Abstract
Spasmodic dysphonia (SD) is a primary focal dystonia of unknown pathophysiology, characterized by involuntary spasms in the laryngeal muscles during speech production. We examined two rare cases of postmortem brainstem tissue from SD patients compared to four controls. In SD patients, small clusters of inflammation were found in the reticular formation surrounding solitary tract, spinal trigeminal and ambigual nuclei, inferior olive and pyramids. Mild neuronal degeneration and depigmentation were observed in the substantia nigra and locus coeruleus. No abnormal protein accumulations and no demyelination or axonal degeneration were found. These neuropathological findings may provide insights into the pathophysiology of SD.
Introduction
Spasmodic dysphonia (SD) is a primary focal dystonia, most frequently characterized by involuntary spasms in the closing muscles of the vocal folds during speech production. SD symptoms usually appear in mid-life, progress gradually and remain chronic for life. The neuropathological bases of SD and other forms of primary focal dystonia are poorly understood, partly, due to a rare availability of the postmortem tissue from these patients. Only few studies have been, to date, conducted to examine the neuropathological abnormalities in a limited number of dystonic patients. Neuropathological case reports in patients with Meige’s syndrome1-3, cranial and cervical dystonia4 have found a mild neuronal loss in the substantia nigra, locus coeruleus, dorsal raphe nucleus, tectum and dentate nucleus1,3 and infrequent Lewy bodies in the substantia nigra4, nucleus basalis of Meynert and nucleus ambiguus1,2. We recently found focal axonal degeneration and demyelination in the genu of the internal capsule and clusters of mineral accumulations, containing calcium, phosphorus and iron, in the internal capsule, lentiform nucleus and cerebellum in one SD patient5. However, it remains unknown whether the brainstem neuropathological changes are also present in SD, although several neurophysiological studies have pointed to brainstem abnormalities in these patients6,7.
As a continuation of our previous study5, in this case report we examined rarely available postmortem brainstem tissue from two SD patients compared to four controls to identify brainstem changes in SD patients, which may contribute to the pathophysiology of this disorder.
Materials and Methods
Postmortem tissue of the brainstem was obtained from two SD patients (Case 1 - female, 85 y.o.; Case 2 - male, 65 y.o.; mean age 70.5 years) and four controls (mean age 68.5 years, age range 50-85 years, 3 females/1 male). Antemortem, both patients were diagnosed with adductor type of SD presented with moderate to severe voice breaks due to spasms in the closing muscles of the vocal folds during vowel production. Case 1 had also adductor vocal tremor during speech production. The onset of the disorder was at age of 56 and 36 years, respectively. Both patients were diagnosed and treated at the National Institute of Neurological Disorders and Stroke with botulinum toxin injections into their laryngeal muscles at regular intervals for several years. Clinical characteristics of patients and controls are summarized in Table 1A. The male patient’s (Case 2) neuropathological findings in the supramedullar brain regions were reported in a previous study5. We did not have an access to the supramedullar brain tissue from the female patient (Case 1); only brainstem tissue of the Case 1 was available to us for neuropathological examination. The study was approved by the Institutional Review Board of the National Institute of Neurological Disorders and Stroke, National Institutes of Health.
Table 1.
Clinico-neuropathological characteristics
| (A) | |||||
|---|---|---|---|---|---|
| Case | Gender | Diagnosis | Age at onset |
Age at death |
Cause of death |
| 1 | F | ADSD, vocal tremor |
56 | 85 | left temporal lobe infarct* |
| 2 | M | ADSD | 36 | 65 | complications during cardiac bypass surgery |
| 3 | M | Control | n/a | 50 | diffuse pulmonary hemorrhage |
| 4 | F | Control | n/a | 58 | disseminated intravascular coagulation |
| 5 | F | Control | n/a | 81 | respiratory failure due to small-cell lung cancer |
| 6 | F | Control | n/a | 85 | dehydration with hypernatremia |
| (B) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case | Cell morphology |
Neuronal degeneration |
Depigmentation | Demyelination | Axonal degeneration |
Microglia/ macrophage activation |
Leukocyte/ lymphocyte activation |
α- synuclein pathology |
Tau pathology |
Ubiquitin pathology |
| 1 ADSD |
Normal | SNpc ++ LC + |
SNpc + LC ++ |
None | None | Sol ++ Sp5 + NA + RF ++ IO ++ Pyr ++ |
None | None | None | None |
| 2 ADSD |
Normal | SNpc + LC + |
SNpc + LC + |
None | None | Sol + Sp5 + RF ++ Pyr ++ |
None | None | None | None |
The left temporal lobe infarct had most remote rostral location and, therefore, did not involve brain regions known to control voice and speech production. Hence, the cause of death in this patient should not have affected the findings of neuropathological changes in the brainstem.
SNpc - substantial nigra, pars compacta; LC — locus coeruleus; Sol — solitary tract nucleus; Sp5 — spinal trigeminal nucleus; NA — nucleus ambiguus; RF — reticular formation; IO- inferior olive; Pyr — pyramids; n/a — not applicable; + mild; ++ moderate.
Postmortem brainstem tissue from all subjects was fixed in 10% formalin solution, sectioned transversely into 6 blocks (thickness 1 cm) for gross examination and then embedded in paraffin. For histopathological examination, each block was sectioned into consequent 10 paraffin sections at 5 μm and stained with hematoxylin and eosin (H&E), luxol-fast blue/periodic acid Schiff (LFB/PAS) and Bielschowski silver stains to assess changes in cell morphology, myelin, and axonal course, respectively. Additionally, subsequent sections were stained with mouse monoclonal antibodies to human microglia/macrophage lineage (1:100, CD68, Clone KP1, Dako, Carpinteria, CA), human leukocyte/lymphocyte lineage (1:400, CD45, Leucocyte Common Antigen, Clone 2B11+PD7/26, Dako, Carpinteria, CA), alpha-synuclein (1:100, Oncogene Research Products, San Diego, CA), tau (1:1500, Clone Tau-2, Chemicon International Inc., CA), and ubiquitin (1:100, Novocastra). Primary antibodies were omitted for negative controls. For reaction product visualization, sections were incubated with a refined avidin-biotin kit (LSAB+ System-HRP, Dako, Carpinteria, CA). Stained sections were evaluated without prior knowledge of the subjects’ identity (blinded) using light microscopy (Olympus BX51, Olympus Inc., Japan). Abnormalities were defined as mild, moderate, severe, or none. Mild abnormalities were defined as occasional yet definitive pathological changes, i.e. degeneration of single cells, in the well-preserved neuroanatomical region. Moderate abnormalities were defined as significant alterations, encompassing 10 to 50% of the affected brain region. Severe abnormalities were defined as total or subtotal destruction of a specific neuroanatomical region of interest.
Results
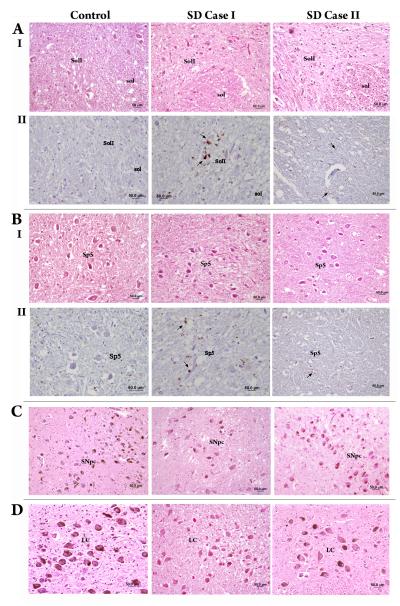
Gross neuropathological examination of the brainstem revealed no abnormalities in either patients or controls. Detailed microscopic examination was performed to investigate the multiple levels of the brainstem nuclei involved in the sensorimotor control of voice production. Normal morphology of the lower brainstem nuclei was identified in both patients and controls (Fig. 1AI,BI). No accumulations of abnormal proteins, such as tau, ubiquitin, or alpha-synuclein, and no signs of demyelination or axonal degeneration were found in either SD patients or controls. However, in both SD cases, small clusters of microglia/macrophages activation were found in the reticular formation surrounding the solitary tract and spinal trigeminal nuclei and in the pyramids (Fig. 1AII,BII). In SD Case 1, these clusters were also observed in the reticular formation around the nucleus ambiguus and in the inferior olive. Mild neuronal degeneration and depigmentation were observed in the pars compact of the substantia nigra and the locus coeruleus in both SD cases (Fig. 1C,D). These brainstem changes were predominant in SD Case 1, present in SD Case 2 and not found in any of the controls (Table 1B).
Figure 1.
Microphotographs of the brainstem regions in the controls and both SD Cases show normal cell morphology in the interstitial part of the solitary tract nucleus (SolI) (AI) and the spinal trigeminal nucleus (Sp5) (BI) (H&E stain); clusters of microglial/macrophage activation (arrows) in the reticular formation surrounding the SolI (AII) and Sp5 (BII) (CD68/KP1 immunostain); and mild neuronal degeneration and depigmentation in the substantial nigra, pars compacta (C) and the locus coeruleus (D) (H&E stain), respectively.
Discussion
The major findings of the present case study are (1) the absence of significant cellular morphology changes in the brainstem neuronal populations, (2) the presence of small clusters of microglia/macrophages activation in the reticular formation surrounding the lower brainstem nuclei involved in the control of laryngeal functions, and (3) mild neuronal degeneration and depigmentation in the substantia nigra and the locus coeruleus in SD patients compared to controls.
The findings of normal cell morphology and the absence of abnormal protein accumulations, such as tau, ubiquitin, and alpha-synuclein, in the brainstem nuclei of SD patients are in line with neuropathological reports in some other forms of primary focal dystonia1-3, 4. In contrast, ubiquitin-positive perinuclear inclusion bodies were found in the midbrain reticular formation, periaqueductal gray, pedunculopontine and cuneiform nuclei of patients with DYT1 dystonia8, suggesting the possibility of different neuropathological processes underlying the pathophysiology of focal and generalized primary dystonias.
The presence of small clusters of microglia/macrophages activation in the brainstem reticular formation of SD patients may represent a disorder-specific neuropathological process, because of their location in the vicinity of the nuclei responsible for sensory (solitary tract and spinal trigeminal nuclei) and motor (nucleus ambiguus) control of voice production. Upregulation of microglia/macrophages in the brain tissue is known to be associated with a large range of neurological pathologies, from trauma to autoimmune conditions. The presence of activated microglia as clusters of focal inflammation is usually a common finding in neurodegenerative disorders, such as Parkinson’s and Alzheimer’s diseases9. However, little knowledge is, to date, available about the molecular processes underlying microglial activation and the exact biological consequences that may result from their upregulation within CNS tissue10. In the present case, we suggest two possibilities for the presence of focal microglia/macrophages activation in the brainstem of SD patients. Based on the previous findings of neurophysiological brainstem abnormalities in SD6,7, clusters of microglia/macrophages activation may represent loci of focal brainstem inflammation that alter sensorimotor processing at the level of the brainstem and modulate the ascending inputs to the supramedullar regions controlling voice and speech production in the SD patients. When compared to our previous findings of demyelination and axonal degeneration in the right genu of the internal capsule5, the brainstem clusters of focal inflammation may represent an independent finding, both of which, in turn, may collectively contribute to the pathophysiology of SD.
Alternatively, based on the location of microglia/macrophage clusters around the brainstem nuclei involved in the laryngeal control and the pyramids, their focal activation may reflect reactive changes surrounding the terminals of degenerative nerve fibers descending within the corticobulbar tract from the laryngeal motor cortex through the genu of the internal capsule to the brainstem nuclei11 in these patients. The fact that we did not find axonal degeneration and demyelination in the brainstem of SD patients in the present study may be explained by very sparse distribution of these descending fibers in the brainstem and by relative inferiority of conventional histological methods to stains for myelin and axonal course compared to more advanced immunohistochemical methods using KP1(CD68) marker. However, our findings of clusters of microglia/macrophage activation in the pyramids in both SD patients substantiate the recent observation of focal axonal degeneration and demyelination within the corticobulbar tract at the level of the genu of the internal capsule5 in the same male patient reported here and suggest that reactive changes in the brainstem nuclei in SD patients may reflect the neuropathological abnormalities descending from the higher brain structures.
Other neuropathological findings in the brainstem of both SD patients included mild neuronal degeneration and depigmentation of the pars compact of the substantia nigra and the locus coeruleus. Although these changes may be due to a normal aging process, they are also similar to those reported in patients with idiopathic cervical dystonia3, Meige’s syndrome1 and torsion dystonia12. Hence, the involvement of the substantia nigra and the locus coeruleus may represent a common neuropathogenic process in both early- and late-onset primary dystonias.
In conclusion, subtle abnormalities in the brainstem of SD patients, such as clusters of microglia/macrophage activation in the reticular formation surrounding the lower brainstem nuclei and mild degeneration and depigmentation in the substantia nigra and the locus coeruleus, most likely represent disorder-specific abnormalities secondary to the changes in the supramedullar regions in this disorder. Future studies will require access to a larger number of postmortem brain specimens from SD patients for statistical analysis between patients and controls to elucidate the significance of subtle abnormalities between the groups and to identify the direct relationships between the brainstem and supramedullar abnormalities in this disorder.
Acknowledgment
We are grateful to Viktoria Baker, Laboratory of Pathology, NCI, for histology preparations, and to Dr. Mark Raffeld and Cynthia Harris, Molecular Pathology Section, NCI, for immunohistochemistry preparations. Tissue specimens were obtained from the Human Brain and Spinal Fluid Resource Center, VAMC, Los Angeles, CA, sponsored by NINDS/NIMH, National Multiple Sclerosis Society, VA Greater Los Angeles Healthcare System and Veterans Health Services and Research Administration, Department of Veterans Affairs.
Supported by the Intramural Program of the National Institute of Neurological Disorders and Stroke, NIH
Footnotes
Statement of the conflict: The authors report no conflict of interest
Financial disclosure: None
Presented at the Annual Meeting of the American Academy of Otolaryngology-Head and Neck Surgery, Los Angeles, CA, September 25-28, 2005
References
- 1.Kulisevsky J, Marti MJ, Ferrer I, Tolosa E. Meige syndrome: neuropathology of a case. Mov Disord. 1988;3:170–175. doi: 10.1002/mds.870030209. [DOI] [PubMed] [Google Scholar]
- 2.Mark MH, Sage JI, Dickson DW, et al. Meige syndrome in the spectrum of Lewy body disease. Neurology. 1994;44:1432–1436. doi: 10.1212/wnl.44.8.1432. [DOI] [PubMed] [Google Scholar]
- 3.Zweig RM, Hedreen JC. Brain stem pathology in cranial dystonia. Adv Neurol. 1988;49:395–407. [PubMed] [Google Scholar]
- 4.Holton JL, Schneider SA, Ganesharajah T, et al. Neuropathology of primary adult-onset dystonia. Neurology. 2008;70:695–699. doi: 10.1212/01.wnl.0000302175.76229.f0. [DOI] [PubMed] [Google Scholar]
- 5.Simonyan K, Tovar-Moll F, Ostuni J, et al. Focal white matter changes in spasmodic dysphonia: a combined diffusion tensor imaging and neuropathological study. Brain. 2008;131:447–459. doi: 10.1093/brain/awm303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deleyiannis FW, Gillespie M, Bielamowicz S, Yamashita T, Ludlow CL. Laryngeal long latency response conditioning in abductor spasmodic dysphonia. Ann Otol Rhinol Laryngol. 1999;108:612–619. doi: 10.1177/000348949910800615. [DOI] [PubMed] [Google Scholar]
- 7.Ludlow CL, Schulz GM, Yamashita T, Deleyiannis FW. Abnormalities in long latency responses to superior laryngeal nerve stimulation in adductor spasmodic dysphonia. Ann Otol Rhinol Laryngol. 1995;104:928–935. doi: 10.1177/000348949510401203. [DOI] [PubMed] [Google Scholar]
- 8.McNaught KS, Kapustin A, Jackson T, et al. Brainstem pathology in DYT1 primary torsion dystonia. Ann Neurol. 2004;56:540–547. doi: 10.1002/ana.20225. [DOI] [PubMed] [Google Scholar]
- 9.McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- 10.Croisier E, Moran LB, Dexter DT, Pearce RK, Graeber MB. Microglial inflammation in the parkinsonian substantia nigra: relationship to alpha-synuclein deposition. J Neuroinflammation. 2005;2:14. doi: 10.1186/1742-2094-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jurgens U. Neural pathways underlying vocal control. Neurosci Biobehav Rev. 2002;26:235–258. doi: 10.1016/s0149-7634(01)00068-9. [DOI] [PubMed] [Google Scholar]
- 12.Zweig RM, Hedreen JC, Jankel WR, Casanova MF, Whitehouse PJ, Price DL. Pathology in brainstem regions of individuals with primary dystonia. Neurology. 1988;38:702–706. doi: 10.1212/wnl.38.5.702. [DOI] [PubMed] [Google Scholar]