Abstract
Formation of the epithelial barrier and apico-basal cell polarity represent two characteristics and mutually dependent features of differentiated epithelial monolayers. They are controlled by special adhesive structures, tight junctions (TJs), and polarity protein complexes that define the apical and the basolateral plasma membrane. The functional interplay between TJs and polarity complexes remains poorly understood. We investigated the role of Scribble, a basolateral polarity protein and known tumor suppressor, in regulating TJs in human intestinal epithelium. Scribble was enriched at TJs in T84 and SK-CO15 intestinal epithelial cell monolayers and sections of normal human colonic mucosa. siRNA-mediated knockdown of Scribble in SK-CO15 cells attenuated development of epithelial barrier and inhibited TJ reassembly independently of other basolateral polarity proteins Lgl-1 and Dlg-1. Scribble selectively co-imunoprecipitated with TJ protein ZO-1, and ZO-1 was important for Scribble recruitment to intercellular junctions and TJ reassembly. Lastly, Scribble was mislocalized from TJs and its expression down-regulated in interferon-γ-treated T84 cell monolayers and inflamed human intestinal mucosa in vivo. We conclude that Scribble is an important regulator of TJ functions and plasticity in the intestinal epithelium. Down-regulation of Scribble may mediate mucosal barrier breakdown during intestinal inflammation.
The epithelial cell layer in the gut plays two crucial physiological functions. One function involves the formation of the physical barrier that separates body compartments from the gut lumen and protects underlying tissues from pathogen invasion and other harmful external stimuli.1,2 Another function involves the regulation of bidirectional passages of solutes and macromolecules, which is essential for nutrients supply and removal of body waste.3–5 Both barrier integrity and vectorial transport in the intestinal epithelium are regulated by specialized cellular structures known as tight junctions (TJs). TJs represent a complex network of protein fibrils within the plasma membrane, which encircle the apical region of the epithelial cell perimeter in close proximity to the gut lumen.6 TJ fibrils are composed of adhesive transmembrane proteins, which associate with ensembles of scaffolding proteins at the cytosolic face of the membrane. The paracellular barrier is created by homotypical interactions between transmembrane TJ components of contacting epithelial cells such as occludin, members of the claudin family and junctional adhesion molecule-A (JAM-A).6–8 These cell-cell adhesions are enhanced and regulated by cytosolic scaffolds such as members of zonula occludens (ZO) family and AF-6/afadin, which cluster and stabilize transmembrane TJ components at the plasma membrane.6–8 Although other junctional complexes at the plasma membrane, viz., adherens junctions (AJs) and desmosomes also mediate cell-cell adhesions, TJs play a unique role in sealing the paracellular space and creating the epithelial barrier.1,6,7
The mature TJs not only mediate barrier function of the intestinal epithelium, but also contribute to the formation and maintenance of the apico-basal cell polarity.9–11 Such cell polarity implies that the apical and basolateral domains of the plasma membrane differ in the composition of transporters, channels and receptors; therefore ensuring directionality of secretion and adsorption processes in epithelial cells.12,13 TJs regulate the epithelial cell polarity by creating a fence that prevents intermixing of protein and lipid constituents of the apical and basolateral plasma membrane domains.9–11 However, TJs alone are not sufficient for the apico-basal polarization of epithelial cells. In this process apical junctions cooperate with specialized protein polarity complexes that control the “identity” of distinct plasma membrane domains.
Epithelial cells have three major evolutionarily conserved polarity complexes that were initially identified and named in model invertebrates. They are known as Crumbs (composed by Crumbs, PALS, and PATJ proteins), Par (Par3, Par6, and atypical protein kinase C) and Scribble (including Scribble, Disks Large (Dlg) and Lethal Giant Larvae (Lgl)) complexes.11,14–16 Crumbs and Par cooperate to define the apical plasma membrane, whereas Scribble is critical for establishment of the basolateral membrane domain.11,14–16 A number of studies have demonstrated a functional interplay between epithelial junctions and the polarity complexes, where these entities mutually affect each other. Thus, several members of the Crumbs and Par complexes were shown to regulate TJ assembly via either direct binding to TJ components (ZO-1 and claudins) or indirect mechanisms, involving modulation of vesicle trafficking and actin cytoskeleton remodeling.11,14–16 In contrast, the role of the Scribble polarity complex in the regulation of epithelial TJs is not well understood,17,18 although such a role is supported by several lines of evidence. For example, mutations in any member of this complex in Drosophila resulted in dramatic disorganization of epithelial architecture that included loss of columnar cell shape and cell-cell adhesions.19–21 Furthermore, several reports have linked decreased protein levels of mammalian Scribble and Lgl with progression and invasiveness of epithelial tumors,22–24 which is also accompanied by down-regulation of TJs.25 Two recent studies have addressed the role of Scribble in the regulation cell-cell adhesions in mammalian epithelia; however their results appear to be inconsistent. Indeed, siRNA-mediated depletion of this protein in Madin-Darby canine kidney (MDCK) epithelial cells resulted in altered cell morphology and disorganized E-cadherin-based AJs.26 However, no changes in cell morphology or AJ structure were observed following the silencing of Scribble expression in MCF10A human mammary epithelial cells.27 Such inconsistent results may reflect tissue- specific effects of Scribble depletion, and they indicate that more work is needed to establish functional links between Scribble and TJs in human epithelia under normal physiological conditions and in disease states.
In this study, we examined the role of Scribble in the regulation of the intestinal epithelial barrier and reorganization of TJs. Our results demonstrate that Scribble is important for TJ barrier function and assembly, and that it may regulate junctions by interacting with the TJ scaffold, ZO-1. We also report that Scribble is mislocalized and its expression down-regulated in the intestinal epithelium by inflammatory conditions in vitro and in vivo.
Materials and Methods
Antibodies and Other Reagents
The following primary polyclonal (pAb) and monoclonal (mAb) antibodies were used to detect junctional, polarity and signaling proteins: anti-occludin, ZO-1, E-cadherin, and β-catenin mAbs and pAbs (Invitrogen, Carlsbad, CA); anti-α-Scribble pAb (Santa Cruz Biotechnology, Santa Cruz, CA); anti-Dlg-1 and AF-6 mAbs (BD PharMingen, San Diego, CA); anti-ERK1/2, phosphorylated ERK1/2, JNK1/2, phosphorylated JNK, p38, and phosphorylated p38 pAbs (Cell Signaling Technology Inc., Beverly, MA); anti-tubulin mAb and anti-actin pAb (Sigma-Aldrich, St. Louis, MO). Monoclonal α-Scribble antibody (7C6.D10) has been previously described.28 Monoclonal antibodies against Lgl-1 (mAb17-35.1.1 and mAb17–17.1.1) were obtained from corresponding hybridoma cell lines generated by fusion of P3X63-Ag8.653 mouse myeloma cells with spleen cells from BALB mice immunized with GST-Lgl-1 (residues 882-1034) fusion protein. Alexa 488 or Alexa 568 dye conjugated donkey anti-rabbit and goat anti-mouse secondary antibodies were obtained from Invitrogen; horseradish peroxidase-conjugated goat anti-rabbit and anti-mouse secondary antibodies were obtained from Jackson Immunoresearch Laboratories (West Grove, PA). Fluorescein isothiocyanate-labeled dextran (4 kd) and all other reagents were obtained from Sigma-Aldrich.
Cell Culture and Calcium Switch Model
T84 (American Type Culture Collection, Manassas, VA) and SK-CO-15 (a gift from Dr. E. Rodriguez-Boulan, Weill Medical College of Cornell University, NY) transformed human colonic epithelial cell lines were cultured as previously described.29–32 HPAF-II human pancreatic epithelial cells (American Type Culture Collection) were cultured in RPMI medium supplemented with 10 mmol/L HEPES, 10% fetal bovine serum and sodium pyruvate,33 whereas 16HBE14o- human bronchial epithelial cells34 (a gift from Dr. D.C. Gruenert, University of California San Francisco, CA) were cultured in minimum essential medium supplemented with 10 mmol/L HEPES, 10% fetal bovine serum and glutamine. For immunolabeling experiments, epithelial cells were grown on either collagen-coated, permeable polycarbonate filters of 0.4 μm pore size (Costar, Cambridge, MA) or on collagen-coated coverslips. For biochemical experiments, the cells were cultured on six-well plastic plates. To study formation of epithelial TJs and AJs, confluent SK-CO15 monolayers were first depolarized by overnight incubation in low-calcium medium (calcium-free Eagle's minimum essential medium for suspension culture (Sigma-Aldrich) supplemented with 10 mmol/L HEPES, 14 mmol/L NaHCO3, 40 μg/ml penicillin, 100 μg/ml streptomycin, 5 μmol/L CaCl2, and 10% dialyzed fetal bovine serum, pH 7.4). To induce junctional reassembly, the cells were returned to normal cell culture media with high (∼1.8 mmol/L) calcium concentration for indicated times at 37°C (referred hereafter as calcium repletion). To induce a rapid disassembly of AJs and TJs, T84, and SK-CO15 cells were incubated for 1 hour in the low-calcium medium devoid of CaCl2 and supplemented with 2 mmol/L ethylene glycol bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid.
Immunofluorescence Labeling and Image Analysis
Cell monolayers were either fixed/permeabilized in 100% methanol (−20°C for 20 minutes) or fixed with 4% paraformaldehyde (20 minutes at room temperature) and permeabilized with 0.5% Triton X-100 (15 minutes). Fixed cells were blocked in HEPES-buffered Hanks’ balanced salt solution (HBSS+) containing 1% bovine serum albumin (blocking buffer) for 60 minutes at room temperature and incubated for another 60 minutes with primary antibodies diluted in blocking buffer. Cells were then washed, incubated for 60 minutes with Alexa dye-conjugated secondary antibodies, rinsed with blocking buffer and mounted on slides with ProLong Antifade medium (Invitrogen). For tissue labeling, frozen sections (5 μm thickness) of human colonic mucosa were mounted on glass coverslips, air-dried, fixed in 100% ethanol (−20°C for 20 minutes), and immunolabeled as described above. Stained cell monolayers and tissue sections were examined using a Zeiss LSM510 laser scanning confocal microscope (Zeiss Microimaging Inc., Thornwood, NY) coupled to a Zeiss 100M Axiovert and 63× or 100× Pan-Apochromat oil lenses. The fluorescent dyes were imaged sequentially in frame-interlace mode to eliminate cross talk between channels. Images were processed using Zeiss LSM5 image browser software and Adobe Photoshop. Images shown are representative of at least 3 experiments, with multiple images taken per slide.
Immunoblotting
Cells were homogenized in a RIPA lysis buffer [20 mmol/L Tris, 50 mmol/L NaCl, 2 mmol/L EDTA, 2 mmol/L ethylene glycol bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid, 1% sodium deoxycholate, 1% Triton X-100, and 0.1% sodium dodecyl sulfate (SDS), pH 7.4], containing a protease inhibitor cocktail (1:100, Sigma) and phosphatase inhibitor cocktails 1 and 2 (both at 1:200, Sigma). Lysates were cleared by centrifugation (10 minutes at 14,000 × g), diluted with 2X SDS sample buffer and boiled. SDS-polyacrylamide gel electrophoresis and immunoblotting were conducted by standard protocols with equal amount of total protein (10 or 20 μg) per lane. Results shown are representative immunoblots of three independent experiments. Protein expression was quantified by densitometric analysis of at least three immunoblot images each representing independent experiments, using Scion Image (Scion, Frederick, MD) and UN-SCAN-IT digitizing software (Silk Scientific, Orem, UT).
Immunoprecipitation
Confluent or subjected to 5 hours of calcium repletion SK-CO15 cell monolayers were homogenized in RIPA buffer supplemented with protease and phosphatase inhibitors. Cell debris-free supernatants (500 μl) were precleared with Protein A-coupled Sepharose beads (Amersham Biosciences, Buckinghamshire, UK) for 60 minutes at 4°C followed by overnight incubation at 4°C with 5 μg of either anti-Scribble pAb or control rabbit IgG (Jackson Laboratories). Immunocomplexes were recovered by incubation with Protein A-Sepharose beads for 3 hours at 4°C with constant rotation. The washed beads were boiled for 5 minutes in 80 μl of 2X SDS sample buffer and equal volumes of supernatants (20 μl) were analyzed by electrophoresis and immunoblotting as described above.
RNA Interference
siRNA-mediated knockdown of Scribble, ZO-1, Lgl-1, and Dlg-1 was performed as previously described29,35,36 using isoform-specific siRNA SmartPools (Dharmacon, Lafayette, CO). Cyclophilin B siRNA SmartPool was used as a control. SK-CO15 cells were transfected using the DharmaFect 1 transfection reagent (Dharmacon) in Opti-MEM I medium (Invitrogen) according to manufacturer's protocol with a final siRNA concentration of 50 or 100 nmol/L. Cells were used in experiments 3 to 4 days post-transfection.
Epithelial Barrier Permeability Measurements
Transepithelial electrical resistance (TEER) was measured using an EVOMX voltohmmeter (World Precision Instruments, Sarasota, FL). The resistance of cell-free collagen-coated filters was subtracted from each experimental point. For dextran flux measurements, SK cells cultured on 5-μm pore size filters were washed in HBSS+ pre-warmed to 37°C. Fluorescein isothiocyanate-dextran was added to the upper chamber at a concentration of 1 mg/ml, with a total volume of 150 μl, whereas 650 μl of pre-warmed HBSS+ was added to the bottom chamber. Basolateral samples of 50 μl were taken over a 3-hour time course of incubation at 37°C, and fluorescence intensity was analyzed on a plate reader (Fluostar; BMG Labtechnologies, Durham, NC). The amount of dextran that diffused through the cell monolayer into the bottom chamber was calculated based on a standard curve and expressed as μg/h/cm2.
Statistics
Numerical values from individual experiments were pooled and expressed as mean ± SEM (SE) throughout. Obtained numbers were compared by a single-tailed Student's t-test, with statistical significance assumed at P < 0.05.
Results
siRNA-Mediated Silencing of Scribble Expression Attenuated Development of the Paracellular Barrier and Delayed TJ Reassembly
The role of Scribble in regulation of the intestinal epithelial barrier was studied using human colonic epithelial cell lines T84 and SK-CO15. When grown on permeable membrane support, both cell types form well-polarized cell monolayers with prominent apical junctions and tight paracellular barrier.29,31,37,38 SK-CO15 but not T84 cells are amendable for siRNA-mediated gene knockdown.29,33,35,36 On the other hand, T84 but not SK-CO15 cells readily respond to proinflammatory cytokines with TJ disassembly.37 These unique features of T84 and SK-CO15 cells make them complementary models to study regulation of intestinal epithelial junctions in normal and inflammatory conditions.
Given previous data that intracellular localization is critical for Scribble functions,39–42 we first analyzed if Scribble is localized at TJs in model human intestinal epithelium. Polarized T84 and SK-CO15 cell monolayers grown on permeable membrane support were fixed and double immunolabeled for Scribble and TJ proteins occludin and ZO-1. The en face (x-y) confocal image presented in Figure 1A and B, shows a “chicken-wire” staining pattern for Scribble in both intestinal epithelial cell lines, which significantly overlaps with occludin and ZO-1 labeling at TJs (arrows). Likewise, the reconstructed x-z plane of these images demonstrates that Scribble labeling is restricted to the apical portion of the lateral plasma membrane, where it colocalizes with occludin and ZO-1(arrowheads). Similar colocalization of Scribble and ZO-1 was also observed in HPAF-II human pancreatic and 16HBE14o- human bronchial epithelial cell monolayers (see Supplemental Figure 1 at http://ajp.amjpathol.org), which indicates that Scribble localization in tight junctions is not a peculiar feature of intestinal epithelial cells. Because immunofluorescence analysis does not spatially separate TJ and AJ complexes in polarized epithelial cell monolayers, we sought to obtain additional evidence of Scribble localization at TJs. Given our previous observation that AJ and TJ proteins segregate into distinct endosomal compartments after their internalization triggered by extracellular calcium depletion,31 we next compared the effect of calcium depletion on intracellular distribution of Scribble and occludin. As shown in Supplemental Figure 2 at http://ajp.amjpathol.org, internalized Scribble colocalizes with occludin and ZO-1 in T84 and SK-CO15 cells respectively (arrows), thus reinforcing our conclusion that Scribble localizes in intact TJs of polarized human epithelia.
Figure 1.
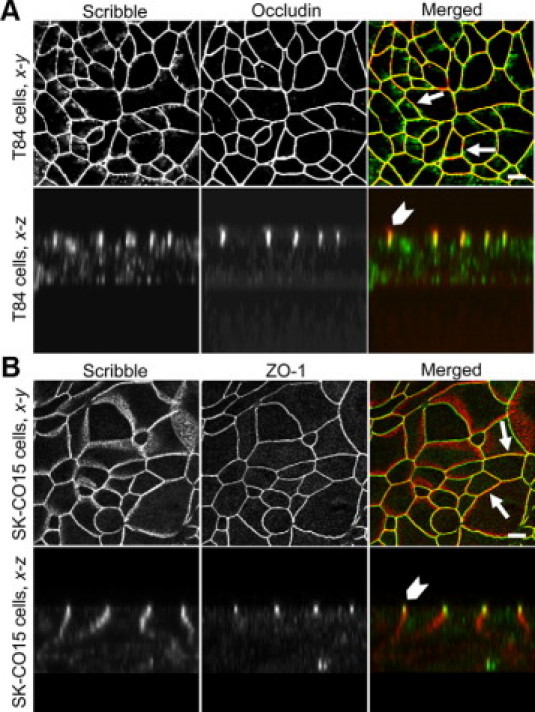
Scribble localizes at TJs in polarized human intestinal epithelial cell monolayers. T84 and SK-CO15 cells were grown on membrane filters until they formed confluent high-resistance monolayers. T84 cells (A) were double-immunolabeled for Scribble (green) and occludin (red), whereas SK-CO15 cells (B) were labeled for Scribble (red) and ZO-1 (green). Note significant colocalization of Scribble and TJ proteins (yellow) in both epithelial cell lines according to x-y (arrows) and reconstructed x-z (arrowheads) confocal images. Scale bar = 10 μm.
Figure 2.
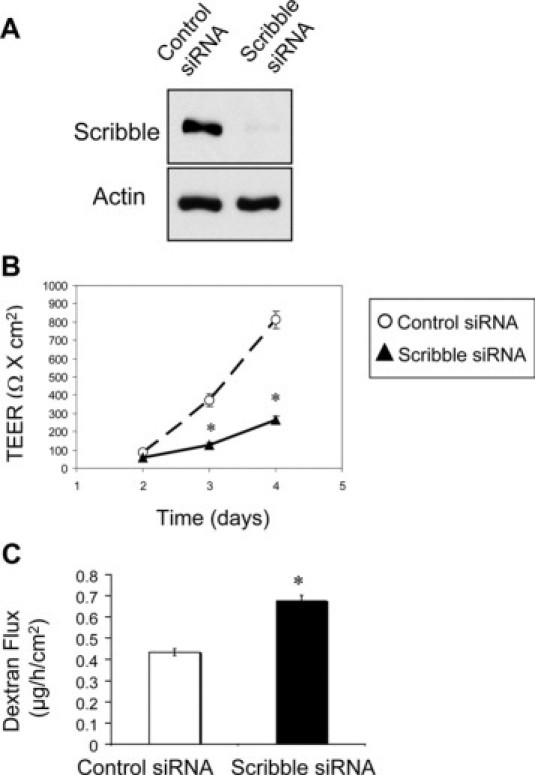
Down-regulation of Scribble attenuates formation of the paracellular barrier in model intestinal epithelium. SK-CO15 cells were transfected with either Scribble-specific or control (cyclophilin B-specific) siRNAs. Development of the paracellular barrier was examined by measuring TEER and fluoresceinated dextran flux. A: Immunoblotting analysis shows siRNA-mediated decrease of Scribble protein level on day 4 post-transfection. Permeability assays show significant attenuation of TEER development (B) and increase in dextran flux (C) in Scribble-depleted cell monolayers on days 2–4 and day 4 post-transfection, respectively. Data are presented as mean ± SE (n = 3); *P < 0.05 compared to control siRNA-transfected cells.
To gain insight into Scribble function at epithelial TJs, we down-regulated its expression using RNA interference in SK-CO15 cells. Figure 2A shows that siRNA-mediated silencing of Scribble expression resulted in an approximately 93% decrease in its protein level by day 4 post-siRNA transfection. Notably, Scribble depletion significantly delayed development of the epithelial barrier. Figure 2B shows that TEER in Scribble-depleted SK-CO15 cell monolayers was significantly lower than in control siRNA-treated cells on days 3 and 4 post-siRNA transfection. Likewise, permeability to fluoresceinated dextran (4000 da) was significantly increased in Scribble-depleted cells compared with control siRNA-transfected SK-CO15 cells (Figure 2C). This functional data provides the first evidence that down-regulation of Scribble compromises barrier properties of the model intestinal epithelium.
Despite inducing functional defects of the epithelial barrier, Scribble knockdown did not affect structure of mature TJs. Indeed, immunofluorescence labeling shows normal localization of either transmembrane (occludin) or cytosolic plaque (ZO-1) TJ proteins in control and Scribble-deficient SK-CO15 cell monolayers (see Supplemental Figure 3 at http://ajp.amjpathol.org). Likewise, immunoblotting analysis failed to detect significant effects of Scribble depletion on total expression of different TJ (occludin, ZO-1, AF-6) and AJ (E-cadherin, β-catenin) proteins (see Supplemental Figure 4 at http://ajp.amjpathol.org). We also investigated if Scribble knockdown affects development of the apico-basal cell polarity, but did not observe mislocalization of the apical membrane-associated marker ezrin in Scribble-depleted SK-CO15 cells (see Supplemental Figure 5 at http://ajp.amjpathol.org).
Previous studies suggested that several junctional components and regulators, while being dispensable for integrity of the mature TJs, are involved in junctional assembly.29,43,44 Therefore we next examined if Scribble knockdown affects reassembly of intestinal epithelial TJs. We used a well-established “calcium switch” model that involves reversible disruption of epithelial junctions by extracellular calcium depletion followed by a rapid and orchestrated junctional reassembly triggered by calcium repletion.31,45,46 Figure 3 shows that in control SK-CO15 cell monolayers most TJs were reassembled after 5 hours of calcium repletion. This was evident by the appearance of continuous junctional labeling of occludin and ZO-1 (Figure 3, arrows). In contrast, Scribble-depleted cell monolayers demonstrated short and disconnected areas of occludin and ZO-1 labeling at the cell-cell contacts, which indicates a significant delay in their TJ reassembly (Figure 3, arrowheads). To clarify whether such a delay is specific for TJs or can be attributed to other junctional complexes, we analyzed the effect of Scribble knockdown on reassembly of epithelial AJs, which is known to precede TJ restoration.46 However, as shown in Supplemental Figure 6 at http://ajp.amjpathol.org, Scribble-depleted and control SK-CO15 cell monolayers similarly reassembled their E-cadherin and β-catenin-based AJs after 1 hour of calcium repletion (arrows).
Figure 3.
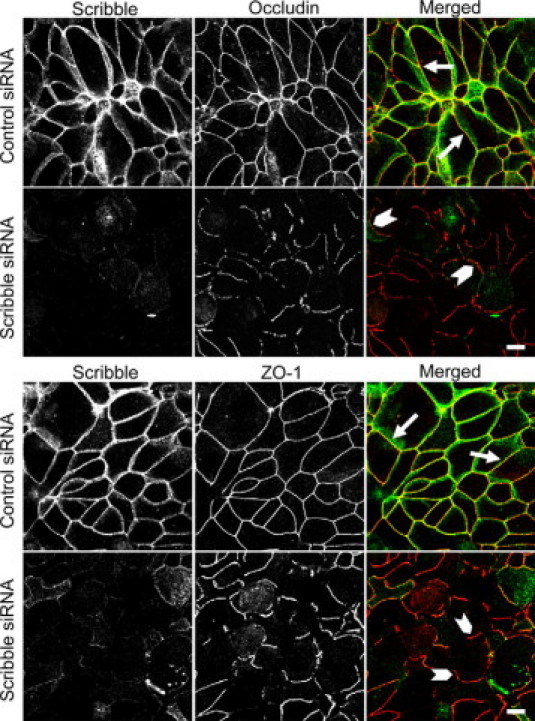
Down-regulation of Scribble attenuates TJ assembly. SK-CO15 cells were transfected with either control or Scribble-specific siRNAs and on day 3 post-transfection were subjected to overnight calcium depletion to disrupt cell-cell adhesion. Reassembly of TJs in control and Scribble-deficient cell monolayers was analyzed after 5 hours of calcium repletion by monitoring the formation of characteristic chicken wire labeling pattern of the TJ proteins occludin and ZO-1 (red). Control SK-CO15 cell monolayers show almost complete restoration of normal TJ localization of occludin and ZO-1 (arrows). By contrast, Scribble-deficient cell monolayers display abnormal discontinuous occludin and ZO-1 labeling at intercellular contacts (arrowheads). Scale bar = 10 μm.
Scribble Interacts with ZO-1 and Is Recruited by ZO-1 to Intercellular Junctions
We next addressed the mechanisms underlying Scribble-dependent regulation of TJ assembly. One potential mechanism is based on recent studies reporting that Scribble modulates activity of mitogen-activated protein kinases (MAPK);47,48 as well as on the known role MAPK signaling in junctional regulation.49,50 To test this mechanism, we analyzed the effects of Scribble depletion on the activation status of three classes of MAPK, such as extracellular signal-activated kinases (ERK) 1/2, c-Jun N-terminal kinases (JNK) and p38 kinase, in SK-CO15 cells. However, immunoblotting analysis using antibodies that selectively recognize phosphorylated (active) forms of ERK1/2, JNK, and p-38 did not show significant difference in the activation status of these kinases in control and Scribble-deficient cells (see Supplemental Figure 7 at http://ajp.amjpathol.org). These data indicate that Scribble-dependent TJ assembly in SK-CO15 cell monolayers does not involve modulation of MAPK activity.
Given the known ability of Scribble to participate in different protein-protein interactions,39,41,42,51 one possibility is that Scribble mediates junctional assembly by directly interacting with TJ proteins, therefore recruiting or stabilizing these proteins at intercellular contacts. To test this mechanism, we examined Scribble interactions with different TJ and AJ components during calcium-dependent junctional reassembly in SK-CO15 cells. Figure 4A shows that endogenous Scribble co-immunoprecipitated with ZO-1, but not occludin, after 5 hours of calcium repletion. Furthermore, we did not observe co-immunoprecipitation of JAM-A, E-cadherin, or β-catenin with Scribble (data not shown). We also performed reverse pulldown experiments and detected Scribble in ZO-1 immunoprecipitates from SK-CO15 cells (Figure 4B). Interestingly, we were able to immunoprecipitate ZO-1 with anti-Scribble antibody only from calcium-repleted SK-CO15 cells but not from confluent cell monolayers. Oppositely, Scribble was pulled-down by anti-ZO-1 antibody from confluent SK-CO15 monolayers but not from the cells subjected to 5 hours of calcium repletion. The reason for such different immunoprecipitation results remain unknown, but they may reflect differences in structure/composition of Scribble-ZO-1 complexes at newly assembled and mature TJs. Importantly, Scribble and ZO-1 significantly colocalized at different stages of junctional biogenesis in SK-CO15 cells including mature TJs (Figure 1), disassembling junctions (see Supplemental Figure 1 at http://ajp.amjpathol.org) and in initial cell-cell contacts formed at the edge of wounded cell monolayers (see Supplemental Figure 8 at http://ajp.amjpathol.org). Such persistent colocalization reinforces our conclusion pertaining to the formation of Scribble-ZO-1 complexes in the model intestinal epithelium.
Figure 4.
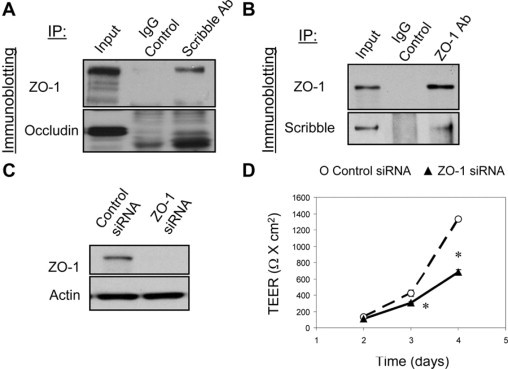
ZO-1 physically associates with Scribble and regulates development of the paracellular barrier. A: Scribble was immunoprecipitated from SK-CO15 cell monolayers after 5 hours of calcium repletion and was analyzed for association with TJ proteins. Immunoblotting analysis shows presence of ZO-1 but not occludin in Scribble immunoprecipitates. B: ZO-1 was immunoprecipitated from confluent SK-CO15 cell monolayers and analyzed for association with Scribble. Immunoblot shows pulldown of Scribble with ZO-1 antibody but not with control IgG. C and D: Effect of silencing of ZO-1 expression on development of the paracellular barrier was analyzed in SK-CO15 cell monolayers. Immunoblotting analysis shows significant down-regulation of ZO-1 expression on day 4 post-siRNA transfection (C). Development of TEER was significantly attenuated in ZO-1-depleted cell monolayers as compared with control siRNA-transfected cells (D). Data are presented as mean ± SE (n = 3); *P < 0.05 compared to control siRNA-transfected cells.
If ZO-1-Scribble interactions are important for junctional regulation, one can predict that knockdown of ZO-1 should phenocopy effects of Scribble depletion on TJ assembly and epithelial permeability. Indeed, siRNA-mediated silencing of ZO-1, which resulted in a more than 90% decrease in its protein expression (Figure 4C), caused delay in development of the paracellular barrier in SK-CO15 cell monolayers (Figure 4D). Furthermore, ZO-1 knockdown attenuated reassembly of occludin-based TJs (Figure 5, top panel) and inhibited Scribble accumulation at intercellular junctions (Figure 5, lower panel). It should be noted that ZO-1 has several binding partners at TJs52 and therefore its depletion may impair junctional assembly via Scribble-independent mechanisms. However similar effects of Scribble and ZO-1 down-regulation on TJ reassembly together with the data on colocalization and co-immunoprecipitation of these proteins strongly support the possibility of their functional interactions in the intestinal epithelium.
Figure 5.
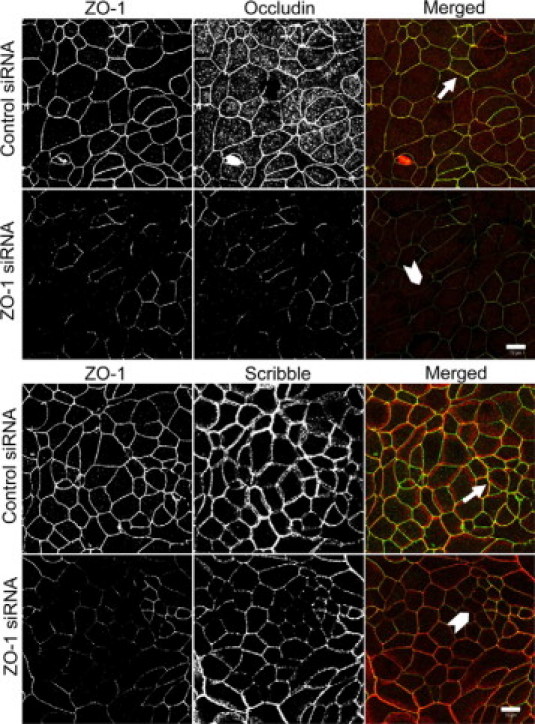
Down-regulation of ZO-1 attenuates assembly of TJs and recruitment of Scribble to apical junctions. SK-CO15 cells transfected with either control or ZO-1-specific siRNAs were subjected to calcium switch on day 3 post-transfection. Cells were fixed 5 hours after calcium repletion and double-immunolabeled for ZO-1 (green) and either occludin or Scribble (red). Control cell monolayers show complete restoration of occludin and Scribble labeling at TJs (arrows). By contrast, TJ reassembly and Scribble recruitment to apical junctions is attenuated in ZO-1-depleted cell monolayers (arrowheads). Scale bar = 10 μm.
Lgl-1 Interacts with Scribble but Has No Effects on TJ Assembly and Barrier Integrity
We next sought to determine whether Scribble regulates the intestinal epithelial barrier as a component of the Scribble/Dlg/Lgl polarity complex. We rationalized that if the Scribble effects on epithelial TJs are mediated by its interactions with Dlg-1 and Lgl-1, then these polarity proteins should be localized at TJs, interact with Scribble, and their depletion should mimic effects of Scribble knockdown on barrier integrity and TJ assembly. Figure 6 shows that Lgl-1 colocalized with occludin in intact (arrows) and internalized (arrowheads) TJs of polarized and calcium-depleted T84 cell monolayers. By contrast, Dlg-1 did not accumulate in TJs but was uniformly distributed along the lateral plasma membrane in polarized T84 cells (see Supplemental Figure 9 at http://ajp.amjpathol.org). Furthermore, internalized Dlg-1 and occludin did not significantly colocalize after disruption of TJs in calcium-depleted cells (see Supplemental Figure 9 at http://ajp.amjpathol.org). We next examined possible interactions between Scribble and other basolateral polarity proteins in SK-CO15 cells. Immunoblotting analysis of Scribble immunoprecipitates, collected after 4 hours of calcium repletion, detected the presence of Lgl-1 but not Dlg-1 protein (Figure 7A), indicating selective binding between Scribble and Lgl-1 during TJ reassembly.
Figure 6.
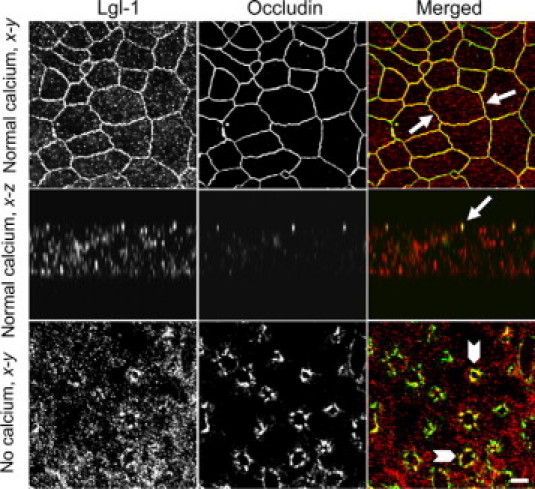
Lgl-1 localizes at TJs in the model intestinal epithelium. Polarized and calcium-depleted for 1 hour T84 cell monolayers were double-immunolabeled for occludin (green) and Lgl-1 (red). Note significant colocalization of occludin and Lgl-1 (yellow) at intact (arrows) and internalized (arrowheads) TJs. Scale bar = 10 μm.
Figure 7.
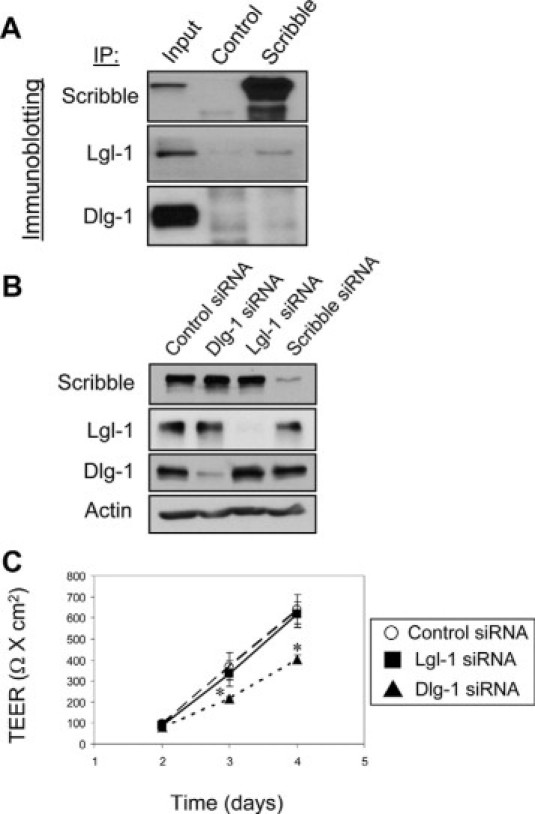
Lgl-1 and Dlg-1 differently associate with Scribble and differently regulate the epithelial barrier. A: Scribble was immunoprecipitated from SK-CO15 cell monolayers after 4 hours of calcium repletion and was analyzed for association with Lgl-1 and Dlg-1. Immunoblotting analysis shows presence of Lgl-1 but not Dlg-1 in Scribble immunoprecipitates. B and C: Effects of Lgl-1 and Dlg-1 knockdown on development of paracellular barrier were analyzed in SK-CO15 cell monolayers. Immunoblotting analysis demonstrates selective down-regulation of Lgl-1 and Dlg-1 expression on day 4 post-siRNA transfection with no effects on each other or Scribble expression (B). TEER measurement shows significant attenuation in development of the paracellular barrier in Dlg-1-depleted but not in Lgl-1-deficient SK-CO15 cell monolayers (C). Data are presented as mean ± SE (n = 3); *P < 0.05 compared to control siRNA-transfected cells.
Using RNA interference approach, we next selectively silenced Lgl-1 and Dlg-1 expression in SK-CO15 cells (Figure 7B). Notably, Lgl-1, and Dlg-1 depletion neither affected the expression of each other, nor decreased the total cellular amount of Scribble. Likewise, Scribble knockdown did not change Lgl-1 and Dlg-1 protein levels (Figure 7B). Down-regulation of Dlg-1 expression modestly attenuated the development of the epithelial barrier, whereas Lgl-1 depletion did not alter the barrier function of SK-CO15 cell monolayers (Figure 7C). We next investigated effects of Lgl-1 and Dlg-1 down-regulation on TJ reassembly. It is noteworthy that Lgl-1 labeling in polarized SK-CO15 cells appeared to be different from its T84 cell labeling shown in Figure 6 and depended on the fixation protocol. Thus, in methanol or ethanol-fixed SK-CO15 cells, Lgl-1 demonstrated predominantly apical labeling (data not shown), and only paraformaldehyde fixation revealed junctional localization of this protein (see Supplemental Figure 10 at http://ajp.amjpathol.org). Nevertheless, Lgl-1 or Dlg-1-deficient SK-CO15 cell monolayers did not show any delay in reestablishment of TJs during 5 hours of calcium repletion (see Supplemental Figure 10 at http://ajp.amjpathol.org,). Overall these data do not support the formation of a trimeric Scribble/Lgl-1/Dlg-1 complex in human intestinal epithelial cells and highlights a unique role of Scribble in regulation of TJ assembly.
Inflammatory Stimuli Mislocalize Scribble and Decrease Its Expression in Intestinal Epithelial Cells
Disruption of the epithelial barrier and TJ disassembly is well documented in intestinal inflammatory disorders.2,53,54 Thus, we next analyzed whether inflammatory stimuli affect association of Scribble with TJs by challenging T84 cell monolayers with known proinflammatory cytokine, interferon (IFN)-γ. As shown in Figure 8A, 48 hours exposure of epithelial cells to IFN-γ transformed occludin labeling from normal chicken-wire pattern into diffuse cytosolic staining, which is indicative of TJ disassembly. Analogous to occludin, TJ localization of Scribble was also diminished in IFN-γ-treated cell monolayers (Figure 8A, arrows). The cytokine treatment not only caused mislocalization of Scribble but also affected its total protein level. Indeed, immunoblotting analysis revealed a ∼47 and 61% decrease in Scribble expression in T84 cells after 48 and 72 hours of IFN-γ treatment (Figure 8B). These in vitro results were complemented by immunofluorescence analysis of Scribble localization in the intestinal mucosa of patients with the chronic inflammatory disorder, Crohn's disease. As shown in Figure 9, Scribble is enriched in occludin-based TJs of normal crypt and surface human intestinal epithelial cells (arrows). In contrast, the overall intensity of Scribble immunolabeling is dramatically decreased in the inflamed mucosa of the patients with acute Crohn's disease (Figure 9). Furthermore, in these tissue sections, we did not detect Scribble localization in TJs (Figure 9). Overall these in vitro and in vivo observations indicate that intestinal inflammation induces down-regulation of junctional Scribble, which is orchestrated with TJ disassembly.
Figure 8.
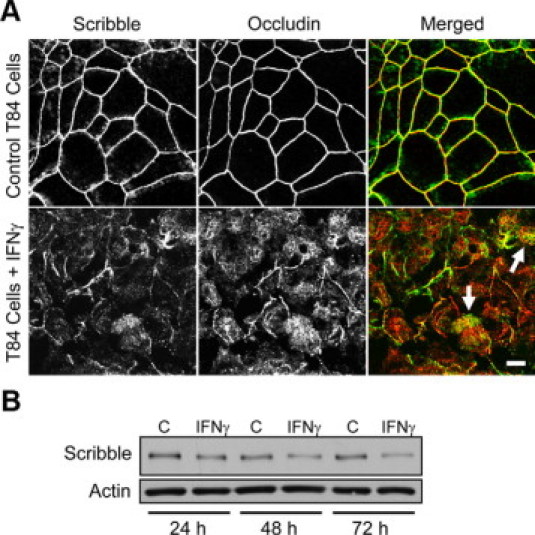
Interferon- γ induces mislocalization and down-regulation of epithelial Scribble in vitro, which parallel TJ disassembly. Polarized T84 cell monolayers were treated with either vehicle or IFN-γ (100 U/ml). A: Cells were fixed after 48 hours of the cytokine exposure and double-immunolabeled for Scribble (green) and occludin (red). Note profound disruption of TJs and disappearance of junctional Scribble in IFN-γ-treated cells (arrows). Scale bar = 10 μm. B: Total cell lysates of vehicle- and IFN-γ-treated T84 cells were analyzed for Scribble expression by immunoblotting. Note that cytokine treatment resulted in significant decrease in Scribble protein expression.
Figure 9.
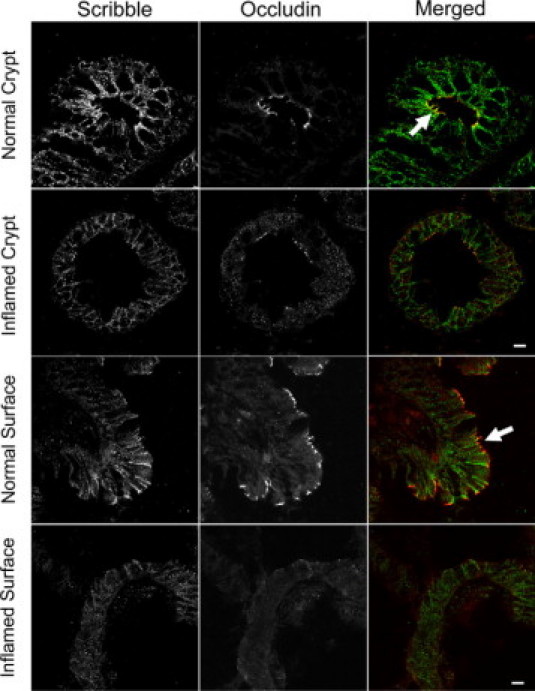
Mucosal inflammation in vivo down-regulates Scribble expression and induces TJ disassembly. Tissue sections of colonic mucosa isolated from a normal human subject and a patient with active Crohn's disease were double-immunolabeled for Scribble (green) and occludin (red). Note significant colocalization (yellow) of Scribble and occludin at intact TJs in normal intestinal mucosa (arrows) and disappearance of junctional Scribble and occludin in inflamed intestinal epithelium of Crohn's disease patient. Scale bar = 20 μm.
Discussion
Formation of epithelial junctions and development of the apico-basal cell polarity are highly-orchestrated and mutually dependent processes. The integrity of TJs is necessary for maintenance of cell polarity, whereas correct segregation of the apical and basolateral plasma membrane domains mediated by different protein polarity complexes is essential for TJ structure and stability.9–11,13 Our study examined the regulation of intestinal epithelial TJs by Scribble, the polarity protein controlling the identity of the basolateral plasma membrane domain.17,18 We report the following new and important observations: 1) Scribble localizes in TJs and controls the integrity of epithelial barrier and TJ assembly in human intestinal epithelium; 2) Scribble interacts with ZO-1 and these interactions may mediate Scribble effects on TJ dynamics and functions; 3) other basolateral polarity proteins such as Dlg-1 and Lgl-1 are dispensable for TJ assembly; and 4) Scribble is mislocalized and expressionally down-regulated during intestinal inflammation.
Our conclusion that Scribble is associated with intestinal epithelial TJs is based on immunolabeling/confocal microscopy data, which demonstrates Scribble colocalization with occludin or ZO-1 in model epithelial cell monolayers (Figure 1 and Supplemental Figures 1 and 2 at http://ajp.amjpathol.org) and tissue sections of normal human colonic mucosa (Figure 9). In addition, IFN-γ treatment, which is known to selectively disrupt TJs while preserving AJ integrity in T84 cells,37 caused orchestrated loss of junctional labeling of Scribble and occludin (Figure 8). These data agree with two recent studies that demonstrated colocalization of Scribble with TJ scaffolds ZO-1 and ZO-2 in corneal and renal epithelia,41,55 but contradict other reports that epithelial Scribble uniformly localizes along the lateral plasma membrane.28,40,56 Our findings are also consistent with Scribble localization at Drosophila septate junctions,20 which are known to be analogs of vertebrate TJs.57 Such variability of Scribble immunolabeling may be due to differences in extent of epithelial cell polarization and/or different experimental conditions of immunofluorescence analysis; but most likely these data reflect the ability of Scribble to participate in multiple protein complexes including different types of intercellular junctions.
The present study provides the first evidence that Scribble regulates the barrier function of TJs. Indeed, according to TEER and dextran flux measurements, depletion of Scribble impaired development of the paracellular barrier in the model intestinal epithelium (Figure 2, B and C). Importantly, loss of Scribble increased epithelial permeability to both small ions and large (∼4000 kd) molecules. This indicates that Scribble may regulate two types of paracellular pores, one of which is selective for small (<4 Å radius) molecules and another that is permeable to large solutes.58 It remains to be investigated whether Scribble enhances the epithelial barrier by decreasing the radius or the number of these paracellular pores.
Despite its involvement in the maturation of the paracellular barrier, Scribble appears to be dispensable once assembly of TJs has been achieved in confluent epithelial cell monolayers. Indeed, silencing Scribble expression did not alter TJ morphology (see Supplemental Figure 3 at http://ajp.amjpathol.org) or the apico-basal polarity (see Supplemental Figure 5 at http://ajp.amjpathol.org) in confluent SK-CO15 cell monolayers, which is consistent with recent data obtained in Scribble-depleted MDCK cells.26 By contrast, our data suggest that Scribble controls the rate of TJ assembly in model intestinal epithelium (Figure 3). This resembles the effects of other junctional or cytoskeletal proteins (ZO-1, E-cadherin, myosin IIA), which depletion reportedly slowed down assembly but failed to prevent eventual formation of TJs.29,43,44 Together, these data indicate that integrity of epithelial TJs is controlled by multiple and duplicative mechanisms, which can compensate the loss of each other. Some of these mechanisms can more efficiently regulate junctional assembly and their role still can be revealed by assays involving a robust and rapid remodeling of TJs.
Interestingly, in intestinal epithelial cells Scribble selectively regulated TJ plasticity and had no effect on mature AJ structure (data not shown) or the velocity of AJ assembly (Suppl. Figure 6 at http://ajp.amjpathol.org). These data agree with a recent study of Scribble knockdown in MCF10A mammary epithelial cells27 but contrasts with the published effects of this protein depletion in MDCK cells.26 The latter study observed defects in normal AJ structure that were manifested by altered junctional labeling and detergent solubility of E-cadherin and β-catenin in Scribble-depleted cells. These contradictory results can be reconciled by assuming tissue specificity of Scribble signaling and functions. For example, Scribble was shown to suppress motility of MDCK cells,26 but reportedly accelerated migration of MCF10A cells.27 Furthermore, in the mammary epithelium Scribble was shown to regulate MAPK activity by suppressing ERK47 and activating JNK.48 By contrast, we did not find such Scribble-dependent modulations of ERK, JNK, and p-38 activity in model intestinal epithelium (see Supplemental Figure 7 at http://ajp.amjpathol.org). More studies are needed to explain this cell/tissue-specific context of Scribble functions.
In line with a recognized role of Scribble in mediating protein-protein interactions, we concluded that Scribble may regulate TJ assembly by binding to a junctional scaffolding protein, ZO-1. This conclusion is based on the observed interactions between endogenous Scribble and ZO-1 (Figure 4, A and B) and colocalization of these proteins at intestinal epithelial TJs (Figures 1 and 3). It is noteworthy that we pull down Scribble in a complex with ZO-1 but not with other junctional proteins from cell lysates prepared by using SDS-containing RIPA buffer. Co-immunoprecipitation in such stringent conditions indicates strong and selective interactions between these proteins. Previously, Scribble binding to another member of zonula occludens family, ZO-2, has been reported.41 However, this binding was observed only after overexpression of exogenous Scribble and ZO-2 and was not detected in endogenous proteins. Hence, our study provides the first evidence of physiologically relevant interactions between endogenous Scribble and TJ protein in human epithelia. These interactions can be involved in different stages of junctional biogenesis. For instance, Scribble may interact with ZO-1 within the TJ cytosolic plaque, therefore preventing its internalization and stabilizing multiprotein complexes of the mature TJs. Alternatively, given recently reported interactions between Scribble and syntaxin-4,59 Scribble may mediate vesicular trafficking of ZO-1 and its delivery to the cell-cell contacts.
It is generally believed that Scribble functions in a complex with other polarity proteins, Dlg-1 and Lgl-1/2.9,11,13,60 The existence of this Scribble/Dlg/Lgl basolateral polarity complex has been suggested based on studies in Drosophila, where mutations of either Scribble, Dlg, or Lgl resulted in phenotypically identical abnormalities in epithelial architecture, and plasma membrane localization of these proteins depended on each other functions.19,20 However the existence of the Scribble/Dlg-1/Lgl complex in mammalian cells remains unproven. If Scribble regulates epithelial TJs by being a component of the trimeric basolateral polarity complex, the following experimental outcome can be predicted: 1) Scribble, Dlg-1, and Lgl-1 similarly localize at epithelial TJs; 2) physical association between these proteins is detectable by immunoprecipitation; 3) expression of each of these proteins may be essential for the expression of the others; 4) down-regulation of Dlg-1 or Lgl-1 phenocopies effects of Scribble depletion on TJ functions and dynamics. However, our experimental data appear to be inconsistent with many of these predictions. Thus, we found that Dlg-1 neither localized at TJs (see Supplemental Figure 9 at http://ajp.amjpathol.org) nor interacted with Scribble (Figure 7A) in colonic epithelial cells. Furthermore, while Dlg-1 depletion attenuated development of the paracellular barrier (Figure 7C), it did not inhibit TJ reassembly (see Supplemental Figure 10 at http://ajp.amjpathol.org). These data agree with a recent study involving Dlg-1-depletion in another human intestinal epithelial cell line, Caco-2 cells61 and suggest that Scribble may regulate TJs in a Dlg-1-independent manner. In contrast to Dlg-1, we found that Lgl-1 selectively accumulated at TJs (Figure 6) and interacted with Scribble in intestinal epithelial cells (Figure 7A). However the functional role of these interactions remains unclear since Lgl-1 depletion had no effect on the barrier integrity and TJ reassembly (Figure 7C and Supplemental Figure 10 at http://ajp.amjpathol.org). Previous studies addressing the role of Lgl in regulation of apical junctions in mammalian epithelia produced controversial results. Thus, Lgl-1 knockout mice demonstrated a dramatic disorganization of junctional complexes in neuroepithelium.62 This indicates that Lgl-1 is essential for junctional integrity. By contrast, co-depletion of Lgl-1 and Lgl-2 isoforms in MDCK cells attenuated TJ disassembly,63 whereas overexpression of Lgl-2 inhibited junctional reassembly.64 These data suggest that Lgl functions as a destabilizer of epithelial TJs. However, our study revealed no obvious role for Lgl-1 in TJ regulation. Such uncertainty surrounding the functions of Lgl-1 is likely to reflect the functional redundancy among the basolateral polarity proteins. Notably, our data and previous reports strongly suggest that these polarity proteins are not equally redundant, and the loss of Scribble function in human intestinal epithelium cannot be fully compensated by Dlg and Lgl-1.
Another novel and important result of this study is the dramatic effects of intestinal inflammation on Scribble localization and expression in vitro and in vivo. Indeed, exposure of T84 cell monolayers to IFN-γ displaced Scribble from epithelial TJs and decreased its protein expression in parallel with TJ disassembly (Figure 8). Likewise, inflamed intestinal mucosa of Crohn's disease patients was characterized by decreased Scribble expression and abnormal TJ architecture (Figure 9). These data suggest that cytokine-induced Scribble depletion may contribute to the breakdown of the epithelial barrier during intestinal inflammation and/or attenuate barrier recovery during epithelial restitution. Such dramatic effects of inflammation on human epithelial Scribble have not been previously reported, and their mechanisms remain to be investigated. However, Scribble is known to be inactivated by other pathogenic stimuli. For example, cell infection with human papillomavirus stimulates ubiquitination and degradation of this polarity protein.65 Additionally, Scribble level was shown to be decreased in apoptotic cells due to caspase-dependent degradation.66 Importantly, loss of expression and mislocalization of Scribble was observed in human colonic carcinomas.22,23 On the other hand, chronic intestinal inflammation is known to increase risk of the development of gastrointestinal tract cancer.67,68 Since Scribble is a powerful tumor suppressor, it is tempting to speculate that its down-regulation in chronic inflammatory conditions such as inflammatory bowel disease may contribute to the development of intestinal cancer.
In conclusion, we have uncovered a novel mechanism that regulates the integrity and plasticity of the intestinal epithelial barrier. This mechanism involves the known cell polarity regulator and tumor suppressor, Scribble, which associates with epithelial TJs by binding to the TJ scaffolding protein ZO-1. We found that epithelial Scribble is mislocalized and down-regulated during intestinal inflammation. This is likely to be important for barrier disruption in inflamed mucosa and may also contribute to tumor development during chronic intestinal inflammation.
Acknowledgements
We thank Dr. Susan Voss and Mr. Moshe Bachar for excellent technical assistance and Dr. Enrique Rodriguez-Boulan and Dr. Dieter Gruenert for providing SK-CO15 and 16HBE14o-cells.
Footnotes
Supported by National Institutes of Health grants DK 55679 (to A. N.), DK 61379 and DK 72564 (to C.A.P.), Emory epithelial pathobiology research development grant R24DK 064399, and Medical Scientists Training Program T32 GM008169-21 (to C. Y.).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
Contributor Information
Andrei I. Ivanov, Email: andrei_ivanov@urmc.rochester.edu.
Asma Nusrat, Email: anusrat@emory.edu.
Web Extra Material
References
- 1.Madara JL. Regulation of the movement of solutes across tight junctions. Annu Rev Physiol. 1998;60:143–159. doi: 10.1146/annurev.physiol.60.1.143. [DOI] [PubMed] [Google Scholar]
- 2.Turner JR. Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application. Am J Pathol. 2006;169:1901–1909. doi: 10.2353/ajpath.2006.060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basu TK, Donaldson D. Intestinal absorption in health and disease: micronutrients. Best Pract Res Clin Gastroenterol. 2003;17:957–979. doi: 10.1016/s1521-6918(03)00084-2. [DOI] [PubMed] [Google Scholar]
- 4.Lennernas H. Intestinal permeability and its relevance for absorption and elimination. Xenobiotica. 2007;37:1015–1051. doi: 10.1080/00498250701704819. [DOI] [PubMed] [Google Scholar]
- 5.Xue J, Askwith C, Javed NH, Cooke HJ. Autonomic nervous system and secretion across the intestinal mucosal surface. Auton Neurosci. 2007;133:55–63. doi: 10.1016/j.autneu.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 7.Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008;1778:660–669. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paris L, Tonutti L, Vannini C, Bazzoni G. Structural organization of the tight junctions. Biochim Biophys Acta. 2008;1778:646–659. doi: 10.1016/j.bbamem.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Cereijido M, Contreras RG, Shoshani L, Flores-Benitez D, Larre I. Tight junction and polarity interaction in the transporting epithelial phenotype. Biochim Biophys Acta. 2008;1778:770–793. doi: 10.1016/j.bbamem.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Knust E, Bossinger O. Composition and formation of intercellular junctions in epithelial cells. Science. 2002;298:1955–1959. doi: 10.1126/science.1072161. [DOI] [PubMed] [Google Scholar]
- 11.Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207–235. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- 12.Martin-Belmonte F, Mostov K. Regulation of cell polarity during epithelial morphogenesis. Curr Opin Cell Biol. 2008;20:227–234. doi: 10.1016/j.ceb.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Tanos B, Rodriguez-Boulan E. The epithelial polarity program: machineries involved and their hijacking by cancer. Oncogene. 2008;27:6939–6957. doi: 10.1038/onc.2008.345. [DOI] [PubMed] [Google Scholar]
- 14.Assemat E, Bazellieres E, Pallesi-Pocachard E, Le Bivic A, Massey-Harroche D. Polarity complex proteins. Biochim Biophys Acta. 2008;1778:614–630. doi: 10.1016/j.bbamem.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 15.Dow LE, Humbert PO. Polarity regulators and the control of epithelial architecture, cell migration, and tumorigenesis. Int Rev Cytol. 2007;262:253–302. doi: 10.1016/S0074-7696(07)62006-3. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein B, Macara IG. The PAR proteins: fundamental players in animal cell polarization. Dev Cell. 2007;13:609–622. doi: 10.1016/j.devcel.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Humbert PO, Grzeschik NA, Brumby AM, Galea R, Elsum I, Richardson HE. Control of tumourigenesis by the Scribble/Dlg/Lgl polarity module. Oncogene. 2008;27:6888–6907. doi: 10.1038/onc.2008.341. [DOI] [PubMed] [Google Scholar]
- 18.Yamanaka T, Ohno S. Role of Lgl/Dlg/Scribble in the regulation of epithelial junction, polarity and growth. Front Biosci. 2008;13:6693–6707. doi: 10.2741/3182. [DOI] [PubMed] [Google Scholar]
- 19.Bilder D, Li M, Perrimon N. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science. 2000;289:113–116. doi: 10.1126/science.289.5476.113. [DOI] [PubMed] [Google Scholar]
- 20.Bilder D, Perrimon N. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature. 2000;403:676–680. doi: 10.1038/35001108. [DOI] [PubMed] [Google Scholar]
- 21.Woods DF, Bryant PJ. The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell. 1991;66:451–464. doi: 10.1016/0092-8674(81)90009-x. [DOI] [PubMed] [Google Scholar]
- 22.Gardiol D, Zacchi A, Petrera F, Stanta G, Banks L. Human discs large and scrib are localized at the same regions in colon mucosa and changes in their expression patterns are correlated with loss of tissue architecture during malignant progression. Int J Cancer. 2006;119:1285–1290. doi: 10.1002/ijc.21982. [DOI] [PubMed] [Google Scholar]
- 23.Kamei Y, Kito K, Takeuchi T, Imai Y, Murase R, Ueda N, Kobayashi N, Abe Y. Human scribble accumulates in colorectal neoplasia in association with an altered distribution of beta-catenin. Hum Pathol. 2007;38:1273–1281. doi: 10.1016/j.humpath.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 24.Schimanski CC, Schmitz G, Kashyap A, Bosserhoff AK, Bataille F, Schafer SC, Lehr HA, Berger MR, Galle PR, Strand S, Strand D. Reduced expression of Hugl-1, the human homologue of Drosophila tumour suppressor gene lgl, contributes to progression of colorectal cancer. Oncogene. 2005;24:3100–3109. doi: 10.1038/sj.onc.1208520. [DOI] [PubMed] [Google Scholar]
- 25.Martin TA, Jiang WG. Loss of tight junction barrier function and its role in cancer metastasis. Biochim Biophys Acta. 2009;1788:872–891. doi: 10.1016/j.bbamem.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Qin Y, Capaldo C, Gumbiner BM, Macara IG. The mammalian Scribble polarity protein regulates epithelial cell adhesion and migration through E-cadherin. J Cell Biol. 2005;171:1061–1071. doi: 10.1083/jcb.200506094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dow LE, Kauffman JS, Caddy J, Zarbalis K, Peterson AS, Jane SM, Russell SM, Humbert PO. The tumour-suppressor Scribble dictates cell polarity during directed epithelial migration: regulation of Rho GTPase recruitment to the leading edge. Oncogene. 2007;26:2272–2282. doi: 10.1038/sj.onc.1210016. [DOI] [PubMed] [Google Scholar]
- 28.Dow LE, Brumby AM, Muratore R, Coombe ML, Sedelies KA, Trapani JA, Russell SM, Richardson HE, Humbert PO. hScrib is a functional homologue of the Drosophila tumour suppressor Scribble. Oncogene. 2003;22:9225–9230. doi: 10.1038/sj.onc.1207154. [DOI] [PubMed] [Google Scholar]
- 29.Ivanov AI, Bachar M, Babbin BA, Adelstein RS, Nusrat A, Parkos CA. A unique role for nonmuscle myosin heavy chain IIA in regulation of epithelial apical junctions. PLoS One. 2007;2:e658. doi: 10.1371/journal.pone.0000658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ivanov AI, McCall IC, Babbin B, Samarin SN, Nusrat A, Parkos CA. Microtubules regulate disassembly of epithelial apical junctions. BMC Cell Biol. 2006;7:12. doi: 10.1186/1471-2121-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ivanov AI, Nusrat A, Parkos CA. Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment. Mol Biol Cell. 2004;15:176–188. doi: 10.1091/mbc.E03-05-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Bivic A, Real FX, Rodriguez-Boulan E. Vectorial targeting of apical and basolateral plasma membrane proteins in a human adenocarcinoma epithelial cell line. Proc Natl Acad Sci USA. 1989;86:9313–9317. doi: 10.1073/pnas.86.23.9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ivanov AI, Samarin SN, Bachar M, Parkos CA, Nusrat A. Protein kinase C activation disrupts epithelial apical junctions via ROCK-II dependent stimulation of actomyosin contractility. BMC Cell Biol. 2009;10:36. doi: 10.1186/1471-2121-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cozens AL, Yezzi MJ, Kunzelmann K, Ohrui T, Chin L, Eng K, Finkbeiner WE, Widdicombe JH, Gruenert DC. CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am J Respir Cell Mol Biol. 1994;10:38–47. doi: 10.1165/ajrcmb.10.1.7507342. [DOI] [PubMed] [Google Scholar]
- 35.Ivanov AI, Hopkins AM, Brown GT, Gerner-Smidt K, Babbin BA, Parkos CA, Nusrat A. Myosin II regulates the shape of three-dimensional intestinal epithelial cysts. J Cell Sci. 2008;121:1803–1814. doi: 10.1242/jcs.015842. [DOI] [PubMed] [Google Scholar]
- 36.Vassilieva EV, Gerner-Smidt K, Ivanov AI, Nusrat A. Lipid rafts mediate internalization of beta1-integrin in migrating intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2008;295:G965–G976. doi: 10.1152/ajpgi.00082.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, Nusrat A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003;171:6164–6172. doi: 10.4049/jimmunol.171.11.6164. [DOI] [PubMed] [Google Scholar]
- 38.Naydenov NG, Hopkins AM, Ivanov AI. c-Jun N-terminal kinase mediates disassembly of apical junctions in model intestinal epithelia. Cell Cycle. 2009;8:2110–2121. doi: 10.4161/cc.8.13.8928. [DOI] [PubMed] [Google Scholar]
- 39.Kallay LM, McNickle A, Brennwald PJ, Hubbard AL, Braiterman LT. Scribble associates with two polarity proteins. Lgl2 and Vangl2, via distinct molecular domains. J Cell Biochem. 2006;99:647–664. doi: 10.1002/jcb.20992. [DOI] [PubMed] [Google Scholar]
- 40.Legouis R, Jaulin-Bastard F, Schott S, Navarro C, Borg JP, Labouesse M. Basolateral targeting by leucine-rich repeat domains in epithelial cells. EMBO Rep. 2003;4:1096–1102. doi: 10.1038/sj.embor.7400006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Metais JY, Navarro C, Santoni MJ, Audebert S, Borg JP. hScrib interacts with ZO-2 at the cell-cell junctions of epithelial cells. FEBS Lett. 2005;579:3725–3730. doi: 10.1016/j.febslet.2005.05.062. [DOI] [PubMed] [Google Scholar]
- 42.Petit MM, Meulemans SM, Alen P, Ayoubi TA, Jansen E, Van de Ven WJ. The tumor suppressor Scrib interacts with the zyxin-related protein LPP, which shuttles between cell adhesion sites and the nucleus. BMC Cell Biol. 2005;6:1. doi: 10.1186/1471-2121-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Capaldo CT, Macara IG. Depletion of E-cadherin disrupts establishment but not maintenance of cell junctions in Madin-Darby canine kidney epithelial cells. Mol Biol Cell. 2007;18:189–200. doi: 10.1091/mbc.E06-05-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McNeil E, Capaldo CT, Macara IG. Zonula occludens-1 function in the assembly of tight junctions in Madin-Darby canine kidney epithelial cells. Mol Biol Cell. 2006;17:1922–1932. doi: 10.1091/mbc.E05-07-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gonzalez-Mariscal L, Contreras RG, Bolivar JJ, Ponce A, Chavez De Ramirez B, Cereijido M. Role of calcium in tight junction formation between epithelial cells. Am J Physiol. 1990;259:C978–C986. doi: 10.1152/ajpcell.1990.259.6.C978. [DOI] [PubMed] [Google Scholar]
- 46.Ivanov AI, Hunt D, Utech M, Nusrat A, Parkos CA. Differential roles for actin polymerization and a myosin II motor in assembly of the epithelial apical junctional complex. Mol Biol Cell. 2005;16:2636–2650. doi: 10.1091/mbc.E05-01-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dow LE, Elsum IA, King CL, Kinross KM, Richardson HE, Humbert PO. Loss of human Scribble cooperates with H-Ras to promote cell invasion through deregulation of MAPK signalling. Oncogene. 2008;27:5988–6001. doi: 10.1038/onc.2008.219. [DOI] [PubMed] [Google Scholar]
- 48.Zhan L, Rosenberg A, Bergami KC, Yu M, Xuan Z, Jaffe AB, Allred C, Muthuswamy SK. Deregulation of scribble promotes mammary tumorigenesis and reveals a role for cell polarity in carcinoma. Cell. 2008;135:865–878. doi: 10.1016/j.cell.2008.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gonzalez-Mariscal L, Tapia R, Chamorro D. Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta. 2008;1778:729–756. doi: 10.1016/j.bbamem.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 50.Wong CH, Cheng CY. Mitogen-activated protein kinases, adherens junction dynamics, and spermatogenesis: a review of recent data. Dev Biol. 2005;286:1–15. doi: 10.1016/j.ydbio.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 51.Sun Y, Aiga M, Yoshida E, Humbert PO, Bamji SX. Scribble interacts with beta-catenin to localize synaptic vesicles to synapses. Mol Biol Cell. 2009;20:3390–3400. doi: 10.1091/mbc.E08-12-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fanning AS, Anderson JM. Zonula occludens-1 and -2 are cytosolic scaffolds that regulate the assembly of cellular junctions. Ann NY Acad Sci. 2009;1165:113–120. doi: 10.1111/j.1749-6632.2009.04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laukoetter MG, Nava P, Nusrat A. Role of the intestinal barrier in inflammatory bowel disease. World J Gastroenterol. 2008;14:401–407. doi: 10.3748/wjg.14.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mankertz J, Schulzke JD. Altered permeability in inflammatory bowel disease: pathophysiology and clinical implications. Curr Opin Gastroenterol. 2007;23:379–383. doi: 10.1097/MOG.0b013e32816aa392. [DOI] [PubMed] [Google Scholar]
- 55.Nguyen MM, Rivera C, Griep AE. Localization of PDZ domain containing proteins Discs Large-1 and Scribble in the mouse eye. Mol Vis. 2005;11:1183–1199. [PubMed] [Google Scholar]
- 56.Navarro C, Nola S, Audebert S, Santoni MJ, Arsanto JP, Ginestier C, Marchetto S, Jacquemier J, Isnardon D, Le Bivic A, Birnbaum D, Borg JP. Junctional recruitment of mammalian Scribble relies on E-cadherin engagement. Oncogene. 2005;24:4330–4339. doi: 10.1038/sj.onc.1208632. [DOI] [PubMed] [Google Scholar]
- 57.Tepass U, Tanentzapf G, Ward R, Fehon R. Epithelial cell polarity and cell junctions in Drosophila. Annu Rev Genet. 2001;35:747–784. doi: 10.1146/annurev.genet.35.102401.091415. [DOI] [PubMed] [Google Scholar]
- 58.Van Itallie CM, Holmes J, Bridges A, Gookin JL, Coccaro MR, Proctor W, Colegio OR, Anderson JM. The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. J Cell Sci. 2008;121:298–305. doi: 10.1242/jcs.021485. [DOI] [PubMed] [Google Scholar]
- 59.Massimi P, Narayan N, Thomas M, Gammoh N, Strand S, Strand D, Banks L. Regulation of the hDlg/hScrib/Hugl-1 tumour suppressor complex. Exp Cell Res. 2008;314:3306–3317. doi: 10.1016/j.yexcr.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 60.Bilder D, Schober M, Perrimon N. Integrated activity of PDZ protein complexes regulates epithelial polarity. Nat Cell Biol. 2003;5:53–58. doi: 10.1038/ncb897. [DOI] [PubMed] [Google Scholar]
- 61.Stucke VM, Timmerman E, Vandekerckhove J, Gevaert K, Hall A. The MAGUK protein MPP7 binds to the polarity protein hDlg1 and facilitates epithelial tight junction formation. Mol Biol Cell. 2007;18:1744–1755. doi: 10.1091/mbc.E06-11-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klezovitch O, Fernandez TE, Tapscott SJ, Vasioukhin V. Loss of cell polarity causes severe brain dysplasia in Lgl1 knockout mice. Genes Dev. 2004;18:559–571. doi: 10.1101/gad.1178004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamanaka T, Horikoshi Y, Izumi N, Suzuki A, Mizuno K, Ohno S. Lgl mediates apical domain disassembly by suppressing the PAR-3-aPKC-PAR-6 complex to orient apical membrane polarity. J Cell Sci. 2006;119:2107–2118. doi: 10.1242/jcs.02938. [DOI] [PubMed] [Google Scholar]
- 64.Yamanaka T, Horikoshi Y, Sugiyama Y, Ishiyama C, Suzuki A, Hirose T, Iwamatsu A, Shinohara A, Ohno S. Mammalian Lgl forms a protein complex with PAR-6 and aPKC independently of PAR-3 to regulate epithelial cell polarity. Curr Biol. 2003;13:734–743. doi: 10.1016/s0960-9822(03)00244-6. [DOI] [PubMed] [Google Scholar]
- 65.Nakagawa S, Huibregtse JM. Human scribble (Vartul) is targeted for ubiquitin-mediated degradation by the high-risk papillomavirus E6 proteins and the E6AP ubiquitin-protein ligase. Mol Cell Biol. 2000;20:8244–8253. doi: 10.1128/mcb.20.21.8244-8253.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sone K, Nakagawa S, Nakagawa K, Takizawa S, Matsumoto Y, Nagasaka K, Tsuruga T, Hiraike H, Hiraike-Wada O, Miyamoto Y, Oda K, Yasugi T, Kugu K, Yano T, Taketani Y. hScrib, a human homologue of Drosophila neoplastic tumor suppressor, is a novel death substrate targeted by caspase during the process of apoptosis. Genes Cells. 2008;13:771–785. doi: 10.1111/j.1365-2443.2008.01204.x. [DOI] [PubMed] [Google Scholar]
- 67.van der Woude CJ, Kleibeuker JH, Jansen PL, Moshage H. Chronic inflammation, apoptosis and (pre-)malignant lesions in the gastro-intestinal tract. Apoptosis. 2004;9:123–130. doi: 10.1023/B:APPT.0000018794.26438.22. [DOI] [PubMed] [Google Scholar]
- 68.Xie J, Itzkowitz SH. Cancer in inflammatory bowel disease. World J Gastroenterol. 2008;14:378–389. doi: 10.3748/wjg.14.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


