Abstract
The study of intraindividual variability is the study of fluctuations, oscillations, adaptations, and “noise” in behavioral outcomes that manifest on micro-time scales. This paper provides a descriptive frame for the combined study of intraindividual variability and aging/development. At the conceptual level, we highlight that the study of intraindividual variability provides access to dynamic characteristics – construct-level descriptions of individuals' capacities for change (e.g., lability), and dynamic processes – the systematic changes individuals' exhibit in response to endogenous and exogenous influences (e.g., regulation). At the methodological level, we review how quantifications of net intraindividual variability (e.g., iSD) and models of time-structured intraindividual variability (e.g., time-series) are being used to measure and describe dynamic characteristics and processes. At the research design level, we point to the benefits of measurement burst study designs, wherein data are obtained across multiple time scales, for the study of development.
Keywords: intraindividual change, lifespan theory, developmental systems, multiple time scales, regulation, adaptation, behavioral flexibility, processes of change, idiographic, single-subject
A key objective in developmental psychology is to describe or characterize the individual and to explain when and how the observed characteristics of the individual change over time (Baltes, Reese, & Nesselroade, 1977; Ford & Lerner, 1992; Molenaar & Campbell, 2009; see also Valsiner, 1986). From a life-span perspective, we seek to understand the dynamics of gain and loss, the interactions of persons and contexts, the limitations on and potential for adaptation, and the interplay and coordination of component parts and processes as they unfold over multiple time scales and contribute to development (Baltes, Lindenberger, & Staudinger, 2006; Magnusson & Cairns, 1996; Lerner, 1984; Thomae, 1959). The individual is the chosen unit of analysis – a unique, inviolable organism with parts that are to be understood as components of an integrated whole – a complex dynamic system, the many component characteristics and processes of which emerge from a variety of endogenous and exogenous influences (Ford & Lerner, 1992; Magnusson, 2000; Molenaar, 2004; Overton & Reese, 1973; Thelen & Smith, 1998). Emphasized are the variability, complexity, and dynamic (opposed to static) properties of the individual.
The emphasis on individual-level change brings to the forefront theoretical and methodological perspectives for examining how and when individuals change over time or age (Collins & Horn, 1991; Collins & Sayer, 2001; Hertzog & Nesselroade, 2003; Nesselroade, 1990; Nesselroade & Reese, 1973). In this paper we develop a descriptive frame for the combined study of intraindividual variability and aging/development. Drawing on the demonstrations in the literature and the on-going discussions with colleagues near and far, we present a framework that links conceptual and methodological notions of intraindividual variability and indicate why and how the study of intraindividual variability is furthering our understanding of aging/development. Specifically, we (1) highlight that the study of intraindividual variability provides access to dynamic characteristics – construct-level descriptions of individuals' capacities for change (e.g., lability), and dynamic processes – the transformations individuals' undergo in response to endogenous and exogenous influences (e.g., adaptation), (2) review how quantifications of net intraindividual variability (e.g., iSD) and models of time-structured intraindividual variability (e.g., time series) are being used to measure and describe dynamic characteristics and processes, and (3) point to the benefits of measurement burst study designs, wherein data are obtained across multiple time scales, for the study of development.
Intraindividual Variability
First, we clarify some concepts and terminology. As the life-span perspective was building momentum in the 1960s and 1970s, the interface between theoretical and methodological perspectives brought an increased focus on the need to study how individuals' “behavior”1 changed over time (e.g., Harris, 1963; Nesselroade & Baltes, 1979; Thomae, 1959; Wohlwill, 1973). Behaviorists, biologists, contextualists, developmentalists, experimentalists, mathematicians, methodologists, philosophers, psychometricians, and scientists from other disciplines contributed to an ongoing exchange and innovation of concepts, designs, and analytical methods useful for the study of development (e.g., West-Virginia Life-Span Conference Series). In the midst of the ensuing dialectic, Nesselroade (1991) highlighted the distinction between intraindividual change – “more or less enduring changes that are construed as developmental”, and intraindividual variability – “relatively short-term changes that are construed as more or less reversible and that occur more rapidly than the former” (p. 215).
Depicted graphically in Figure 1 by the longer, dark gray lines, intraindividual change is conceptualized as directional changes resulting from long-term processes such as development, maturation, aging, or senescence that manifest on a macro-time scale (e.g., years, decades; also see Figure 8.1 in Nesselroade, 1991). For example, researchers have described distinctive lifetime trajectories for developmental change of individuals' fluid and crystallized intellectual abilities, the former characterized by normative declines through the many decades of adulthood, and the latter by relative stability across the same age span (e.g., Baltes, 1987; Cattell, 1971; Horn, 1982; Schaie, 2005). Typically, this is the way we think about and study aging – within-person changes that accrue with advancing age over the long-term. We shall refer to such long-term changes as aging. We use the word aging as a venue-specific construct synonymous with “development”, keeping in mind that the concepts and methods discussed here apply widely and just as readily to biological, social, psychological, and other developmental phenomena that manifest at all ages.
Figure 1.
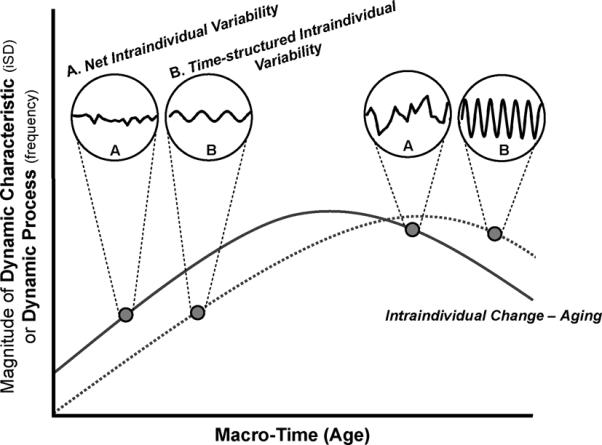
Intraindividual change in net and time-structured intraindividual variability. Net intraindividual variability (A circles) is characterized by changes in micro-time that are not systematically ordered in relation to time, is quantified by measures such as an iSD, and is used to capture individuals' dynamic characteristics (e.g., lability). Time-structured intraindividual variability (B circles) is characterized by patterns of change in micro-time that are systematically ordered with respect to time and/or previous observations, is modeled using time-series or other techniques, and is used to capture dynamic processes (e.g., adaptation). Differences between the circles on the left and the circles on the right indicate that aspects of both types of intraindividual variability (dynamic characteristics / iSD and dynamic processes / frequency) can differ between individuals and/or change with age. Depending on the construct, long-term changes in intraindividual variability may be best characterized by increases, decreases, stability, or other more complex, nonlinear forms of change.
In complement to long-term, intraindividual change, intraindividual variability, depicted in the shorter, black lines within the magnified circles in Figure 1, is conceptualized as fluctuations, inconsistency, instability, oscillations, or “noise” that manifest on micro-time scales (e.g., minutes, hours, days, weeks) as the result of short-term processes. Its study is characterized by repeated measurement of individuals' attributes over many relatively closely spaced occasions. For example, Eizenman and colleagues (1997) obtained weekly reports of individuals' perceived level of control over the course of half a year. Similar to the differences seen between the two sets of magnified circles in Figure 1, these authors found that some individuals were more labile than others. That is, some individuals' control beliefs remained relatively stable from week-to-week (circles on left), while others' fluctuated wildly from week-to-week (circles on right). We shall refer to such short-term changes as intraindividual variability and will, in distinguishing them from aging, associate them with other dynamic concepts such as plasticity, lability, adaptation, and homeostasis – attributes and processes that, theoretically, change with age.
Drawing on existing nomenclatures, especially Fiske and Rice (1955), and subsequent elaborations (e.g., Hultsch, MacDonald & Dixon, 2002; Li, Huxold, & Schmiedek, 2004; Martin & Hofer, 2004; Nesselroade & Ghisletta, 2003; Nesselroade & Ram, 2004; Nesselroade & Schmidt McCollam, 2001; and also earlier discussions by Stern, 1911), we forward a conceptual, and corresponding methodological, distinction between two types of short-term within-person changes. In brief, net intraindividual variability is constituted of short-term within-person changes that are analyzed as being unstructured in relation to time (e.g., characterized by changes that are “randomly” ordered in time).2 In Figure 1, fluctuations of this type are shown in the A circles and are conceptualized as indicators of individuals' dynamic characteristics (e.g., lability, plasticity, robustness). In contrast, time-structured intraindividual variability is characterized by changes that are systematically ordered in time (e.g., lags or cycles). Visually intuitive depictions of one kind of time-structured fluctuations are shown in the B circles. More generally, time-structured fluctuations are conceptualized as indicators of the dynamic processes that underlie individuals' behavior (e.g., adaptation, homeostasis). As can be obtained from comparing the two circles on the left side of Figure 1 (i.e., earlier in macro time) with those on the right side (i.e., later in macro time), both dynamic characteristics and processes can potentially change with advancing age. The figure depicts both types of short-term change as increasing over the long-term, but this may not always be the case. Depending on the construct, long-term changes in intraindividual variability may be best characterized by increases, decreases, stability, or other more complex, nonlinear forms of change (for discussion, see MacDonald, Li & Bäckman, 2009; Williams, Strauss, Hultsch, & Hunter, 2007).
Dynamic Characteristics and Processes
Theoretical accounts of individual development include descriptions of both individuals' inherent capacity for change and the change processes that individuals' engage as they respond to endogenous and exogenous influences (e.g., Ford & Lerner, 1992; Magnusson, 2000; Overton & Reese, 1973; Thelen & Smith, 1998). In this section, we highlight how the study of intraindividual variability provides access to the constructs used to describe individuals' capacity for change – dynamic characteristics, and systematic patterns of change that describe behavioral transformations – dynamic processes.3 To illustrate, we draw on examples from the psychological aging literature.
Dynamic Characteristics
Borrowing and expanding on terminology and concepts used in other disciplines (e.g., physics, chemistry, biology), psychologists use a number of constructs to describe individuals' inherent capacity or potential for change (or stability).4 For example, plasticity refers to an entity's (individual's) capability of, or susceptibility to, being molded, shaped, modified, or otherwise changed (Baltes, 1987; Gottlieb, 1998; Lerner 1984). Similarly, the constructs lability and rigidity describe either individuals' proneness to or inability to change across contexts (Cattell, 1966; Leary, 1957). Robustness is the ability of an individual or system to maintain its functionality across a wide range of conditions (e.g., stresses, pressures; Hammerstein et al., 2006). On the one hand, these constructs describe “trait-like” capacities or abilities. At a given point in macro-time, they are considered fixed, inherent characteristics of the individual that may be quantified and examined. Individuals are described as labile or rigid, or may be rank ordered with respect to their inherent plasticity – in the same way that individuals are described as young or old or are rank ordered with respect to age or other interindividual differences. On the other hand, these constructs are truly about within-person change, or more precisely individuals' potential for change (or stability). As such, attributes such as plasticity, lability, rigidity, and robustness require a conceptualization and description of the diversity of function and behavior that can be expressed by an individual.
How do we measure dynamic characteristics? We use flexibility as an illustrative example. The main objective in describing individuals' flexibility is to quantify the range of behaviors they exhibit (or might exhibit) across a variety of situations. Individuals who have a tendency to behave in the same way no matter the social situation would be considered inflexible, while individuals who do behave differently across social situations might be considered more flexible (for the moment ignoring the appropriateness of the behavior). Analytically, the repeated measurements obtained from a single individual across a variety of situations are collected. Then, measures of central tendency and dispersion are used to describe that individuals' set of behaviors. Relevant to the current example, dispersion across categories of behavior can be quantified, through calculation of Shannon's entropy (an index used in ecology to quantify the extent to which multiple species live in the same area – “biodiversity”; Morin, 1999). Using a stream of behavioral observations obtained in real time as individuals encounter a variety of social situations, the entropy index can be used as a measure of a person's flexibility – a manifest indicator of a (latent) dynamic characteristic.
Applying the same principles to other aspects of human experience and behavior, we can ask questions such as: How much new information can an individual assimilate and/or accommodate (e.g., developmental reserve capacity: Kliegl, Smith, & Baltes, 1990)? To what extent can an individual maintain consistency of task performance despite abnormalities in neuronal or biological integrity (Li, Huxold, & Schmiedek, 2004; Slifkin & Newell, 1998)? In each case, we might measure the range of behaviors exhibited across a variety of conditions. In aging research, for example, inconsistency, an indicator of neurobiological integrity, is often measured via variation in the latency of responses across multiple trials of a simple choice reaction time task completed over a few minutes (Hultsch & MacDonald, 2004). In the motor domain, postural control has been examined via measurements of the extent to which individuals deviate from an upright stance over a few seconds (i.e., variation in center of pressure; Woollacott & Shumway-Cook, 2002). In research on emotions, individuals' potential for experiencing mixed emotions, poignancy, is measured through quantification of the co-variation of emotions across hours or days (Carstensen et al., 2000). Even in the study of personality, behavioral signatures, individuals' potential or likelihood of behaving in particular ways in particular situation, are indicated by the variation of behaviors expressed across a week or more of randomly encountered contexts (Mischel & Shoda, 1995). These and other manifestations of intraindividual variability have provided the raw material needed for describing specific dynamic properties of the individual.
Dynamic Characteristics and Aging
Once extracted from features of short-term change, the specific aspects of individual functioning indicated by these dynamic constructs can be examined with respect to aging. Questions to ask include: Are differences in level of robustness related to differences in age? Does an individual's robustness decrease with age? Do some individuals lose their robustness faster than others? Are differences or changes in health related to differences or changes in robustness? For example, measuring neurobiological robustness as the total amount of intraindividual variability observed across multiple trials of a cognitive task, Lövdén, Li, Shing, and Lindenberger (2007) found that level of robustness portended the extent of age-related change in intellectual ability. That is, lower levels of robustness were associated with greater subsequent declines in cognitive performance – providing some evidence for the hypothesized neurobiological underpinnings of long-term cognitive decline. As demonstrated by these and other researchers, careful examination and quantification of the diversity of behaviors over the short-term can allow for a more precise accounting and tracking of long-term developmental changes in dynamic characteristics of individual functioning (Nesselroade, 1991; Siegler, 1994).
Dynamic Processes
At another level of inquiry, psychologists and developmentalists make good use of constructs that describe systematic changes in behavior, including regulation, homeostasis, adaptation, accommodation, differentiation, learning, and metamorphosis. Making liberal use of descriptions provided by developmental systems theorists (e.g., Ford, 1987; Ford & Lerner, 1992), processes underlying behavioral change can be considered as being of three types: stability-maintenance processes, incremental change processes, and transformational change processes. There are of course many taxonomies of process. This one was chosen for use here because of its developmental roots and because it suits our current purposes. Other classifications can be applied to the study of variability in similar fashion.
Stability-maintenance processes are those that maintain and restore the systems organizational and functional unity. Examples include maintenance of physical, emotional and cognitive function during or return to equilibrium after endogenous or exogenous perturbation/challenge (e.g., maintaining homeostasis; equilibration, e.g. Piaget 1977). Incremental change processes are those in which an existing characteristic is refined, elaborated, made larger or more complex. It is characterized by relatively smooth directional changes. A classic example that often manifests over the short-term would be reinforcement learning. In contrast to incremental change processes, transformational change processes are marked by discontinuities that involve a relatively rapid reorganization of an existing state or pattern into a qualitatively different state or pattern. Examples include insights in learning, stage transitions, and “crucial shifts” in psychopathology.
The dynamics that govern these processes – how an individual moves from one state or moment in time to the next – can be modeled and quantified by considering the serial patterning or “across-time” relationships among successive observations (e.g., cycles, oscillations, lagged effects; e.g. Nesselroade & Schmidt McCollam, 2000). Behaviorists and modern behavioral analysts, for instance, have long made use of time-series experiments and mathematical models in describing conditioning, reinforcement, extinction, and other change processes (Baum, 1994; Hull, 1943; Pavlov, 1927; Skinner, 1938). Tracking the pattern of successive changes in bar presses, pecks, or cognitive performances from trial-to-trial or session-to-session allows for precise description of many incremental change processes (see Lattal & Perone, 1998; Risley & Wolf, 1973). Similarly, tracking the progression of minute-to-minute changes in cortisol after exposure to a stressful event is necessary for describing how the neuroendocrine system contributes to the return to equilibrium levels or patterns of function – stability-maintenance processes such as allostasis or homeostasis (e.g., Granger & Kivlighan, 2003; Kudielka & Kirschbaum, 2007; McEwen & Stellar, 1993; Seeman & Grunewald, 2006; Sterling & Eyer, 1988).
On the one hand, processes and their outcomes can be considered “trait-like” descriptions of how an individual, at a given point in macro-time, functions. Individual patterns of adaption might be described as adaptive or maladaptive processes or may be rank ordered with respect to the speed or quality of the processes – in the same way that individuals are described as young or old or are rank ordered with respect to age or other interindividual differences. On the other hand, these constructs are truly about within-person change. They describe a series of transactions or activities that systematically connect prior states to future states – dynamic processes (Martin & Hofer, 2004). As such, broad constructs such as adaptation or self-regulation require a conceptualization and description of the systematic patterns of change over time (Cole, Martin, & Dennis, 2004).
How do we measure dynamic processes? Well, we need parameters that describe systematic patterns of change. Consider, for example, models used to describe warm-blooded animals' thermo-regulation, a stability-maintenance process. When discrepancies arise between an individual's current temperature and his or her ideal, equilibrium temperature, action is taken (e.g., shivering or sweating) that reduces these discrepancies until the equilibrium state is attained. The systematic pattern of changes observed during return to equilibrium are taken as the manifest indicators of the thermo-regulation process and modeled accordingly (e.g., using mathematical models like a damped linear oscillator, e.g. Boker, 2001; Chow et al., 2005; Nesselroade & Boker, 1994). The obtained model parameters provide a mathematical description (qualitative and/or quantitative) of when and how the process is likely to proceed.
Applying the same principles to human behavior, dynamic processes such as psychological adaptation or self-regulation can be extracted from the regularity or order seen across repeated observations over the short-term (Martin & Hofer, 2004, Nesselroade & Boker, 1994). For example, in describing the process of bereavement, Bisconti, Bergeman, and Boker (2004) obtained information about processes of adaptation to loss by modeling the time-ordered oscillations of emotional well-being present in the daily reports that recently bereaved widows provided over 12 weeks of study. Specifically, a dynamical systems model (in this case a damped linear oscillator model) of a stability-maintenance process was invoked to describe the systematic patterns of change wherein individuals' day-to-day oscillations in emotion were regulated towards equilibrium. As this and other studies demonstrate, the time-ordered repeated measures nature of intraindividual variability provides the opportunity for modeling and quantifying the intrinsic dynamics that govern how an individual (or dyad) moves from one state or moment in time to the next.
Dynamic Processes and Aging
Again, once obtained and modeled, the specific aspects of the dynamic processes supporting individual functioning that are indicated by model parameters can be examined with respect to aging. Consistent with the typical examination of age differences and age-related changes, we can hypothesize about and track how the dynamic processes producing individual behavior over the short term change over the long term. Questions might take the form: Does the speed of the adaptation process change with age? Are age-related differences or changes in health related to qualitative differences in the effectiveness of a stability-maintenance process? For example, Brauer, Woollacott and Shumway-Cook (2002) found differential patterns of moment-to-moment task performance among younger and older adults attempting to maintain postural control while completing another attention-demanding cognitive task. Specifically, the ordering of compensatory movements and responses differed, indicating differential progression of stability-maintenance processes with age and impairment. Somewhat parallel notions emerge in life-span theories of developmental regulation and coping, which suggest that the dynamics of adaptation processes change qualitatively with age. In particular, while stability-maintenance processes may play a primary role in adaptation at younger ages, transformational change processes that involve a lowering of equilibrium set-points become increasingly prominent at older ages (e.g., accommodation: Brandstädter & Renner, 1990; compensatory secondary control: Heckhausen & Schulz, 1995). As elaborated by these and other researchers, careful modeling and examination of the systematic patterns of behavior exhibited over the short-term can allow for a more precise accounting and tracking of long-term developmental changes in dynamic processes underlying individual functioning (Molenaar, 2004; Nesselroade & Schmidt McCollam, 2000; Van Geert, & van Dijk, 2002). In the final section of this article, we will discuss how measurement burst study designs may facilitate such multiple-time scale inquiries. Before doing so, though, we turn to the analytical methods.
Time-Structured and Net Intraindividual Variability
In the preceding section, we drew a conceptual distinction between constructs used to describe individuals' inherent capacity for change – dynamic characteristics (e.g., lability, plasticity, robustness) and the systematic patterns of change that describe behavioral transformations – dynamic processes (e.g. adaptation). In this section, we tether this conceptual distinction to a corresponding methodological distinction between measures of net intraindividual variability and models of time-structured intraindividual variability. It must be acknowledged that there are infinitely many ways to carve up the conceptual and methodological space (for just a few, see Fiske & Rice, 1955; Ford & Lerner, 1992; Hofer & Sliwinski, 2006; Li, Schmiedek, & Huxold, 2004; Lindenberger & van Oertzen, 2006; Martin & Hofer, 2004; Nesselroade & Ram, 2004; Ram & Gerstorf, 2009). Here, we have prioritized the tethering of intraindividual variability concepts and methods, with the objective of identifying a heuristic framework that may be useful when selecting measures and models that are appropriately suited for the rendering of particular “theoretical” concepts, and, vice versa, when interpreting analytical results and mapping them onto substantive theory.
We follow most closely in the footsteps Fiske and Rice (1955) laid down in their classic review of intraindividual response variability, and attempt to push forward in a way that accommodates and meshes their framework with many of the conceptual and methodological innovations that have occurred in the decades since. Fiske and Rice defined pure variability as variability across repeated assessments where (a) the individual was exposed at each occasion to the same stimulus or to objectively indistinguishable stimuli, and (b) the total situation in which the responses are made is the same on all occasions. Said differently, pure intraindividual variability is variability that manifests in a static, unchanging, stable context. As they admitted, “It is doubtful whether such an abstract case ever exists. (p. 217)” From there, Fiske and Rice distinguished Type I (spontaneous) and Type II (reactive) intraindividual variability based on how the data were structured with respect to time. Specifically, Type I intraindividual variability requires conformity to the additional assumptions that (c) the order of responses is immaterial, meaning that the data show no systematic trend over time (e.g., due to processes such as learning, fatigue, etc.) and (d) that behavior at any occasion t is not affected by or related to either the response at t–1 or any other previous (or future) occasion. Data that violate this latter assumption and that contain lagged effects or cycles (patterns other than a monotonic function of time) were considered to be manifestations of Type II (reactive) intraindividual variability.
Coming from a developmental perspective where one of the fundamental principles is that both the individual and his or her environment are always changing (e.g., Baltes, Reese, & Nesselroade, 1977; Bronfenbrenner, 1979; Ford & Lerner, 1992) we are, in part, adapting Fiske and Rice's (1955) notions of intraindividual variability for use outside the “vacuum” they described.5 Our framework takes for granted the idea that the individual and his or her context both change over time. Straightforwardly, a series of repeated observations of a person and the relevant context (endogenous and/or exogenous) can be collected into a multivariate time series of scores, y(t), with elements describing the person and elements describing the surrounding context. Methodologically, then, the task is to “decompose” this stream of information into interpretable components – some of which we can map onto dynamic characteristics and others we can map onto dynamic processes.
Building on Fiske & Rice's (1955) distinction between intraindividual variability that is unstructured (Type I) or structured (Type II) with respect to time, we use a general scheme where the total intraindividual variability (intravar) contained in y(t) is “portioned” into two parts by the investigator, with total intravar = time-structured intravar + net intravar.
Net intraindividual variability is constituted of short-term within-person changes that are treated as being unstructured in relation to time (e.g., characterized by randomly ordered changes, as in the A circles in Figure 1) and will be tethered to dynamic characteristics. In contrast, time-structured intraindividual variability is constituted of short-term within-person changes that are analyzed as systematically structured as a function of time and/or prior states (e.g., characterized by regular patterns like those shown in the B circles of Figure 1) and will be tethered to dynamic processes. Key to the distinction is that with net intraindividual variability, the ordering of the occasions is treated as immaterial, whereas with time-structured intraindividual variability the serial ordering of the repeated measurements is of material interest. To be clear, the ordering of repeated assessments in a given study is inherently time-structured (e.g., occasion 1 occurs before occasion 2). However, it is at the discretion of the researcher to select an analytic approach that treats the data as time-structured or not. Such decisions about if and how to decompose total variability into time-structured and net portions and their relative size are typically based on a number of different considerations, including conceptual arguments and study design – a point we will discuss in more detail below. Also, our distinctions are designed for application to within-person variability. The structure of between-person differences is not addressed specifically, nor do we presume that the distinction has a straightforward between-person variability analogue. At most, we suggest that summary descriptions of an individuals' time-structured and net intraindividual variability (measured or modeled individual by individual) can be used in a secondary analysis of between-person differences. Our focus is on how the methodological landscape can be viewed in relation to the dynamic characteristics and processes that manifest at the level of the individual.
Time-Structured Intraindividual Variability
Dynamic processes (e.g., stability maintenance, incremental, or transformational) are indicated by repeatedly observed systematic patterns of change (e.g. Ford & Lerner, 1992). The main objective in describing them is to identify, extract, and measure the systematic serial ordering within a stream of observed behavior (within-person). Methodologically, the repeated measurements obtained from a single individual are examined for systematic time-related structures. The data are modeled in relation to time. The parameters obtained from the time-structured model are used to describe specific aspects of the dynamic process of interest. For example, the rate of linear change obtained from a regression model wherein repeated measures of cognitive performance are regressed on an index of time (e.g., trial number) would provide a quantification of the speed of an incremental change process (e.g., learning).
Structured with respect to time
The important distinction between time-structured and net variability is how the data are structured and/or treated with respect to time. By definition, time-structured variability focuses on the temporal relations in the data. The objective is to use the repeated measures to describe and understand how a person's state at one occasion, t, is related to or leads to the person's state at subsequent occasions, t + 1, t + 2, …, t + h. The time-locked, serial ordering of the data is used to describe and understand dynamic processes. For example, Carstensen, Gottman, and Levenson (1995), in describing regulatory and control processes occurring while members of older married couples interacted with one another, modeled theoretically interesting sequences of global affect, some indicating stability-maintenance processes (e.g., positive, neutral, and negative continuance), others incremental change processes (negative startup, de-escalation). Each type of process was modeled by a specific pattern of how an individuals' affect changed from one moment to the next, from t to t + 1 to t + 2, and so on (see also Ferrer & Nesselroade, 2003; Sbarra & Ferrer, 2006).
Note that the time metric is open. Time can be indexed in a number of different ways, including calendar time, time from some universal event (e.g., the bombing of Hiroshima), or an individually-defined event (e.g., birth or death). Further, the progression or serial ordering of time can also be conceptualized and tracked in different ways. That is, the relevant ordering of observations may be in relation to the progression of clock time, in relation to progression of psychological or social time, or in relation to a series of individually defined events (e.g., successive visits to the laboratory or interacting with person A, then with person B, etc.; for aging-related discussions, see Baars & Visser, 2007; Featherman & Petersen, 1986; Hertzog & Nesselroade, 2003; Li & Schmiedek, 2002; Schroots & Birren, 1990; Settersten & Mayer 1997). The only basic requirement is that the observations have a specific ordering, but the metric is of additional use (especially with respect to causality) if it retains the special quality that time always moves forward.
Measures and Models
The methods used to measure and model time-structured intraindividual variability all make heavy use of the time-ordered nature of the data. The objective is to construct a statistical model that adequately describes systematic time-dependent structures in the data and that allows for the prediction of future behaviors or outcomes. Estimates of specific parameters from the selected model of time-structured intraindividual variability might then be used as an indicator of substantively important characteristics of specific within-person processes. These models include auto-regressive models, moving average models, and spectral analysis – or more generally, time-series analysis in the time domain (e.g., auto-regressive; see Box & Jenkins, 1976; Shumway & Stoffer, 2006) and frequency domain (e.g., spectral; see Jenkins & Watts, 1968; Koopmans, 1995; Warner, 1998). Additional models are drawn from the tools used to describe linear and non-linear dynamic systems (Tong, 1993; Gottman, Murray, Swanson, Tyson, & Swanson, 2002; van der Maas & Molenaar, 1992).
Extensions to multivariate time-structured intraindividual variability are also available. They include dynamic factor analysis (Molenaar, 1985), vector auto-regressive and moving average models (Browne & Nesselroade, 2005; Shumway & Stoffer, 2006), hidden Markov models (Elliot, Aggoun, & Moore, 1995; Rabiner, 1989), multivariate spectral analysis (Jenkins & Watts, 1968), coupled differential equation models (Boker, 2001), and state-space models (Ho, Shumway, & Ombao, 2006; Molenaar & Ram, in press). Although these multivariate models have not yet been applied widely in the psychology of aging, there are some exemplars.
Chow, Nesselroade, Shifren, and McArdle (2004; see also Shifren, Hooker, Wood and Nesselroade, 1997) used dynamic factor models to extract systematic patterns of change in the emotional states experienced by a sample of Parkinson's patients across 70 days. These researchers used the time-ordered nature of the repeated measures data to identify the extent to which individuals' current mood states systematically persisted from day-to-day. In particular, they obtained a description of the stability-maintenance processes engaged by a subset of individuals who maintained positive mood in the face of a debilitating disease characterized by symptoms that fluctuate unpredictably from day-to-day.
There is little doubt that the technological advances in intensive, real-time data collection and highly speeded data manipulation and calculation, and the long base of theorizing about dynamic processes in relation to aging will lead to much more applications of such methods (Stone et al., 2007). For example, vector autoregressive methods and multivariate spectral analysis are being used in the modeling of functional connectivity among brain regions (e.g., Kim et al, 2007; Müller et al., 2001). Combining such models with intensive longitudinal data collected across many minutes, hours, days, or weeks allows for capture of the dynamic processes occurring at other micro-time scales. Such examinations are now within reach (see Walls & Schaefer, 2006).
Net Intraindividual Variability
Dynamic characteristics (e.g., flexibility, lability) are often indicated by the range of behaviors individuals exhibit (or might exhibit) across a variety of situations or stimuli. For example, a dancers' flexibility is indicated by the diversity of positions into which they can contort their bodies. The main objective in describing an individuals' flexibility, or other dynamic characteristic, is to identify, extract, and measure the diversity of behaviors exhibited over time. Methodologically, the repeated measurements obtained from a single individual are collected into a distribution or ensemble of scores. Then, assuming exchangeability of observations, standard measures of central tendency and dispersion are used to describe that individual's total ensemble of behaviors.
Of specific interest with respect to quantifying dynamic characteristics describing an individual's capacity for change are indices of dispersion that capture information about where the ends of the distribution are. These are the measures that locate the “extremes” of behavior exhibited by that individual. For example, an intraindividual standard deviation (iSD), calculated on the distribution of scores obtained across repeated measurements of a single individual, describes the extent to which his or her scores tend to vary around the mean score. A large iSD would indicate that the individual had a wide range of behaviors (e.g., high flexibility), whereas a small iSD would indicate a narrow range of behaviors (e.g., low flexibility). Calculated separately for the distributions obtained from multiple individuals, the iSD (or other measures of dispersion) can be treated as a measure of interindividual differences in capacity for change (e.g., lability, plasticity, robustness) and can be examined with respect to other interindividual differences, including gender, age, and other abilities or capacities using standard correlational, ANOVA, regression, or other methods typically applied to between-person analyses.
Unstructured with respect to time
As previously noted, the key distinction between time-structured and net intraindividual variability is how the data are structured and/or treated with respect to time. Measures and models of net intraindividual variability assume exchangeability of observations or more precisely, locally independent and identically distributed observations (i.e., the usual iid assumption). That is, the observations are assumed to be independent assessments, with time simple treated as a nominal variable/identifier that holds the multivariate observations at a given occasion intact (similar to the way in which a random id number is usually used to keep an individual's data organized within a data file).
Consider that the concept of flexibility does not have to do with the process by which the dancer moves from one contorted position to another. Rather, it is simply about the range of possible positions (the level to which one leg can be raised while standing on the other, the extent to which the back bends, etc.). In this sense, when measuring flexibility, the serial or time-structured ordering of the contortions does not matter, only their overall diversity. Whether a flexible person can engage in the process of dance is a separate question.
To be clear, a key assumption in the calculation of indices of net intrainidividual variability (e.g., the iSD) is that the repeated observations forming the within-person distribution are independent and identically distributed. The benefit of these assumptions is that the repeated observations can then be straightforwardly “compressed” into a single distribution and quantified by a few summary statistics (e.g., iSD) – scores that represent substantively important dynamic characteristics of the individual (e.g., lability, flexibility, robustness). For example, the mean, variance, skewness, and kurtosis of the distribution (1st, 2nd, 3rd, and 4th moments) provide a relatively comprehensive description of how that individual's behaviors are dispersed across the range of possible scores.
The potential costs of the needed assumptions include incongruence between the dynamic characteristics of human behavior we seek to observe and examine and the statistical viability of measuring and modeling specific aspects of intraindividual variability. Given the conceptual priority developmentalists place on how individuals change with respect to time, it may be difficult to imagine that repeated observations of the same person are ever truly independent. Given organismic continuity, observations obtained at time t are likely to be related in some way to observation t + 1, t + 2, …, t + h. Although a few indices of dispersion make use of the time-ordered nature of repeated measurements (e.g., mean square of successive differences: Leiderman & Shapiro, 1962; von Neumann et al., 1941; probability of acute change, see Jahng, Wood, & Trull, 2008), most do not.
In principle, before calculating statistics that require the independence assumption all time-related patterns should be removed or covaried. Time dependencies in the data should be accounted for and set aside. For example, the data might be subjected to “de-trending”, “filtering”, or “pre-whitening” procedures that remove time-related trends and systematic oscillations (e.g., seasonal trends or cycles; see Shumway & Stoffer, 2006). The result is intraindividual variability that is “residual”, net of all time-structured variability – time-series data that conform to the iid assumptions and allow for statistically viable calculation of most dispersion indices. Thus, our use of the term net intraindividual variability (similar usage as in net revenue or net profit).
Measures and Models
Assuming independent and identically distributed observations, a plethora of summary statistics can be used to quantify how the repeated observations obtained from a single individual are dispersed or distributed across scores or categories. From these, one should choose the index that most appropriately coincides with the theoretical construct it is intended to measure.
Of the many indices and models available, the intraindividual standard deviation (iSD) is by far the most popular index of intraindividual variability in psychological aging research and has been used as a measure of a wide range of dynamic characteristics (e.g., lability, robustness, flexibility). Although the iSD has proven particularly useful, there are also other quantifications of dispersion and models available. Other univariate measures for continuous variables include the variance, root mean square, absolute range (max – min), interquartile range, median absolute deviation, mean difference, average deviation, coefficient of variation (variance/mean), signal-to-noise ratio (mean/variance), quartile coefficient of dispersion, relative mean difference. Correspondent indices for count data include the index of dispersion (IDV; also called coefficient of dispersion or variance to mean ratio), and for categorical data, indices of qualitative variation (see Wilcox, 1973) and entropy (Shannon, 1950). In some domains, skewness and kurtosis also provide useful indices for quantifying individuals' dynamic characteristics (see Newell & Hancock, 1984).
Net intraindividual variability measures can also be used to describe multivariate data. The amount or extent of association among multiple variables assessed in tandem repeatedly from a single individual or entity (multivariate time series) is often called intraindividual covariation or coupled within-person variation (e.g. Fiske & Rice, 1955 for the former, Hofer & Sliwinski, 2006 for the latter). In the bivariate case, the objective is the same as above – to quantify the amount of observed covariation between the two variables. Analogues to the univariate measures listed previously would include the various within-person correlation coefficients (e.g., polychoric, Pearson, etc.) and, for categorical data, within-person odds ratios. Again, the measures of intraindividual covariation (or more generally, association) are used as measures of individuals' dynamic characteristics. For example, within-person correlations between positive and negative affect have been used as a measure of poignancy, individuals' capacity to experience mixed emotions (e.g., Carstensen et al., 2000; Ersner-Hershfield et al., 2008). Similarly, within-person associations between repeated measures of stress and negative affect have been used to measure individuals' affective reactivity (e.g., Bolger, DeLongis, Kessler & Schilling, 1989). Multilevel modeling has provided a useful and increasingly popular framework for examining between-person differences in bivariate within-person associations (see Bolger et al., 2003). Note, however that within the multilevel regression framework, precedence is implicitly given to one or the other of two variables. One variable is treated as an outcome, the other as a predictor. Care should be taken when interpreting results with respect to the intended univariate or bivariate nature of one's theoretical construct.
Some recent additions to the lexicon of measures of explicitly bivariate net intraindividual variability include “pulse” and “spin”, wherein circular statistics are used to characterize the variability in a stream of behaviors sampled from a space defined by two orthogonal dimensions (or a circumplex; see Moskowitz & Zuroff, 2004, 2005). For example, Moskowitz and Zuroff (2004) used the bivariate (or circular) measures of dispersion to measure individual's behavioral flexibility with respect to behavioral extremity (pulse) and interpersonal style (spin). Similar applications to repeated measures of individuals' core affect, as defined by dimensions of valence (pleasure-displeasure) and arousal (activation-deactivation) make use of pulse and spin to measure individuals' emotional variability (Kuppens et al., 2007).
Moving beyond two variables, the main method for examining multivariate net intraindividual variability is P-technique analysis, wherein (detrended) multivariate time-series obtained from single individuals are quantified using correlational analyses (see Nesselroade & Ford, 1985). In short, P-technique methods model the intraindividual variation of and covariation among many measures using data reduction methods (e.g., common factor models; Cattell, Cattell, & Rhymer, 1947) or other quantifications (e.g., network graphs; Fair et al., 2008). The obtained summary measures (e.g., number of principal components with large eigenvalues) provide an indication of the structure of short-term change occurring across multiple variables – and can be used as an indicator of an individual's dynamic characteristics. For example, Ong and Bergeman (2004) used P-technique analyses (intraindividual principal components) to quantify the intraindividual structure of day-to-day emotional experiences as an index of individuals' capacity to distinguish between pleasant and unpleasant feeling states, emotional complexity (considered an indicator of adaptational effectiveness; for other applications, see Carstensen et al., 2000; Ram, Carstensen & Nesselroade, 2009; Quinn & Martin, 1999; Wessman & Ricks, 1966). As the application of daily-diary methodologies reaches further up the adult lifespan and the duration of those studies increase we see the application of P-technique as holding great promise for the measurement and quantification of dynamic characteristics that are expressed in a multivariate manner (see Jones & Nesselroade, 1990; Nesselroade & Ford, 1985; Nesselroade & Jones, 1991 for discussion of application to study of aging).
Aligning Constructs and Methods
In summarizing this section on the methods being used to examine time-structured and net intraindividual variability, we highlight the need for care in the alignment of theoretical conceptions and the methods meant to render them operational (e.g. Bergeman & Wallace, 2006; Collins, 2006). Proceeding from Fiske and Rice's (1955) footsteps, we have formulated distinct types of intraindividual variability based on fundamental statistical principles about how the repeated measures data of single individuals are structured and/or treated with respect to time. The distinction between net and time-structured intraindividual variability is a methodological one, wherein one set of measures and models requires or assumes that data conform to iid assumptions (time only being used as a categorical identifier) and the other set explicitly models data with time-related dependencies.
We have tethered this distinction to two sets of constructs, dynamic characteristics and dynamic processes. Formulaic calculation of the iSD and other measures of net intraindividual variability rests on the assumption that the observations are random draws from a single distribution. As a consequence, the use of such indices implies that the ordering of occasions is immaterial and that the same summary information would be obtained even when the data are reshuffled with respect to time. Thus, the constructs these measures represent are inherently about the possible range of behavior, not the progression of behavior. In contrast, when crucial aspects of the dynamic concept to be measured include, are defined by, or imply systematic progression or patterns of behavior, then models of time-structured intraindividual variability may be more appropriate. For example, negative feedback processes imply an ordering wherein discrepancies between current and preferred states reduce as time progresses forward (e.g. Ford & Lerner, 1992, Ch. 5). Appropriate measures of such regulatory processes, in essence, require specificity of the time course and time-ordered patterning of short-term change – and imply specific models of time-structured intraindividual variability (e.g., damped oscillators; e.g., Bisconti, Bergeman, & Boker, 2004).
As noted in the lists of measures and models above, there are many tools available for quantifying and studying both time-structured and net intraindividual variability. The same data can be analyzed in many ways, each of which invoke a particular set of assumptions and can be used to indicate one or more possible constructs. Said differently, there are many ways to divide one's “data stream” into time-structured and unstructured (net) portions (multiple pieces of each also being possible). For example, in obtaining net intraindividual variability, the time-structured aspects of the data might be “filtered” out using sinusoidal functions (Fourier decomposition) or polynomial functions (Taylor-series expansion) or some combination of both. Each “filter” carries with it an implicit or explicit view on the various types of processes that contributed to the stream of observed behaviors. What is removed by one filter may be passed over by another. At the extremes, the researcher has the possibility to treat any set of intraindividual variability data as a manifestation of completely, in principle, deterministic processes (total intravar = time-strucutred intravar), or as a completely, in principle, stochastic or random (total intravar = net intravar). Thus, theory plays an exquisitely crucial role in how we choose from among the many analytical possibilities.6
So does study design – particularly the timing and spacing of observations. Kept in mind at all times must be the fact that the frequency and length of observation has severe implications on if and how the data may have been “pre-filtered” during collection (see Adolph et al., 2008). Too few occasions and one lacks reliability (see last section of Schmiedek, Lövden, & Lindenberger, 2009). Too many and the choice of filters becomes more difficult. Too small an interval between observations and one only sees stability, too large an interval and one misses the phenomena (Boker & Nesselroade, 2002). The end result is that the study may lead one toward a set of measures and models that may or may not align with or indicate the intended dynamic characteristics or dynamic processes. See the Boker, Molenaar, and Nesselroade's (2009) comment for a cogent discussion of this problem.
Further, as with all research, the particular constructs and variables one is examining must be considered as but one portion of a system, an incomplete model of individual's total functionality. Intraindividual variability measures and models must be interpreted within a broader framework of interindividual and contextual differences, historical changes, and so on (e.g., Baltes, Resse, & Nesselroade, 1977; Hofer & Sliwinski, 2006; Sliwinski & Mogle, 2008). In sum, intraindividual variability research requires a lot of hard choices, many of which we, as a field, are not yet in a particularly informed position to make.
Viewing these difficulties as part of the usual challenges faced in behavioral research, the point we wish to underscore is that there are many measures and models that can be used to extract and describe short-term change. These methods offer the possibility to articulate and examine a multitude of conceptually interesting dynamic characteristics and processes. As the study of intraindividual variability proceeds and expands, and more concepts and methods are developed and used, we have the opportunity to outline precise definitions of the individual characteristics and processes of interest and to carefully tether them to the quantitative and qualitative assumptions on which the correspondent indices or models and inquiries are based. In our view, the tighter and more efficient the tie, the better.
Intraindividual Variability and Aging
Returning to the concepts introduced at the outset, the study of intraindividual variability is characterized by intensive repeated measurements at relatively fast, “micro”, time scales (e.g., seconds, hours, days, weeks). It offers the possibility to measure and model dynamic characteristics and dynamic processes. In complement, the study of intraindividual changes that proceed on relatively slow, “macro” time scales (e.g., years, decades) offers the opportunity to describe and examine long-term processes such as development, maturation, aging, or senescence.
At the micro-time scale researchers in many areas make use of methods wherein multiple reports or assessments are obtained over a relatively short span of time, including diary, ecological momentary assessment (EMA), multi-trial (e.g., reaction time tasks), ambulatory, (practically) continuous assessment (e.g., EEG) and other intensive longitudinal study designs (Bolger, Davis, & Rafaeli, 2003; Csikszentmihalyi & Larson, 1987; Shiffman, Stone, & Hufford, 2008; Walls & Schaeffer, 2006). Aging researchers in particular have been making good use of such designs to examine intraindividual variability in affect, activities of daily living, physical activities, social exchanges, cognitive and physiological function, and other variables (see Cain, Depp, & Jeste, 2009; Hultsch & Macdonald, 2004). But this is only part of the story of aging.
Combining “micro-time” research designs with more “macro-time” designs for examining intraindividual change, researchers can examine questions about the aging of individuals' dynamic characteristics and processes. Nesselroade (1991) suggested making use of a measurement-burst design that involves measuring individuals on multiple time scales. At the micro-time level, observations are obtained from one or more individuals at closely spaced intervals (e.g., seconds, minutes, hours, weeks) – a “burst” of measurement. At the macro-time level, these same individuals are measured again at a wider interval (e.g., months, years), each time providing another “burst” of information from which measures of the individual's dynamic characteristics and processes can be extracted. Thus, the multi-time-scale repeated measures design combines the benefits of short-term longitudinal studies and the study of net and time-structured intraindividual variability with those of long-term longitudinal studies and the study of aging.
To illustrate, consider the bursts of measurement depicted in the A circles of Figure 1. Both bursts of intraindividual variability were obtained from the same person. The burst on the left was assessed at an earlier age and showed fluctuations characterized by low levels of dispersion. The burst on the right was assessed at a later age, at which time the person exhibited a more disperse set of short-term fluctuations in their behavior. The long line connecting the bursts obtained from this individual implies intraindividual change, aging, of the dynamic characteristic measured in the burst (e.g., age-related increases in inconsistency). In parallel, the bursts of measurement depicted in the B circles imply intraindividual, age-related change in the dynamic process indicated by the amplitude and frequency of oscillations captured by a sinusoidal model of time-structured intraindividual variability.
Reviewing and elaborating the potential of measurement-burst designs for investigating human behavior, Sliwinski (2008) highlighted that the design's multi-time scale features augment the information obtained from conventional (single time-scale) studies. These include improved precision and power for estimating long-term change (e.g., using intraindividual variability to distinguish and separate “noise”); greater capability to distinguish between changes and change processes that operate over different time scales (e.g., distinguishing short-term learning or forgetting processes from long-term aging processes); and the ability to track long-term changes (aging) in what we have called net intraindividual variability. In line with our presentation of the study of intraindividual variability as affording the opportunity to measure and model both net and time-structured intraindividual variability, we add that the measurement-burst design also provides the opportunity to track long-term changes in the dynamic processes that underlie behavior.
Of course, burst designs also come with problems. Sliwinski (2008) noted some of the drawbacks, including: the complexity and cost of the infrastructures required for coordinating and collecting many, many repeated measures on multiple time-scales; the heavy burdens on participants and the resulting difficulties in retaining them; and the tough issues and choices that must be made regarding the relative spacing of occasions and techniques for analysis. We see this last issue as one of the primary obstacles in designing a successful burst study. When, how often, and what should we measure? Good answers are not readily available beyond – measure as often as possible for as long as possible (e.g. Adolph et al., 2008). Unable to conduct intensive measurements of all variables, all the time (and still having a participant or two left) we are faced with the difficult task of making very specific predictions about when and how the constructs and processes of interest interact and manifest. Believing or acknowledging that human behavior is “co-constructed” (Baltes, Reuter-Lorenz, & Rösler, 2006; Li, 2003) by processes occurring at many levels and across multiple time scales in some senses demands a new level of theoretical precision. In many cases, we will be wrong, and the data will lead us astray. But, with faith and a diligent accounting and reporting of how behavior manifests over micro-, macro-, exo- and other time scales might just bring together some of the pieces of the puzzle needed for further understanding of the variety and complexities of development.
Synopsis
As a part of this collection of articles on intraindividual variability and aging, our purpose has been to introduce some concepts and indicate why and how the study of short-term intraindividual variability can be used as a tool for examining the aging of dynamic properties of human behavior. In doing so, we hope to have illustrated three points: (1) studying variability provides access to an individual's capacities for change (dynamic characteristics) and systematic transformations (dynamic processes); (2) such dynamic characteristics and processes can effectively be rendered operational by measures of net intraindividual variability and models of time-structured intraindividual variability; and (3) an integrated and combined study of multiple time metrics using measurement burst designs allows for examining the aging of dynamic characteristics and processes and other key developmental questions.
Across a wide variety of domains, the study of intraindividual variability is vibrant and pushing forward our understanding of the dynamic nature of individual functioning and its age-related change. Along with the ubiquitous multi-trial and continuous recording of behavior obtained at faster time scales in many studies of cognitive and/or motor performance and psychophysiology, daily dairy and other studies of intraindividual variability take us many steps along what is hopefully a long avenue of inquiry that extends from rather “static” and stable representations – single-occasion still-photo pictures describing where individuals are in the world – to more dynamic representations – moving pictures that provide a rich and dynamic picture of how individuals can or are travelling through time and space. With diligence and planning, we might even then be able to splice those clips together to describe, explain, predict and potentially modify the complex stories that unfold over a life span – development in process.
Acknowledgments
The authors gratefully acknowledge the support provided by the National Institute on Aging (NIA) RC1-AG035645; NIA R21-AG032379; and NIA R21-AG033109; the Max Planck Institute for Human Development, Berlin; and the Penn State Children, Youth, & Families Consortium. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. Thanks to Lauren Molloy and Frank Infurna for help in collecting and organizing literature, and to Ron Spiro and the reviewers for their thoughtful contributions and shaping. Special thanks to Annette Brose, Peter Molenaar, and John R. Nesselroade for the wonderful conversations from which exciting ideas continually emerge.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/journals/pag
We use behavior as a catch-all term for the many aspects of human function, physiology, experience, thought, action, etc.
We note that these types of fluctuations may not necessarily be “random”, but very well may reflect reactions to endogenous or exogenous factors. We thus use the term random to highlight that such fluctuations are treated as being unstructured in relation to time. Often, the amount of “noise” left in a model depends upon the analytic approach chosen.
Despite the possible redundancy of the adjective modifier, we use the term “dynamic processes” to highlight that the processes we are considering require manifest changes (variance > 0). In physics and chemistry, both quasi-static processes and steady-state processes are defined. The former are processes where changes accrue infinitesimally slowly and the latter are processes where “inputs” exactly equal “outputs”. They might be considered “pure” stability-maintenance processes. Methodologically, “pure” stability at the manifest level does not lend itself to statistical analysis (because there is no variance). “Dynamic” processes, though, allow for (perhaps require) deviation or perturbation from equilibrium, and thus provide the variability needed for statistical analysis.
Note that throughout the remainder of the paper we use `change' and `variability' to describe observable phenomena, and terms such as `lability', `plasticity', and `development' to describe the substantive constructs researchers tether to those phenomena. Following the above definitions, we use `short-term change' and `intraindividual variability' synonymously.
Fiske and Rice (1955) also delineated Type III intraindividual variability, where the stimulus or situation differs across occasions, but they did not treat this type in depth and were somewhat ambiguous as to whether in this type of intraindividual variability the ordering of occasions was immaterial or not.
As well, one needs to take great care to model the temporal dependencies appropriately. Although in some cases model misspecification is not detrimental for recovery of parameters that adequately represent the data (see Molenaar & Nesselroade, 2009), in many other cases (i.e., most cases outside of the 1-lag state-space model), ignoring the time-dependencies is very detrimental. Spurious structures (e.g., latent factors) and relationships among variables appear without ever raising an alarm.
References
- Allaire JC, Marsiske M. Intraindividual variability may not always indicate vulnerability in elders' cognitive performance. Psychology and Aging. 2005;20:390–401. doi: 10.1037/0882-7974.20.3.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baars J, Visser H, editors. Aging and time: Multidisciplinary perspectives. Baywood; Amityville, NY: 2007. [Google Scholar]
- Baltes PB. Theoretical propositions of lifespan developmental psychology: On the dynamics between growth and decline. Developmental Psychology. 1987;23:611–696. [Google Scholar]
- Baltes PB, Lindenberger U, Staudinger UM. Lifespan theory in developmental psychology. In: Lerner RM, editor. Handbook of child psychology Vol. 1: Theoretical models of human development. 6th ed. Wiley; New York, NY: 2006. pp. 569–664. [Google Scholar]
- Baltes PB, Reese HW, Nesselroade JR. Lifespan developmental psychology: Introduction to research methods. Brooks/Cole; Monterrey, CA: 1977. [Google Scholar]
- Baltes PB, Reuter-Lorenz PA, Rösler F, editors. Lifespan development and the brain: The perspective of biocultural co-constructivism. Cambridge University Press; New York: 2006. [Google Scholar]
- Baum WM. Understanding behaviorism: Science, behavior, and culture. Harper-Collins; New York: 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeman CS, Wallace KA. The theory-methods interface. In: Bergeman CS, Boker SM, editors. Methodological issues in aging research. Erlbaum; Mahwah, NJ: 2006. pp. 19–42. [Google Scholar]
- Bisconti TL, Bergeman CS, Boker S. Emotion regulation in recently bereaved widows: A dynamical systems approach. Journal of Gerontology: Psychological Sciences. 2004;59B:P168–P176. doi: 10.1093/geronb/59.4.p158. [DOI] [PubMed] [Google Scholar]
- Boker S. Differential structural equation models of intraindividual variability. In: Collins LM, Sayer AG, editors. New methods for the analysis of change. American Psychological Association; Washington, D. C.: 2001. pp. 5–27. [Google Scholar]
- Boker SM, Nesselroade JR. A method for modeling the intrinsic dynamics of intraindividual variability: Recovering the parameters of simulated oscillators in multi-wave panel data. Multivariate Behavioral Research. 2002;37:127–160. doi: 10.1207/S15327906MBR3701_06. [DOI] [PubMed] [Google Scholar]
- Boker S, Molenaar PCM, Nesselroade JR. Issues in intraindividual variability: Individual differences in equilibria and dynamics over multiple time scales. Psychology & Aging. 2009;24:???–???. doi: 10.1037/a0017912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger N, Davis A, Rafaeli E. Diary methods: Capturing life as it is lived. Annual Review of Psychology. 2003;54:579–616. doi: 10.1146/annurev.psych.54.101601.145030. [DOI] [PubMed] [Google Scholar]
- Bolger N, DeLongis A, Kessler RC, Schilling EA. Effects of daily stress on negative mood. Journal of Personality & Social Psychology. 1989;57:808–818. doi: 10.1037//0022-3514.57.5.808. [DOI] [PubMed] [Google Scholar]
- Box GEP, Jenkins GM. Time series analysis: Forecasting and control. rev. ed. Holden-Day; San Francisco, CA: 1976. [Google Scholar]
- Brandtstädter J, Renner G. Tenacious goal pursuit and flexible goal adjustment: Explication and age-related analysis of assimilative and accommodative strategies of coping. Psychology and Aging. 1990;5:58–67. doi: 10.1037//0882-7974.5.1.58. [DOI] [PubMed] [Google Scholar]
- Brauer SG, Woollacott M, Shumway-Cooke A. The influence of a concurrent cognitive task on the compensatory stepping response to a perturbation in balance-impaired and healthy elders. Gait and Posture. 2002;15:83–93. doi: 10.1016/s0966-6362(01)00163-1. [DOI] [PubMed] [Google Scholar]
- Browne MW, Nesselroade JR. Representing psychological processes with dynamic factor models: Some promising uses and extensions of ARMA time series models. In: Maydeu-Olivares A, McArdle JJ, editors. Psychometrics: A festschrift to Roderick P. McDonald. Erlbaum; Mahwah, NJ: 2005. pp. 415–452. [Google Scholar]
- Cain AE, Depp CA, Jeste DV. Ecological momentary assessment in aging research: A critical assessment. Journal of Psychiatry Research. 2009;43:987–996. doi: 10.1016/j.jpsychires.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen LL, Gottman JM, Levenson RW. Emotional behavior in long-term marriage. Psychology and Aging. 1995;10:140–149. doi: 10.1037//0882-7974.10.1.140. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, Pasupathi M, Mayr U, Nesselroade JR. Emotional experience in everyday life across the adult lifespan. Journal of Personality and Social Psychology. 2000;79:644–655. [PubMed] [Google Scholar]
- Cattell RB. Patterns of change: Measurement in relation to state-dimension, trait change, lability, and process concepts. In: Cattell RB, editor. Handbook of multivariate experimental psychology. Rand McNally; Chicago: 1966. [Google Scholar]
- Cattell RB. Abilities: Their structure, growth, and action. Houghton Mifflin; Boston: 1971. [Google Scholar]
- Cattell RB, Cattell AKS, Rhymer RM. P-technique demonstrated in determining psychophysical source traits in a normal individual. Psychometrika. 1947;12:267–288. doi: 10.1007/BF02288941. [DOI] [PubMed] [Google Scholar]
- Chow SM, Nesselroade JR, Shifren K, McArdle JJ. Dynamic structure of emotions among individuals with Parkinson's disease. Structural Equation Modeling. 2004;11:560–582. [Google Scholar]
- Chow SM, Ram N, Boker SM, Fujita F, CLore G. Capturing weekly fluctuation in emotion using a latent differential structural approach. Emotion. 2005;5:208–225. doi: 10.1037/1528-3542.5.2.208. [DOI] [PubMed] [Google Scholar]
- Cole PM, Martin SE, Dennis TA. Emotion regulation as a scientific construct: Challenges and directions for child development research. Child Development. 2004;75:317–333. doi: 10.1111/j.1467-8624.2004.00673.x. [DOI] [PubMed] [Google Scholar]
- Collins LM. Analysis of longitudinal data: The integration of theoretical model, temporal design, and statistical model. Annual Review of Psychology. 2006;57:505–528. doi: 10.1146/annurev.psych.57.102904.190146. [DOI] [PubMed] [Google Scholar]
- Collins LM, Horn JL. Best methods for the analysis of change. American Psychological Association; Washington, DC: 1991. [Google Scholar]
- Collins LM, Sayer A. New methods for the analysis of change. American Psychological Association; Washington, DC: 2001. [Google Scholar]
- Csikszentmihalyi M, Larson R. Validity and reliability of the experience-sampling method. Journal of Nervous and Mental Disease. 1987;175:526–536. doi: 10.1097/00005053-198709000-00004. [DOI] [PubMed] [Google Scholar]
- Eizenman DR, Nesselroade JR, Featherman DL, Rowe JW. Intraindividual variability in perceived control in an older sample: The MacArthur successful aging studies. Psychology and Aging. 1997;12:489–502. doi: 10.1037//0882-7974.12.3.489. [DOI] [PubMed] [Google Scholar]
- Elliott RJ, Aggoun L, Moore JB. Hidden Markov models: Estimation and control. Springer; New York: 1995. [Google Scholar]
- Ersner-Hershfield H, Mikels JA, Sullivan S, Carstensen LL. Poignancy: Mixed emotional experience in the face of a meaningful ending. Journal of Personality and Social Psychology. 2008;94:158–67. doi: 10.1037/0022-3514.94.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM. The maturing architecture of the brain's default network. Proceeding of the National Academy of Science. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherman DL, Petersen T. Markers of aging: Modeling the clocks that time us. Research on Aging. 1986;8:339–365. doi: 10.1177/0164027586008003001. [DOI] [PubMed] [Google Scholar]
- Ferrer E, Nesselroade JR. Modeling affective processes in dyadic relations via dynamic factor analysis. Emotion. 2003;3:344–360. doi: 10.1037/1528-3542.3.4.344. [DOI] [PubMed] [Google Scholar]
- Fiske DW, Rice L. Intra-individual response variability. Psychological Bulletin. 1955;52:217–250. doi: 10.1037/h0045276. [DOI] [PubMed] [Google Scholar]
- Ford DH. Humans as self-constructing living systems: A developmental perspective on personality and behavior. Erlbaum; Hillsdale, NJ: 1987. [Google Scholar]
- Ford DH, Lerner RM. Developmental systems theory: An integrative approach. Sage; Newbury Park, CA: 1992. [Google Scholar]
- Gottlieb G. Normally occuring environmental and behavioral influences on gene activity: From central dogma to probalistic epigenesis. Psychological Review. 1998;105:792–802. doi: 10.1037/0033-295x.105.4.792-802. [DOI] [PubMed] [Google Scholar]
- Gottman J, Murray J, Swanson C, Tyson R, Swanson K. The mathematics of marriage: Dynamic nonlinear models. MIT Press; Cambridge, MA: 2002. [Google Scholar]
- Granger DA, Kivlighan KT. Integrating biological, behavioral, and social levels of analysis in early child development: Progress, problems, and prospects. Child Development. 2003;74:1058–1063. doi: 10.1111/1467-8624.00590. [DOI] [PubMed] [Google Scholar]
- Hammerstein P, Hagen EH, Herz AVM, Herzel H. Robustness: A key to evolutionary design. Biological theory: Integrating development, evolution and cognition. 2006;1:90–93. [Google Scholar]
- Harris CW, editor. Problems in measuring change. University of Wisconsin Press; Madison, WI: 1963. [Google Scholar]
- Heckhausen J, Schulz R. A life-span theory of control. Psychological Review. 1995;102:284–304. doi: 10.1037/0033-295x.102.2.284. [DOI] [PubMed] [Google Scholar]
- Hertzog C, Nesselroade JR. Assessing psychological change in adulthood: An overview of methodological issues. Psychology and Aging. 2003;18:639–657. doi: 10.1037/0882-7974.18.4.639. [DOI] [PubMed] [Google Scholar]
- Ho M-HR, Shumway R, Ombao H. The state-space approach to modeling dynamic processes. In: Walls TA, Schafer JL, Schafer JL, editors. Models for intensive longitudinal data. Oxford University Press; New York: 2006. pp. 148–194. [Google Scholar]
- Hofer SM, Sliwinski MJ. Design and analysis of longitudinal studies of aging. In: Birren JE, Schaie KW, editors. Handbook of the psychology of aging. 6th Edition Academic Press; San Diego: 2006. pp. 15–37. [Google Scholar]
- Hooker K. Change and stability in self during the transition to retirement: An intraindividual study using P-technique factor analysis. International Journal of Behavioral Development. 1991;14:209–233. [Google Scholar]
- Horn JL. The theory of fluid and crystallized intelligence in relation to concepts of cognitive psychology and aging in adulthood. In: Craik FIM, Trehub S, editors. Aging and cognitive processes. Plenum; New York: 1982. pp. 77–117. [Google Scholar]
- Hull C. Principles of behavior. Appleton-Century-Crofts; New York: 1943. [Google Scholar]
- Hultsch DF, MacDonald SWS. Intraindividual variability in performance as a theoretical window onto cognitive aging. In: Dixon RA, Bäckman L, Nilsson L-G, editors. New frontiers in cognitive aging. Oxford University Press; New York: 2004. pp. 65–88. [Google Scholar]
- Hultsch DF, MacDonald SWS, Dixon RA. Variability in reaction time performance of younger and older adults. Journal of Gerontology: Psychological Sciences. 2002;57B:101–115. doi: 10.1093/geronb/57.2.p101. [DOI] [PubMed] [Google Scholar]
- Jahng S, Wood PK, Trull TJ. Analysis of affective stability in ecological momentary assessment: Indices using successive difference and group comparison using multilevel modeling. Psychological Methods. 2008;13:354–375. doi: 10.1037/a0014173. [DOI] [PubMed] [Google Scholar]
- Jenkins GM, Watts DG. Spectral Analysis and its applications. Holden Day; San Francisco: 1968. [Google Scholar]
- Jones CJ, Nesselroade JR. Multivariate, replicated, single-subject designs and P-technique factor analysis: A selective review of the literature. Experimental Aging Research. 1990;16:171–183. doi: 10.1080/03610739008253874. [DOI] [PubMed] [Google Scholar]
- Kim J, Zhu W, Chang L, Bentler PM, Ernst T. Unified structural equation modeling approach for the analysis of multisubject, multivariate, functional fMRI data. Human Brain Mapping. 2007;28:85–93. doi: 10.1002/hbm.20259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleban MH, Lawton MP, Nesselroade JR, Parmelee P. The structure and variation in affect among depressed and nondepressed elderly. Journal of Gerontology: Psychological Sciences. 1992;47B:P190–P198. doi: 10.1093/geronj/47.3.p190. [DOI] [PubMed] [Google Scholar]
- Kliegl R, Smith J, Baltes PB. On the locus and process of magnification of age differences during mnemonic training. Developmental Psychology. 1990;26:894–904. [Google Scholar]
- Koopmans LH. The spectral analysis of time series. 2nd ed. Academic Press; San Diego, CA: 1995. [Google Scholar]
- Kudielka BM, Kirschbaum C. Biological bases of the stress response. In: Al'Absi M, editor. Stress and addiction: Biological and psychological mechanisms. Elsevier Academic Press; San Diego, CA: 2007. pp. 3–19. [Google Scholar]
- Kuppens P, Van Mechelen I, Nezlek JB, Dossche D, Timmermans T. Individual differences in core affect variability and their relationship to personality and adjustment. Emotion. 2007;7:262–274. doi: 10.1037/1528-3542.7.2.262. [DOI] [PubMed] [Google Scholar]
- Larson R, Zuzanek J, Mannell R. Being alone versus being with people: disengagement in the daily experience of older adults. Journal of Gerontology. 1985;40:375–381. doi: 10.1093/geronj/40.3.375. [DOI] [PubMed] [Google Scholar]
- Lattal KA, Perone M, editors. Handbook of research methods in human operant behavior. Plenum Press; New York: 1998. [Google Scholar]
- Leary T. Interpersonal diagnosis of personality: A functional theory and methodology for personality evaluation. Wiley; New York: 1957. [Google Scholar]
- Leiderman PH, Shapiro D. Application of a time series statistic to physiology and psychology. Science. 1962;138:141–142. doi: 10.1126/science.138.3537.141. [DOI] [PubMed] [Google Scholar]
- Lerner RM. On the nature of human plasticity. Cambridge University Press; New York, NY: 1984. [Google Scholar]
- Li S, Schmiedek F. Age is not necessarily aging: Another step towards understanding the `clocks' that time aging. Gerontology. 2002;48:5–12. doi: 10.1159/000048917. [DOI] [PubMed] [Google Scholar]
- Li S-C. Biocultural orchestration of developmental plasticity across levels: The interplay of biology and culture in shaping the mind and behavior across the life span. Psychological Bulletin. 2003;129:171–194. doi: 10.1037/0033-2909.129.2.171. [DOI] [PubMed] [Google Scholar]
- Li S-C, Schmiedek F, Huxold O, Rocke C, Smith J, Lindenberger U. Working memory plasticity in old age: Practice gain, transfer, and maintenance. Psychology and Aging. 2008;23:731–742. doi: 10.1037/a0014343. [DOI] [PubMed] [Google Scholar]
- Li S-C, Huxhold O, Schmiedek F. Aging and attenuated processing robustness: Evidence from cognitive and sensorimotor functioning. Gerontology. 2004;50:28–34. doi: 10.1159/000074386. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Oertzen T. v., Craik FIM, Bialystok E. Lifespan cognition: Mechanisms of change. Oxford University Press; Oxford, UK: 2006. Variability in cognitive aging: From taxonomy to theory; pp. 297–314. [Google Scholar]
- Lövdén M, Li S-C, Shing YL, Lindenberger U. Within-person trial-to-trial variability precedes and predicts cognitive decline in old and advanced old age: Longitudinal data from the Berlin Aging Study. Neuropsychologia. 2007;45:2827–2838. doi: 10.1016/j.neuropsychologia.2007.05.005. [DOI] [PubMed] [Google Scholar]
- MacDonald SWS, Nyberg L, Bäckman L. Intra-individual variability in behavior: Links to brain structure, neurotransmission and neuronal activity. Trends in Neurosciences. 2006;29:474–480. doi: 10.1016/j.tins.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Magnusson D. The individual as the organizing principle in psychological inquiry: A holistic approach. In: Bergman LR, Cairns RB, Nilsson L–G, Nystedt, editors. Developmental science and the holistic approach. Erlbaum; Mahwah, NJ: 2000. pp. 33–47. [Google Scholar]
- Magnusson D, Cairns RB. Developmental science: Toward a unified framework. In: Cairns RB, Elder GH, Costello EJ, editors. Developmental science. Cambridge University Press; New York, NY: 1996. pp. 7–30. [Google Scholar]
- Martin M, Hofer SM. Intraindividual variability, change, and aging: Conceptual and analytical issues. Gerontology. 2004;50:7–11. doi: 10.1159/000074382. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Stellar E. Stress and the individual: Mechanisms leading to disease. Archives of Internal Medicine. 1993;153:2093–2101. [PubMed] [Google Scholar]
- Mischel W, Shoda Y. A cognitive-affective system of personality: Reconceptualizing of situations, dispositions, dynamics, and invariance in personality structure. Psychological Review. 1995;102:246–268. doi: 10.1037/0033-295x.102.2.246. [DOI] [PubMed] [Google Scholar]
- Molenaar PCM, Nesselroade JR. The recoverability of P--technique factor analysis. Multivariate Behavioral Research. 2009;44:130–141. doi: 10.1080/00273170802620204. [DOI] [PubMed] [Google Scholar]
- Molenaar PCM. A dynamic factor model for the analysis of multivariate time series. Psychometrika. 1985;50:181–202. [Google Scholar]
- Molenaar PCM. A Manifesto on psychology as idiographic science: Bringing the person back into scientific psychology, this time forever. Measurement: Interdisciplinary research and perspectives. 2004;2:201–218. [Google Scholar]
- Molenaar PCM, Ram N. Advances in dynamic factor analysis of psychological processes. In: Valsiner JA, editor. Dynamic process methodology in the social and developmental sciences. Springer; New York: in press. [Google Scholar]
- Molenaar PCM, Campbell CG. The new person-specific paradigm in psychology. Current Directions in Psychology. 2009;18:112–117. [Google Scholar]
- Morin PJ. Community ecology. Blackwell Science; Malden, MA: 1999. [Google Scholar]
- Moskowitz DS, Hershberger SL. Modeling intraindividual variability with repeated measures data: Advances and techniques. Erlbaum; Mahwah, NJ: 2001. [Google Scholar]
- Moskowitz DS, Zuroff DC. Flux, pulse, and spin: Dynamic additions to the personality lexicon. Journal of Personality and Social Psychology. 2004;86:880–893. doi: 10.1037/0022-3514.86.6.880. [DOI] [PubMed] [Google Scholar]
- Moskowitz DS, Zuroff DC. Robust predictors of flux, pulse, and spin. Journal of Research in Personality. 2005;39:130–147. [Google Scholar]
- Müller K, Lohmann G, Bosch V, von Cramon DY. On multivariate spectral analysis of fMRI time series. NeuroImage. 2001;14:347–356. doi: 10.1006/nimg.2001.0804. [DOI] [PubMed] [Google Scholar]
- Nesselroade JR. Adult personality and development: Issues in assessing constancy and change. In: Rabin AE, Zucker RA, Emmons RA, Frank S, editors. Studying persons and lives. Springer; New York: 1990. pp. 41–85. [Google Scholar]
- Nesselroade JR. The warp and woof of the developmental fabric. In: Downs R, Liben L, Palermo D, editors. Visions of development, the environment, and aesthetics: The legacy of Joachim F. Wohlwill. Erlbaum; Hillsdale, NJ: 1991. pp. 213–240. [Google Scholar]
- Nesselroade JR, Baltes PB, editors. Longitudinal research in the study of behavior and development. Academic Press; New York: 1979. [Google Scholar]
- Nesselroade JR, Boker SM. Assessing constancy and change. In: Heatherton TD, Weinberger JL, editors. Can personality change? American Psychological Association; Washington, DC: 1994. pp. 121–147. [Google Scholar]
- Nesselroade JR, Ford DH. P-technique comes of age: Multivariate, replicated, single-subject designs for research on older adults. Research on Aging. 1985;7:46–80. doi: 10.1177/0164027585007001003. [DOI] [PubMed] [Google Scholar]
- Nesselroade JR, Jones CJ. Multi-modal selection effects in the study if adult development: A perspective on multivariate, replicated, single-subject, repeated measures designs. Experimental Aging Research. 1991;17:21–27. doi: 10.1080/03610739108253883. [DOI] [PubMed] [Google Scholar]
- Nesselroade JR, Ram N. Studying intraindividual variability: What we have learned that will help us understand lives in context. Research in Human Development. 2004;1:9–29. [Google Scholar]
- Nesselroade JR, Reese HW. Life-span developmental psychology: Methodological issues. Academic Press; New York: 1973. [Google Scholar]
- Nesselroade JR, Schmidt McCollam KM. Putting the process in developmental processes. International Journal of Behavioral Development. 2000;24:295–300. [Google Scholar]
- Nesselroade JR, Ghisletta P. Structuring and measuring change over the lifespan. In: Staudinger UM, Lindenberger U, editors. Understanding human development. Kluwer Academic; Boston: 2003. pp. 317–337. [Google Scholar]
- Newell KM, Hancock PA. Forgotten moments: A note on skewness and kurtosis as influential factors in inferences extrapolated from response distributions. Journal of Motor Behavior. 1984;16:320–335. doi: 10.1080/00222895.1984.10735324. [DOI] [PubMed] [Google Scholar]
- Ong AD, Bergeman CS. The complexity of emotions in later life. Journal of Gerontology: Psychological Sciences. 2004;59B:P117–P122. doi: 10.1093/geronb/59.3.p117. [DOI] [PubMed] [Google Scholar]
- Overton WF, Reese HW. Models of development: Methodological implications. In: Nesselroade JR, Reese HW, editors. Life-span developmental psychology: Methodological issues. Academic Press; New York: 1973. pp. 65–86. [Google Scholar]
- Pavlov I. Conditioned reflexes. Oxford University Press; London: 1927. [Google Scholar]
- Piaget J. The development of thought: Equilibration of cognitive structures. The Viking Press; New York: 1977. [Google Scholar]
- Quinn ME, Martin P. Intra-individual change and inter-individual differences in negative mood states of older women. International Journal of Behavioral Development. 1999;23:685–701. [Google Scholar]
- Quinn ME, Johnson MA, Martin P. Intraindividual change and interindividual differences in factors influencing older women's health-seeking behavior. Health Care for Women International. 1996;17:187–196. doi: 10.1080/07399339609516232. [DOI] [PubMed] [Google Scholar]
- Rabbitt P, Osman P, Moore B, Stollery B. There are stable differences in performance variability, both from moment to moment and from day to day. The Quarterly Journal of Experimental Psychology. 2001;54A:981–1003. doi: 10.1080/713756013. [DOI] [PubMed] [Google Scholar]
- Rabiner LR. A tutorial on hidden Markov models and selected applications in speech recognition. Proceedings of the IEEE. 1989;77:257–286. [Google Scholar]
- Ram N, Carstensen LL, Nesselroade JR. Age differences in the structure of emotional experience across the adult lifespan: Articulating individual-level theory via individual-level method. 2009. Manuscript submitted for publication. [Google Scholar]
- Ram N, Gerstorf D, Fauth E, Zarit S, Malberg B. Aging, disablement, and dying: Using time-as-process and time-as-resources metrics to chart late-life change. Research in Human Development. doi: 10.1080/15427600903578151. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risley TR, Wolf MM. Strategies for analyzing behavioral change over time. In: Nesselroade JR, Reese HW, editors. Life-span developmental psychology: Methodological issues. Academic Press; New York: 1973. pp. 175–183. [Google Scholar]
- Sbarra DA, Ferrer E. The structure and process of emotional experience following nonmarital relationship dissolution: Dynamic factor analyses of love, anger, and sadness. Emotion. 2006;6:224–238. doi: 10.1037/1528-3542.6.2.224. [DOI] [PubMed] [Google Scholar]
- Schaie KW. Developmental influences on intelligence: The Seattle Longitudinal Study. Oxford University Press; New York: 2005. [Google Scholar]
- Schmiedek F, Lövden M, Lindenberger U. On the relation of mean reaction time and intraindividual reaction time variability. Psychology & Aging. 2009;24:???–???. doi: 10.1037/a0017799. [DOI] [PubMed] [Google Scholar]
- Schroots JJF, Birren JE. Concepts of time and aging in science. In: Birren JE, Schaie KW, editors. Handbook of the psychology of aging. 3rd ed. Academic Press; San Diego, CA: 1990. pp. 45–64. [Google Scholar]
- Seeman TE, Gruenewald TL. Allostasis and allostatic load over the life course. In: Eaton WW, editor. Medical and psychiatric comorbidity over the course of life. American Psychiatric Publishing; Arlington, VA: 2006. pp. 179–196. [Google Scholar]
- Settersten RA, Mayer KU. The measurement of age, age structuring, and the life course. Annual Review of Sociology. 1997;23:233–261. [Google Scholar]
- Shannon CE. Prediction and entropy of printed English. The Bell System Technical Journal. 1950;30:50–64. [Google Scholar]
- Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annual Review of Clinical Psychology. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- Shifren K, Hooker K, Wood P, Nesselroade JR. Structure and variation of mood in individuals with Parkinson's Disease: A dynamic factor analysis. Psychology and Aging. 1997;12:328–339. doi: 10.1037//0882-7974.12.2.328. [DOI] [PubMed] [Google Scholar]
- Shumway RH, Stoffer DS. 2nd ed. Springer; New York: 2006. Time series analysis and its applications. [Google Scholar]
- Siegler RS. Cognitive variability: A key to understanding cognitive development. Current Directions in Psychological Science. 1994;3:1–5. [Google Scholar]
- Skinner BF. The behavior of organisms. Appleton-Century-Crofts; New York: 1938. [Google Scholar]
- Slifkin AB, Newell KM. Is variability in human performance a reflection of system noise? Current Directions in Psychological Science. 1998;7:170–177. [Google Scholar]
- Sliwinski M. Measurement-burst designs for social health research. Social and Personality Psychology Compass. 2008;2:245–261. [Google Scholar]
- Sliwinski MJ, Mogle J. Time-based and process-based approaches to analysis of longitudinal data. In: Hofer SM, Alwin DF, editors. Handbook on cognitive aging: Interdisciplinary perspectives. Sage; Thousand Oaks: 2008. pp. 477–491. [Google Scholar]
- Sterling P, Eyer J. Allostasis: A new paradigm to explain arousal pathology. In: Fisher S, Reason J, editors. Handbook of life stress, cognition and health. Wiley; New York: 1988. pp. 629–649. [Google Scholar]
- Stern W. Differentielle Psychologie: Ihre Methodischen Grundlagen (Differential psychology: Methodological foundations) Barth; Leipzig, Germany: 1911. [Google Scholar]
- Stone AA, Shiffman S. Capturing momentary, self-reported data: A proposal for reporting guidelines. Annals of Behavioral Medicine. 2002;24:236–243. doi: 10.1207/S15324796ABM2403_09. [DOI] [PubMed] [Google Scholar]
- Stone A, Shiffman S, Atienza A, Nebeling L, editors. The science of real-time data capture. Oxford University Press; New York: 2007. [Google Scholar]
- Thelen E, Smith LB. A dynamic systems approach to the development of cognition and action. MIT Press; Cambridge, MA: 1994. [Google Scholar]
- Thelen E, Smith LB. Dynamic systems theories. In: Damon W, Lerner RM, editors. Handbook of child psychology. 5th edition Vol. 1. Wiley; New York: 1998. pp. 258–312. [Google Scholar]
- Thomae H, editor. Handbuch der Psychologie, Vol 3.: Entwicklungpsychologie (Handbook of Psychology: Developmental Psychology) Hogrefe & Huber; Göttigen, Germany: 1959. [Google Scholar]
- Tong H. Non-linear time series: A dynamical systems approach. Oxford University Press; New York: 1993. [Google Scholar]
- Valsiner J. Where is the individual subject in scientific psychology? In: Valsiner J, editor. The individual subject and scientific psychology. Plenum; New York: 1986. pp. 1–14. [Google Scholar]
- van der Maas HLJ, Molenaar PCM. Stagewise cognitive development an applicalion of catastrophe theory. Psychological Review. 1992;99:395–417. doi: 10.1037/0033-295x.99.3.395. [DOI] [PubMed] [Google Scholar]
- van Geert P, van Dijk M. Focus on variability: New tools to study intra-individual variability in developmental data. Infant Behavior & Development. 2002;25:340–374. [Google Scholar]
- von Neumann J, Kent RH, Bellison HR, Hart BI. The mean square successive differences. Annals of Mathematical Statistics. 1941;12:153–162. [Google Scholar]
- Walls TA, Schafer JL, editors. Models for intensive longitudinal data. Oxford University Press; New York: 2006. [Google Scholar]
- Warner RM. Spectral analysis of time-series data. Guilford Press; New York: 1998. [Google Scholar]
- Wessman AE, Ricks DF. Mood and personality. Holt, Rinehart, and Winston; New York: 1966. [Google Scholar]
- Wilcox AR. Indices of qualitative variation and political measurement. The Western Political Quarterly. 1973;26:325–343. [Google Scholar]
- Williams BR, Strauss EH, Hultsch DF, Hunter MA. Reaction time inconsistency in a spatial Stroop task: Age-related differences through childhood and adulthood. Aging, Neuropsychology, and Cognition. 2007;14:417–439. doi: 10.1080/13825580600584590. [DOI] [PubMed] [Google Scholar]
- Wohlwill JF. The study of behavioral development. Academic Press; New York: 1973. [Google Scholar]
- Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: A review of an emerging area of research. Gait and Posture. 2002;16:1–14. doi: 10.1016/s0966-6362(01)00156-4. [DOI] [PubMed] [Google Scholar]


