Abstract
Urokinase receptor (uPAR) is a widely recognized target for potential treatment of cancer. The development of uPAR inhibitors has been going on for over a decade. Despite the identification and validation of many highly potent hits using screening or medicinal approaches, none of them has been moved further along the drug discovery pipeline. The development of uPAR inhibitors exemplifies several challenges now faced by drug discovery. These include 1) hydrophobicity and thus poor bioavailability of the inhibitors from screening approaches; 2) specificity of the inhibitor, where a peptidyl inhibitor causes conformational change of the receptor; 3) species specificity, where some inhibitors developed based on the human receptor do not inhibit the murine receptor and thus cannot be validated in mouse models. The recently determined crystal structures of uPAR in complex with its ligand or inhibitor not only provide the structural insight to understand these challenges but also offer a potential solution for further inhibitor development and thus illustrate the importance of structural information in facilitating drug discovery.
Drug discovery was historically driven by serendipity and by screening approaches [1]. The last decade has witnessed an increasing implementation of screening approaches in drug discovery due to the advent of high throughput screening (HTS) technology. For many pharmaceutical companies, HTS has become an essential component to identify drug leads [2]. Robotic compound handling and assaying facilities now perform various tasks such as retrieval of compounds from storage, sample preparation and plating, and the running of assays of ever-increasing complexity. Such automated systems can handle tens of thousands of compounds per day. As a result, HTS has become a focus in the drug discovery process. Herein we present the drug development of urokinase receptor as an example to illustrate several challenges still faced by drug discovery, and suggest that the availability of crystal structure of the target protein can greatly facilitate the drug discovery.
Urokinase receptor as a target for the treatment of cancer
Urokinase-type plasminogen activator (uPA) is one of the two primary endogenous systems that mediate plasminogen activation into plasmin [3]. The cellular receptor of uPA, uPAR, binds with both zymogen uPA (pro-uPA) and activated uPA with a high affinity (KD < 1nM), promotes the activation of pro-uPA, and localizes the proteolytic activities of uPA onto cell surface [4, 5]. Plasmin generated by uPA has broad proteolytic specificity, capable of directly degrading several extracellular matrix proteins and activating the matrix metalloproteases (MMPs), collegenase and stromolysin-1, from their zymogen forms (pro-MMPs), thus implicating uPA-uPAR's role in cell adhesion and migration. Besides this role of promoting protease activity, uPAR can also affects migration, adhesion, differentiation and proliferation through intracellular signaling. Lacking transmembrane and cytosolic segments in its protein structure, uPAR is believed to carry out its signal transduction role by interacting with an array of extracellular partners, including vitronectin (VN), several integrins, G-protein-coupled receptors, and uPAR-associated protein [6]. Important regulators of the uPA-uPAR system include plasminogen activator inhibitor 1 (PAI1), an inhibitor of uPA, and α2-antiplasmin, an inhibitor of solution phase plasmin. Other regulators of the uPA-uPAR system include α2-macroglobulin receptor and low-density lipoprotein (LDL)-receptor-related protein (LRP) that can interact with both PAI1 and uPAR [7]. LRP can recognize the uPAR-uPA-PAI1 complex and induce the internalization of the complex, leading to the cytosolic degradation of uPA, but not uPAR, which is then recycled back to cell surface.
The critical role of uPA/uPAR in tumor biology is widely recognized [6, 8-10]. uPAR is overexpressed in a variety of tumor cell lines. At the tissue level, uPAR is also found to have significantly higher concentrations in several human malignant tumors compared with the corresponding normal tissues. Higher uPAR expression can also commonly be found in tumor-associated stromal cells, including macrophages, mast cells, and endothelial cells [11]. Patients with lung cancer [12], ovarian [13], breast and colorectal cancer [14], as well as other type of cancers, have higher plasma levels of soluble uPAR than in healthy individuals. High levels of uPAR in plasma or tumor tissue lysates were found to be a marker of poor prognosis in many human tumor cell types [15, 16]. Over-expression of uPAR in human neuroglioma [17], osteosarcoma [18], epidermoid carcinoma [19] and rat mammary cancer cells [20] resulted in increased tumorigenicity and tumor invasiveness in vitro and in vivo.
Down-regulation of uPAR expression by using anti-gene techniques (antisense, ribozyme, DNAzyme and siRNA) has been shown to reverse invasive behavior, induce substantial tumor regression, or even total inhibition of tumor metastasis of several cancers including squamous cell carcinoma, glioblastoma, colon, prostate, and non-small cell lung cancer [10]. Recombinant soluble uPAR inhibited the invasiveness of ovarian cancer cells by competing with surface uPAR for ligand binding [21]. Adenovirus-mediated delivery of ATF, an uPAR-uPA antagonist, suppresses angiogenesis-dependent tumor growth, lung dissemination and liver metastasis in mice [22]. Another uPAR antagonist, the GFD domain of uPA fused with the Fc portion of human IgG, inhibits angiogenesis and primary tumor growth in syngeneic mice in vivo [23]. An anti-uPAR mAb has shown remarkably efficacy inhibiting primary tumor growth, peritoneal invasion and liver metastasis of an orthotopically transplanted pancreatic carcinoma cell line [24]. Gene deletion of uPAR seems not to affect normal physical development, normal reproduction, normal thrombosis, vascular remodeling, and angiogenesis as shown in a mouse gene knockout study in which uPAR-null mice were born and survived to adulthood with no overt phenotypic abnormalities [25]. These findings suggest that uPAR inhibitors might be useful therapeutically for the prevention of invasion and metastasis of certain human cancers.
Barriers to therapeutic intervention: Hydrophobicity of the current small molecular uPAR inhibitors
Since the discovery of uPAR [26-28], several classes of uPAR inhibitors have been developed, including monoclonal antibodies [24, 29], recombinant fusion proteins [30-32], peptidyl inhibitors [33-38], and small molecule inhibitors [39]. In this paper, we will focus on the small molecular inhibitors including peptidyl inhibitors.
Two kinds of small molecular peptidyl uPAR inhibitors have been developed including linear peptides and cyclic peptides. A bacteriophage peptide display library was used to generate peptides that inhibit the uPAR-uPA interaction [33]. This resulted in a large number of 15-mer inhibitory peptides with IC50-values in the range from 10 nM to 10 μM for the uPAR-uPA interaction. A 17-mer peptide, AEPMPHSLNFSQYLWYT, displayed the highest binding affinity towards uPAR. The functional important segment of the peptide was further narrowed down to SLNFSQYLWS [40]. Further studies on structure-activity relationships and affinity maturation by combinatorial chemistry identified a novel 9-mer inhibitory peptide denoted as AE105 (D-Cha-F-s-r-Y-L-W-S, where capital letters denote amino acid in L-configuration, lower case letters denote D-configuration amino acids, and Cha represents cyclo-L-hexylalanine) and has a KD of ~0.4 nM for uPAR binding [34, 35]. This peptide competes with ATF (amino terminal fragment, uPA 1-143) binding to uPAR-expressing cells with an IC50 of 10 nM. Recently, the peptide was also coupled to an α-emitter (213Bi) through a K-G-S-G-G spacer, and the labeled AE105 probe (213Bi-P-P4D) showed 17 nM IC50 to human ovarian cancer cells OVMZ-6 and specific uptake to this tumor implanted in nude mice in vivo [41].
Magdolen and co-workers [36-38] developed a cyclic peptidyl uPAR inhibitor based on the fact that residues in a beta-hairpin loop (18-32) of uPA is the major binding determinant of its receptor [4]. After extensive synthesis and testing, they obtained a cyclic peptide, cyclo21;29[D-Cys21]-uPA21_30[S21C;H29C], that showed an IC50 of 40 nM toward uPAR [38]. Both the cyclic structure (maintained by the Cys21-Cys29 disulfide bond) and the chirality of D-Cys21 are important for the high affinity binding of uPAR. The cyclic peptide was shown to reduce the overall tumor burden of an orthotopic xenograft of human ovarian cyst-adenomacarcinoma cell line in nude mice [42].
Although peptides tend to have short half-lives in plasma, the above mentioned peptide inhibitors are quite stable in plasma. Even so, small molecular organic compounds are still a major goal for inhibitor development. Several small molecule organic inhibitors for uPAR were identified by traditional screening of chemical libraries (for a review, see [39]). Some inhibitors have IC50 values in the lower nanomolar range. However, no further development/evaluation of these inhibitors can be found in the literature. A common characteristic of all these peptidyl and small molecule inhibitors is that they are all quite hydrophobic, and thus, may have poor bioavailability.
Barriers to therapeutic intervention: how to cross species boundaries
Remarkable species specificity exists in the uPA-uPAR interactions among human and other species. Binding of human soluble receptor with murine uPA is 68-fold weaker than that with human uPA. Similarly, interaction between murine receptor and human uPA is 46-fold weaker than that within murine pair [43]. In addition, the human uPAR inhibitors developed so far also show species specificity, e.g., the linear peptidyl inhibitors developed based on human uPAR did not bind to the murine uPAR or to the uPARs of three species of monkeys [4]. Since the efficacy of human uPAR inhibitors is commonly validated in mouse model systems, such species specificity of inhibitors presents a barrier for the uPAR inhibitor development.
Barriers to therapeutic intervention: inhibitors induced uPAR conformational change
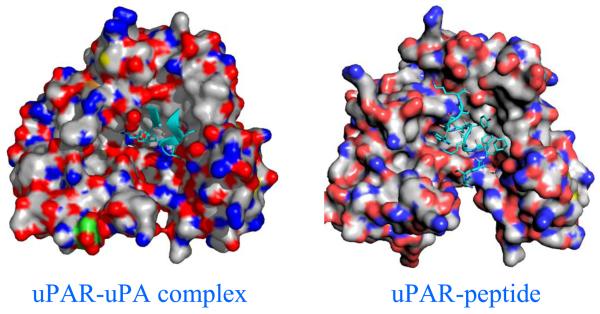
The crystal structures of soluble uPAR in complex with ATF fragment of uPA [44], and with a peptidyl inhibitor (AE147) [35] were recently determined. Comparison of suPAR conformations between suPAR-ATF and suPAR-peptide (AE147) structures indicates that D1 domain of uPAR rotates by 20.5° if D2 and D3 domains in two uPAR structures are superimposed (Fig. 2). This demonstrates not only the inter-domain conformational flexibility of uPAR, but also the possibility of inhibitor-induced uPAR conformational changes. Even though the peptide inhibitor is highly potent, such inhibitor-induced conformational perturbation on uPAR is not an ideal result for inhibitor development, because such changes may affect uPAR's interaction with other ligands, leading to lower specificity to the targeted function. uPAR is a surface receptor capable of interacting with an array of other proteins. Besides uPA, uPAR also interacts with several cell surface proteins (e.g., integrins and GPCR [45] or matrix proteins (e.g., vitronectin [46]). uPAR uses its central cavity to bind to its primary ligand (uPA) and its outer surface to interact with vitronectin [47]. The ligand occupancy on uPAR central cavity enhances the binding affinity between uPAR and vitronectin [48]. Therefore, an ideal inhibitor of uPAR-uPA interaction should occupy the central cavity, should not change the receptor conformation and, thus, should not affect uPAR interaction with its other ligand.
Figure 2.
Inhibitor-induced receptor conformational changes. On the left panel is the crystal structure of uPAR in complex with its native uPA ligand (only GFD domain of the ligand, in cyan, is shown in the figure). Three domains of uPAR (D1, lower right; D2, upper center; D3, lower left) form a central cavity to accommodate the uPA ligand. On the right panel is the structure of uPAR in complex with a peptidyl inhibitor (AE147, in cyan) showing the D1 (lower right) domain separated from the D3 (lower left) domain.
Structural insights for uPAR-uPA inhibitor development
uPAR-uPA interaction has been a target of intensive inhibitor development since the first report of high affinity antagonists in 1994 [33]. Several classes of potent inhibitors, including antibodies, peptides, or organic compounds, have been identified since then. However, most inhibitors do not move beyond the preclinical stage, and not many follow up reports can be found. Three barriers for uPAR-uPA inhibitor development are highlighted here. Recently determined crystal structures of uPAR complexes [44, 47, 49] provide structural insight to these barriers, might offer solutions to overcome these barriers, and might revive the interest in the development of uPAR-uPA inhibitors.
It took years of effort to produce large quantities of homogeneous suPAR recombinant protein and to generate usable crystals of uPAR-ligand complexes [50, 51]. Such capability will provide one of the methods to study inhibitor-induced uPAR conformational changes by crystallizing uPAR-inhibitor complexes and studying their crystal structures.
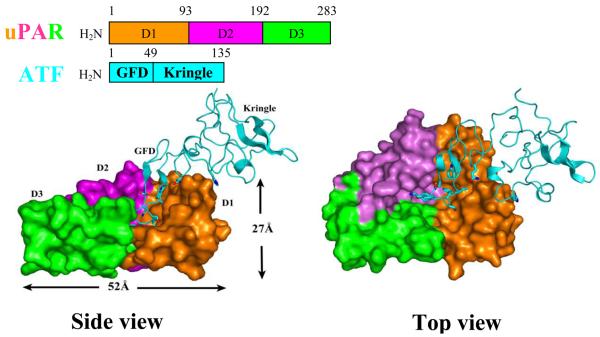
Human urokinase receptor (uPAR) is a glycosylated membrane protein composed of three homologous domains (D1, D2 and D3) and is linked to the cell membrane through a carboxyl-terminal glycosylphosphatidylinositol (GPI) anchor [43]. The crystal structure of uPAR-ATF complex shows (Fig. 3) that the three individual domains in uPAR are packed closely into each other, resulting in a unique cone-shaped central cavity surrounded by these three domains. The central cavity has a wide opening (25 Å) and significant depth (14 Å). uPA consists of three domains: a small N-terminal growth factor domain (GFD, 47 residues), a kringle domain, and a protease domain at its carboxy terminus. uPA inserts its GFD domain into the uPAR cavity and contacts with all three uPAR domains, whereas the kringle domain of urokinase has only few and apparently weak interactions with the receptor.
Figure 3.
Overall structure of uPAR in complex with its uPA ligand. uPAR is in surface representation in two views with three domains colored differently, while the amino-terminal fragment (ATF) of uPA is shown as a cyan ribbon. Three domains of uPAR pack closely to each other, resulting in a cavity surrounded by all three domains. The cavity has a wide opening of 25 Å and a depth of 14 Å. ATF inserts its growth factor domain into the uPAR cavity and contacts with all three uPAR domains, as shown in the top view picture on the right hand side.
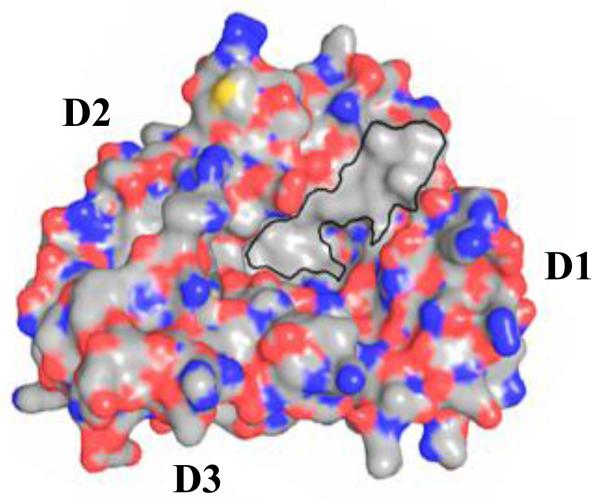
A distinguishing feature of the uPAR central cavity is a large hydrophobic patch on one side of the cavity (413 Å2, Fig. 4), which is formed by the aliphatic hydrophobic residues from both D1 and D2 domains. Exposure of a large hydrophobic area to the aqueous environment is energetically disfavored. In the ligand-bound form, this hydrophobic patch is completely covered up by interaction with ATF. Thus, the uPA ligand might play an important role in stabilizing the receptor into the observed conformation [52]. The existence of this large hydrophobic area also explains why the small organic inhibitors discovered through HTS tend to be hydrophobic.
Figure 4.
A large hydrophobic area exists in the uPAR central cavity. uPAR is in surface representation where carbon, oxygen and nitrogen atoms are colored in gray, red and blue, respectively.
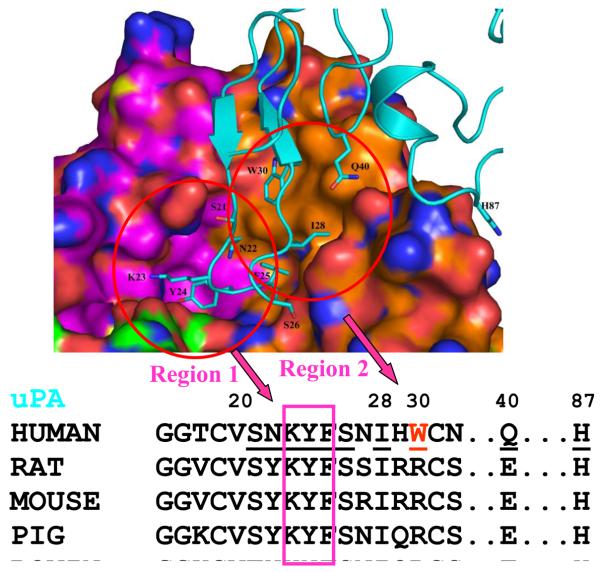
The interface between uPAR and ATF can be classified into two regions based on the curvatures of uPAR surface. As illustrated in Fig. 5, region 1 is a large pocket formed mainly by uPAR D2 domain residues and accommodates several uPA GFD residues, especially Lys23, Tyr24, and Phe25. This region is buried deep in the uPAR cavity and has both hydrophobic and polar interactions between uPA and its receptor. Region 2 of the uPAR-ATF interface is relatively flat, formed mainly by the residues in uPAR D1 domain, and is hydrophobic in nature. This region interacts with the ATF hydrophobic residues, Phe25, Ile28 and Trp30. Based on the structure of uPAR-ATF and the sequence of uPA (see Fig. 5), it can be seen that region 2 of uPAR (especially, Trp30 and Gln40 of uPA in this region) is responsible for the species specificity. The uPA residues in the region 1 are quite conserved among different species in sequence, and mostly likely, also on a structural level.
Figure 5.
The uPAR-uPA interface can be classified into two regions (left and right circles). The receptor is in surface representation where carbon atoms of three domain of uPAR are colored orange, cyan and green, respectively. The uPA ligand (cyan) is in stick representation. Nitrogen atoms are colored in blue and oxygen atoms are in red. Region 1 includes uPA residues 23-KYF-25. Region 2 contains mainly uPA residues I28, W30, and N40.
New opportunities in the development of uPAR inhibitors
The human uPAR inhibitors developed so far are from either screening or medicinal chemistry approaches and face various problems (see above). The recently determined crystal structures of uPAR in complex with uPA (1-135)[49] or with a peptidyl inhibitor (AE147) [44] provide new opportunities for the development of human uPAR inhibitors.
These structures facilitate the understanding of the structural origin of the species specificity and show that the screening method tends to generate hits toward the hydrophobic patch in the suPAR central cavity. This is consistent with a detailed analysis that shows HTS lead generation tends to produce hits that are high-molecular-weight and hydrophobic moieties, whereas high-quality lead compounds and the majority of drugs are generally low-molecular-weight, soluble entities [53, 54].
Is it possible to develop small organic compounds to intervene with uPAR-uPA interaction and to overcome the above-mentioned barriers? We propose that the region 1 of uPAR (Fig. 5) be the targeting area for the design of small organic inhibitors to intervene with urokinase-receptor interaction and to overcome these barriers. A primary reason for this proposal is that the uPAR-uPA interaction at this region has both hydrophobic and hydrophilic characters and thus may allow inhibitors that are not too hydrophobic. In addition, inhibitors targeting at this area can bypass the species specificity problem, allowing the inhibitors developed based on human proteins can be validated in mouse models.
In summary, the case of uPAR inhibitor development provides an example to highlight the barriers and the challenges for drug discovery, especially for the screening approach, and suggest that the availability of crystal structures can greatly facilitate drug discovery.
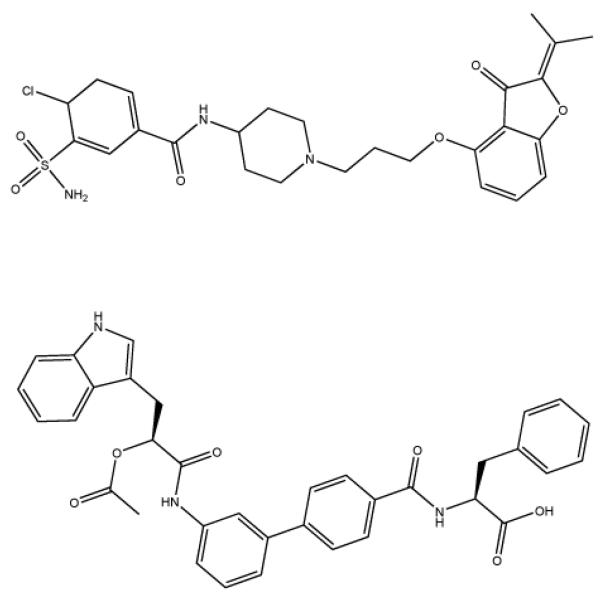
Figure 1.
Small molecule organic uPAR inhibitors that have IC50 values in the low nanomolar range.
Acknowledgements
Our research is supported by grants from Fujian province (2009I0029), NSFC (30811130467, 30625011), CAS (KSCX2-YW-R-082, SZD08003), and NIH (RO1 HL086584).
References
- 1.Drews J. Drug discovery: a historical perspective. Science. 2000;287(5460):1960–4. doi: 10.1126/science.287.5460.1960. [DOI] [PubMed] [Google Scholar]
- 2.Cox B, Denyer JC, Binnie A, Donnelly MC, Evans B, Green DV, Lewis JA, Mander TH, Merritt AT, Valler MJ, Watson SP. Application of high-throughput screening techniques to drug discovery. Prog Med Chem. 2000;37:83–133. doi: 10.1016/s0079-6468(08)70058-4. [DOI] [PubMed] [Google Scholar]
- 3.Andreasen PA, Egelund R, Petersen HH. The plasminogen activation system in tumor growth, invasion, and metastasis. Cell Mol Life Sci. 2000;57(1):25–40. doi: 10.1007/s000180050497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ploug M. Structure-function relationships in the interaction between the urokinase-type plasminogen activator and its receptor. Curr Pharm Des. 2003;9(19):1499–528. doi: 10.2174/1381612033454630. [DOI] [PubMed] [Google Scholar]
- 5.Behrendt N. The urokinase receptor (uPAR) and the uPAR-associated protein (uPARAP/Endo180): membrane proteins engaged in matrix turnover during tissue remodeling. Biological chemistry. 2004;385(2):103–36. doi: 10.1515/BC.2004.031. [DOI] [PubMed] [Google Scholar]
- 6.Blasi F, Carmeliet P. uPAR: a versatile signalling orchestrator. Nat Rev Mol Cell Biol. 2002;3(12):932–43. doi: 10.1038/nrm977. [DOI] [PubMed] [Google Scholar]
- 7.Czekay RP, Kuemmel TA, Orlando RA, Farquhar MG. Direct binding of occupied urokinase receptor (uPAR) to LDL receptor-related protein is required for endocytosis of uPAR and regulation of cell surface urokinase activity. Molecular biology of the cell. 2001;12(5):1467–79. doi: 10.1091/mbc.12.5.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazar AP. The urokinase plasminogen activator receptor (uPAR) as a target for the diagnosis and therapy of cancer. Anticancer Drugs. 2001;12(5):387–400. doi: 10.1097/00001813-200106000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Reuning U, Sperl S, Kopitz C, Kessler H, Kruger A, Schmitt M, Magdolen V. Urokinase-type plasminogen activator (uPA) and its receptor (uPAR): development of antagonists of uPA/uPAR interaction and their effects in vitro and in vivo. Curr Pharm Des. 2003;9(19):1529–43. doi: 10.2174/1381612033454612. [DOI] [PubMed] [Google Scholar]
- 10.Pillay V, Dass CR, Choong PF. The urokinase plasminogen activator receptor as a gene therapy target for cancer. Trends Biotechnol. 2007;25(1):33–9. doi: 10.1016/j.tibtech.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Nielsen BS, Rank F, Illemann M, Lund LR, Dano K. Stromal cells associated with early invasive foci in human mammary ductal carcinoma in situ coexpress urokinase and urokinase receptor. International journal of cancer. 2007;120(10):2086–95. doi: 10.1002/ijc.22340. [DOI] [PubMed] [Google Scholar]
- 12.Pappot H, Hoyer-Hansen G, Ronne E, Hansen HH, Brunner N, Dano K, Grondahl-Hansen J. Elevated plasma levels of urokinase plasminogen activator receptor in non-small cell lung cancer patients. Eur J Cancer. 1997;33(6):867–72. doi: 10.1016/s0959-8049(96)00523-0. [DOI] [PubMed] [Google Scholar]
- 13.Pedersen N, Schmitt M, Ronne E, Nicoletti MI, Hoyer-Hansen G, Conese M, Giavazzi R, Dano K, Kuhn W, Janicke F, et al. A ligand-free, soluble urokinase receptor is present in the ascitic fluid from patients with ovarian cancer. The Journal of clinical investigation. 1993;92(5):2160–7. doi: 10.1172/JCI116817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stephens RW, Pedersen AN, Nielsen HJ, Hamers MJ, Hoyer-Hansen G, Ronne E, Dybkjaer E, Dano K, Brunner N. ELISA determination of soluble urokinase receptor in blood from healthy donors and cancer patients. Clin Chem. 1997;43(10):1868–76. [PubMed] [Google Scholar]
- 15.Stephens RW, Nielsen HJ, Christensen IJ, Thorlacius-Ussing O, Sørensen S, Danø K, Brünner N. Plasma urokinase receptor levels in patients with colorectal cancer: relationship to prognosis. Journal of the National Cancer Institute. 1999;91(10):869–74. doi: 10.1093/jnci/91.10.869. [DOI] [PubMed] [Google Scholar]
- 16.Foekens JA, Peters HA, Look MP, Portengen H, Schmitt M, Kramer MD, Brunner N, Janicke F, Meijer-van Gelder ME, Henzen-Logmans SC, van Putten WL, Klijn JG. The urokinase system of plasminogen activation and prognosis in 2780 breast cancer patients. Cancer research. 2000;60(3):636–43. [PubMed] [Google Scholar]
- 17.Mohanam S, Chintala SK, Mohan PM, Sawaya R, Lagos GK, Gokaslan ZL, Kouraklis GP, Rao JS. Increased invasion of neuroglioma cells transfected with urokinase plasminogen activator receptor cDNA. Int J Oncol. 1998;13(6):1285–90. doi: 10.3892/ijo.13.6.1285. [DOI] [PubMed] [Google Scholar]
- 18.Kariko K, Kuo A, Boyd D, Okada SS, Cines DB, Barnathan ES. Overexpression of urokinase receptor increases matrix invasion without altering cell migration in a human osteosarcoma cell line. Cancer Res. 1993;53(13):3109–17. [PubMed] [Google Scholar]
- 19.Lyu MA, Choi YK, Park BN, Kim BJ, Park IK, Hyun BH, Kook YH. Over-expression of urokinase receptor in human epidermoid-carcinoma cell line (HEp3) increases tumorigenicity on chorio-allantoic membrane and in severe-combined-immunodeficient mice. Int J Cancer. 1998;77(2):257–63. doi: 10.1002/(sici)1097-0215(19980717)77:2<257::aid-ijc15>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 20.Xing RH, Rabbani SA. Overexpression of urokinase receptor in breast cancer cells results in increased tumor invasion, growth and metastasis. Int J Cancer. 1996;67(3):423–9. doi: 10.1002/(SICI)1097-0215(19960729)67:3<423::AID-IJC18>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 21.Wilhelm O, Weidle U, Hohl S, Rettenberger P, Schmitt M, Graeff H. Recombinant soluble urokinase receptor as a scavenger for urokinase-type plasminogen activator (uPA). Inhibition of proliferation and invasion of human ovarian cancer cells. FEBS Lett. 1994;337(2):131–4. doi: 10.1016/0014-5793(94)80259-9. [DOI] [PubMed] [Google Scholar]
- 22.Festuccia C, Dolo V, Guerra F, Violini S, Muzi P, Pavan A, Bologna M. Plasminogen activator system modulates invasive capacity and proliferation in prostatic tumor cells. Clinical & experimental metastasis. 1998;16(6):513–28. doi: 10.1023/a:1006590217724. [DOI] [PubMed] [Google Scholar]
- 23.Min HY, Doyle LV, Vitt CR, Zandonella CL, Stratton-Thomas JR, Shuman MA, Rosenberg S. Urokinase receptor antagonists inhibit angiogenesis and primary tumor growth in syngeneic mice. Cancer Res. 1996;56(10):2428–33. [PubMed] [Google Scholar]
- 24.Bauer TW, Liu W, Fan F, Camp ER, Yang A, Somcio RJ, Bucana CD, Callahan J, Parry GC, Evans DB, Boyd DD, Mazar AP, Ellis LM. Targeting of urokinase plasminogen activator receptor in human pancreatic carcinoma cells inhibits c-Met- and insulin-like growth factor-I receptor-mediated migration and invasion and orthotopic tumor growth in mice. Cancer research. 2005;65(17):7775–81. doi: 10.1158/0008-5472.CAN-05-0946. [DOI] [PubMed] [Google Scholar]
- 25.Bugge TH, Suh TT, Flick MJ, Daugherty CC, Romer J, Solberg H, Ellis V, Dano K, Degen JL. The receptor for urokinase-type plasminogen activator is not essential for mouse development or fertility. The Journal of biological chemistry. 1995;270(28):16886–94. doi: 10.1074/jbc.270.28.16886. [DOI] [PubMed] [Google Scholar]
- 26.Stoppelli MP, Corti A, Soffientini A, Cassani G, Blasi F, Assoian RK. Differentiation-enhanced binding of the amino-terminal fragment of human urokinase plasminogen activator to a specific receptor on U937 monocytes. Proceedings of the National Academy of Sciences of the United States of America. 1985;82(15):4939–43. doi: 10.1073/pnas.82.15.4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vassalli JD, Baccino D, Belin D. A cellular binding site for the Mr 55,000 form of the human plasminogen activator, urokinase. The Journal of cell biology. 1985;100(1):86–92. doi: 10.1083/jcb.100.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nielsen LS, Kellerman GM, Behrendt N, Picone R, Danø K, Blasi F. A 55,000-60,000 Mr receptor protein for urokinase-type plasminogen activator. Identification in human tumor cell lines and partial purification. The Journal of biological chemistry. 1988;263(5):2358–63. [PubMed] [Google Scholar]
- 29.Pass J, Jögi A, Lund IK, Rønø B, Rasch MG, Gårdsvoll H, Lund LR, Ploug M, Rømer J, Danø K, Høyer-Hansen G. Murine monoclonal antibodies against murine uPA receptor produced in gene-deficient mice: Inhibitory effects on receptor mediated uPA activity in vitro and in vivo. Thrombosis and haemostasis. 2007;97(6):1013–22. [PubMed] [Google Scholar]
- 30.Quax PH, Lamfers ML, Lardenoye JH, Grimbergen JM, de Vries MR, Slomp J, de Ruiter MC, Kockx MM, Verheijen JH, van Hinsbergh VW. Adenoviral expression of a urokinase receptor-targeted protease inhibitor inhibits neointima formation in murine and human blood vessels. Circulation. 2001;103(4):562–9. doi: 10.1161/01.cir.103.4.562. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi H, Sugino D, She MY, Ohi H, Hirashima Y, Shinohara H, Fujie M, Shibata K, Terao T. A bifunctional hybrid molecule of the amino-terminal fragment of urokinase and domain II of bikunin efficiently inhibits tumor cell invasion and metastasis. European journal of biochemistry / FEBS. 1998;253(3):817–26. doi: 10.1046/j.1432-1327.1998.2530817.x. [DOI] [PubMed] [Google Scholar]
- 32.Muehlenweg B, Assfalg-Machleidt I, Parrado SG, Burgle M, Creutzburg S, Schmitt M, Auerswald EA, Machleidt W, Magdolen V. A novel type of bifunctional inhibitor directed against proteolytic activity and receptor/ligand interaction. Cystatin with a urokinase receptor binding site. The Journal of biological chemistry. 2000;275(43):33562–6. doi: 10.1074/jbc.C000383200. [DOI] [PubMed] [Google Scholar]
- 33.Goodson RJ, Doyle MV, Kaufman SE, Rosenberg S. High-affinity urokinase receptor antagonists identified with bacteriophage peptide display. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(15):7129–33. doi: 10.1073/pnas.91.15.7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jorgensen TJ, Gardsvoll H, Dano K, Roepstorff P, Ploug M. Dynamics of urokinase receptor interaction with Peptide antagonists studied by amide hydrogen exchange and mass spectrometry. Biochemistry. 2004;43(47):15044–57. doi: 10.1021/bi048706j. [DOI] [PubMed] [Google Scholar]
- 35.Ploug M, Ostergaard S, Gardsvoll H, Kovalski K, Holst-Hansen C, Holm A, Ossowski L, Dano K. Peptide-derived antagonists of the urokinase receptor. affinity maturation by combinatorial chemistry, identification of functional epitopes, and inhibitory effect on cancer cell intravasation. Biochemistry. 2001;40(40):12157–68. doi: 10.1021/bi010662g. [DOI] [PubMed] [Google Scholar]
- 36.Burgle M, Koppitz M, Riemer C, Kessler H, Konig B, Weidle UH, Kellermann J, Lottspeich F, Graeff H, Schmitt M, Goretzki L, Reuning U, Wilhelm O, Magdolen V. Inhibition of the interaction of urokinase-type plasminogen activator (uPA) with its receptor (uPAR) by synthetic peptides. Biological chemistry. 1997;378(34):231–7. doi: 10.1515/bchm.1997.378.3-4.231. [DOI] [PubMed] [Google Scholar]
- 37.Magdolen V, Burgle M, de Prada NA, Schmiedeberg N, Riemer C, Schroeck F, Kellermann J, Degitz K, Wilhelm OG, Schmitt M, Kessler H. Cyclo19,31[D-Cys19]-uPA19-31 is a potent competitive antagonist of the interaction of urokinase-type plasminogen activator with its receptor (CD87) Biological chemistry. 2001;382(8):1197–205. doi: 10.1515/BC.2001.150. [DOI] [PubMed] [Google Scholar]
- 38.Schmiedeberg N, Schmitt M, Rolz C, Truffault V, Sukopp M, Burgle M, Wilhelm OG, Schmalix W, Magdolen V, Kessler H. Synthesis, solution structure, and biological evaluation of urokinase type plasminogen activator (uPA)-derived receptor binding domain mimetics. J Med Chem. 2002;45(23):4984–94. doi: 10.1021/jm020254q. [DOI] [PubMed] [Google Scholar]
- 39.Rosenberg S. New developments in the urokinase-type plasminogen activator system. Expert Opin Ther Targets. 2001;5(6):711–722. doi: 10.1517/14728222.5.6.711. [DOI] [PubMed] [Google Scholar]
- 40.Ploug M, Ostergaard S, Hansen LB, Holm A, Dano K. Photoaffinity labeling of the human receptor for urokinase-type plasminogen activator using a decapeptide antagonist. Evidence for a composite ligand-binding site and a short interdomain separation. Biochemistry. 1998;37(11):3612–22. doi: 10.1021/bi972787k. [DOI] [PubMed] [Google Scholar]
- 41.Knor S, Sato S, Huber T, Morgenstern A, Bruchertseifer F, Schmitt M, Kessler H, Senekowitsch-Schmidtke R, Magdolen V, Seidl C. Development and evaluation of peptidic ligands targeting tumour-associated urokinase plasminogen activator receptor (uPAR) for use in alpha-emitter therapy for disseminated ovarian cancer. European journal of nuclear medicine and molecular imaging. 2008;35(1):53–64. doi: 10.1007/s00259-007-0582-3. [DOI] [PubMed] [Google Scholar]
- 42.Sato S, Kopitz C, Schmalix WA, Muehlenweg B, Kessler H, Schmitt M, Kruger A, Magdolen V. High-affinity urokinase-derived cyclic peptides inhibiting urokinase/urokinase receptor-interaction: effects on tumor growth and spread. FEBS Lett. 2002;528(13):212–6. doi: 10.1016/s0014-5793(02)03311-2. [DOI] [PubMed] [Google Scholar]
- 43.Ploug M, Ronne E, Behrendt N, Jensen AL, Blasi F, Dano K. Cellular receptor for urokinase plasminogen activator. Carboxyl-terminal processing and membrane anchoring by glycosyl-phosphatidylinositol. The Journal of biological chemistry. 1991;266(3):1926–33. [PubMed] [Google Scholar]
- 44.Llinas P, Le Du MH, Gardsvoll H, Dano K, Ploug M, Gilquin B, Stura EA, Menez A. Crystal structure of the human urokinase plasminogen activator receptor bound to an antagonist peptide. Embo J. 2005;24(9):1655–63. doi: 10.1038/sj.emboj.7600635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Engelholm LH, Behrendt N. Differential binding of urokinase and peptide antagonists to the urokinase receptor: evidence from characterization of the receptor in four primate species. Biological chemistry. 2001;382(3):435–42. doi: 10.1515/BC.2001.053. [DOI] [PubMed] [Google Scholar]
- 46.Wei Y, Waltz DA, Rao N, Drummond RJ, Rosenberg S, Chapman HA. Identification of the Urokinase Receptor as All Adhesion Receptor for Vitronectin. Journal of Biological Chemistry. 1994;269(51):32380–32388. [PubMed] [Google Scholar]
- 47.Huai Q, Zhou A, Lin L, Mazar AP, Parry GC, Callahan J, Shaw DE, Furie B, Furie BC, Huang M. Crystal structures of two human vitronectin, urokinase and urokinase receptor complexes. Nature structural & molecular biology. 2008;15(4):422–3. doi: 10.1038/nsmb.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gardsvoll H, Ploug M. Mapping of the vitronectin-binding site on the urokinase receptor: involvement of a coherent receptor interface consisting of residues from both domain I and the flanking interdomain linker region. The Journal of biological chemistry. 2007;282(18):13561–72. doi: 10.1074/jbc.M610184200. [DOI] [PubMed] [Google Scholar]
- 49.Huai Q, Mazar AP, Kuo A, Parry GC, Shaw DE, Callahan J, Li Y, Yuan C, Bian C, Chen L, Furie B, Furie BC, Cines DB, Huang M. Structure of human urokinase plasminogen activator in complex with its receptor. Science. 2006;311(5761):656–9. doi: 10.1126/science.1121143. [DOI] [PubMed] [Google Scholar]
- 50.Huang MD, Mazar AP, Parry G, Higazi AA, Kuo A, Cines DB. Crystallization of soluble urokinase receptor (suPAR) in complex with urokinase amino-terminal fragment (1-143) Acta Crystallographica Section D-Biological Crystallography. 2005;61:697–700. doi: 10.1107/S0907444905014174. [DOI] [PubMed] [Google Scholar]
- 51.Yuan C, Huai Q, Bian CB, Huang M. The expression, purification and crystallization of monomeric soluble human urokinase receptor. Progress in Biochemistry and Biophysics. 2006;33:277–281. [Google Scholar]
- 52.Yuan C, Huang M. Does the urokinase receptor exist in a latent form? Cell Mol Life Sci. 2007;64(9):1033–7. doi: 10.1007/s00018-007-6498-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oprea TI, Bologa CG, Edwards BS, Prossnitz ER, Sklar LA. Post-high-throughput screening analysis: an empirical compound prioritization scheme. J Biomol Screen. 2005;10(5):419–26. doi: 10.1177/1087057104272660. [DOI] [PubMed] [Google Scholar]
- 54.Hann MM, Oprea TI. Pursuing the leadlikeness concept in pharmaceutical research. Curr Opin Chem Biol. 2004;8(3):255–63. doi: 10.1016/j.cbpa.2004.04.003. [DOI] [PubMed] [Google Scholar]