Abstract
Targeted gene disruption or expression often encounters lethality. Conditional approaches, permitting manipulation at desired stages, are required to overcome this problem in order to analyze gene function in later developmental processes. Wnt1 has been shown to be expressed in neural crest precursors at the dorsal midline region. However, its expression was not detected in emigrated neural crest cells, the descendants of Wnt1-expressing precursors. We have developed mouse transgenic systems to manipulate gene activity in the Wnt1-expressing precursors and their derivatives by integrating the tetracycline-dependent activation and Cre-mediated recombination methods. A new Wnt1-rtTA strain, carrying rtTA under control of Wnt1 regulatory elements, has been created for gene manipulation in a spatiotemporal-specific fashion. Together with our previously developed Wnt1-Cre/R26STOPrtTA model, these systems permit conditional gene expression and ablation in pre-migratory and/or post-migratory neural crest cells. This study demonstrated the versatility of our mouse models to achieve gene manipulation in early neural development.
Keywords: Wnt, rtTA, Cre, gene expression and deletion, neural development, mouse genetics
Introduction
Craniofacial morphogenesis is regulated by complex interactions between the surface and neural ectoderms, endoderm, paraxial mesoderm and cranial neural crest (CNC) (Francis-West et al., 1998). This process is highly dependent on the patterning information of emigrant CNC cells (Kontges and Lumsden, 1996; Le Douarin et al., 2007). During early embryogenesis, the precursors arise in the lateral margin of the neural fold at the boundary between surface and neural ectoderm (LaBonne and Bronner-Fraser, 1999; Steventon et al., 2005; Crane and Trainor, 2006; Delfino-Machin et al., 2007). Upon closure of the neural tube, these cells migrate ventrolaterally to populate the head and neck regions (Kuriyama and Mayor, 2008). The migration process requires CNC cells to undergo epithelial-mesenchymal transition (EMT), which is also a key step in tumor progression toward metastasis (Levayer and Lecuit, 2008). CNC cells give rise to a variety of facial tissues and structures, including nerves, ganglia, connective tissues, cartilages and bones (Le Douarin and Kalcheim, 1999). As a result, the majority of craniofacial deformities are caused by deficiencies in CNC. Elucidating the mechanism underlying CNC development is essential to gain important insights into the mechanism underlying EMT in cancer metastasis and the molecular basis of congenital diseases.
Using genetic analysis in mice, genes or gene families important for craniofacial morphogenesis have been identified. Mice with mutations of these genes often exhibit severe craniofacial deformities. Affected animals show defects in the development of facial tissues and structures, suggesting the mutated genes might be associated with development of CNC. However, relatively little is known about the mechanism underlying CNC development. The development of experimental systems capable of analyzing the morphogenetic regulatory network during formation, migration and differentiation of CNC cells will greatly advance our knowledge base of these developmental processes.
The evolutionary conserved Wnt signaling pathway is critical for development of the neural crest (Yanfeng et al., 2003; Raible and Ragland, 2005). Members of the Wnt family are expressed in the developing brain and neural tube (Parr et al., 1993). Wnt1 and Wnt3a are both expressed in the dorsolateral region of the neural tube that gives rise to CNC. The expression of Wnt1 is restricted to the midbrain whereas Wnt3a is found in both mid/hindbrain regions (McMahon et al., 1992). Each signal is essential for embryonic development, Wnt1 for midbrain patterning (McMahon et al., 1992) and Wnt3a for the formation of somite, tailbud and hippocampus (Takada et al., 1994; Greco et al., 1996; Lee et al., 2000). However, inactivation of either the mouse Wnt1 or Wnt3a gene did not cause defects in craniofacial development (McMahon and Bradley, 1990; Takada et al., 1994). Mice in which both the Wnt1 and Wnt3a genes have been eliminated showed a marked deficiency in CNC derivatives that originate from the dorsal neural tube (Ikeya et al., 1997). Wnt signaling appears to regulate the expansion of CNC progenitors. Later, this was confirmed by lineage tracing analysis using a genetic labeling technique (Chai et al., 2000; Jiang et al., 2000). In mice carrying both the Wnt1-Cre transgene (Danielian et al., 1998) and R26RlacZ reporter allele (Soriano, 1999), the lacZ gene is expressed in CNC derivatives in addition to the region where Wnt1 is normally expressed. These data indicate that Wnt1 expressing cells from the dorsal neural tube migrate to craniofacial regions where Wnt1 expression is then terminated. The nature of this Wnt1 expression pattern during CNC development permits creation of experimental systems to manipulate gene activity in pre-migratory and/or post-migratory cells. By integrating the Cre-mediated recombination and tetracycline-dependent activation methods, the present study demonstrates the successful establishment of such advanced models for gene manipulation in mice.
Results
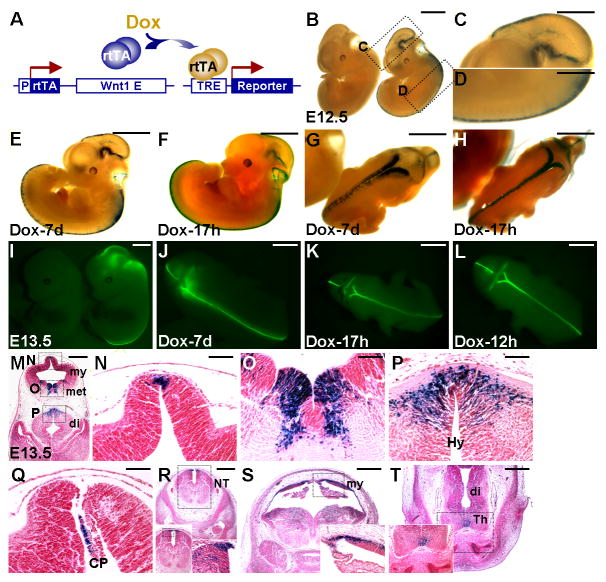
For conditional gene expression, the tetracycline-dependent activation system has been widely used that requires three essential components. First is the transcription factor tTA or rtTA, which can be expressed in a spatial-specific manner. Second, the tetracycline response elements (TRE) are used to control the expression of target genes. The presence of the third component, doxycycline (Dox), administered at a desired time, activates target gene expression in a temporal-specific fashion. To develop a mouse model permitting manipulation of gene activity in CNC precursors, we generated a new Wnt1-rtTA transgenic strain. An improved version of rtTA, rtTA2s-M2 (Urlinger et al., 2000), was inserted into a Wnt1 expression vector, containing its promoter and enhancer (Figure 1A). Mice carrying the Wnt1-rtTA transgene were generated by injecting the expression construct. To test if Wnt1-rtTA strain provides a tool for conditional gene expression in the developing embryo, mice carrying the Wnt1-rtTA transgene were crossed with TRE-lacZ mice (Yu et al., 2005b; Yu et al., 2007), where the expression of lacZ is under control of TRE, to obtain double transgenic animals (ratio: 1/4). Their pregnant mother was treated with Dox for 7 days to induce expression of the lacZ reporter in the E12.5 embryos (Figure 1B–D). Compared with the endogenous Wnt1 expression pattern (McMahon and Bradley, 1990) and that of the Wnt1-lacZ transgenic animal (Danielian and McMahon, 1996) reported previously, the results showed that expression of the lacZ reporter was successfully targeted to the dorsal midline of the developing brain and neural tube during embryogenesis (Figure 1B–D). Similar expression patterns were obtained when the Wnt1-rtTA transgene was crossed into reporter mice carrying TRE-H2BGFP, a transgenic line containing TRE to regulate the expression of a fusion protein between histone H2B and GFP (Tumbar et al., 2004). In the E12.5 (data not shown) and E13.5 (Figure 1I–L) double transgenic embryos (ratio: 1/4), the Wnt1-expressing cells exhibited strong expression of the H2BGFP fusion protein in the mid/hind brain and neural tube regions.
Figure 1.
Wnt1-rtTA mouse strain permits targeted gene expression in Wnt1-expressing neural precursors. (A) A schematic representation of the tetracycline-dependent gene activation by Wnt1-rtTA mouse strain. The Wnt1 promoter (P) and enhancer (E) direct the expression of rtTA in the Wnt1-expressing precursors. The presence of Dox induces the binding of rtTA to TRE, activating the reporter (lacZ or GFP) expression. (B) Embryos carrying Wnt1-rtTA and TRE-lacZ transgenes (double transgenics, right; control, left) enable conditional expression of the lacZ gene in the Wnt1-expressing cells of developing brain and neural tube. Embryos were recovered at E12.5 and analyzed by β-gal staining in whole mounts (B–H) and sections (M–T). The lacZ reporter is highly stimulated in the dorsal midline of developing neural tube and mid/hind brain upon Dox induction (B–D, M–T). Inducible expression of the lacZ reporter is detected in the double transgenic embryos with 7 days (E, G) or 17 hours (F, H) of Dox treatment. (I–L) Whole mount fluorescent analysis revealed an inducible expression of the GFP reporter in double transgenic embryos for Wnt1-rtTA and TRE-H2BGFP at E13.5. The GFP reporter is expressed in the double transgenic embryos with 7 days (I, J), 17 hours (K) or 12 hours (L) of Dox treatment. Enlargements of the insets in panels B and M are shown in C, D, N, O, P. Insets exhibit enlargement of the stained regions (R–T). CP, choroid plexus; di, diencephalon; Hy, hypothalamus; met, metencephalon; my, myelencephalon; NT, neural tube; Th, thalamus. Scale bars, 2 mm (B, C, I); 1 mm (D, E–H, J–L); 0.5 mm (M, R–T); 0.1 mm (N–Q).
To examine the kinetics of inducible gene expression, we treated the pregnant mother with Dox for various time periods. We detected fairly comparable expression of the lacZ reporter between 7 days and 17 hours (Figure 1E–H). Using TRE-H2BGFP, we found that the GFP reporter also displayed similar expression patterns between 7 days and 12 hours (Figure J–K). Although the reported expression is stimulated within several hours of Dox treatment, a 2-day induction ensures that target gene expression is at the optimal level. Our finding indicates that the Dox-inducible expression of the target gene can be achieved in a relatively short period in animals.
Sections of the β-gal stained embryos (Figure 1M–T) revealed that the Wnt1-expressing cells in diencephalon (Figure 1P and T), metencephalon (Figure 1O), myelencephalon (Figure 1N and S), choroid plexus (Figure 1Q) and neural tube (Figure 1R) were positive for β-gal staining. The results demonstrated that we were able to use the Wnt1-rtTA strain for conditional gene expression in the Wnt1-expressing cells during embryonic neural development.
We next examined if Wnt1-rtTA could be used for inducible gene ablation and expression by combining the tetracycline-dependent activation and Cre-mediated recombination methods. Our strategy is to use the Wnt1-rtTA transgene for spatiotemporal specific expression of Cre under control of TRE (Figure 2A). The expressed Cre can then be used for Cre-mediated recombination for conditional gene ablation or activation. For conditional gene ablation, Cre is able to excise a gene coding sequence, flanked by two loxP sites (gene specific floxed allele). For conditional gene activation, Wnt1-rtTA and TRE-Cre are required to cross with an allele, which contains the loxP site flanking STOP cassette to control target gene expression. Upon Cre-mediated recombination, the target gene is expressed by removal of the STOP cassette.
Figure 2.
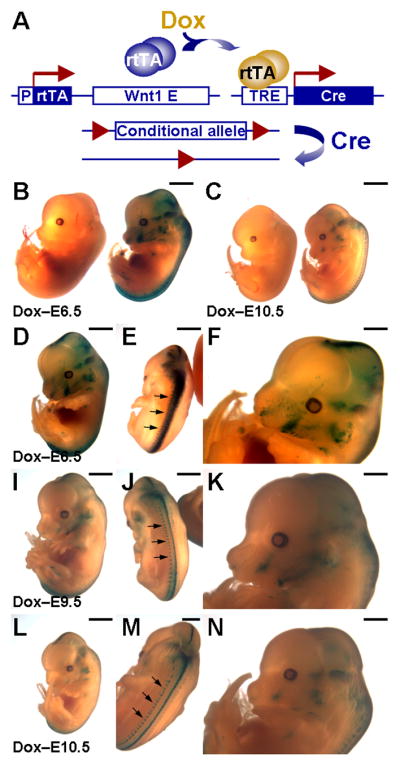
Cre-mediated recombination in the Wnt1-expressing neural precursors is induced by tetracycline-dependent activation. (A) A diagram illustrates our strategy for spatiotemporal specific-recombination in the Wnt1-expressing cells. Expression of rtTA in the Wnt1-expressing cells permits the Dox-inducible activation of TRE-Cre. The Cre-mediated recombination, which deletes DNA sequences flanked by two loxP sites, occurs in the Wnt1-expressing neural precursors, thereby achieving either gene expression or ablation. Note that the conditional allele, containing the loxP site flanking sequence, could be a gene specific floxed allele (for conditional gene ablation) or an allele permitting target gene expression upon Cre-mediated recombination (for conditional gene expression). (B, C) Embryos carrying Wnt1-rtTA and TRE-Cre transgenes (double transgenics, right; control: carrying Wnt1-rtTA, left) enable conditional excision of the sequence flanked by two loxP sites upon Dox treatment, leading to the expression of a lacZ reporter (R26RlacZ). The expression of Cre is stimulated by Dox at E6.5 (D–F), E9.5 (I–K) and E10.5 (L–N), followed by harvesting of embryos at E13.5. Scale bars, 2 mm (B, C); 1 mm (D–N).
Using the R26RlacZ strain as a conditional allele (Soriano, 1999), we tested the feasibility of our strategy. The Wnt1-rtTA transgene was first bred with the TRE-Cre (Radomska et al., 2002) to obtain mice carrying both transgenes (ratio: 1/4). These double transgenics were then crossed to the R26RlacZ homozygous mice to get embryos carrying all three transgenes (ratio: 1/8). Because of Wnt1 expression in CNC precursors, the Dox-dependent induction of Cre activated the expression of lacZ from the R26RlacZ allele. The lacZ reporter was then expressed not only in these precursors but also in their descendents because it was under control of the ROSA26 promoter independent of the Wnt1 regulatory element. Therefore, β-gal staining should be positive in post-migratory CNC cells even though the Wnt1 regulatory element was silenced. Indeed, we found the neural crest derivatives, including craniofacial regions, expressed the lacZ reporter when Dox was administered at E6.5 (Figure 2B, D–F). A slight leaky expression was observed around the neck and spinal cord area in some of the TRE-Cre; R26RlacZ double transgenic embryos. This is likely due to the positional effect of the TRE-Cre transgene because the TRE-lacZ and TRE-H2BGFP transgenes do not seem to have obvious leaky problems. We also were not able to induce a robust expression of lacZ in the CNC lineage which was shown by the Wnt1-Cre; R26RlacZ genetic labeling system (Chai et al., 2000). This might also be attributed to the limitation caused by the TRE-Cre transgenic line. Nonetheless, the data demonstrated the success of using the Wnt1-rtTA/TRE-Cre system for excision of the loxP flanked DNA sequence. The data also imply that this system can be utilized for conditional gene ablation and expression in CNC development.
Next, we determined if Dox-dependent activation of Cre induced after the initiation of neural crest migration will restrict the stimulation of the target gene expression in the CNC derivatives. When Dox was administered at E9.5 (Figure 2I–K) and E10.5 (Figure 2C, L–N), we observed a gradual reduction of Cre-mediated recombination in the CNC derivatives (Figure 2D, I and L). The Cre-dependent expression of lacZ was still detected in the dorsal midline of CNS, but highly reduced in the craniofacial area (Figure 2F, K and N) and dorsal root ganglia (Figure 2E, J and M). Thus suggests that the timing of Dox induction determines the Cre-mediated recombination that occurs in the population of neural crest precursors.
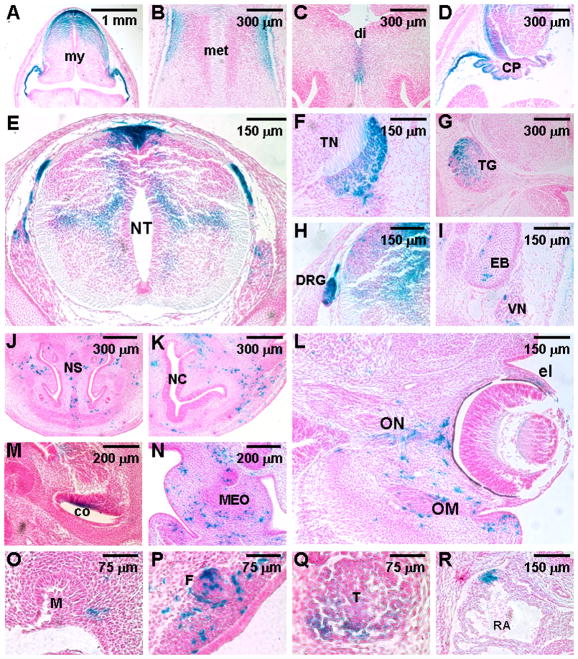
To identify the cell populations which are positive for expression of the reporter and permit manipulation of gene activity using this system, the β-gal stained embryo with Dox administered on E6.5 (Figure 2D–F) was analyzed in sections. In addition to the Wnt1-expressing cells in CNS (Figure 3A–E), we found that the lacZ reporter is expressed in their derivatives, including migrating neural progenitors within spinal cord (Figure 3E), trigeminal nerve and ganglion (Figure 3F, G), dorsal root ganglion (Figure 3H), exoccipital bone and vagus nerve (Figure 3I), nasal septum (Figure 3J), nasal capsule (Figure 3K), ear (Figure 3M, N), optic nerve and ocular muscle (Figure 3L), tooth (Figure 3O), follicle (Figure 3P), thymic rudiment (Figure 3Q), and heart (Figure 3R). On the other hand, the β-gal stained embryo with Dox administered on E10.5 (Figure 2L–N) displayed comparable expression of the reporter in the myelencephalon, metencephalon, diencephalon, choroid plexus and dorsal neural tube, similar to those shown in Figure 3A–E. However, the lacZ expression was significantly altered in the neural crest derivatives. Drastic reduction was evident in the trigeminal nerve and ganglion, dorsal root ganglion, middle ear ossicle and thymic rudiment with very few positive cells (data not shown). No detectable β-gal signal was found in the migrating neural progenitors within spinal cord, exoccipital bone and vagus nerve, nasal septum, nasal capsule, cochlea, ocular muscle, optic nerve, tooth, follicle, and heart (data not shown).
Figure 3.
Inducible gene expression is targeted to the Wnt1-expressing cells and their derivatives using Wnt1-rtTA and TRE-Cre transgenes. Sections of the β-gal stained embryo shown in Figure 2D reveal that the target gene expression is stimulated in the Wnt1-expressing precursors and their derivatives, including those in hindbrain (A, D), midbrain (B, C), neural tube (E), trigeminal nerve (F) and ganglion (G), dorsal root ganglion (H), exoccipital bone and vagus nerve (I), nasal septum (J), nasal capsule (K), ear (M, N), eye (L), molar (O), follicle (P), thymic rudiment (Q), heart (R). CP, choroid plexus; co, cochlea; di, diencephalon; DRG, dorsal root ganglion; e, ear; el, eyelid; EB, exoccipital bone; F, follicle; met, metencephalon; my, myelencephalon; M, molar; MEO, middle ear ossicle; NC, nasal cavity; NS, nasal septum; NT, neural tube; OM, ocular muscle; ON, optic nerve; RA, right atrium; T, thymic rudiment; TG, trigeminal ganglion; TN, trigeminal nerve; VN, vagus nerve. Scale bars, 1 mm (A); 300 μm (B–D, G, J, K); 150 μm (E, F, H, I, L, R); 200 μm (M, N); 75 μm (O–Q).
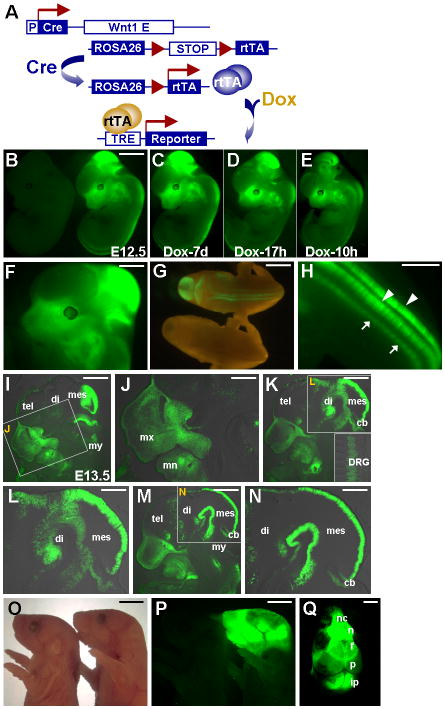
We also compared the newly developed Wnt1-rtTA model with the Wnt1-Cre; R26STOPrtTA expression system that we created previously (Yu et al., 2005b). In the Wnt1-Cre; R26STOPrtTA system, rtTA is expressed in both CNC progenitors and their descendents. In animals also carrying a target gene under control of TRE, its expression can be activated in development of the CNC lineage in a spatiotemporal specific fashion upon Dox treatment (Figure 4A). To enhance the efficiency of obtaining our desired genotypes, we first established a mouse strain containing TRE-H2BGFP in the R26STOPrtTA homozygous background. These mice were then bred with the Wnt1-Cre mice to obtain the Wnt1-Cre; R26STOPrtTA; TRE-H2BGFP embryos (ratio: 1/4). When we obtained the Wnt1-Cre; R26STOPrtTA; TRE-H2BGFP embryos treated by Dox, fluorescent signal is observed in the CNC precursors and their derivatives at the dorsal midline and craniofacial regions, respectively (Figure 4B, F–H). The GFP expression is detected in maxilla, mandible, telencephalon, diencephalon, mesencephalon, myelencephalon, cerebellum, dorsal root ganglia (Figure 4I–N). To examine the kinetics of inducible gene expression, we treated the pregnant mother with Dox for various time periods. We detected fairly comparable expression of the H2BGFP reporter between 7 days and 10 hours (Figure 4C–E). The GFP signal is clearly visible after a short treatment of Dox for 10 hr although the expression level seems relatively reduced (Figure 4E). To achieve the optimal induction as shown in Figure 4C, a 2-day treatment is more than enough (data not shown). In addition, the reporter was also expressed in the skull structures containing derivatives of CNC, including nasal cartilage, nasal bone, frontal bone and interparietal bone (Figure 4O–Q). However, parietal bone, which is mesodermal in origin, only showed background level. The results showed a proof of principle that the Wnt1-rtTA (Figure 1) and Wnt1-Cre; R26STOPrtTA (Figure 4) systems can be used for inducible gene expression in pre-migratory CNC precursors, and both pre-migratory and post-migratory CNC cells, respectively. The target gene expression can be stimulated effectively by the addition of Dox in a relatively short period in animals.
Figure 4.
Neural crest lineage-specific gene activation in an inducible fashion. (A) A scheme represents a strategy for manipulating gene expression in neural crest lineage. Neural crest lineage specificity is provided by Cre-mediated excision of the loxP-flanked STOP sequence, and further temporal activation is controlled by Dox administration. The expression of rtTA in the Wnt1-expressing cells is controlled by the Cre-mediated excision of the loxP-flanked STOP sequence. The rtTA gene, expressed in all Wnt1-expressing cells and their descendants, enables inducible gene expression by the tetracycline-dependent activation system. (B) Whole mount GFP analysis of the E12.5 control (R26STOPrtTA; TRE-H2BGFP double transgenics, left) and Wnt1-Cre; R26STOPrtTA; TRE-H2BGFP triple transgenic embryos (right), with Dox treatment. (C–E) Inducible expression of the H2BGFP reporter in the triple transgenic embryos with varying lengths of Dox treatment (C, 7d; D, 17h; E, 10h). (F) Enlargement of the Dox-induced embryo from panel B, showing targeted gene expression in the Wnt1-expressing neural precursors (mid/hind brain) and their derivatives/neural crest cells (craniofacial regions). (G) Dorsal view of embryos exhibits the GFP expression in the developing neural tube of the triple transgenic animal (upper), but not the control (R26STOPrtTA; TRE-H2BGFP double transgenics, lower). (H) Enlargement of the GFP positive embryo displays target gene expression in the neural tube (arrowheads) and neural crest derivatives, dorsal root ganglions (arrows). (I–N) Sections of the GFP positive E13.5 embryos show conditional gene expression in developing neural tube as well as neural crest derivatives, facial processes. The reporter is highly expressed in developing mid/hind brain, including mesencephalon (mes), myelencephalon (my), diencephalon (di) and cerebellum (cb), as well as neural crest derived craniofacial mesenchyme, including maxillary (mx) and mandibular (mn) prominences. Enlargements of the inset in panels I, K and M are shown in J, L and N. An inset displays enlargement of the dorsal root ganglion (DRG) regions in panel K. (O) Whole mount and (P) GFP analysis reveals targeted expression of the H2BGFP reporter in the neural crest-derived craniofacial region of the triple transgenic newborn (right), but not the control (left), with Dox treatment. (Q) GFP analysis of the skull indicates the transgenic expression in the skeletal elements, including the nasal cartilage (nc), nasal bone (n), frontal bone (f) and interparietal (ip) bone, but not in mesoderm-derived parietal bone (p). Scale bars, 2 mm (B, G, O–Q); 1 mm (F, H, I, K, M); 0.5 mm (J, L, N).
Discussion
We have successfully established a new Wnt1-rtTA mouse strain expressing an improved version of rtTA under control of the Wnt1 regulatory element. This study has also demonstrated the usefulness of this strain for gene expression and deletion during embryogenesis. The inducible expression of the target gene was evident as early as ten hours after the Dox administration in the drinking water. An overnight induction for 12 hours is able to recapitulate a 7-day treatment. To ensure that the expression reaches its optimal levels, we recommend a 2-day induction of Dox. It is possible that the time required to activate the gene expression can be even shorter. Thus provides a very sensitive system for conditional gene activation. A reversal of gene expression can also be achieved by the withdrawal of Dox for 7 days (our unpublished data). These types of study depend on the nature of experimental designs. It has been successfully used to identify the label retaining cells exhibiting stem cell-like characteristics in the hair follicles (Tumbar et al., 2004).
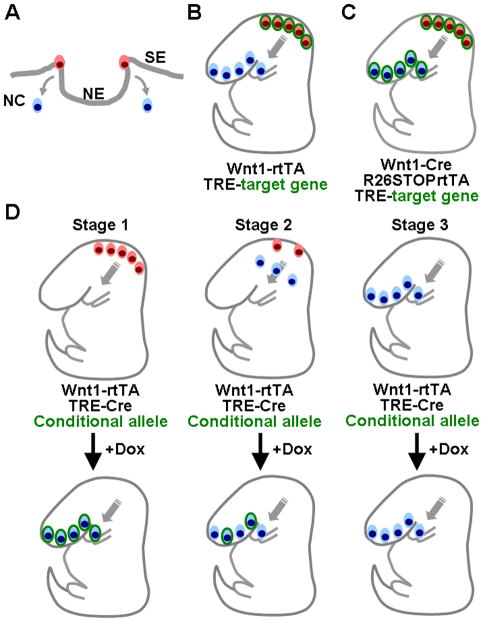
The CNC precursors arise at the lateral margin of the neural fold at the boundary between surface and neural ectoderms (Figure 5A). Upon closure of neural tube, they migrate ventrolaterally to populate the craniofacial regions. The Wnt1-rtTA mice can be used to express the target gene under control of TRE in the precursors of neural epithelial and neural crest cells (Figure 5B). The timing of gene expression is controlled by administration of Dox. Regardless of when the Dox is administered, the target gene is only activated in neural epithelial cells at the dorsal midline of the brain and neural tube. Thus provides a system to manipulate gene expression in a spatiotemporal-specific, and potentially reversible, fashion. In contrast, the expression of Cre is regulated by the Wnt1 regulatory elements in the Wnt1-Cre; R26STOPrtTA mice. The Cre expression is able to permanently delete the STOP cassette in the Wnt1-expressing precursors and then activate the expression of rtTA, controlled by the ROSA26 locus, in both the Wnt1-expressing precursors as well as their derivatives. As our results demonstrated, this system permits inducible gene expression in the dorsal midline regions and the entire neural crest lineage, including precursors located to the dorsal midline and their derivatives in craniofacial regions and dorsal root ganglia, upon Dox treatment (Figure 5C). The advantage of this system is to allow CNC lineage-specific expression and to induce the expression of the target gene at various times in migrating neural crest cells upon Dox treatment.
Figure 5.
Schematic representation illustrates the use of transgenic systems for manipulating gene activity in neural precursors and CNC cells. (A) Neural crest cells arise at the lateral margin of neural fold at the boundary between surface (SE) and neural (NE) ectoderms. They migrate ventrolaterally to populate the head and neck regions upon closure of the neural tube. (B) The Wnt1-rtTA strain expresses rtTA only in the pre-migratory precursors (red cells) in dorsal midline because the Wnt1 regulatory element is not active after emigration of these cells (blue cells). (C) The Cre-mediated recombination, permitting deletion of the loxP-flanked STOP sequence, activates the expression of rtTA under control of ROSA26 promoter in both neural crest precursors and their derivatives in the neural tube and craniofacial primordium, respectively. After the Cre-mediated expression, rtTA is constitutively expressed in the Wnt1-expressing cells and their derivatives, independent of the activity of the Wnt1 regulatory elements. (D) The inducible expression domain of Cre in the Wnt1-rtTA/TRE-Cre system varies by the timing of Dox administration. The presence of Dox prior to the migration of neural crest precursors enables the Cre-mediated recombination to occur in both the dorsal midline and craniofacial regions (Stage 1). When Dox is administered during the migration of CNC, only the cells leaving late will be activated (Stage 2). Cre would not be present in the derivatives of CNC if Dox is added after they have emigrated (Stage 3). Pre-migratory and post-migratory cells are shown in red and blue, respectively. Cells permitting the manipulation of gene activity are highlighted in green.
We have shown the proof of principle that Wnt1-rtTA can be used in combination with TRE-Cre for conditional gene expression and ablation in CNC development although a better TRE-Cre transgenic line should be used. We tested another TRE-Cre strain (Wong et al., 2000) but failed to detect any Cre recombination activity when crossed with Wnt1-rtTA, Axin2-rtTA (Yu et al., 2007) or ROSA26rtTA (Yu et al., 2005b). Although the TRE-Cre line used in this study was somewhat disappointing, it works well with other rtTA transgenic strains, Axin2-rtTA (Yu et al., 2007) and Nestin-rtTA (Yu et al., 2005c). Development of an improved TRE-Cre using a new TRE vector containing seven copies of tetO should overcome the restricted expression in the CNC lineage. A knock-in allele of TRE-Cre in the ROSA26 locus will also avoid the positional effect caused by transgene insertion. We rule out the possibility that the restricted CNC expression is caused by the Wnt1 regulatory element because the same pWEXP3C plasmid was used to generate Wnt1-Cre and Wnt1-rtTA transgenic animals (Jiang et al., 2000).
In the Wnt1-rtTA; TRE-Cre system, the expression of Cre in the dorsal midline and/or craniofacial primordia depends on the timing of Dox induction (Figure 5D). If Dox is administered before emigration of neural crest cells, the Cre-mediated recombination will occur in the precursors, thereby modifying the conditional allele (activating or deleting the target gene) even in the craniofacial regions. However, the addition of Dox at the stage where the emigration of neural crest cells has occurred will result in gene modification restricted to the dorsal midline area. This strategy avoids gene deletion in the Wnt1-dependent CNC derivatives which cannot be achieved using the conventional Wnt1-Cre transgene. In theory, similar results can be achieved by the use of Wnt1-CreER™ mouse strain, which expresses Cre-ER™ fusion protein under control of the Wnt1 regulatory elements (Danielian et al., 1998). However, the ligand-mediated activation of Cre activity works most effectively by drug administration after E9.5 in this system. This might contribute to no activation of the R26RlacZ reporter shown in the craniofacial regions (Danielian et al., 1998). In addition, the biggest problem for this system is that high concentrations of ligand (tamoxifen or 4-OHT) are required to activate the Cre activity. This is necessitated by the fact that the point mutation which allows preferential binding of 4-OHT rather than 17β-estradiol also decreases the binding affinity for 4-OHT. As a result, the dose of ligand required to activate CreER™ is quite close to that interfering with the maintenance of pregnancy. In contrast, the concentration of Dox added in the drinking water does not cause any toxicity in the Wnt1-rtTA; TRE-Cre system. The Dox treated embryos are able to survive to term and continue to develop. We have tested the treatment of this dose for up to several months in mice without causing any obvious problems. By integrating the tetracycline-dependent activation and Cre-mediated recombination systems, this study has shown the versatility of our mouse genetic models to manipulate gene activity in early neural development and craniofacial morphogenesis.
Experimental Procedures
Mouse strains
Plasmid Wnt1-rtTA was constructed by inserting the blunt-ended EcoRI-BamHI fragment of a modified form of rtTA (rtTA2S-M2) (Urlinger et al., 2000) in the EcoRV site of the pWEXP3C plasmid containing the Wnt1 enhancer and promoter (Danielian and McMahon, 1996; Danielian et al., 1998). Methods to the Wnt1-rtTA transgenic mouse strain were reported previously (Hsu et al., 2001; Yu et al., 2007). Mice were genotyped for the presence of the transgene by PCR and Southern blot analyses. For PCR, the primers 5′-gacaaggaaactcgctcaaaag-3′ and 5′-ttgctacttgatgctcctgttc-3′, which are located in the rtTA coding sequences, were used as described (Yu et al., 2007). For Southern analysis (Hsu et al., 2001; Chiu et al., 2008), the full-length cDNA for rtTA was used as a probe to detect the Wnt1-rtTA transgenic mice. Three out of nine Wnt1-rtTA founders tested positive for the integration of the transgene by PCR and Southern analyses. All of them enable Dox-inducible gene activation as determined by crossing the transgenic lines with TRE reporter transgenes. TRE-lacZ, TRE-H2BGFP, TRE-Cre, Wnt1-Cre, R26RlacZ and R26STOPrtTA mice were described previously (Danielian et al., 1998; Soriano, 1999; Tumbar et al., 2004; Yu et al., 2005b; Yu et al., 2005c; Yu et al., 2007). Dox (doxycycline, 2 mg/ml plus 50 mg/ml sucrose) was administered orally in the drinking water as described (Hsu et al., 2001; Yu et al., 2005b; Yu et al., 2007). Care and use of experimental animals described in this work comply with guidelines and policies of the University Committee on Animal Resources at the University of Rochester.
Histology, β-gal staining and GFP analyses
Samples were fixed, paraffin embedded, sectioned, and stained with hematoxylin/eosin for histological evaluation as described (Hsu et al., 2001; Liu et al., 2008). Staining for β-galactosidase activity in embryos was performed as described (Yu et al., 2005a; Yu et al., 2005b; Yu et al., 2007). In brief, specimens were dissected in phosphate buffered saline (PBS), and prefixed in PBS containing 1% formaldehyde, 0.2% glutaraldehyde, 2 mM magnesium chloride, 5 mM EGTA and 0.02% NP-40 at 4°C. Samples were washed three times in PBS containing 0.02% NP-40 at room temperature for 30 min before they were stained in PBS containing 1 mg/ml of X-Gal, 5 mM potassium ferricyanide, 2mM potassium ferrocyanide, 2mM magnesium chloride, 0.01% Sodium deoxycholate and 0.02% NP-40 at 30°C. The stained specimens were photographed for whole mount analyses. For analyses in sections, samples were subsequently fixed and processed for paraffin sections and nuclear fast red counterstaining. Whole mount GFP analysis was performed using fluorescence stereomicroscopy (Nikon) to visualize the embryo (Yu et al., 2005b; Yu et al., 2007; Chiu et al., 2008). Embryos were then embedded and processed for frozen sections and fluorescence analysis (Liu et al., 2007).
Acknowledgments
We thank Andy McMahon, Lisa Monteggia and David Rowitch for reagents and C-S Victor Lin and Brain H Son for technical assistance. This work is supported by a National Institutes of Health grant DE015654 to W.H.
This work is support by National Institutes of Health: DE015654
List of Abbreviations
- CNC
cranial neural crest
- CNS
central nervous system
- Dox
doxycycline
- TRE
tetracycline response elements
References
- Chai Y, Jiang X, Ito Y, Bringas P, Jr, Han J, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- Chiu SY, Asai N, Costantini F, Hsu W. SUMO-Specific Protease 2 Is Essential for Modulating p53-Mdm2 in Development of Trophoblast Stem Cell Niches and Lineages. PLoS Biol. 2008;6:e310. doi: 10.1371/journal.pbio.0060310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane JF, Trainor PA. Neural crest stem and progenitor cells. Annu Rev Cell Dev Biol. 2006;22:267–286. doi: 10.1146/annurev.cellbio.22.010305.103814. [DOI] [PubMed] [Google Scholar]
- Danielian PS, McMahon AP. Engrailed-1 as a target of the Wnt-1 signalling pathway in vertebrate midbrain development. Nature. 1996;383:332–334. doi: 10.1038/383332a0. [DOI] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- Delfino-Machin M, Chipperfield TR, Rodrigues FS, Kelsh RN. The proliferating field of neural crest stem cells. Dev Dyn. 2007;236:3242–3254. doi: 10.1002/dvdy.21314. [DOI] [PubMed] [Google Scholar]
- Francis-West P, Ladher R, Barlow A, Graveson A. Signalling interactions during facial development. Mech Dev. 1998;75:3–28. doi: 10.1016/s0925-4773(98)00082-3. [DOI] [PubMed] [Google Scholar]
- Greco TL, Takada S, Newhouse MM, McMahon JA, McMahon AP, Camper SA. Analysis of the vestigial tail mutation demonstrates that Wnt-3a gene dosage regulates mouse axial development. Genes Dev. 1996;10:313–324. doi: 10.1101/gad.10.3.313. [DOI] [PubMed] [Google Scholar]
- Hsu W, Shakya R, Costantini F. Impaired mammary gland and lymphoid development caused by inducible expression of Axin in transgenic mice. J Cell Biol. 2001;155:1055–1064. doi: 10.1083/jcb.200107066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeya M, Lee SM, Johnson JE, McMahon AP, Takada S. Wnt signalling required for expansion of neural crest and CNS progenitors. Nature. 1997;389:966–970. doi: 10.1038/40146. [DOI] [PubMed] [Google Scholar]
- Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cardiac neural crest. Development. 2000;127:1607–1616. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- Kontges G, Lumsden A. Rhombencephalic neural crest segmentation is preserved throughout craniofacial ontogeny. Development. 1996;122:3229–3242. doi: 10.1242/dev.122.10.3229. [DOI] [PubMed] [Google Scholar]
- Kuriyama S, Mayor R. Molecular analysis of neural crest migration. Philos Trans R Soc Lond B Biol Sci. 2008;363:1349–1362. doi: 10.1098/rstb.2007.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBonne C, Bronner-Fraser M. Molecular mechanisms of neural crest formation. Annu Rev Cell Dev Biol. 1999;15:81–112. doi: 10.1146/annurev.cellbio.15.1.81. [DOI] [PubMed] [Google Scholar]
- Le Douarin N, Kalcheim C. The neural crest. xxiii. Cambridge, U.K.; New York, NY: Cambridge University Press; 1999. p. 445. [Google Scholar]
- Le Douarin NM, Brito JM, Creuzet S. Role of the neural crest in face and brain development. Brain Res Rev. 2007;55:237–247. doi: 10.1016/j.brainresrev.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Lee SM, Tole S, Grove E, McMahon AP. A local Wnt-3a signal is required for development of the mammalian hippocampus. Development. 2000;127:457–467. doi: 10.1242/dev.127.3.457. [DOI] [PubMed] [Google Scholar]
- Levayer R, Lecuit T. Breaking down EMT. Nat Cell Biol. 2008;10:757–759. doi: 10.1038/ncb0708-757. [DOI] [PubMed] [Google Scholar]
- Liu B, Yu HM, Hsu W. Craniosynostosis caused by Axin2 deficiency is mediated through distinct functions of beta-catenin in proliferation and differentiation. Dev Biol. 2007;301:298–308. doi: 10.1016/j.physletb.2003.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Yu HM, Huang J, Hsu W. Co-opted JNK/SAPK signaling in Wnt/beta-catenin-induced tumorigenesis. Neoplasia. 2008;10:1004–1013. doi: 10.1593/neo.08548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon AP, Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 1990;62:1073–1085. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- McMahon AP, Joyner AL, Bradley A, McMahon JA. The midbrain-hindbrain phenotype of Wnt-1-/Wnt-1- mice results from stepwise deletion of engrailed-expressing cells by 9.5 days postcoitum. Cell. 1992;69:581–595. doi: 10.1016/0092-8674(92)90222-x. [DOI] [PubMed] [Google Scholar]
- Parr BA, Shea MJ, Vassileva G, McMahon AP. Mouse Wnt genes exhibit discrete domains of expression in the early embryonic CNS and limb buds. Development. 1993;119:247–261. doi: 10.1242/dev.119.1.247. [DOI] [PubMed] [Google Scholar]
- Radomska HS, Gonzalez DA, Okuno Y, Iwasaki H, Nagy A, Akashi K, Tenen DG, Huettner CS. Transgenic targeting with regulatory elements of the human CD34 gene. Blood. 2002;100:4410–4419. doi: 10.1182/blood-2002-02-0355. [DOI] [PubMed] [Google Scholar]
- Raible DW, Ragland JW. Reiterated Wnt and BMP signals in neural crest development. Semin Cell Dev Biol. 2005;16:673–682. doi: 10.1016/j.semcdb.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Steventon B, Carmona-Fontaine C, Mayor R. Genetic network during neural crest induction: from cell specification to cell survival. Semin Cell Dev Biol. 2005;16:647–654. doi: 10.1016/j.semcdb.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Takada S, Stark KL, Shea MJ, Vassileva G, McMahon JA, McMahon AP. Wnt-3a regulates somite and tailbud formation in the mouse embryo. Genes Dev. 1994;8:174–189. doi: 10.1101/gad.8.2.174. [DOI] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urlinger S, Baron U, Thellmann M, Hasan MT, Bujard H, Hillen W. Exploring the sequence space for tetracycline-dependent transcriptional activators: novel mutations yield expanded range and sensitivity. Proc Natl Acad Sci U S A. 2000;97:7963–7968. doi: 10.1073/pnas.130192197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MH, Saam JR, Stappenbeck TS, Rexer CH, Gordon JI. Genetic mosaic analysis based on Cre recombinase and navigated laser capture microdissection. Proc Natl Acad Sci U S A. 2000;97:12601–12606. doi: 10.1073/pnas.230237997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanfeng W, Saint-Jeannet JP, Klein PS. Wnt-frizzled signaling in the induction and differentiation of the neural crest. Bioessays. 2003;25:317–325. doi: 10.1002/bies.10255. [DOI] [PubMed] [Google Scholar]
- Yu HM, Jerchow B, Sheu TJ, Liu B, Costantini F, Puzas JE, Birchmeier W, Hsu W. The role of Axin2 in calvarial morphogenesis and craniosynostosis. Development. 2005a;132:1995–2005. doi: 10.1242/dev.01786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HM, Liu B, Chiu SY, Costantini F, Hsu W. Development of a unique system for spatiotemporal and lineage-specific gene expression in mice. Proc Natl Acad Sci U S A. 2005b;102:8615–8620. doi: 10.1073/pnas.0500124102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HM, Liu B, Costantini F, Hsu W. Impaired neural development caused by inducible expression of Axin in transgenic mice. Mech Dev. 2007;124:146–156. doi: 10.1016/j.mod.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu TS, Dandekar M, Monteggia LM, Parada LF, Kernie SG. Temporally regulated expression of Cre recombinase in neural stem cells. Genesis. 2005c;41:147–153. doi: 10.1002/gene.20110. [DOI] [PubMed] [Google Scholar]