Abstract
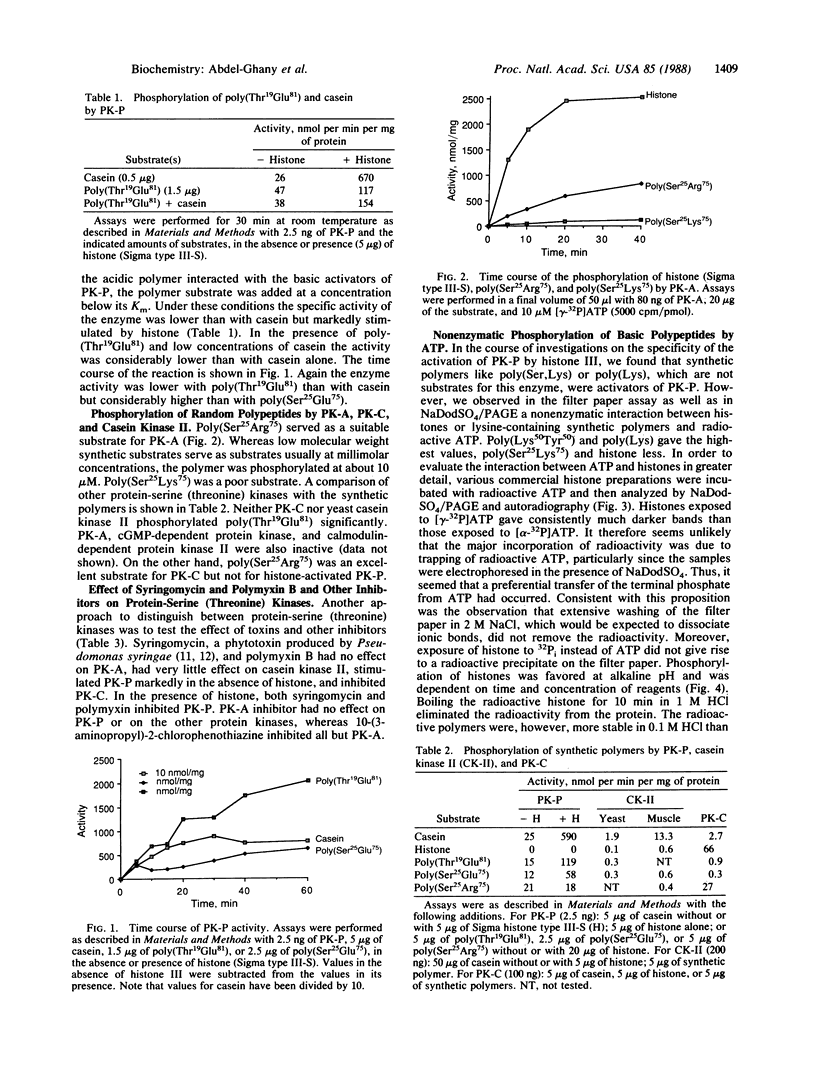
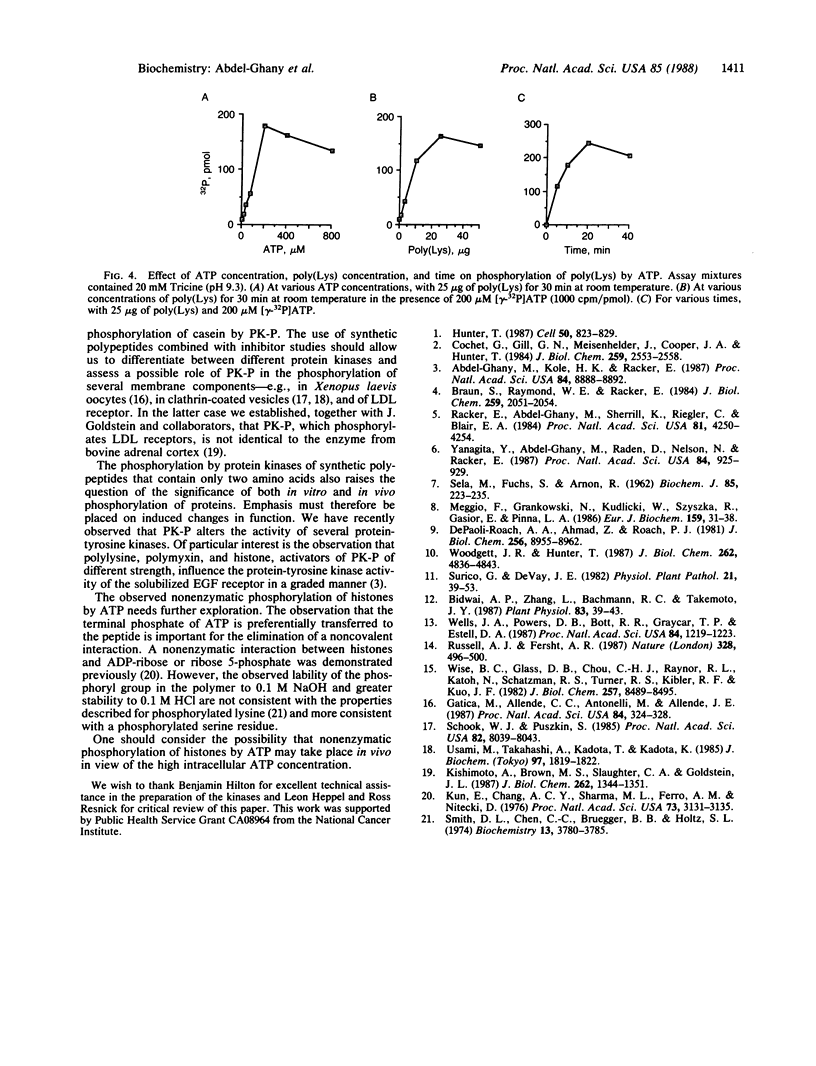
A synthetic random polymer of threonine and glutamate (1:4.4) is readily phosphorylated by protein kinase P but not by five other protein-serine (threonine) kinases. A synthetic random polymer of serine and arginine (1:3) is readily phosphorylated by protein kinase A and protein kinase C but not by protein kinase P. Although the amino acid sequences surrounding the phosphorylated serine (threonine) residue have been demonstrated in studies with small synthetic polypeptides to be decisive factors in the rate at which they are phosphorylated, the findings with the large synthetic polypeptides suggest that in the case of proteins the size, the tertiary structure, and particularly the electrostatic interactions are equally or more important contributing factors. Syringomycin, a toxin from Pseudomonas syringae, and polymyxin B, from Bacillus polymyxa, stimulate protein kinase P, strongly inhibit protein kinase C, and have no effect on protein kinase A. Basic polypeptides with high lysine content are phosphorylated by ATP nonenzymatically.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Ghany M., Kole H. K., Racker E. Effect of protein kinase P on phosphorylations catalyzed by the epidermal growth factor. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8888–8892. doi: 10.1073/pnas.84.24.8888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidwai A. P., Zhang L., Bachmann R. C., Takemoto J. Y. Mechanism of Action of Pseudomonas syringae Phytotoxin, Syringomycin : Stimulation of Red Beet Plasma Membrane ATPase Activity. Plant Physiol. 1987 Jan;83(1):39–43. doi: 10.1104/pp.83.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun S., Raymond W. E., Racker E. Synthetic tyrosine polymers as substrates and inhibitors of tyrosine-specific protein kinases. J Biol Chem. 1984 Feb 25;259(4):2051–2054. [PubMed] [Google Scholar]
- Cochet C., Gill G. N., Meisenhelder J., Cooper J. A., Hunter T. C-kinase phosphorylates the epidermal growth factor receptor and reduces its epidermal growth factor-stimulated tyrosine protein kinase activity. J Biol Chem. 1984 Feb 25;259(4):2553–2558. [PubMed] [Google Scholar]
- DePaoli-Roach A. A., Ahmad Z., Roach P. J. Characterization of a rabbit skeletal muscle protein kinase (PC0.7) able to phosphorylate glycogen synthase and phosvitin. J Biol Chem. 1981 Sep 10;256(17):8955–8962. [PubMed] [Google Scholar]
- Gatica M., Allende C. C., Antonelli M., Allende J. E. Polylysine-containing peptides, including the carboxyl-terminal segment of the human c-Ki-ras 2 protein, affect the activity of some key membrane enzymes. Proc Natl Acad Sci U S A. 1987 Jan;84(2):324–328. doi: 10.1073/pnas.84.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. A thousand and one protein kinases. Cell. 1987 Sep 11;50(6):823–829. doi: 10.1016/0092-8674(87)90509-5. [DOI] [PubMed] [Google Scholar]
- Kishimoto A., Brown M. S., Slaughter C. A., Goldstein J. L. Phosphorylation of serine 833 in cytoplasmic domain of low density lipoprotein receptor by a high molecular weight enzyme resembling casein kinase II. J Biol Chem. 1987 Jan 25;262(3):1344–1351. [PubMed] [Google Scholar]
- Kun E., Chang A. C., Sharma M. L., Ferro A. M., Nitecki D. Covalent modification of proteins by metabolites of NAD+. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3131–3135. doi: 10.1073/pnas.73.9.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meggio F., Grankowski N., Kudlicki W., Szyszka R., Gasior E., Pinna L. A. Structure and properties of casein kinase-2 from Saccharomyces cerevisiae. A comparison with the liver enzyme. Eur J Biochem. 1986 Aug 15;159(1):31–38. doi: 10.1111/j.1432-1033.1986.tb09829.x. [DOI] [PubMed] [Google Scholar]
- Racker E., Abdel-Ghany M., Sherrill K., Riegler C., Blair E. A. New protein kinase from plasma membrane of Ehrlich ascites tumor cells activated by natural polypeptides. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4250–4254. doi: 10.1073/pnas.81.14.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell A. J., Fersht A. R. Rational modification of enzyme catalysis by engineering surface charge. Nature. 1987 Aug 6;328(6130):496–500. doi: 10.1038/328496a0. [DOI] [PubMed] [Google Scholar]
- SELA M., FUCHS S., ARNON R. Studies on the chemical basis of the antigenicity of proteins. 5. Synthesis, characterization and immunogenicity of some multichain and linear polypeptides containing tyrosine. Biochem J. 1962 Oct;85:223–235. doi: 10.1042/bj0850223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schook W. J., Puszkin S. Brain clathrin light chain 2 can be phosphorylated by a coated vesicle kinase. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8039–8043. doi: 10.1073/pnas.82.23.8039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. L., Chen C. C., Bruegger B. B., Holtz S. L., Halpern R. M., Smith R. A. Characterization of protein kinases forming acid-labile histone phosphates in Walker-256 carcinosarcoma cell nuclei. Biochemistry. 1974 Aug 27;13(18):3780–3785. doi: 10.1021/bi00715a025. [DOI] [PubMed] [Google Scholar]
- Usami M., Takahashi A., Kadota T., Katoda K. Phosphorylation of a clathrin light chain of coated vesicles in the presence of histones. J Biochem. 1985 Jun;97(6):1819–1822. doi: 10.1093/oxfordjournals.jbchem.a135243. [DOI] [PubMed] [Google Scholar]
- Wells J. A., Powers D. B., Bott R. R., Graycar T. P., Estell D. A. Designing substrate specificity by protein engineering of electrostatic interactions. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1219–1223. doi: 10.1073/pnas.84.5.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise B. C., Glass D. B., Chou C. H., Raynor R. L., Katoh N., Schatzman R. C., Turner R. S., Kibler R. F., Kuo J. F. Phospholipid-sensitive Ca2+-dependent protein kinase from heart. II. Substrate specificity and inhibition by various agents. J Biol Chem. 1982 Jul 25;257(14):8489–8495. [PubMed] [Google Scholar]
- Woodgett J. R., Hunter T. Isolation and characterization of two distinct forms of protein kinase C. J Biol Chem. 1987 Apr 5;262(10):4836–4843. [PubMed] [Google Scholar]
- Yanagita Y., Abdel-Ghany M., Raden D., Nelson N., Racker E. Polypeptide-dependent protein kinase from bakers' yeast. Proc Natl Acad Sci U S A. 1987 Feb;84(4):925–929. doi: 10.1073/pnas.84.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]




