Abstract
Hepatocytes are a key target for gene therapy of inborn errors of metabolism as well as of acquired diseases such as liver cancer and hepatitis. Gene transfer efficiency into hepatocytes is significantly determined by histological and functional aspects of liver sinusoidal cells. On the one hand, uptake of vectors by Kupffer cells and liver sinusoidal endothelial cells may limit hepatocyte transduction. On the other hand, the presence of fenestrae in liver sinusoidal endothelial cells provides direct access to the space of Disse and allows vectors to bind to receptors on the microvillous surface of hepatocytes. Nevertheless, the diameter of fenestrae may restrict the passage of vectors according to their size. On the basis of lege artis measurements of the diameter of fenestrae in different species, we show that the diameter of fenestrae affects the distribution of transgene DNA between sinusoidal and parenchymal liver cells after adenoviral transfer. The small diameter of fenestrae in humans may underlie low efficiency of adenoviral transfer into hepatocytes in men. The disappearance of the unique morphological features of liver sinusoidal endothelial cells in pathological conditions like liver cirrhosis and liver cancer may further affect gene transfer efficiency. Preclinical gene transfer studies should consider species differences in the structure and function of liver sinusoidal cells as important determinants of gene transfer efficiency into hepatocytes.
The liver is a central organ in many metabolic processes. Numerous inherited metabolic disorders have their origin in the liver. Therefore, hepatocytes are a key target for gene transfer directed at correction of inborn errors of metabolism and of hemophilia. Inborn errors of metabolism may lead to accumulation of toxic products in hepatocytes and extensive hepatotoxicity, as observed in disorders like α1-antitrypsin deficiency, type I tyrosinemia, or Wilson disease. In other metabolic diseases, such as in Crigler-Najjar syndrome type I, ornithine transcarbamylase deficiency, familial hypercholesterolemia, and hemophilia A and B, manifestations are primarily extrahepatic. In addition, the liver is a target for gene therapy of acquired diseases such as liver cancer and hepatitis.
Insights into the determinants of gene transfer efficiency to hepatocytes are therefore required to evaluate the potential of gene therapy for inborn errors of metabolism and for acquired liver diseases. These determinants include innate and adaptive immune responses, cellular and biochemical determinants of hepatocyte transduction such as ligand receptor interactions, and anatomical and histological factors. In this minireview, we focus predominantly on the role of liver sinusoidal cells and sinusoidal fenestrae as determinants of the efficiency in hepatocyte-directed gene transfer.
Liver Sinusoidal Cells
Histologically, the liver is divided into lobuli, hexagonal functional units formed by hepatocytes and sinusoids surrounding a central vein. Neighboring lobules are surrounded by portal triads, consisting of branches of the bile duct, the portal vein, and the hepatic artery. From the hilus, continuous branching of the hepatic artery and portal vein results in an intricate network of intertwining capillaries called sinusoids. Sinusoidal cells, which are a compilation of endothelial cells, Kupffer cells (resident liver macrophages), fat-storing cells (also called stellate cells or Ito cells), and pit cells (natural killer cells), constitute ∼33% of the number of resident liver cells, with parenchymal liver cells or hepatocytes comprising the remaining cells.1,2 Liver sinusoidal endothelial cells comprise 70%, Kupffer cells 20%, stellate cells 10%, and pit cells <1% of the number of sinusoidal cells.1,2 In the context of hepatocyte-directed gene transfer, we focus here on the role of Kupffer cells and liver sinusoidal endothelial cells, which together constitute the reticulo-endothelial cells of the liver. Sinusoidal endothelial cells, with a diameter of 7 to 9 μm2, are scavenger cells that are able to internalize particles up to 0.23 μm under physiological conditions in vivo.3 Larger particles are taken up by Kupffer cells,3 which have a diameter of 10 to 15 μm2. Since most gene transfer vectors have a diameter <0.23 μm, uptake of vectors by both Kupffer cells and liver sinusoidal endothelial cells may attenuate the efficiency of hepatocyte-directed gene transfer.
Most experimental work on the role of liver reticulo-endothelial cells in relation to hepatocyte transduction has been performed with adenoviral vectors. The relevance of these observations for other types of vectors and other modes of gene transfer will also be highlighted in this minireview.
In Vitro and in Vivo Transduction by Adenoviral Vectors
The presence of fenestrae in liver sinusoidal endothelial cells provides direct access for vectors to the space of Disse and to the microvillous surface of hepatocytes. Indeed, efficient hepatocyte transduction by adenoviral vectors requires that two clearly distinct conditions are met. First, adenoviral vectors should be able to migrate to the space of Disse via sufficiently large sinusoidal fenestrae. Second, vectors in the space of Disse must be able to bind to cellular receptors on hepatocytes for internalization and transduction. Both anatomical access of vectors to the space of Disse and efficient interaction of vectors with hepatocyte receptors are necessary for hepatocyte transduction in vivo. Before going into the anatomical access of vectors to the space of Disse, we will first discuss differences between adenoviral transduction in vitro and transduction of hepatocytes in vivo.
Fifity-one adenovirus serotypes have been identified that infect humans; these serotypes are classified into six species (A–F). In vitro, uptake of most Ad serotypes belonging to species A, C, D, E, and F is initiated by binding of the adenovirus fiber proteins to coxsackie and adenovirus receptors on the cell surface.4,5 CD46, a complement regulatory protein that is ubiquitously expressed in humans,6 but only in the testis in mice,7 is a cellular receptor for group B adenoviruses.6
Human species C adenovirus serotype (Ad)5 is the most common viral vector used in clinical studies worldwide.8 In vitro, Ad5 infects cells via fiber binding to coxsackie and adenovirus receptor, followed by binding of an arginine-glycine-aspartic acid (RGD) motif on the Ad5 penton base with cellular integrins (mainly αvβ3 and αvβ5), which initiates receptor-mediated endocytosis via clathrin-coated pits.9,10 However, coxsackie and adenovirus receptor-binding ablation11 and αv integrin-binding ablation11,12 do not significantly reduce liver transduction by adenoviral vectors in vivo. In contrast, the major mechanism of hepatocyte transduction following adenoviral gene transfer in vivo has been suggested to be direct binding of Ad to hepatic heparan sulfate proteoglycans via the KKTK motif within the fiber shaft domain.13,14 Indeed, mutation of the KKTK motif in the Ad5 fiber shaft renders the fiber inflexible and prevents internalization of Ad5 through steric hindrance.15,16 However, in vitro and in vivo infectivity studies of Ad5-based vectors possessing long Ad31 (species A)- or Ad41 (species F)-derived fiber shaft domains that lack the KKTK motif have shown that these vectors transduce hepatocytes with similar efficiency compared with Ad5 vectors,17 consistent with a noncritical role of the KKTK motif in hepatocyte transduction.
An important difference between in vitro and in vivo transduction of liver cells after i.v. injection is that adenoviral vectors are in contact with blood proteins in vivo. Treatment of mice with the vitamin K antagonist warfarin, which inactivates several proteins of the coagulation cascade (factors II, VII, IX, and X) as well as the anticoagulant protein C, abrogates transduction of hepatocytes by Ad5 vectors.18–21 These studies suggested that a coagulation protein or coagulation proteins have a bridging function in the entry of liver cells by adenoviral vectors. Indeed, only factor X can rescue liver transduction in warfarin-anticoagulated mice.22 Recently, it has been shown that the γ-carboxyglutamic acid domain of factor X binds in a calcium-dependent manner to hexon protein in Ad58,22,23 and that this binding occurs at the cup formed by the center of each hexon trimer. Moreover, Ads with a high affinity for factor X, such as the species C serotypes Ad2 and Ad5, have been shown to efficiently transduce hepatocytes following i.v. administration.24,25 In contrast, species B Ad35 and species D Ad26 either bind to factor X weakly or not at all, and these serotypes fail to transduce hepatocytes.22,26,27
More specifically, factor X binds to the adenovirus hexon hypervariable regions. Liver infection by the factor X-Ad5 complex is mediated through a heparin-binding exosite in the factor X serine protease domain. Substitution of hexon hypervariable region 5 or hexon hypervariable region 7 from Ad5 with sequences from the nonfactor X binding serotype Ad26 substantially lowered factor X binding and liver transduction in vivo.28 In addition, an Ad5 mutant containing an insertion in hexon hypervariable region 5 was shown to bind factor X in vitro with 10,000-fold reduced affinity compared with unmodified vector and failed to deliver the red fluorescent protein transgene in vivo.8 Taken together, factor X binding to hexon trimer is a necessary prerequisite for hepatocyte transduction in vivo.22
Liver Trapping of Adenoviral Vectors
Previous studies have shown that different adenoviral serotypes are rapidly sequestered in the liver after intravenous delivery, independent of their potential to effectively transduce hepatocytes.26,29,30 Trapping of adenoviral vectors in the liver is comparable between wild-type mice and mice treated with warfarin, which shows that factor X-facilitated adenoviral vector entry into hepatocytes is not required for trapping of vectors in the liver.20 However, demonstration of liver sequestration using whole livers does not distinguish between the presence of vectors extracellularly (in the vascular lumen of the sinusoids or in the space of Disse) or intracellularly (in the nonparenchymal liver cells of the parenchymal liver cells).
Cellular uptake of adenoviral vectors after systemic gene transfer occurs predominantly in nonparenchymal liver cells (ie, mainly liver sinusoidal endothelial cells and Kupffer cells).31–34 Kupffer cells may bind adenoviral vectors via multiple mechanisms including scavenger receptor-A, complement, and natural antibodies.35–37 In contrast to hepatocytes, uptake of adenoviral vectors by Kupffer cells is independent of factor X.36,37 In addition, interactions of adenoviral vectors with platelets in blood may contribute significantly to sequestration in the reticulo-endothelial system of the liver.35 Nevertheless, the exact mechanisms of adenoviral vector uptake in Kupffer cells have not been elucidated. Indeed, the amount of Ad vector DNA after i.v. administration was nearly identical in wild-type mice and scavenger receptor-A-deficient mice,37 consistent with the presence of multiple pathways leading to Kupffer cell sequestration.36
Recently, Di Paolo et al37 showed that simultaneous treatment of mice with warfarin and clodronate liposomes, which deplete Kupffer cells, results in only a minor reduction of sequestration of adenoviral vectors in the liver 1 hour after gene transfer. Transmission electron microsopy showed the presence of vectors in the space of Disse, consistent with anatomical sequestration of vectors. We suggest that the presence of fenestrae is crucial in this anatomical targeting of adenoviral vectors. This implies that molecular strategies directed at liver detargeting of adenoviral vectors should take into account the existence of anatomical targeting to the liver.
Uptake of Gene Transfer Vectors by Reticulo-Endothelial Cells of the Liver Reduces Hepatocyte Transduction
Both Kupffer cells and liver sinusoidal endothelial cells take up the large majority of adenoviral vectors after systemic gene transfer.34 Indeed, uptake of vectors by nonparenchymal liver cells (ie, mainly liver sinusoidal endothelial cells and Kupffer cells) inversely correlates with transduction of parenchymal liver cells.34 The transgene DNA copy number in the nonparenchymal liver cells at 1 hour after transfer in BALB/c mice was nearly sixfold higher than in C57BL/6 mice.34 This difference in scavenging of vectors between these strains is a major determinant of the approximately threefold higher transgene DNA levels in hepatocytes and higher transgene expression levels in C57BL/6 mice compared with BALB/c mice.34 On the basis of more refined experiments with isolation of Kupffer cells and liver sinusoidal endothelial cells, we showed that the transgene DNA copy number per diploid genome at 1 hour after transfer in C57BL/6 mice was 2.9-fold higher in liver sinusoidal endothelial cells than in Kupffer cells.34 In contrast, the copy number in Kupffer cells was 2.6-fold higher than in liver sinusoidal endothelial cells in BALB/c mice. These data indicate that the relative contribution of liver sinusoidal endothelial cells and Kupffer cells to adenoviral vector clearance may be highly dependent on the specific genetic context.
One explanation for this difference of uptake of adenoviral vectors by the liver reticulo-endothelial cells of C57BL/6 and BALB/c mice may be the differential modulation of the function of these cells by humoral factors produced by splenocytes. Indeed, a significantly reduced transgene DNA copy number was observed in the liver reticulo-endothelial cells 1 hour after adenoviral transfer in splenectomized BALB/c mice and in BALB/c rag-2−/− mice compared with control BALB/c mice.34 This was accompanied by a significantly higher transgene DNA copy number in hepatocytes of splenectomized BALB/c mice and of BALB/c rag-2−/− mice than in hepatocytes of wild-type BALB/c mice.34 Splenectomy in BALB/c rag-2−/− mice did not result in an additive effect.34 This suggests that humoral factors produced by spleen lymphocytes may affect the clearance of adenoviral vectors by liver reticulo-endothelial cells in BALB/c mice. In contrast, no such effects on intrahepatic transgene DNA distribution were observed in splenectomized C57BL/6 mice and in C57BL/6 rag-1−/− mice, suggesting highly heterogeneous effects of humoral factors produced by spleen lymphocytes on liver reticulo-endothelial cells.
Further evidence for a major role of liver reticulo-endothelial cells as a determinant of hepatocyte transduction comes from experiments with clodronate liposomes. Depletion of Kupffer cells and macrophages in the spleen by i.v. administration of clodronate liposomes results in significantly increased transgene DNA levels in parenchymal liver cells34 and in increased transgene expression.33,34,38,39 Since liver sinusoidal endothelial cell function may be modified by Kupffer cells,40,41 it cannot be excluded that part of the effect of clodronate liposomes is due to reduced activation of liver sinusoidal endothelial cells by Kupffer cells. Besides clodronate liposomes, preadministration of polyinosinic acid, a scavenger receptor A ligand, before gene transfer has been shown to prevent sequestration of adenoviral vectors in Kupffer cells and to enhance parenchymal liver cell transduction.42 Transient saturation of the reticulo-endothelial system with phosphatidylcholine liposomes or with Intralipid also reduces uptake of vectors in the nonparenchymal liver cells and augments hepatocyte transduction.34 Taken together, various interventions that result in reduced uptake of adenoviral vectors in liver reticulo-endothelial cells consistently enhance hepatocyte transduction.
Liver Sinusoidal Endothelial Fenestrae
Liver sinusoids are highly specialized capillaries with two critical features: the thin endothelium contains open fenestrae and a basal lamina is lacking.43 Fenestrae are clustered in sieve plates and provide an open pathway between the sinusoidal lumen and the space of Disse, in which numerous microvilli from parenchymal liver cells protrude.43 Sinusoidal fenestrae have no diaphragm, and visualization requires perfusion fixation with glutaraldehyde. Scanning electron microscopy analysis has shown that sinusoidal fenestrae comprise 6 to 8% of the sinusoidal surface.44 Compared with the centrilobular area, the diameter of fenestrae is larger, but the frequency of fenestrae is lower in the periportal area.44,45
The open communication between the sinusoidal lumen and the space of Disse through fenestrae represents a unique route that provides direct access for gene transfer vectors to the surface of hepatocytes. However, fenestrae act as a sieve and will mechanically restrict the transport of gene transfer vectors according to their size. Thus, two parameters must be taken into account when considering the access of gene transfer vectors to hepatocytes: the diameter of both fenestrae and gene transfer vectors.
Species Variation of the Average Diameter of Fenestrae
Fenestrae generally measure between 100 and 200 nm, although significant species-specific differences in their size exist.43,44 However, the interpretation of the existing literature on species variations of the size of sinusoidal fenestrae is hampered by differences in preparatory methods applied by different investigators. Standardized protocols within one group of investigators are therefore a conditio sine qua non for reliable species and strain comparisons. A direct comparative study of the diameter of sinusoidal fenestrae in five species using scanning electron microscopy was performed by Higashi et al.46 The average diameter of sinusoidal endothelial fenestrae in this study was 45 nm in cows, 52 nm in sheep, 66 nm in guinea pigs, 82 nm in pigs, and 131 nm in dogs.46 However, these results are based on scanning electron microscopy preparations and are therefore subject to a shrinkage effect in the order of 30% caused by dehydration and drying of the tissue.
Accurate measurements of fenestrae can only be obtained by gradually replacing cellular water by plastic during preparation for transmission electron microscopy. Previous studies have shown that this method of preparation leads to accurate measurements of cellular details, such as fenestrae. Visualization of fenestrae in transmission electron microscopy sections requires that endothelial cells and their sieve plates are cut tangentially so that fenestrae become visible as holes. Using this technology and standardized protocols, we have previously shown that the average diameter of fenestrae is significantly larger in Sprague Dawley rats (150 nm in the pericentral area and 175 nm in the periportal area)44 and C57BL/6 mice (141 nm)47 than in New Zealand White rabbits (103 nm),47 Fauve de Bourgogne rabbits (105 nm),48 and humans with a healthy liver (107 nm).49 The diameter in Dutch Belt rabbits was intermediate (124 nm).48 Taken together, this species comparison demonstrates that the diameter of fenestrae in humans is similar to New Zealand White rabbits and significantly smaller compared with mice and rats, two species that are most frequently used in gene transfer studies.
A representative scanning electron micrograph showing sinusoidal fenestrae, mostly grouped in sieve plates, in normal human liver is shown in Figure 1, A and B, shows a transmission electron micrograph of a human liver sinusoid. Because the endothelial lining is cut tangentially, fenestrae appear as complete holes in the endothelium (Figure 1B). Transmission electron microscopy studies consistently show that the interindividual variation of the average diameter of fenestrae within the same species or strain is low, as indicated by coefficients of variation between 3 and 8%. In contrast, as will be discussed in the next paragraph, the intraindividual variation of diameters of fenestrae is high.
Figure 1.
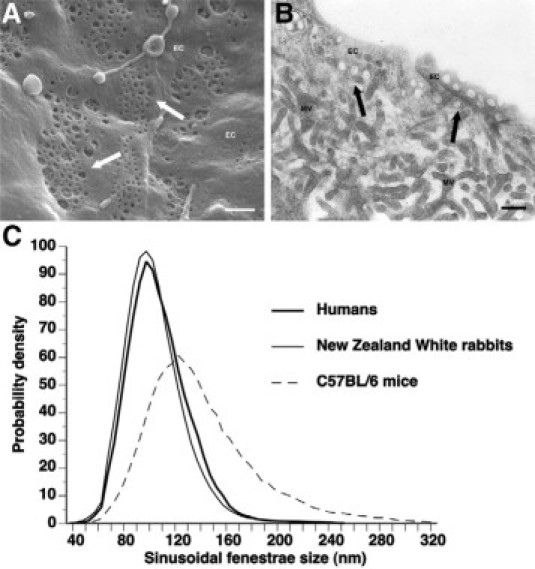
A: Scanning electron micrograph of the endothelial lining of a human liver sinusoid. Sinusoidal fenestrae, mostly grouped in sieve plates, appear as openings in the endothelial lining and are indicated by arrows. EC, endothelial cell. Scale bar represents 500 nm. B: Transmission electron micrograph of fenestrae in a human liver sinusoid. The endothelial lining is cut tangentially and shows the occurrence of fenestrae as complete holes in the endothelium. Fenestrae are indicated by arrows. EC, endothelial cell; MV, microvillous surface of hepatocytes. The right top corner of the picture shows the lumen of the sinusoid. Scale bar represents 250 nm. C: Distribution of sinusoidal liver fenestrae in humans, New Zealand White rabbits, and C57BL/6 mice. Data constitute an extrapolation to an infinite number of measurements of previously published frequency distribution histograms.47,49
Intraindividual Variation of the Diameter of Fenestrae
The intraindividual variation of the diameter of fenestrae is an important parameter that may complicate investigations on the relation between the diameter of fenestrae and gene transfer efficiency to hepatocytes. Figure 1C shows the distribution curves for humans, New Zealand White rabbits, and C57BL/6 mice. These plots were generated by extrapolating the data of frequency distribution histograms47,49 with a finite number of observations in each individual to curves corresponding to an infinite number of observations. The distribution curves in Figure 1C show a nearly perfect overlap for humans and New Zealand White rabbits, whereas the distribution in C57BL/6 mice is significantly different compared with humans.
The schematic drawing of a sinusoid in Figure 2 illustrates that two opposing processes will determine the entrance of vectors into the space of Disse: on the one hand passage through sinusoidal fenestrae and on the other hand endocytosis by Kupffer cells and endothelial cells. Species and strain differences in transendothelial passage will be determined by intrinsic differences of the function of liver reticulo-endothelial cells (eg, C57BL/6 versus BALB/c mice) as well as the rate passage of vectors through fenestrae. On the basis of these considerations, one can predict that the ratio of transgene DNA copy number in parenchymal liver cells versus the copy number in sinusoidal liver cells will correlate positively with the diameter of fenestrae. After reviewing data on the diameters of different gene transfer vectors, we will present several lines of experimental evidence that support the critical role of the diameter of fenestrae in hepatocyte transduction.
Figure 2.
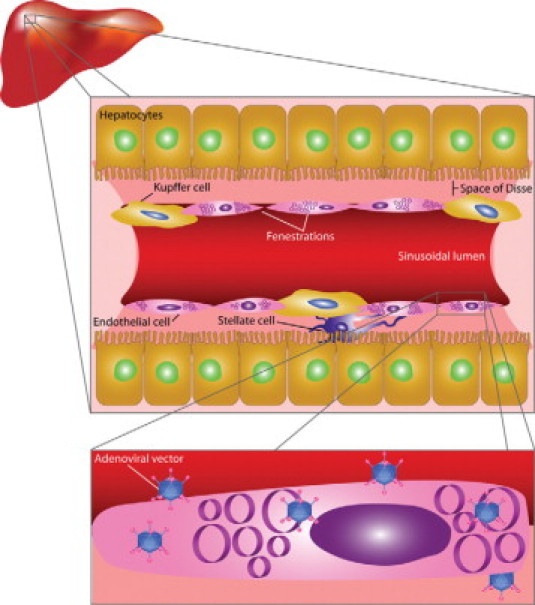
Schematic drawing of a liver sinusoid. The sinusoidal wall is formed by liver sinusoidal endothelial cells and juxtaposed Kupffer cells. Whereas endocytosis of gene transfer vectors by Kupffer cells and liver sinusoidal endothelial cells limits hepatocyte transduction, the presence of fenestrae in liver sinusoidal endothelial cells provides a direct access to the space of Disse and the microvillous surface of hepatocytes and may allow transcellular migration of vectors. As indicated in the bottom panel, the diameter of fenestrae may restrict the passage of gene transfer vectors according to their size.
Diameters of Gene Transfer Vectors
To put the importance of the size of fenestrae for hepatocyte-directed gene transfer into perspective, accurate knowledge of the diameter of gene transfer vectors is required. To avoid bias in the measurement of the diameter of adenoviral vectors, we previously vitrified a sample of adenoviral vectors using Vitrobot technology and determined the diameter by cryoelectron microscopy.47 Ad5 virions were shown to have a diameter of 93 nm with protruding fibers of 30 nm.47 Using the same imaging techniques, the diameter of a vesicular stomatitis virus-G-pseudotyped, HIV-1-derived lentiviral vector was found to be 150 nm.47 Adeno-associated viral serotype 2 vectors have an average diameter of 22 nm.50 Herpes simplex virions have been reported to be as large as 180 nm.51 The diameter of liposomes used for nonviral gene transfer varies between 50 and 1000 nm and is highly dependent on production parameters.52
Experimental Evidence for a Critical Role of Sinusoidal Fenestrae in Hepatocyte Transduction following Adenoviral Gene Transfer
On the basis of our prior studies in different strains of rabbits and in different species,47–49 the correlation coefficient between the average diameter of sinusoidal fenestrae in these different strains and species and human apo A-I expression at day 7 after transfer with an adenoviral vector containing a hepatocyte-specific expression cassette was found to be 0.94 (P < 0.01). This strongly suggests that the diameter of sinusoidal fenestrae is an important determinant of gene transfer efficiency to hepatocytes.
To demonstrate that the difference of human apo A-I plasma levels reflects differences of transgene DNA levels in parenchymal liver cells, we isolated parenchymal and nonparenchymal liver cells at day 3 after transfer in C57BL/6 mice and New Zealand White rabbits. Transgene DNA levels in parenchymal liver cells were much higher in C57BL/6 mice than in New Zealand White rabbits, whereas the reverse pattern was observed in nonparenchymal liver cells.47 Considering the small average diameter of fenestrae in New Zealand White rabbits (103 nm), it appears that the sinusoidal wall constitutes a histological barrier for adenoviral vectors in this species, leading to increased uptake by liver reticulo-endothelial cells. In contrast, the larger fenestrae in C57BL/6 mice (141 nm) facilitate access to hepatocytes, leading to increased uptake into hepatocytes and to reduced scavenging by Kupffer cells and liver sinusoidal endothelial cells. In other words, the size of fenestrae determines the distribution of vectors between sinusoidal and parenchymal liver cells.
Although the relation between the diameter of sinusoidal fenestrae and transgene expression after adenoviral gene transfer may be confounded by substantial differences in genetic background, we showed that interventions that increase the diameter of fenestrae result in significantly increased transgene expression in New Zealand White rabbits.47,48 These intervention studies support the view that the correlation between the diameter of fenestrae and transgene expression after adenoviral transfer reflects a causal relationship.
On the basis of the high degree of similarity of the distribution of the diameter of fenestrae between humans and New Zealand White rabbits (Figure 1C), one would predict a low efficiency of gene transfer into hepatocytes after adenoviral transfer in humans. In the ornithine transcarbamylase deficiency trial, low levels of gene transfer in hepatocytes were indeed observed.53 The authors concluded that the level of transgene expression was lower than what would have been predicted based on preclinical animal models.53 Although histological alterations of the livers in patients with partial ornithine transcarbamylase deficiency may have contributed to low hepatocyte transduction, we speculate that a much smaller size of fenestrae in humans compared with mice and rats is likely the most critical factor in the observed species difference of hepatocyte transduction. On the other hand, the small diameter of fenestrae in humans may be beneficial for the efficacy of molecular strategies directed at liver detargeting of adenoviral vectors since anatomical targeting to the liver will be limited.
Recently, Brunetti-Pierri et al54 developed a minimally invasive procedure that significantly improves the efficiency of hepatocyte-directed transfer in nonhuman primates. A balloon occlusion catheter was percutaneously positioned in the inferior vena cava to occlude hepatic venous outflow.54 Gene transfer of gutted vectors was performed via a percutaneously placed hepatic artery catheter with an infusion time of 7.5 or 15 minutes. This procedure resulted in ∼10-fold higher transgene expression levels compared with systemic gene transfer. Increased intrahepatic pressure following occlusion of hepatic outflow of the liver may increase the diameter of fenestrae, similar as observed following hydrodynamic injections in mice,55 and this may contribute to the beneficial effects of this procedure in monkeys.
Potential Relevance of Sinusoidal Fenestrae for Other Modes of Hepatocyte-Directed Gene Transfer
On the basis of the data obtained with adenoviral vectors, it is likely that the large diameter of lentiviral vectors is an important limitation for hepatocyte-directed gene transfer and may restrict passage of vectors even in mice and rats. Indeed, gene transfer efficiency in mice and rats is low after in vivo lentiviral gene transfer.56–58 Although other factors like technological challenges to obtain high titer vector stocks may play a role, it is likely that the large diameter of lentiviral vectors is a limitation for hepatocyte-directed gene transfer. Because this anatomical limitation does not exist for adeno-associated viral vectors, gene transfer efficiency into hepatocytes with this type of vectors will be solely dependent on cellular and molecular determinants of hepatocyte transduction.
Fenestrae may also play a role in naked DNA transfer. Liu et al59 showed that the murine liver can rapidly extract up to 25 μg of plasmid DNA from the blood during a single pass after simple i.v. injection. Moreover, this study showed that naked DNA is primarily taken up by the liver endothelial cells, but not by Kupffer cells, and that transfection of hepatocytes can be improved by mechanical massage of the liver, which increases the size of liver sinusoidal fenestrae.59 Substantial amounts of plasmid DNA are degraded by nucleases in the blood following simple i.v. injection, which can be overcome by hydrodynamic gene transfer.
Fenestrae have also been proposed to play a role in the transport of naked DNA into hepatocytes during hydrodynamic gene transfer.55 Although the exact mechanism of hepatocyte transfection following hydrodynamic gene transfer remains to be elucidated, a general consensus is that the injected volume induces right heart volume overload. This results in a retrograde flow through the vena cava and in particular in a retrograde flow into the hepatic veins. As a result, intrahepatic pressure increases, and the DNA containing solution is forced out of the hepatic sinusoids into the parenchymal liver cells. After systemic hydrodynamic gene transfer in mice and rats, the majority of the injected DNA (ie, >90%) can be retrieved in the liver.60 In addition, microscopic analysis has indicated that transfected hepatocytes are predominantly located in the pericentral region.61 This predilection may be explained by the fact that sinusoids are wider and straighter and contain more fenestrae per unit of surface in the pericentral area than in the periportal area.45,60
The question remains of how nucleic acids are taken up by hepatocytes. Initially, it was postulated that injected DNA was taken up via a receptor-mediated process.62 At present, multiple lines of evidence, including the quick and random uptake of various structurally unrelated molecules by hepatocytes,55,61,63–65 the rapid onset of liver transaminases in the blood,64,66 and electronmicroscopic observations55,61 all support a mechanism wherein transient membrane defects result in DNA uptake by hepatocytes, so-called “hydroporation.”55 Alternatively, Crespo et al67 proposed, based on observations of large numbers of endocytotic vesicles in the absence of membrane defects, that cellular uptake of nucleic acids occurs via a microfluid uptake process. Further studies are warranted to delineate the importance of each of these pathways in the uptake and expression of nucleic acids by hepatocytes following hydrodynamic gene transfer.
Sinusoidal Fenestrae and Hepatocyte Transduction in Diseased Livers
The unique morphological features of liver sinusoidal endothelial cells may change in pathological conditions. Liver fibrosis and cirrhosis lead to a decreased number of fenestrae,68 and capillarization and perisinusoidal fibrosis leads to the development of a basal lamina, found to be absent in normal sinusoids. A significant reduction in the number of fenestrae and porosity of the sinusoidal endothelial cells was observed in alcoholic liver disease without cirrhosis.69 In a comparative study, decreased transduction by adenoviral vectors has been observed in cirrhotic rat livers compared with normal livers.70 Furthermore, hydrodynamic gene transfer was significantly less efficient in rats with a fibrotic liver compared with rats with a healthy liver.71 Sinusoidal capillarization also occurs in hepatocellular carcinoma.72 This may constitute a major obstacle for efficient gene therapy for liver cancers.
Conclusion
Preclinical viral and nonviral gene transfer studies should consider scavenging of vectors by liver reticulo-endothelial cells and the diameter of sinusoidal fenestrae as important determinants of gene transfer efficiency into hepatocytes. Although the diameter of fenestrae may be modulated to some extent, there is currently no safe pharmacological intervention that results in a significant enlargement of fenestrae. The small diameter of fenestrae in humans and alterations of liver sinusoidal endothelial cells in liver disease may constitute a significant and potentially insurmountable obstacle for efficient gene transfer into hepatocytes with several vectors. As outlined in this review for in vivo transduction of hepatocytes by adenoviral vectors, both anatomical access of vectors to the space of Disse on the one hand and interaction of vectors with hepatocyte receptors in vivo on the other hand are necessary for efficient hepatocyte transduction in vivo. A model on hepatocyte transduction should therefore take into account that both an anatomical prerequisite and a molecular prerequisite have to be met.
Footnotes
Supported by grants G.0322.06 and G.0533.08 of the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen. The Center for Molecular and Vascular Biology is supported by Excellentiefinanciering KU Leuven (EF/05/013). Frank Jacobs is a postdoctoral fellow of the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen.
References
- 1.Blouin A, Bolender RP, Weibel ER. Distribution of organelles and membranes between hepatocytes and nonhepatocytes in the rat liver parenchyma: a stereological study. J Cell Biol. 1977;72:441–455. doi: 10.1083/jcb.72.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Do H, Healey JF, Waller EK, Lollar P. Expression of factor VIII by murine liver sinusoidal endothelial cells. J Biol Chem. 1999;274:19587–19592. doi: 10.1074/jbc.274.28.19587. [DOI] [PubMed] [Google Scholar]
- 3.Shiratori Y, Tananka M, Kawase T, Shiina S, Komatsu Y, Omata M. Quantification of sinusoidal cell function in vivo. Semin Liver Dis. 1993;13:39–49. doi: 10.1055/s-2007-1007336. [DOI] [PubMed] [Google Scholar]
- 4.Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 5.Tomko RP, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaggar A, Shayakhmetov DM, Lieber A. CD46 is a cellular receptor for group B adenoviruses. Nat Med. 2003;9:1408–1412. doi: 10.1038/nm952. [DOI] [PubMed] [Google Scholar]
- 7.Tsujimura A, Shida K, Kitamura M, Nomura M, Takeda J, Tanaka H, Matsumoto M, Matsumiya K, Okuyama A, Nishimune Y, Okabe M, Seya T. Molecular cloning of a murine homologue of membrane cofactor protein (CD46): preferential expression in testicular germ cells. Biochem J. 1998;330(Pt 1):163–168. doi: 10.1042/bj3300163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalyuzhniy O, Di Paolo NC, Silvestry M, Hofherr SE, Barry MA, Stewart PL, Shayakhmetov DM. Adenovirus serotype 5 hexon is critical for virus infection of hepatocytes in vivo. Proc Natl Acad Sci USA. 2008;105:5483–5488. doi: 10.1073/pnas.0711757105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 10.Lowenstein PR. With a little help from my f(X)riends! The basis of Ad5-mediated transduction of the liver revealed. Mol Ther. 2008;16:1004–1006. doi: 10.1038/mt.2008.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizuguchi H, Koizumi N, Hosono T, Ishii-Watabe A, Uchida E, Utoguchi N, Watanabe Y, Hayakawa T. CAR- or αv integrin-binding ablated adenovirus vectors, but not fiber-modified vectors containing RGD peptide, do not change the systemic gene transfer properties in mice. Gene Ther. 2002;9:769–776. doi: 10.1038/sj.gt.3301701. [DOI] [PubMed] [Google Scholar]
- 12.Hautala T, Grunst T, Fabrega A, Freimuth P, Welsh MJ. An interaction between penton base and αv integrins plays a minimal role in adenovirus-mediated gene transfer to hepatocytes in vitro and in vivo. Gene Ther. 1998;5:1259–1264. doi: 10.1038/sj.gt.3300722. [DOI] [PubMed] [Google Scholar]
- 13.Smith TA, Idamakanti N, Rollence ML, Marshall-Neff J, Kim J, Mulgrew K, Nemerow GR, Kaleko M, Stevenson SC. Adenovirus serotype 5 fiber shaft influences in vivo gene transfer in mice. Hum Gene Ther. 2003;14:777–787. doi: 10.1089/104303403765255165. [DOI] [PubMed] [Google Scholar]
- 14.Smith TA, Idamakanti N, Marshall-Neff J, Rollence ML, Wright P, Kaloss M, King L, Mech C, Dinges L, Iverson WO, Sherer AD, Markovits JE, Lyons RM, Kaleko M, Stevenson SC. Receptor interactions involved in adenoviral-mediated gene delivery after systemic administration in non-human primates. Hum Gene Ther. 2003;14:1595–1604. doi: 10.1089/104303403322542248. [DOI] [PubMed] [Google Scholar]
- 15.Kritz AB, Nicol CG, Dishart KL, Nelson R, Holbeck S, Von Seggern DJ, Work LM, McVey JH, Nicklin SA, Baker AH. Adenovirus 5 fibers mutated at the putative HSPG-binding site show restricted retargeting with targeting peptides in the HI loop. Mol Ther. 2007;15:741–749. doi: 10.1038/sj.mt.6300094. [DOI] [PubMed] [Google Scholar]
- 16.Bayo-Puxan N, Cascallo M, Gros A, Huch M, Fillat C, Alemany R. Role of the putative heparan sulfate glycosaminoglycan-binding site of the adenovirus type 5 fiber shaft on liver detargeting and knob-mediated retargeting. J Gen Virol. 2006;87:2487–2495. doi: 10.1099/vir.0.81889-0. [DOI] [PubMed] [Google Scholar]
- 17.Di Paolo NC, Kalyuzhniy O, Shayakhmetov DM. Fiber shaft-chimeric adenovirus vectors lacking the KKTK motif efficiently infect liver cells in vivo. J Virol. 2007;81:12249–12259. doi: 10.1128/JVI.01584-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parker AL, Waddington SN, Nicol CG, Shayakhmetov DM, Buckley SM, Denby L, Kemball-Cook G, Ni S, Lieber A, McVey JH, Nicklin SA, Baker AH. Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes. Blood. 2006;108:2554–2561. doi: 10.1182/blood-2006-04-008532. [DOI] [PubMed] [Google Scholar]
- 19.Parker AL, McVey JH, Doctor JH, Lopez-Franco O, Waddington SN, Havenga MJ, Nicklin SA, Baker AH. Influence of coagulation factor zymogens on the infectivity of adenoviruses pseudotyped with fibers from subgroup D. J Virol. 2007;81:3627–3631. doi: 10.1128/JVI.02786-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waddington SN, Parker AL, Havenga M, Nicklin SA, Buckley SM, McVey JH, Baker AH. Targeting of adenovirus serotype 5 (Ad5) and 5/47 pseudotyped vectors in vivo: fundamental involvement of coagulation factors and redundancy of CAR binding by Ad5. J Virol. 2007;81:9568–9571. doi: 10.1128/JVI.00663-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shayakhmetov DM, Gaggar A, Ni S, Li ZY, Lieber A. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J Virol. 2005;79:7478–7491. doi: 10.1128/JVI.79.12.7478-7491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waddington SN, McVey JH, Bhella D, Parker AL, Barker K, Atoda H, Pink R, Buckley SM, Greig JA, Denby L, Custers J, Morita T, Francischetti IM, Monteiro RQ, Barouch DH, van Rooijen N, Napoli C, Havenga MJ, Nicklin SA, Baker AH. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008;132:397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 23.Vigant F, Descamps D, Jullienne B, Esselin S, Connault E, Opolon P, Tordjmann T, Vigne E, Perricaudet M, Benihoud K. Substitution of hexon hypervariable region 5 of adenovirus serotype 5 abrogates blood factor binding and limits gene transfer to liver. Mol Ther. 2008;16:1474–1480. doi: 10.1038/mt.2008.132. [DOI] [PubMed] [Google Scholar]
- 24.Morral N, O'Neal W, Rice K, Leland M, Kaplan J, Piedra PA, Zhou H, Parks RJ, Velji R, Aguilar-Cordova E, Wadsworth S, Graham FL, Kochanek S, Carey KD, Beaudet AL. Administration of helper-dependent adenoviral vectors and sequential delivery of different vector serotype for long-term liver-directed gene transfer in baboons. Proc Natl Acad Sci USA. 1999;96:12816–12821. doi: 10.1073/pnas.96.22.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parks R, Evelegh C, Graham F. Use of helper-dependent adenoviral vectors of alternative serotypes permits repeat vector administration. Gene Ther. 1999;6:1565–1573. doi: 10.1038/sj.gt.3300995. [DOI] [PubMed] [Google Scholar]
- 26.Sakurai F, Mizuguchi H, Yamaguchi T, Hayakawa T. Characterization of in vitro and in vivo gene transfer properties of adenovirus serotype 35 vector. Mol Ther. 2003;8:813–821. doi: 10.1016/s1525-0016(03)00243-0. [DOI] [PubMed] [Google Scholar]
- 27.Seshidhar Reddy P, Ganesh S, Limbach MP, Brann T, Pinkstaff A, Kaloss M, Kaleko M, Connelly S. Development of adenovirus serotype 35 as a gene transfer vector. Virology. 2003;311:384–393. doi: 10.1016/s0042-6822(03)00161-2. [DOI] [PubMed] [Google Scholar]
- 28.Alba R, Bradshaw AC, Parker AL, Bhella D, Waddington SN, Nicklin SA, van Rooijen N, Custers J, Goudsmit J, Barouch DH, McVey JH, Baker AH. Identification of coagulation factor (F)X binding sites on the adenovirus serotype 5 hexon: effect of mutagenesis on FX interactions and gene transfer. Blood. 2009;114:965–971. doi: 10.1182/blood-2009-03-208835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stone D, Ni S, Li ZY, Gaggar A, DiPaolo N, Feng Q, Sandig V, Lieber A. Development and assessment of human adenovirus type 11 as a gene transfer vector. J Virol. 2005;79:5090–5104. doi: 10.1128/JVI.79.8.5090-5104.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stone D, Liu Y, Li ZY, Tuve S, Strauss R, Lieber A. Comparison of adenoviruses from species B, C, E, and F after intravenous delivery. Mol Ther. 2007;15:2146–2153. doi: 10.1038/sj.mt.6300319. [DOI] [PubMed] [Google Scholar]
- 31.Alemany R, Suzuki K, Curiel DT. Blood clearance rates of adenovirus type 5 in mice. J Gen Virol. 2000;81:2605–2609. doi: 10.1099/0022-1317-81-11-2605. [DOI] [PubMed] [Google Scholar]
- 32.Tao N, Gao GP, Parr M, Johnston J, Baradet T, Wilson JM, Barsoum J, Fawell SE. Sequestration of adenoviral vector by Kupffer cells leads to a nonlinear dose response of transduction in liver. Mol Ther. 2001;3:28–35. doi: 10.1006/mthe.2000.0227. [DOI] [PubMed] [Google Scholar]
- 33.Wolff G, Worgall S, van Rooijen N, Song WR, Harvey BG, Crystal RG. Enhancement of in vivo adenovirus-mediated gene transfer and expression by prior depletion of tissue macrophages in the target organ. J Virol. 1997;71:624–629. doi: 10.1128/jvi.71.1.624-629.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snoeys J, Mertens G, Lievens J, van Berkel T, Collen D, Biessen EA, De Geest B. Lipid emulsions potently increase transgene expression in hepatocytes after adenoviral transfer. Mol Ther. 2006;13:98–107. doi: 10.1016/j.ymthe.2005.06.477. [DOI] [PubMed] [Google Scholar]
- 35.Stone D, Liu Y, Shayakhmetov D, Li ZY, Ni S, Lieber A. Adenovirus-platelet interaction in blood causes virus sequestration to the reticuloendothelial system of the liver. J Virol. 2007;81:4866–4871. doi: 10.1128/JVI.02819-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu Z, Tian J, Smith JS, Byrnes AP. Clearance of adenovirus by Kupffer cells is mediated by scavenger receptors, natural antibodies, and complement. J Virol. 2008;82:11705–11713. doi: 10.1128/JVI.01320-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Paolo NC, van Rooijen N, Shayakhmetov DM. Redundant and synergistic mechanisms control the sequestration of blood-born adenovirus in the liver. Mol Ther. 2009;17:675–684. doi: 10.1038/mt.2008.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuzmin AI, Finegold MJ, Eisensmith RC. Macrophage depletion increases the safety, efficacy and persistence of adenovirus-mediated gene transfer in vivo. Gene Ther. 1997;4:309–316. doi: 10.1038/sj.gt.3300377. [DOI] [PubMed] [Google Scholar]
- 39.Schiedner G, Hertel S, Johnston M, Dries V, van Rooijen N, Kochanek S. Selective depletion or blockade of Kupffer cells leads to enhanced and prolonged hepatic transgene expression using high-capacity adenoviral vectors. Mol Ther. 2003;7:35–43. doi: 10.1016/s1525-0016(02)00017-5. [DOI] [PubMed] [Google Scholar]
- 40.Niwano M, Arii S, Monden K, Ishiguro S, Nakamura T, Mizumoto M, Takeda Y, Fujioka M, Imamura M. Amelioration of sinusoidal endothelial cell damage by Kupffer cell blockade during cold preservation of rat liver. J Surg Res. 1997;72:36–48. doi: 10.1006/jsre.1997.5162. [DOI] [PubMed] [Google Scholar]
- 41.Deaciuc IV, Bagby GJ, Niesman MR, Skrepnik N, Spitzer JJ. Modulation of hepatic sinusoidal endothelial cell function by Kupffer cells: an example of intercellular communication in the liver. Hepatology. 1994;19:464–470. [PubMed] [Google Scholar]
- 42.Haisma HJ, Kamps JA, Kamps GK, Plantinga JA, Rots MG, Bellu AR. Polyinosinic acid enhances delivery of adenovirus vectors in vivo by preventing sequestration in liver macrophages. J Gen Virol. 2008;89:1097–1105. doi: 10.1099/vir.0.83495-0. [DOI] [PubMed] [Google Scholar]
- 43.Wisse E. An electron microscopic study of the fenestrated endothelial lining of rat liver sinusoids. J Ultrastruct Res. 1970;31:125–150. doi: 10.1016/s0022-5320(70)90150-4. [DOI] [PubMed] [Google Scholar]
- 44.Wisse E, De Zanger RB, Charels K, Van Der Smissen P, McCuskey RS. The liver sieve: considerations concerning the structure and function of endothelial fenestrae, the sinusoidal wall and the space of Disse. Hepatology. 1985;5:683–692. doi: 10.1002/hep.1840050427. [DOI] [PubMed] [Google Scholar]
- 45.Wisse E, De Zanger RB, Jacobs R, McCuskey RS. Scanning electron microscope observations on the structure of portal veins, sinusoids and central veins in rat liver. Scan Electron Microsc. 1983:1441–1452. [PubMed] [Google Scholar]
- 46.Higashi N, Ueda H, Yamada O, Oikawa S, Koiwa M, Tangkawattana P, Takehana K. Micromorphological characteristics of hepatic sinusoidal endothelial cells and their basal laminae in five different animal species. Okajimas Folia Anat Jpn. 2002;79:135–142. doi: 10.2535/ofaj.79.135. [DOI] [PubMed] [Google Scholar]
- 47.Snoeys J, Lievens J, Wisse E, Jacobs F, Duimel H, Collen D, Frederik P, De Geest B. Species differences in transgene DNA uptake in hepatocytes after adenoviral transfer correlate with the size of endothelial fenestrae. Gene Ther. 2007;14:604–612. doi: 10.1038/sj.gt.3302899. [DOI] [PubMed] [Google Scholar]
- 48.Lievens J, Snoeys J, Vekemans K, Van Linthout S, de Zanger R, Collen D, Wisse E, De Geest B. The size of sinusoidal fenestrae is a critical determinant of hepatocyte transduction after adenoviral gene transfer. Gene Ther. 2004;11:1523–1531. doi: 10.1038/sj.gt.3302326. [DOI] [PubMed] [Google Scholar]
- 49.Wisse E, Jacobs F, Topal B, Frederik P, De Geest B. The size of endothelial fenestrae in human liver sinusoids: implications for hepatocyte-directed gene transfer. Gene Ther. 2008;15:1193–1199. doi: 10.1038/gt.2008.60. [DOI] [PubMed] [Google Scholar]
- 50.Chen H. Comparative observation of the recombinant adeno-associated virus 2 using transmission electron microscopy and atomic force microscopy. Microsc Microanal. 2007;13:384–389. doi: 10.1017/S1431927607070808. [DOI] [PubMed] [Google Scholar]
- 51.Szilagyi JF, Berriman J. Herpes simplex virus L particles contain spherical membrane-enclosed inclusion vesicles. J Gen Virol. 1994;75(Pt 7):1749–1753. doi: 10.1099/0022-1317-75-7-1749. [DOI] [PubMed] [Google Scholar]
- 52.Banerjee R. Liposomes: applications in medicine. J Biomater Appl. 2001;16:3–21. doi: 10.1106/RA7U-1V9C-RV7C-8QXL. [DOI] [PubMed] [Google Scholar]
- 53.Raper SE, Yudkoff M, Chirmule N, Gao GP, Nunes F, Haskal ZJ, Furth EE, Propert KJ, Robinson MB, Magosin S, Simoes H, Speicher L, Hughes J, Tazelaar J, Wivel NA, Wilson JM, Batshaw ML. A pilot study of in vivo liver-directed gene transfer with an adenoviral vector in partial ornithine transcarbamylase deficiency. Hum Gene Ther. 2002;13:163–175. doi: 10.1089/10430340152712719. [DOI] [PubMed] [Google Scholar]
- 54.Brunetti-Pierri N, Stapleton GE, Law M, Breinholt J, Palmer DJ, Zuo Y, Grove NC, Finegold MJ, Rice K, Beaudet AL, Mullins CE, Ng P. Efficient, long-term hepatic gene transfer using clinically relevant HDAd doses by balloon occlusion catheter delivery in nonhuman primates. Mol Ther. 2009;17:327–333. doi: 10.1038/mt.2008.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang G, Gao X, Song YK, Vollmer R, Stolz DB, Gasiorowski JZ, Dean DA, Liu D. Hydroporation as the mechanism of hydrodynamic delivery. Gene Ther. 2004;11:675–682. doi: 10.1038/sj.gt.3302210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Follenzi A, Sabatino G, Lombardo A, Boccaccio C, Naldini L. Efficient gene delivery and targeted expression to hepatocytes in vivo by improved lentiviral vectors. Hum Gene Ther. 2002;13:243–260. doi: 10.1089/10430340252769770. [DOI] [PubMed] [Google Scholar]
- 57.Kang Y, Xie L, Tran DT, Stein CS, Hickey M, Davidson BL, McCray PB., Jr Persistent expression of factor VIII in vivo following nonprimate lentiviral gene transfer. Blood. 2005;106:1552–1558. doi: 10.1182/blood-2004-11-4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nguyen TH, Aubert D, Bellodi-Privato M, Flageul M, Pichard V, Jaidane-Abdelghani Z, Myara A, Ferry N. Critical assessment of lifelong phenotype correction in hyperbilirubinemic Gunn rats after retroviral mediated gene transfer. Gene Ther. 2007;14:1270–1277. doi: 10.1038/sj.gt.3302993. [DOI] [PubMed] [Google Scholar]
- 59.Liu F, Shollenberger LM, Conwell CC, Yuan X, Huang L. Mechanism of naked DNA clearance after intravenous injection. J Gene Med. 2007;9:613–619. doi: 10.1002/jgm.1054. [DOI] [PubMed] [Google Scholar]
- 60.Herweijer H, Wolff JA. Gene therapy progress and prospects: hydrodynamic gene delivery. Gene Ther. 2007;14:99–107. doi: 10.1038/sj.gt.3302891. [DOI] [PubMed] [Google Scholar]
- 61.Suda T, Gao X, Stolz DB, Liu D. Structural impact of hydrodynamic injection on mouse liver. Gene Ther. 2007;14:129–137. doi: 10.1038/sj.gt.3302865. [DOI] [PubMed] [Google Scholar]
- 62.Budker V, Budker T, Zhang G, Subbotin V, Loomis A, Wolff JA. Hypothesis: naked plasmid DNA is taken up by cells in vivo by a receptor-mediated process. J Gene Med. 2000;2:76–88. doi: 10.1002/(SICI)1521-2254(200003/04)2:2<76::AID-JGM97>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 63.Kobayashi N, Kuramoto T, Yamaoka K, Hashida M, Takakura Y. Hepatic uptake and gene expression mechanisms following intravenous administration of plasmid DNA by conventional and hydrodynamics-based procedures. J Pharmacol Exp Ther. 2001;297:853–860. [PubMed] [Google Scholar]
- 64.Kobayashi N, Nishikawa M, Hirata K, Takakura Y. Hydrodynamics-based procedure involves transient hyperpermeability in the hepatic cellular membrane: implication of a nonspecific process in efficient intracellular gene delivery. J Gene Med. 2004;6:584–592. doi: 10.1002/jgm.541. [DOI] [PubMed] [Google Scholar]
- 65.Yoshino H, Hashizume K, Kobayashi E. Naked plasmid DNA transfer to the porcine liver using rapid injection with large volume. Gene Ther. 2006;13:1696–1702. doi: 10.1038/sj.gt.3302833. [DOI] [PubMed] [Google Scholar]
- 66.Liu F, Song Y, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999;6:1258–1266. doi: 10.1038/sj.gt.3300947. [DOI] [PubMed] [Google Scholar]
- 67.Crespo A, Peydro A, Dasi F, Benet M, Calvete JJ, Revert F, Alino SF. Hydrodynamic liver gene transfer mechanism involves transient sinusoidal blood stasis and massive hepatocyte endocytic vesicles. Gene Ther. 2005;12:927–935. doi: 10.1038/sj.gt.3302469. [DOI] [PubMed] [Google Scholar]
- 68.Neubauer K, Saile B, Ramadori G. Liver fibrosis and altered matrix synthesis. Can J Gastroenterol. 2001;15:187–193. doi: 10.1155/2001/870205. [DOI] [PubMed] [Google Scholar]
- 69.Horn T, Christoffersen P, Henriksen JH. Alcoholic liver injury: defenestration in noncirrhotic livers—a scanning electron microscopic study. Hepatology. 1987;7:77–82. doi: 10.1002/hep.1840070117. [DOI] [PubMed] [Google Scholar]
- 70.Garcia-Banuelos J, Siller-Lopez F, Miranda A, Aguilar LK, Aguilar-Cordova E, Armendariz-Borunda J. Cirrhotic rat livers with extensive fibrosis can be safely transduced with clinical-grade adenoviral vectors: evidence of cirrhosis reversion. Gene Ther. 2002;9:127–134. doi: 10.1038/sj.gt.3301647. [DOI] [PubMed] [Google Scholar]
- 71.Yeikilis R, Gal S, Kopeiko N, Paizi M, Pines M, Braet F, Spira G. Hydrodynamics based transfection in normal and fibrotic rats. World J Gastroenterol. 2006;12:6149–6155. doi: 10.3748/wjg.v12.i38.6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ichida T, Hata K, Yamada S, Hatano T, Miyagiwa M, Miyabayashi C, Matsui S, Wisse E. Subcellular abnormalities of liver sinusoidal lesions in human hepatocellular carcinoma. J Submicrosc Cytol Pathol. 1990;22:221–229. [PubMed] [Google Scholar]


