Abstract
The mRNA of bacteriophage T4 contains a strikingly abundant intercistronic hairpin. Within the 55 kilobases of known T4 sequence, the hexanucleotide sequence CTTCGG is found 13 times in the DNA strand equivalent to mRNA sequences. In 12 of those occurrences, the sequence is flanked by inverted repeats predictive of RNA hairpins with UUCG in the loop. Avian myeloblastosis virus reverse transcriptase, which can traverse hairpins of larger calculated stability, terminates efficiently at these CUUCGG hairpins. Thermal denaturation studies of model hairpins show that the loop sequence UUCG dramatically stabilizes RNA hairpins when compared to a control sequence. These data, when combined with previously described parameters of helix stability, suggest that T4 has utilized this loop sequence to optimize the stability of intercistronic hairpins. The stability of CUUCGG hairpins is also utilized in the RNAs of many organisms besides T4.
Full text
PDF

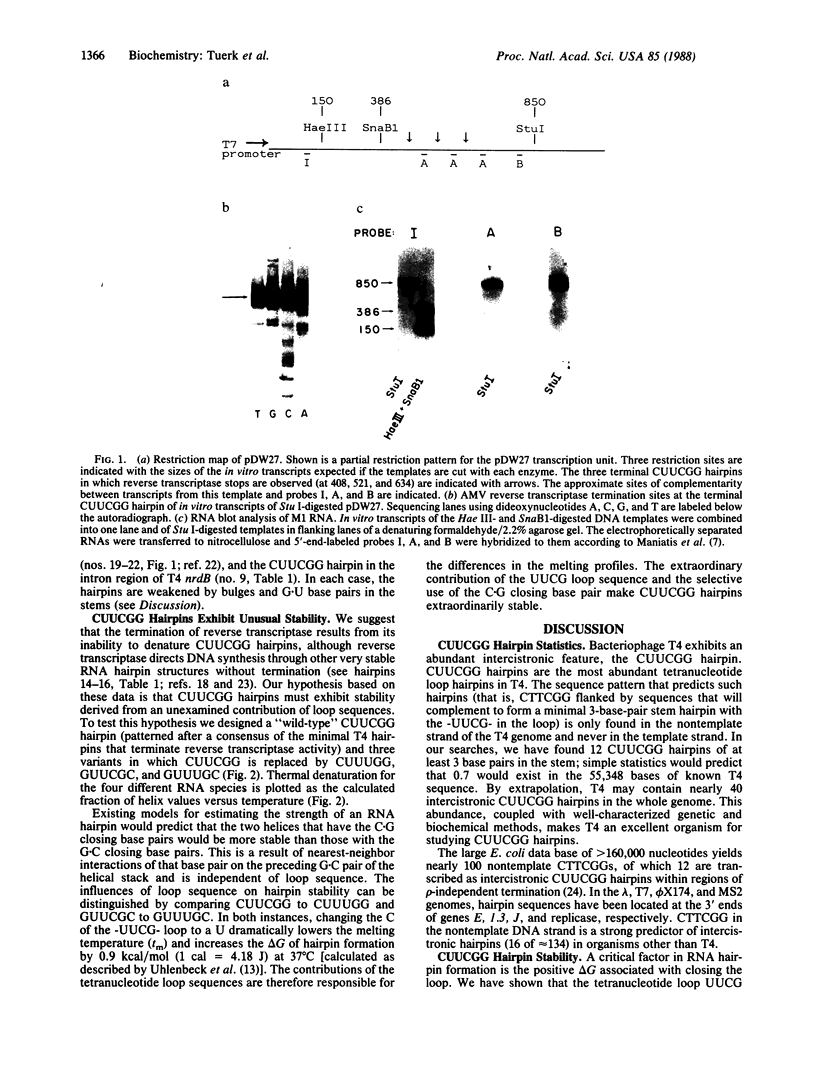
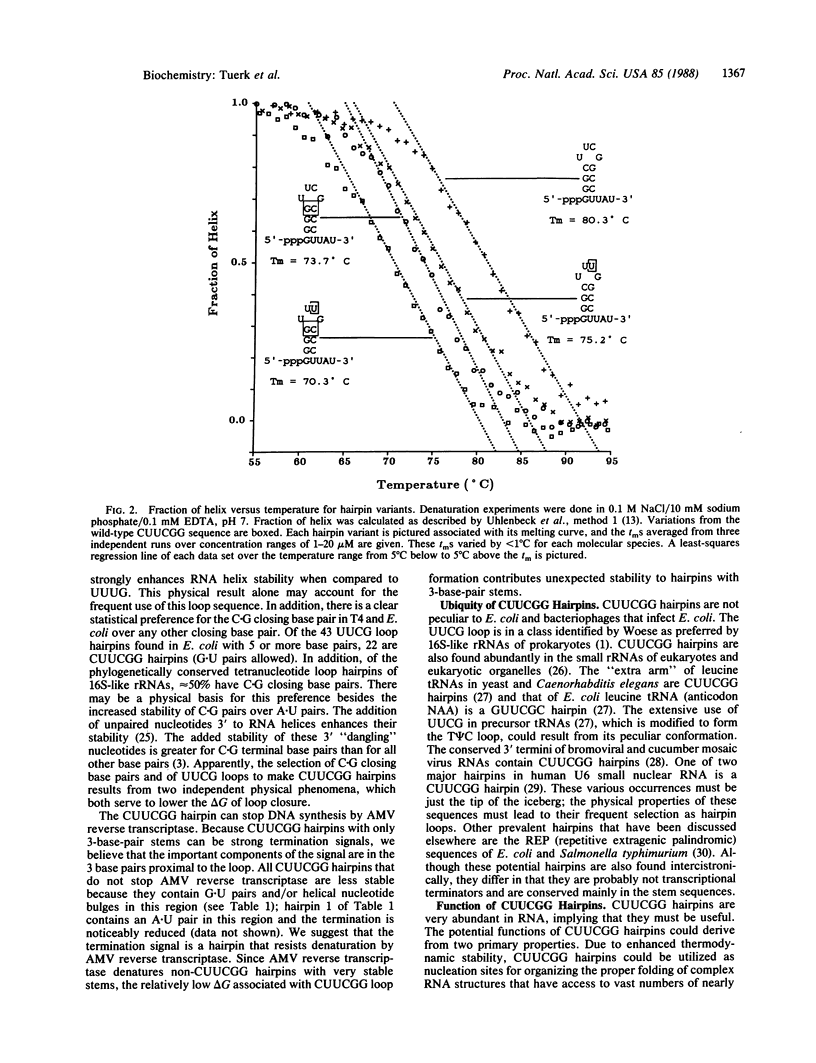

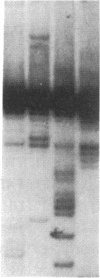
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist P., Dasgupta R., Kaesberg P. Near identity of 3- RNA secondary structure in bromoviruses and cucumber mosaic virus. Cell. 1981 Jan;23(1):183–189. doi: 10.1016/0092-8674(81)90283-x. [DOI] [PubMed] [Google Scholar]
- Black D. L., Steitz J. A. Pre-mRNA splicing in vitro requires intact U4/U6 small nuclear ribonucleoprotein. Cell. 1986 Aug 29;46(5):697–704. doi: 10.1016/0092-8674(86)90345-4. [DOI] [PubMed] [Google Scholar]
- Briat J. F., Chamberlin M. J. Identification and characterization of a new transcriptional termination factor from Escherichia coli. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7373–7377. doi: 10.1073/pnas.81.23.7373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey J., Cameron V., de Haseth P. L., Uhlenbeck O. C. Sequence-specific interaction of R17 coat protein with its ribonucleic acid binding site. Biochemistry. 1983 May 24;22(11):2601–2610. doi: 10.1021/bi00280a002. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Complete nucleotide sequence of bacteriophage T7 DNA and the locations of T7 genetic elements. J Mol Biol. 1983 Jun 5;166(4):477–535. doi: 10.1016/s0022-2836(83)80282-4. [DOI] [PubMed] [Google Scholar]
- Freier S. M., Kierzek R., Jaeger J. A., Sugimoto N., Caruthers M. H., Neilson T., Turner D. H. Improved free-energy parameters for predictions of RNA duplex stability. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9373–9377. doi: 10.1073/pnas.83.24.9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauss P., Gayle M., Winter R. B., Gold L. The bacteriophage T4 dexA gene: sequence and analysis of a gene conditionally required for DNA replication. Mol Gen Genet. 1987 Jan;206(1):24–34. doi: 10.1007/BF00326532. [DOI] [PubMed] [Google Scholar]
- Guerrier-Takada C., Altman S. Structure in solution of M1 RNA, the catalytic subunit of ribonuclease P from Escherichia coli. Biochemistry. 1984 Dec 18;23(26):6327–6334. doi: 10.1021/bi00321a006. [DOI] [PubMed] [Google Scholar]
- Hayashi M. N., Hayashi M., Müller U. R. Role for the J-F intercistronic region of bacteriophages phi X174 and G4 in stability of mRNA. J Virol. 1983 Oct;48(1):186–196. doi: 10.1128/jvi.48.1.186-196.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W. M. Nucleotide sequence of a type II DNA topoisomerase gene. Bacteriophage T4 gene 39. Nucleic Acids Res. 1986 Oct 10;14(19):7751–7765. doi: 10.1093/nar/14.19.7751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W. M. The 52-protein subunit of T4 DNA topoisomerase is homologous to the gyrA-protein of gyrase. Nucleic Acids Res. 1986 Sep 25;14(18):7379–7390. [PMC free article] [PubMed] [Google Scholar]
- Huysmans E., De Wachter R. Compilation of small ribosomal subunit RNA sequences. Nucleic Acids Res. 1986;14 (Suppl):r73–118. doi: 10.1093/nar/14.suppl.r73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPheeters D. S., Christensen A., Young E. T., Stormo G., Gold L. Translational regulation of expression of the bacteriophage T4 lysozyme gene. Nucleic Acids Res. 1986 Jul 25;14(14):5813–5826. doi: 10.1093/nar/14.14.5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midgley C. A., Murray N. E. T4 polynucleotide kinase; cloning of the gene (pseT) and amplification of its product. EMBO J. 1985 Oct;4(10):2695–2703. doi: 10.1002/j.1460-2075.1985.tb03989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazed D., Stern S., Noller H. F. Rapid chemical probing of conformation in 16 S ribosomal RNA and 30 S ribosomal subunits using primer extension. J Mol Biol. 1986 Feb 5;187(3):399–416. doi: 10.1016/0022-2836(86)90441-9. [DOI] [PubMed] [Google Scholar]
- Oliver D. B., Crowther R. A. DNA sequence of the tail fibre genes 36 and 37 of bacteriophage T4. J Mol Biol. 1981 Dec 15;153(3):545–568. doi: 10.1016/0022-2836(81)90407-1. [DOI] [PubMed] [Google Scholar]
- Reed R. E., Altman S. Repeated sequences and open reading frames in the 3' flanking region of the gene for the RNA subunit of Escherichia coli ribonuclease P. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5359–5363. doi: 10.1073/pnas.80.17.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed R. E., Baer M. F., Guerrier-Takada C., Donis-Keller H., Altman S. Nucleotide sequence of the gene encoding the RNA subunit (M1 RNA) of ribonuclease P from Escherichia coli. Cell. 1982 Sep;30(2):627–636. doi: 10.1016/0092-8674(82)90259-8. [DOI] [PubMed] [Google Scholar]
- Romaniuk P. J., Lowary P., Wu H. N., Stormo G., Uhlenbeck O. C. RNA binding site of R17 coat protein. Biochemistry. 1987 Mar 24;26(6):1563–1568. doi: 10.1021/bi00380a011. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sakamoto H., Kimura N., Shimura Y. Processing of transcription products of the gene encoding the RNA component of RNase P. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6187–6191. doi: 10.1073/pnas.80.20.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Hong G. F., Hill D. F., Petersen G. B. Nucleotide sequence of bacteriophage lambda DNA. J Mol Biol. 1982 Dec 25;162(4):729–773. doi: 10.1016/0022-2836(82)90546-0. [DOI] [PubMed] [Google Scholar]
- Schneider T. D., Stormo G. D., Haemer J. S., Gold L. A design for computer nucleic-acid-sequence storage, retrieval, and manipulation. Nucleic Acids Res. 1982 May 11;10(9):3013–3024. doi: 10.1093/nar/10.9.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider T. D., Stormo G. D., Yarus M. A., Gold L. Delila system tools. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):129–140. doi: 10.1093/nar/12.1part1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinedling S., Gayle M., Pribnow D., Gold L. Mutations affecting translation of the bacteriophage T4 rIIB gene cloned in Escherichia coli. Mol Gen Genet. 1987 May;207(2-3):224–232. doi: 10.1007/BF00331582. [DOI] [PubMed] [Google Scholar]
- Uhlenbeck O. C., Borer P. N., Dengler B., Tinoco I., Jr Stability of RNA hairpin loops: A 6 -C m -U 6 . J Mol Biol. 1973 Feb 5;73(4):483–496. doi: 10.1016/0022-2836(73)90095-8. [DOI] [PubMed] [Google Scholar]
- Vandenberghe A., Min Jou W., Fiers W. 3'-Terminal nucletide sequence (n equals 361) of bacteriophage MS2 RNA. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2559–2562. doi: 10.1073/pnas.72.7.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Gutell R., Gupta R., Noller H. F. Detailed analysis of the higher-order structure of 16S-like ribosomal ribonucleic acids. Microbiol Rev. 1983 Dec;47(4):621–669. doi: 10.1128/mr.47.4.621-669.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaug A. J., Been M. D., Cech T. R. The Tetrahymena ribozyme acts like an RNA restriction endonuclease. Nature. 1986 Dec 4;324(6096):429–433. doi: 10.1038/324429a0. [DOI] [PubMed] [Google Scholar]