bile duct, who was admitted to our department on October 13, 2008. Imaging showed an asymmetry dilation of the biliary tree, different bile signals in the biliary tree, a multiloculated lesion and an extrahepatic bile duct lesion with internal septation. A regular left hemihepatectomy en bloc was performed with resection of the entire tumor, during which a tumor embolus protruding into the extrahepatic bile duct and originating from biliary duct of segment 4 was revealed. Microscopically, the multiloculated tumor was confirmed to be a biliary cystadenoma with an epithelial lining composed of biliary-type cuboidal cells and surrounded by an ovarian-like stroma. An aggressive en bloc resection was recommended for the multiloculated lesion. Imaging workup, clinicians and surgeons need to be aware of this different presentation.
Keywords: Intrahepatic biliary cystadenoma, Growth manner, Tumor embolus
INTRODUCTION
Intrahepatic biliary cystadenoma (IBC) is a rare benign epithelial tumor characterized by unicystic or multicystic growth, accounting for less than 5% of solitary non-parasitic cysts of biliary origin[1,2]. IBC was first described in 1892 by Keen[3], followed by Edmondson as a multilocular cystic lesion lined by mucus-secreting cuboidal or columnar epithelium accompanying a densely cellular “ovarian-like” stroma[4]. The most common symptoms of IBC patients at presentation are abdominal pain and mass in which 35% have obstructive jaundice[1,5]. Knowledge about the pathogenesis of IBC is limited. IBC manifested as a tumor embolus in the extrahepatic bile duct occurs rarely. In addition, no other case report has yet described its clinicopathological features. We report a case of IBC with a tumor embolus in the extrahepatic bile duct with its clinicopathological features described and its diagnostic approaches and surgical procedures discussed.
CASE REPORT
A 56-year-old woman with hypertension, diabetes and chronic B hepatitis was transferred to our hospital on October 13, 2008 due to a 2-wk history of right hypochondrial pain and spontaneously remitted jaundice. She denied nausea, vomiting, fever, shoulder or back pain. She was admitted to a local hospital but not received surgical treatment. Before admission, her peak bilirubin level was 434 μmol/L and magnetic resonance cholangiopancreatography (MRCP) demonstrated a multiloculated cystic lesion in segment 4, measuring 5.5 cm in diameter, and an obviously dilated left intra- and extra-hepatic biliary tree (Figure 1) but no evidence of choledocholithiasis. The signal was different between extrahepatic and marginal bile ducts on T2-weighted magnetic resonance images (MRI) showing thin septa in bile duct (Figure 2). The patient had no history of tobacco smoking, alcohol abuse or recent travel or previous surgery.
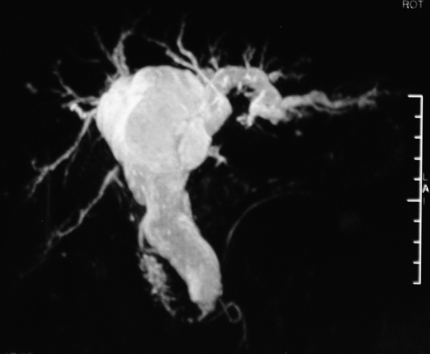
Figure 1.
Magnetic resonance cholangiopancreatography (MRCP) showing the dilation of extrahepatic and intrahepatic bile ducts lacking of asymmetry.
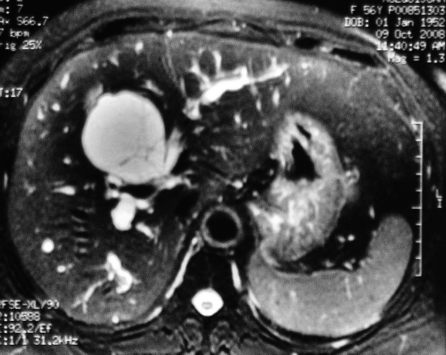
Figure 2.
Magnetic resonance imaging (MRI) showing different bile signals between the right and left intrahepatic ducts in a multiloculated lesion with internal septation.
After admission, she demonstrated obstructive jaundice features, her bilirubin level declined spontaneously to 103.8 μmol/L, carbohydrate antigen 19-9 (CA19-9) was elevated to 92.1 μg/L, and carcinoembryonic antigen (CEA) level was normal. Abdominal ultrasound showed a dilated biliary tree and a multilocular cyst in segment 4. The extrahepatic bile duct (1.8 cm in diameter) was filled with fluid-like matters. Prior to a planned left hepatectomy, percutanous transhepatic biliary drainage (PTBD) of the right lobe was performed to decompress the right biliary system with an 8.5F tube. The TB level was 71 μmol/L and 35.8 μmol/L, respectively, before PTBD and hepatectomy. Volumetric computed tomography (CT) scan revealed that the ratio of remnant liver to total liver volume was 63.2%, with no change in cystic tumor size and no dilation of the right bile ducts, and CT scans showed a small septum in the extrahepatic bile duct, thus the cystic tumor was diagnosed as an intrahepatic tumor infiltrating the extrahepatic bile duct.
The patient underwent choledochotomy under general anesthesia on November 5, 2008, during which no palpable mass was found on the liver surface, but a tumor embolus originating from the intrahepatic duct of segment 4 with smooth surface that protruded into the extrahepatic bile duct was observed without mucus-secretion (Figures 3 and 4). Coexistence of a confluence variation in separate posterior right bile ducts and a variation in the replaced right hepatic artery from superior mesenteric artery was observed. A multiloculated cyst in segment 4 adhering to the bile duct wall of the anterior right lobe was found. So a regular left hemihepatectomy en bloc was performed with resection of the entire tumor, segment 5 and the wall of 8 bile ducts. The common hepatic duct was transected to facilitate reconstruction of the orifice wall of the anterior right bile duct, and Roux-en-Y anastomosis between the hilar bile duct and jejunum was performed. Microscopically, the multiloculated tumor infiltrating the left hepatic duct was confirmed to be a biliary cystadenoma with an epithelial lining composed of biliary-type cuboidal cells and surrounded by an ovarian-like stroma (Figure 5). The bile duct margin was negative. The patient had an uneventful postoperative recovery. Follow-up imaging within twelve months showed no signs of local or distant tumor recurrence.
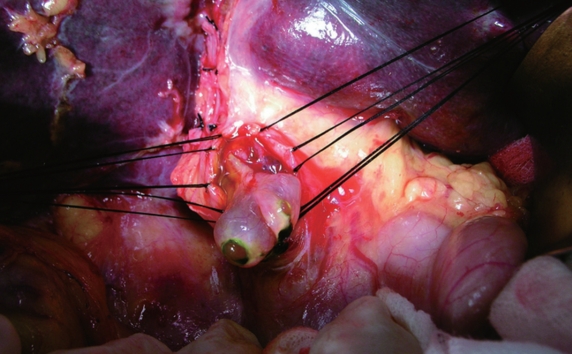
Figure 3.
A tumor embolus originating from left intrahepatic duct with smooth surface protrudes the extrahepatic bile duct.
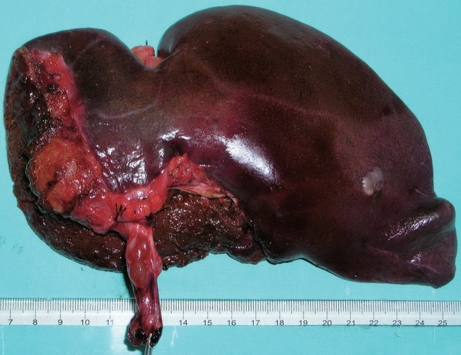
Figure 4.
Macroscopy revealing a multilocular cystic lesion containing serous fluid with tumor embolus protruding into the left hepatic duct and the extrahepatic bile duct.
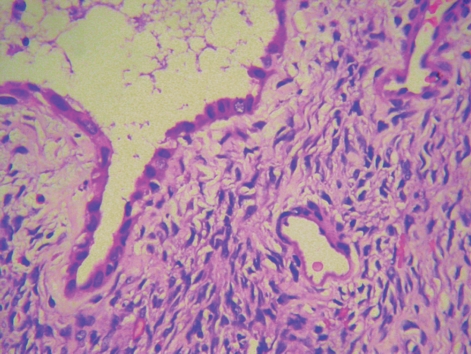
Figure 5.
Postoperative pathology showing epithelial lining composed of biliary-type cuboidal cells surrounded by an ovarian-like stroma (400 ×).
DISCUSSION
IBC is an uncommon tumor of biliary tract, but IBC with a tumor embolus in the extrahepatic bile duct is rare. The clinical features of IBC, especially with a tumor embolus in the extrahepatic bile duct, are unclear. No risk factors for IBC have been identified, although its predominance in females suggests a hormonal factor for its etiology. The lesion grows slowly but it is believed to be premalignant[6]. Clinically, it can be differentially diagnosed from other cystic hepatic lesions, such as simple cysts, liver abscesses, cystic degeneration of a liver neoplasm, Caroli’s disease, etc.[6,7].
Imaging studies are of great importance in its diagnosis. Characteristic ultrasound, CT and MRI findings, including a multiloculated lesion with internal septation, a thickened and irregular wall, mural nodules and papillary projections, calcifications, and wall enhancements, have been well described[7,8]. However, IBC with a tumor embolus extending into the extrahepatic bile duct is uncommon and demonstrates some specific clinicopathological features, such as jaundice with spontaneous remission or recurrence[9]. Generally, jaundice often occurs when a big tumor embolus fills the extrahepatic bile duct. When the pressure of intra-biliary tract is high, the bile duct is dilated and the pressure of cystadenoma is counteracted, remitting the obstructive jaundice. The MRCP imaging manifestations of IBC, with a tumor embolus protruding into the extrahepatic bile duct, are different from those of IBC without a tumor embolus. In our case, MRCP showed different bile signals in the peripheral and extrahepatic bile ducts, while MRI and CT showed distinctive thin septa in the extrahepatic bile duct. In IBC with a tumor embolus in the extrahepatic bile duct, the extrahepatic bile duct was dilated much greater than the intrahepatic bile duct, and septa in the extrahepatic bile duct should be the main feature which is different from that of mucin-producing IBC without a tumor embolus, although their manifestations on MRCP are similar. Cystadenoma with an epithelial lining composed of biliary-type cuboidal cells is surrounded by an ovarian-like stroma. Before surgery for our patient, PTBD was performed with no mucin or serous fluid observed in the drainage. Since the tumor did not communicate with the bile duct tree, and was filled with serous fluid, intraductal papillary neoplasm of bile duct (IPNB) and biliary papillomatosis were excluded. This tumor develops intraductally but rarely infiltrates the distal bile duct. The base point of this tumor infiltrating the extrahepatic bile duct is intrahepatic, and the lower end of its embolus is dissociated, thus facilitating complete resection of IBC with a tumor embolus as that without a tumor embolus[10-12]. Complete resection of the tumor provides the chance of cure. An early preoperative diagnosis of IBC is essential to improve the prognosis and survival of such patients.
In conclusion, IBC has a special growth manner[9,10,13-15], but its clinical features have not been fully illustrated. Imaging workup, clinicians and surgeons need to be aware of its different presentations, such as recurrent jaundice, different bile signals on MRI, distinctive thin septa in extrahepatic bile duct, and asymmetry dilation of bile ducts. An aggressive complete resection of the lesion is recommended. Large randomized controlled trials are warranted.
Footnotes
Peer reviewer: Terumi Kamisawa, MD, PhD, Department of Internal Medicine, Tokyo Metropolitan Komagome Hospital, 3-18-22 Honkomagome, Bunkyo-ku, Tokyo, 113-8677, Japan
S- Editor Wang YR L- Editor Wang XL E- Editor Lin YP
References
- 1.Tsiftsis D, Christodoulakis M, de Bree E, Sanidas E. Primary intrahepatic biliary cystadenomatous tumors. J Surg Oncol. 1997;64:341–346. doi: 10.1002/(sici)1096-9098(199704)64:4<341::aid-jso17>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 2.Kubota E, Katsumi K, Iida M, Kishimoto A, Ban Y, Nakata K, Takahashi N, Kobayashi K, Andoh K, Takamatsu S, et al. Biliary cystadenocarcinoma followed up as benign cystadenoma for 10 years. J Gastroenterol. 2003;38:278–282. doi: 10.1007/s005350300048. [DOI] [PubMed] [Google Scholar]
- 3.Henson SW, Gray HK, Dockey MB. Benign tumors of the liver. VI. Multilocular cystadenomas. Surg Gynecol Obstet. 1957;104:551–554. [PubMed] [Google Scholar]
- 4.Edmondson HA. Tumors of the liver and intrahepatic bile ducts. In: Atlas of tumor pathology, fasc , editors. 25, first series. Washington, DC: Armed forces Institute of Pathology; 1958. [Google Scholar]
- 5.Davies W, Chow M, Nagorney D. Extrahepatic biliary cystadenomas and cystadenocarcinoma. Report of seven cases and review of the literature. Ann Surg. 1995;222:619–625. doi: 10.1097/00000658-199511000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teoh AY, Ng SS, Lee KF, Lai PB. Biliary cystadenoma and other complicated cystic lesions of the liver: diagnostic and therapeutic challenges. World J Surg. 2006;30:1560–1566. doi: 10.1007/s00268-005-0461-7. [DOI] [PubMed] [Google Scholar]
- 7.Korobkin M, Stephens DH, Lee JK, Stanley RJ, Fishman EK, Francis IR, Alpern MB, Rynties M. Biliary cystadenoma and cystadenocarcinoma: CT and sonographic findings. AJR Am J Roentgenol. 1989;153:507–511. doi: 10.2214/ajr.153.3.507. [DOI] [PubMed] [Google Scholar]
- 8.Mortelé KJ, Ros PR. Cystic focal liver lesions in the adult: differential CT and MR imaging features. Radiographics. 2001;21:895–910. doi: 10.1148/radiographics.21.4.g01jl16895. [DOI] [PubMed] [Google Scholar]
- 9.Beretta E, De Franchis R, Staudacher C, Faravelli A, Primignani M, Vecchi M, Conti E, Di Carlo V. Biliary cystadenoma: an uncommon cause of recurrent cholestatic jaundice. Am J Gastroenterol. 1986;81:138–140. [PubMed] [Google Scholar]
- 10.Thomas KT, Welch D, Trueblood A, Sulur P, Wise P, Gorden DL, Chari RS, Wright JK Jr, Washington K, Pinson CW. Effective treatment of biliary cystadenoma. Ann Surg. 2005;241:769–773; discussion 773-775. doi: 10.1097/01.sla.0000161982.57360.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koffron A, Rao S, Ferrario M, Abecassis M. Intrahepatic biliary cystadenoma: role of cyst fluid analysis and surgical management in the laparoscopic era. Surgery. 2004;136:926–936. doi: 10.1016/j.surg.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 12.Vogt DP, Henderson JM, Chmielewski E. Cystadenoma and cystadenocarcinoma of the liver: a single center experience. J Am Coll Surg. 2005;200:727–733. doi: 10.1016/j.jamcollsurg.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Sutton CD, White SA, Berry DP, Dennison AR. Intrahepatic biliary cystadenoma causing luminal common bile duct obstruction. Dig Surg. 2000;17:297–299. doi: 10.1159/000018857. [DOI] [PubMed] [Google Scholar]
- 14.Baudin G, Novellas S, Buratti MS, Saint-Paul MC, Chevallier P, Gugenheim J, Bruneton JN. Atypical MRI features of a biliary cystadenoma revealed by jaundice. Clin Imaging. 2006;30:413–415. doi: 10.1016/j.clinimag.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Erdogan D, Busch OR, Rauws EA, van Delden OM, Gouma DJ, van-Gulik TM. Obstructive jaundice due to hepatobiliary cystadenoma or cystadenocarcinoma. World J Gastroenterol. 2006;12:5735–5738. doi: 10.3748/wjg.v12.i35.5735. [DOI] [PMC free article] [PubMed] [Google Scholar]