Abstract
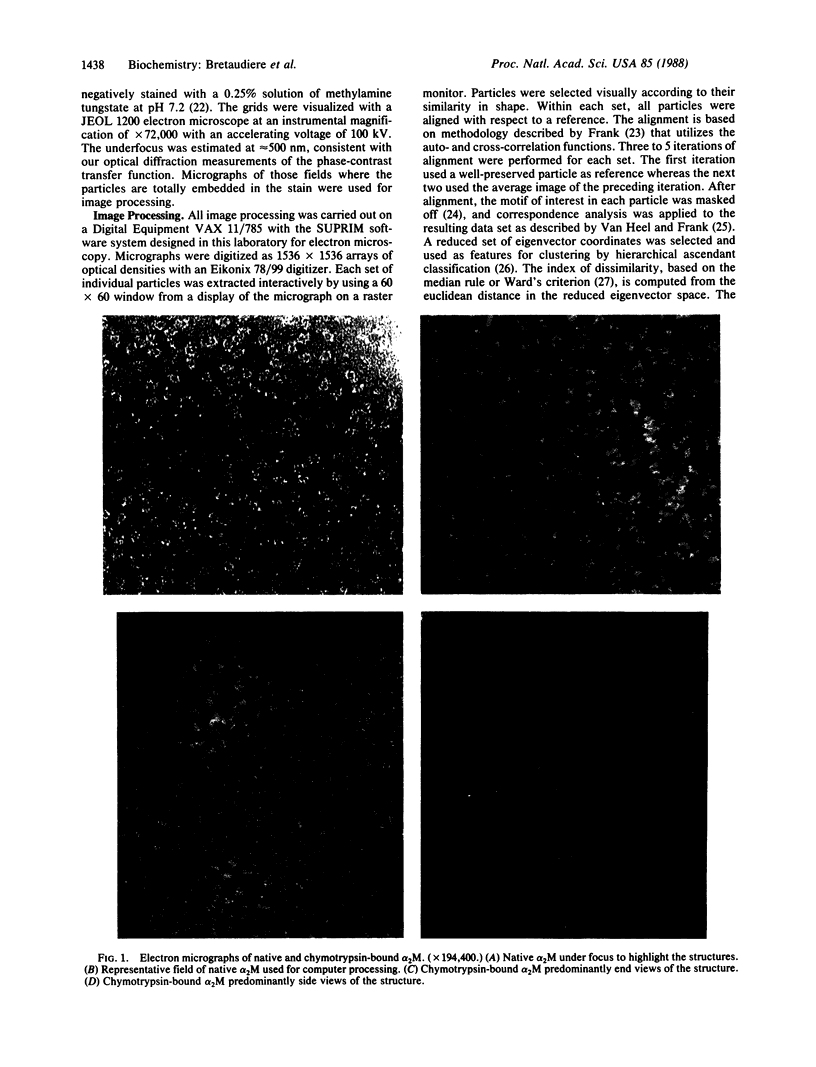
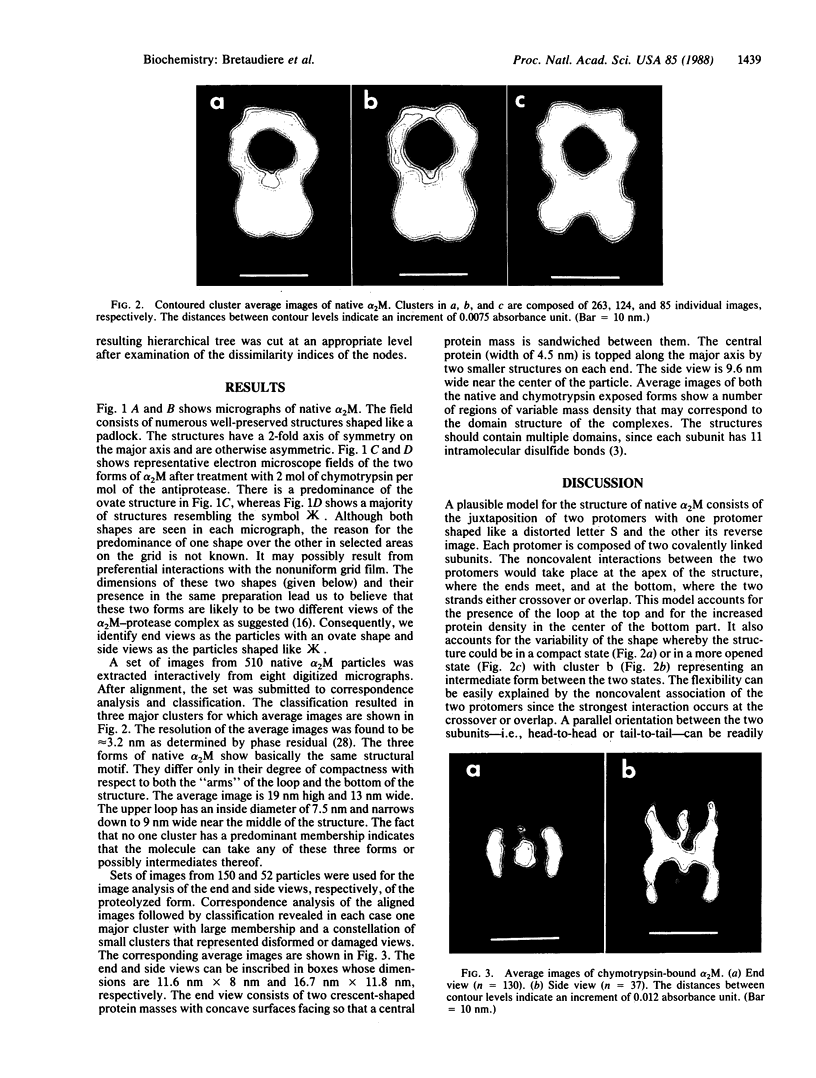
Well-preserved structures of native and alpha-chymotrypsin-bound alpha 2-macroglobulin were obtained by electron microscopy. Computer processing of these images has shown that the native structure has the shape of a padlock 19 nm long. It is proposed that the native alpha 2-macroglobulin consists of the juxtaposition of two protomers with one protomer shaped like a distorted letter "S" and with the other its reverse image, to form a binding site between the two protomers near the bottom of the complex. On cleavage of the subunits with chymotrypsin, the native structure condenses to 16.7 nm and rearranges so that the interaction between the protomers is near the middle. Two images of the alpha 2-macroglobulin-chymotrypsin conjugate were obtained. We suggest that these images represent the end and side view of this complex. Based on the manner in which the native structure is assembled, we propose that the proteolyzed form of alpha 2-macroglobulin is functionally asymmetric in that both protease binding sites reside on the same half of the complex.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett A. J., Brown M. A., Sayers C. A. The electrophoretically 'slow' and 'fast' forms of the alpha 2-macroglobulin molecule. Biochem J. 1979 Aug 1;181(2):401–418. doi: 10.1042/bj1810401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Starkey P. M. The interaction of alpha 2-macroglobulin with proteinases. Characteristics and specificity of the reaction, and a hypothesis concerning its molecular mechanism. Biochem J. 1973 Aug;133(4):709–724. doi: 10.1042/bj1330709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birktoft J. J., Blow D. M. Structure of crystalline -chymotrypsin. V. The atomic structure of tosyl- -chymotrypsin at 2 A resolution. J Mol Biol. 1972 Jul 21;68(2):187–240. doi: 10.1016/0022-2836(72)90210-0. [DOI] [PubMed] [Google Scholar]
- Bloth B., Chesebro B., Svehag S. E. Ultrastructural studies of human and rabbit alpha-M-globulins. J Exp Med. 1968 Apr 1;127(4):749–756. doi: 10.1084/jem.127.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman S. R., Gonias S. L., Pizzo S. V. Model of alpha 2-macroglobulin structure and function. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5700–5704. doi: 10.1073/pnas.82.17.5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank J., Verschoor A., Boublik M. Computer averaging of electron micrographs of 40S ribosomal subunits. Science. 1981 Dec 18;214(4527):1353–1355. doi: 10.1126/science.7313694. [DOI] [PubMed] [Google Scholar]
- Frank J., Verschoor A., Boublik M. Multivariate statistical analysis of ribosome electron micrographs. L and R lateral views of the 40 S subunit from HeLa cells. J Mol Biol. 1982 Oct 15;161(1):107–133. doi: 10.1016/0022-2836(82)90281-9. [DOI] [PubMed] [Google Scholar]
- Gonias S. L., Pizzo S. V. Reaction of human alpha 2-macroglobulin half-molecules with plasmin as a probe of protease binding site structure. Biochemistry. 1983 Oct 11;22(21):4933–4940. doi: 10.1021/bi00290a009. [DOI] [PubMed] [Google Scholar]
- Gonias S. L., Reynolds J. A., Pizzo S. V. Physical properties of human alpha 2-macroglobulin following reaction with methylamine and trypsin. Biochim Biophys Acta. 1982 Aug 10;705(3):306–314. doi: 10.1016/0167-4838(82)90252-7. [DOI] [PubMed] [Google Scholar]
- Howell J. B., Beck T., Bates B., Hunter M. J. Interaction of alpha 2-macroglobulin with trypsin, chymotrypsin, plasmin, and papain. Arch Biochem Biophys. 1983 Feb 15;221(1):261–270. doi: 10.1016/0003-9861(83)90143-1. [DOI] [PubMed] [Google Scholar]
- Jacquot-Armand Y., Krebs G. The interaction of a naphthalene dye with 2 -macroglobulin, free or bound to trypsin. Biochim Biophys Acta. 1973 Mar 23;303(1):128–137. doi: 10.1016/0005-2795(73)90154-2. [DOI] [PubMed] [Google Scholar]
- Jensen P. E., Sottrup-Jensen L. Primary structure of human alpha 2-macroglobulin. Complete disulfide bridge assignment and localization of two interchain bridges in the dimeric proteinase binding unit. J Biol Chem. 1986 Dec 5;261(34):15863–15869. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morelis P., Ambrosioni J. C., Got R., Fontanges R. Observation au microscope électronique du complexe formé par l'alpha-1-macroglobuline de sérum de lapin avec la trypsine. C R Acad Sci Hebd Seances Acad Sci D. 1969 Oct 13;269(15):1453–1454. [PubMed] [Google Scholar]
- Nishigai M., Osada T., Ikai A. Structural changes in alpha-2- and ovomacroglobulins studied by gel chromatography and electron microscopy. Biochim Biophys Acta. 1985 Oct 4;831(2):236–241. doi: 10.1016/0167-4838(85)90040-8. [DOI] [PubMed] [Google Scholar]
- Oliver R. M. Negative stain electron microscopy of protein macromolecules. Methods Enzymol. 1973;27:616–672. doi: 10.1016/s0076-6879(73)27029-5. [DOI] [PubMed] [Google Scholar]
- Osterberg R., Pap S. Structure of alpha 2-macroglobulin in solution and its interaction with proteases: an X-ray scattering study using the contrast variation method. Ann N Y Acad Sci. 1983;421:98–111. doi: 10.1111/j.1749-6632.1983.tb18096.x. [DOI] [PubMed] [Google Scholar]
- Pochon F., Amand B., Lavalette D., Bieth J. Rotational relaxation of free and protease-bound alpha2-macroglobulin. J Biol Chem. 1978 Oct 25;253(20):7496–7499. [PubMed] [Google Scholar]
- Pochon F., Bieth J. G. Separation of free and chymotrypsin-bound alpha 2-macroglobulin by affinity chromatography. Its use to demonstrate that the two chymotrypsin-binding sites of alpha 2-macroglobulin are equivalent and independent. J Biol Chem. 1982 Jun 25;257(12):6683–6685. [PubMed] [Google Scholar]
- Pochon F., Favaudon V., Tourbez-Perrin M., Bieth J. Localization of the two protease binding sites in human alpha 2-macroglobulin. J Biol Chem. 1981 Jan 25;256(2):547–550. [PubMed] [Google Scholar]
- Roberts R. C. Protease inhibitors of human plasma. Alpha-2-macroglobulin. J Med. 1985;16(1-3):129–224. [PubMed] [Google Scholar]
- Schramm H. J., Schramm W. Computer averaging of single molecules of alpha 2-macroglobulin and the alpha 2-macroglobulin/trypsin complex. Hoppe Seylers Z Physiol Chem. 1982 Aug;363(8):803–812. doi: 10.1515/bchm2.1982.363.2.803. [DOI] [PubMed] [Google Scholar]
- Sottrup-Jensen L., Stepanik T. M., Kristensen T., Wierzbicki D. M., Jones C. M., Lønblad P. B., Magnusson S., Petersen T. E. Primary structure of human alpha 2-macroglobulin. V. The complete structure. J Biol Chem. 1984 Jul 10;259(13):8318–8327. [PubMed] [Google Scholar]
- Stoops J. K., Wakil S. J., Uberbacher E. C., Bunick G. J. Small-angle neutron-scattering and electron microscope studies of the chicken liver fatty acid synthase. J Biol Chem. 1987 Jul 25;262(21):10246–10251. [PubMed] [Google Scholar]
- Tapon-Bretaudiére J., Bros A., Couture-Tosi E., Delain E. Electron microscopy of the conformational changes of alpha 2-macroglobulin from human plasma. EMBO J. 1985 Jan;4(1):85–89. doi: 10.1002/j.1460-2075.1985.tb02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heel M., Frank J. Use of multivariate statistics in analysing the images of biological macromolecules. Ultramicroscopy. 1981;6(2):187–194. doi: 10.1016/0304-3991(81)90059-0. [DOI] [PubMed] [Google Scholar]