Abstract
Stress affects the pathophysiology of cutaneous immune reactions, including contact hypersensitivity (CH) in individuals sensitized with sensitizing hapten, where local endothelial cell activation plays a critical role. To clarify the effects of stress in cutaneous immune reactions, we selected a CH model using annoying sound as a stress. Furthermore, we conducted the stress experiments by using selectin-deficient mice to determine the involvement of selectin molecules regarding local endothelial activation. Auditory stress augmented CH responses in the present study. Namely, ear thickness and mast cell numbers were significantly increased in stressed CH mice. mRNA expression of preprotachykinin-A, a precursor of substance-P; interferon-γ; interleukin (IL)-4; IL-6; and tumor necrosis factor-α significantly increased in stressed CH mice. Furthermore, stressed L-selectin-deficient mice showed significant decreases in all parameters mentioned above relative to stressed wild-type mice in CH response. Meanwhile, treatment with anti-L-selectin Ab resulted in a significant decrease in ear thickness and mRNA levels of interferon-γ, IL-4, IL-6, and tumor necrosis factor-α, but failed to significantly reduce preprotachykinin-A mRNA levels and mast cell numbers. Our results indicated that auditory stress enhances CH response and that the augmentation of this CH response might be mediated through L-selectin, but not through P- or E-selectin pathways.
Contact hypersensitivity (CH) is one of the delayed-type hypersensitivity (DTH) reactions, which are antigen (Ag)-specific, cell-mediated immune responses. CH is a cutaneous immune reaction in individuals sensitized with sensitizing hapten, where local endothelial cell activation plays a critical role. In acute CH models, inflammatory responses are elicited by a single epicutaneous application of a contact-sensitizing agent in animals previously sensitized with the same Ag.1 L-selectin-deficient mice exhibited reduced CH responses under these conditions.2 Meanwhile, repeated application of a contact-sensitizing agent in mice induces chronic CH responses that are clinically relevant to human skin allergic diseases including atopic dermatitis.3 Sometimes, dermatologists note that some skin diseases such as atopic dermatitis become worse seemingly because of some kinds of stress stimuli, and that the removal of such stress can improve the skin condition of such patients. There have been many reports concerning stress in experimental animal models.4–6 Among them, Dhabhar et al5 mentioned that a stress-induced trafficking of leukocytes to the skin may mediate skin DTH reactions. However, the exact mechanism is still unclear as to the involvement of stress in the exacerbation of skin conditions.
Leukocyte recruitment from the circulation into a site of inflammation involves adhesive interactions between the leukocyte and the vascular endothelium, and has been implicated in the pathology of various inflammatory diseases. Leukocytes first tether and roll on vascular cells, before they are activated to adhere firmly and subsequently to immigrate into the extravascular spaces. The selectin family, which mediates tethering and rolling of leukocytes, consists of three cell-surface molecules expressed by leukocytes (L-selectin), vascular endothelium (E- and P-selectin), and platelets (P-selectin).7 The interaction of adhesion molecules during the process of leukocyte migration into inflammatory sites is complex and highly regulated. Although L-selectin deficiency is reported to reduce airway hyperresponsiveness in a murine asthma model induced by repeated Ag exposure through the respiratory mucosa,8 the contribution of the selectin family to the exacerbation of cutaneous inflammation by stress stimuli remains unknown.
Therefore, we conducted the present study to evaluate the effects of stress stimuli on CH reactions and to clarify the involvement of the selectin family in the stress-induced modifications of CH reactions.
Materials and Methods
Mice
L-selectin-deficient (selectin−/−) mice were produced as described elsewhere.9 P-selectin−/−, E-selectin−/−, and wild-type C57BL/6 mice were obtained from the Jackson Laboratory (Bar Harbor, ME). All of the mice were healthy and fertile and did not display evidence of infection or disease and were backcrossed between 10 generations as to the C57BL/6 genetic background. The mice used for this study were 8 to 10 weeks old. All mice were housed in a pathogen-free barrier facility and screened regularly for pathogens. All studies and procedures were approved by the Committee on Animal Experimentation of Nagasaki University Graduate School of Biomedical Sciences.
Induction of CH
The mice were sensitized three times with 20 μl of a 3% oxazolone solution (4-ethyoxymethylene-2-phenyloxazolone; Sigma-Aldrich, St. Louis, MO) in acetone/sesamin seed oil (4:1) applied to the abdomen for a week. Starting 7 days after sensitization, 20 μl of 0.5% oxazolone was applied to the right ear. Two days later, elicited ears were examined macroscopically and microscopically.
Ear thickness was measured by using a dial thickness gauge (Ozaki Seisakusho Co., Tokyo, Japan) under light ether anesthesia 48 hours after elicitation. Each ear lobe was measured three times and the mean of those values was used for analysis.
Stress Application to Mice
The mice were divided into two groups: those housed in a special room installed with sound equipment generating 400 Hz noise and vibrations for 2 seconds at 25-second intervals during the whole experiment; and those housed just after the elicitation for 2 days in a special room. This sound generator is manufactured to scare away moles and wild mice (Tokyo Yunikomu Co., Tokyo, Japan). The manufacturer claims that this generator emits an annoying sound for small rodents into the ground and that the area covered by this sound stress makes a circle with a radius of 8 m, fooling moles and wild mice into thinking there is a danger of an earthquake. Thus, it was conjectured that the sound generator gave mice emotional stress in the present study.
Blocking Study by Monoclonal Antibodies
For a blocking study, monoclonal antibody (mAb) to L-selectin was injected intravenously into wild-type mice just before the sensitization and elicitation. mAb used in this blocking study meant mAb to murine L-selectin (MEL14, rat IgG2a, 30 g per mouse; BD PharMingen, San Diego, CA). The concentration of this mAb was required to inhibit L-selectin-dependent leukocyte recruitment in vivo as previously described.10 Irrelevant isotype-matched, purified rat IgG2a mAb (R35-95), served as controls (30 μg per mouse; BD PharMingen).
Inhibition Study by Neurokinin Receptor Antagonist
For an inhibition study, neurokinin 1 receptor (NK1R) antagonist was injected intraperitoneally into wild-type mice 30 minutes before the elicitation.11 “Spantide I” (S0274, [D-Arg,1 D-Trp,7,9 Leu11]-Substance P [SP] acetate salt, 2 Mol) was adopted as a NK1R antagonist in the inhibition study (Sigma-Aldrich). The concentration of this drug was required to inhibit the function of NK1R in vivo as previously described.12
Grouping
Seven groups of 8 to 10 mice each were created in the present study: (1) Ag-elicited mice without sensitization ([control] wild-type mice sensitized three times with vehicle oil for 1 week and elicited with 0.5% oxazolone solution); (2) CH without stress ([unstressed] wild-type mice sensitized three times with 3% oxazolone solution for 1 week and elicited with 0.5% oxazolone solution); (3) CH with stress only at elicitation phase ([single stressed] wild-type mice sensitized three times with 3% oxazolone solution for 1 week, elicited with 0.5% oxazolone solution and immediately housed in a special room for 2 days); (4) CH with stress both at sensitization and elicitation phases ([double stressed] wild-type mice sensitized three times with 3% oxazolone solution over a period of 1 week and elicited with 0.5% oxazolone solution; all procedures were performed in a special room equipped with a sound generator); (5) CH with stress both at sensitization and elicitation phases in P-, E-, and L-selectin−/− mice ([double stressed P-, E-, and L-selectin] P-selectin−/−, E-selectin−/−, and L-selectin−/− mice sensitized three times with 3% oxazolone solution over a period of 1 week and elicited with 0.5% oxazolone solution; all procedures were performed in a special room equipped with a sound generator); (6) CH with stress both at sensitization and elicitation phases in wild-type mice injected with anti-L-selectin Ab ([double stressed anti-L-selectin Ab] wild-type mice sensitized three times with 3% oxazolone solution over a period of 1 week and elicited with 0.5% oxazolone solution. The injection of mAb to murine L-selectin or isotype-matched, purified rat IgG2a mAb was performed intravenously just before the sensitization and elicitation with oxazolone solution. All procedures were performed in a special room equipped with a sound generator); and (7) CH with stress both at sensitization and elicitation phases in wild-type mice injected with NK1R antagonist ([double stressed NK1R antagonist] wild-type mice sensitized three times with 3% oxazolone solution over a period of 1 week and elicited with 0.5% oxazolone solution. The injection of NK1R antagonist was performed intrapenitoneally 30 minutes before the elicitation with oxazolone solution. All procedures were performed in a special room equipped with a sound generator).
Histological Examination
A central strip of the ear was fixed in 3.5% paraformaldehyde and then paraffin-embedded. Sections (6 μm) were stained with H&E for general histological evaluation and toluidine blue staining for mast cells. Dermal mast cell infiltration was evaluated by counting the number of mast cells present in 12 high-power fields (0.07 mm2). Each section was examined independently by two investigators in a blind manner, and the mean was used for analysis.
RNA Isolation and Real-Time RT-PCR
Total RNA was isolated from the ear of mutant and wild-type mice by using Qiagen RNeasy spin columns (Qiagen, Crawley, UK) and digested by DNease I (Qiagen) to remove chromosomal DNA in accordance with the manufacturer's protocol. Total RNA was reverse-transcribed to cDNA by using a reverse transcription system with random hexamers (Promega, Madison, WI), and mRNA expression of E-, L-, and P-selectin, preprotachykinin (PPT)-A, interleukin (IL)-4, IL-6, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α were analyzed by real-time quantitative RT-PCR by using the TaqMan system (Applied Biosystems, Foster City, CA). Glyceral-dehyde-3-phosphate dehydrogenase (GAPDH) was used to normalize mRNA. We obtained all sequence-specific primers and probes from TaqMan gene expression assays (Applied Biosystems). Real-time PCR was performed on an ABI Prism 7000 sequence detector (Applied Biosystems) according to the manufacturer's instructions. Relative expression of real-time PCR products was determined by using the ΔΔCT technique as previously described.13 In brief, we normalized each set of samples using the difference in threshold cycle (CT) between the target gene and GAPDH: ΔCT = (CT target gene − CT GAPDH). Relative mRNA levels were calculated by the formula 2−ΔΔCT where ΔΔCT = ΔCT sample (η) − ΔCT calibrator (η). Each reaction was repeated at least three times. One of the control samples was chosen as a calibrator sample.
Statistical Analysis
The Mann-Whitney's U-test was used for determining the level of significance of differences in sample means, and all data are shown as mean ± SEM.
Results
Histological Examination of the Ear-Swelling and Leukocyte Infiltration
To assess histological characteristics associated with the ear-swelling, ear biopsies were performed 48 hours after elicitation. After Ag elicitation, the ear biopsies of the unstressed CH mice revealed mild edema in the s.c. tissue. Meanwhile, the biopsies from the single-stressed CH mice revealed more moderate edema and leukocyte infiltration. Furthermore, the biopsies from the double-stressed CH mice revealed prominent edema as compared with the other three groups. Similar results were also obtained for mast cell infiltration in these groups (Figure 1A).
Figure 1.
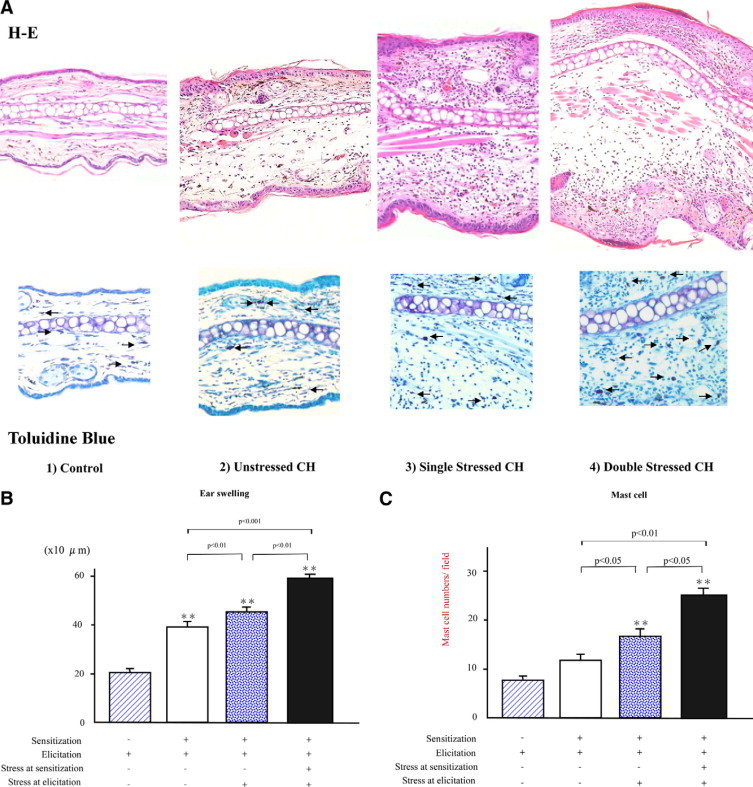
A: Top: Representative histological sections of the ear pinnae from mice without sensitization and CH mice with or without stress 48 hours after oxazolone elicitation. The sections were stained with H&E. The mice were grouped as follows: (1) control; (2) unstressed; (3) single stressed; and (4) double stressed. Original magnification, ×100. More prominent edema and leukocyte infiltration were noticed in the pinnae from the stressed CH mice relative to the unstressed and control mice. Bottom: Representative histological sections showing mast cell accumulation in the ear pinnae, where mast cells were detected as cells with metachromatic staining of granules in toluidine-blue stain (arrows). Original magnification, ×120. B: Total ear thickness 48 hours after oxazolone elicitation in mice without sensitization and in CH mice with or without stress. The ear thickness was measured 48 hours after oxazolone elicitation. All values represent the mean ± SEM of results obtained from 8 to 10 mice in each group. Single-stressed and double-stressed CH mice exhibited significant increases relative to the unstressed CH mice (P < 0.01, P < 0.001, respectively). Furthermore, double-stressed mice exhibited a more significant increase than the single-stressed mice (P < 0.01). **P < 0.001 vs Ag-elicited mice without sensitization. C: Mast cell numbers in the dermis of pinnae from mice without sensitization, the control, unstressed, and stressed CH mice 48 hours after oxazolone elicitation. Mast cell numbers per dermis section in high-power microscopic field (0.07 mm2) were determined by counting in paraffin sections stained with toluidine blue. Both types of stressed CH mice showed significant increases as compared with the unstressed ones (P < 0.05, P < 0.01, respectively). Furthermore, the double-stressed mice exhibited a more significant increase than the single-stressed mice (P < 0.05). All values represent the mean ± SEM of results obtained from 8 to 10 mice in each group. **P < 0.01 vs Ag-elicited mice without sensitization.
Estimation of Ear-Swelling and Leukocyte Infiltration
Ear thickness was measured 48 hours after Ag elicitation in these four groups (Figure 1B). A significant increase in the total ear thickness was recognized in the unstressed CH mice as compared with the controls (1.9-fold increase, P < 0.001). Meanwhile, the single-stressed CH mice showed a significant increase compared with the unstressed CH mice (15% higher, P < 0.01) and furthermore, the double-stressed CH mice revealed a more prominent increase than the unstressed CH mice (50% higher, P < 0.001). Interestingly, the double-stressed CH mice also exhibited a significant increase relative to the single-stressed CH mice (30% higher, P < 0.01).
Similar results were also obtained for mast cell numbers except that there was no significant difference between the unstressed CH mice and the controls (Figure 1C). The mast cell numbers were significantly higher in the single-stressed CH mice than the unstressed CH mice (42% higher, P < 0.05). Furthermore, the double-stressed CH mice exhibited a significant increase in mast cell numbers compared with both the unstressed (100% higher, P < 0.05) and the single-stressed CH mice (50% higher, P < 0.05).
Estimation of CH Response Augmented by Stress
We investigated mRNA expressions of IL-4, IL-6, TNF-α, IFN-γ, and PPT-A, which was a precursor of SP in the double-stressed CH mice. Namely, mRNA expressions of IL-4, IFN-γ, and TNF-α exhibited significant increases in the double-stressed CH mice relative to the unstressed CH mice (Figure 2, 2.9-fold, 4.0-fold, and 4.8-fold, P < 0.01, respectively). Simultaneously, IL-6 mRNA was significantly increased in the double-stressed CH mice (Figure 2, 7.0-fold higher, P < 0.001). Interestingly, the double-stressed CH mice also showed a significantly higher PPT-A level than the unstressed CH mice (Figure 2, 4.0-fold, P < 0.001,). Thus, all five parameters selected showed significantly higher increases in CH responses augmented by auditory stress.
Figure 2.

mRNA expressions of PPT-A, IL-4, IL-6, TNF-α, and IFN-γ in the pinnae taken from the double-stressed CH mice relative to the unstressed mice. Their expressions were analyzed by real-time PCR. Relative expression of real-time PCR products was determined by the Ct method to compare target gene and GAPDH mRNA expressions. All values represent the mean ± SEM, which indicate mRNA expressions in stressed CH mice relative to those in unstressed CH mice 48 hours after elicitation. Stressed CH mice showed significant increases in all parameters relative to the unstressed CH mice (*P < 0.05). These results were obtained from 8 to 10 mice.
Role of Selectin Molecules in CH Response Augmented by Stress
We conducted the same experiments by using E-, P-, and L-selectin−/− mice to examine the role of selectin molecules in CH reactions augmented by auditory stress (Figure 3A). Ear thickness was measured 48 hours after elicitation in E-, P-, and L-selectin−/− mice and compared with wild-type mice in CH responses (Figure 3, A and B). Significant decreases in the total ear thickness were not recognized in P- and E-selectin−/− mice, as compared with the double-stressed wild-type mice. Consistent with these findings, skin mRNA expression of P- and E-selectins was not up-regulated in the double-stressed CH mice relative to the unstressed CH mice (data not shown). By contrast, L-selectin−/− mice exhibited a significant decrease in CH responses in comparison with the double-stressed wild-type mice (Figure 3B, 39% lower, P < 0.001).
Figure 3.

A: Representative histological sections of the ear pinnae from stressed wild-type, E-, P-, and L-selectin−/− mice 48 hours after oxazolone elicitation. The sections were stained with H&E. The improvement of edema and leukocyte infiltration was noticed only in the dermis of ear pinnae from the stressed L-selectin−/− mice as compared with the stressed wild-type mice. Original magnification, ×100. B: Total ear thickness in CH responses in stressed wild-type, E-, P-, and L-selectin−/− mice 48 hours after oxazolon elicitation. All values represent the mean ± SEM of results obtained from 8 to 10 mice. Stressed L-selectin−/− mice only showed a significant improvement in ear thickness as compared with stressed wild-type mice in CH responses (**P < 0.01).
Next, mast cell numbers were also assessed in the Ag-administered ears taken from E-, P-, and L-selectin−/− mice as compared with the double-stressed wild-type mice (Figure 4A). Mast cell numbers were significantly lower in the double-stressed L-selectin−/− mice than the double-stressed wild-type mice (25% lower, P < 0.05).
Figure 4.
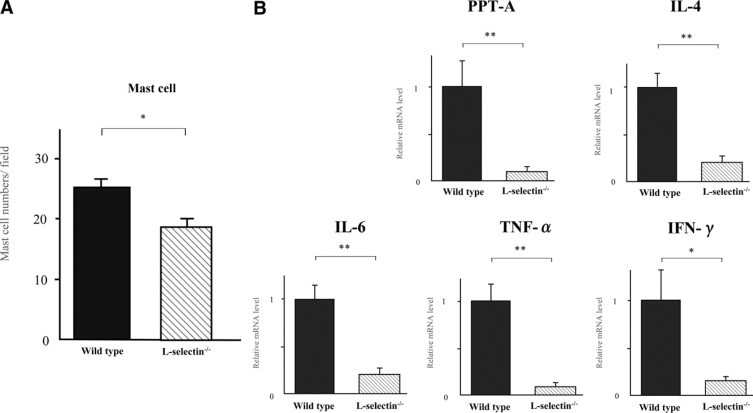
A: Mast cell numbers in the dermis of pinnae from stressed wild-type and L-selectin−/− mice 48 hours after oxazolone elicitation in CH responses. Mast cell numbers per dermis section in high-power microscopic field (0.07 mm2) were determined by counting in paraffin sections stained with toluidine blue. Stressed L-selectin−/− mice showed a significant decrease by 25% as compared with stressed wild-type mice in mast cell numbers (*P < 0.05). B: mRNA expressions of PPT-A, IL-4, IL-6, TNF-α, and IFN-γ in the pinnae from stressed L-selectin−/− mice relative to stressed wild-type mice in CH responses. Their mRNA expressions were analyzed by real-time PCR. Relative expression of real-time PCR products was determined by the Ct method to compare target gene and GAPDH mRNA expression. All values represent the mean ± SEM, which indicate mRNA expressions in stressed CH response in L-selectin−/− mice relative to those in wild-type mice 48 hours after elicitation. Stressed CH response in L-selectin−/− mice showed significant decreases in all parameters selected as compared with those in wild-type mice (**P < 0.01 and *P < 0.05, respectively). These results were obtained from 8 to 10 mice.
We estimated CH responses augmented by auditory stress by the expressions of PPT-A, IL-4, IL-6, TNF-α, and IFN-γ mRNAs (Figure 4B). L-selectin−/− mice showed dramatically significant decreases in PPT-A, IL-4, IL-6, and TNF-α mRNAs compared with stressed wild-type mice (90%, 79%, 79%, and 90% decreased, P < 0.01, respectively). Interestingly, IFN-γ mRNA was also significantly lower in stressed L-selectin−/− mice (86% decreased, P < 0.05). Thus, all five parameters selected showed that the L-selectin−/− mice's responses to auditory stress were far less than wild-type mice.
The Effects of Anti-L-Selectin Ab on CH Response Augmented by Stress
Next, we performed the blocking study by using anti-L-selectin mAb. We intravenously injected anti-murine L-selectin Ab and isotype-matched rat monoclonal IgG into the wild-type mice, just before sensitization and elicitation, respectively.
Histologically, after being stressed, these mice showed a prominent improvement in edema and the accumulation of infiltrating cells relative to the uninjected CH mice (Figure 5A).
Figure 5.
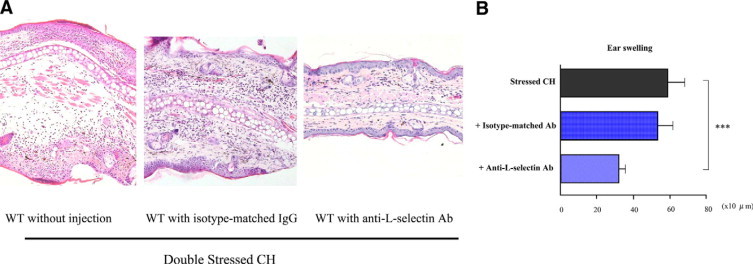
A: Representative histological sections of CH responses in the ear pinnae from stressed wild-type (WT) mice with or without injection of isotye-matched Ab or anti-L-selectin Ab 48 hours after oxazolone elicitation. The sections were stained with H&E. Original magnification, ×100 The improvement of edema and leukocyte infiltration was shown in the dermis of ear pinnae from stressed CH mice injected with anti-L-selectin Ab as compared with stressed CH mice without injection. B: Total ear thickness in stressed CH mice without or with injections of isotype-matched Ab or anti-L-selectin Ab 48 hours after oxazolon elicitation. The ear thickness was measured 48 hours after oxazolone elicitation. All values represent the mean ± SEM of results obtained from 8 to 10 mice. Stressed CH mice injected with anti-L-selectin Ab showed a significant improvement of ear thickness as compared with stressed CH mice without injection (***P < 0.001).
Ear thickness was measured 48 hours after Ag elicitation (Figure 5B), and it was found that the stressed wild-type mice injected with isotype-matched IgG nearly matched the uninjected mice, while the stressed wild-type mice injected with anti-L-selectin Ab mice showed a significant decrease (Figure 5B, 46% lower, P < 0.001). An assessment of the mast cell numbers showed no significant statistical difference between the stressed wild-type mice injected with anti-L-selectin and the uninjected mice (data not shown).
IL-4, IFN-γ, and TNF-α mRNA expressions were significantly lower in the stressed wild-type mice injected with anti-L-selectin Ab than the uninjected ones (Figure 6, 75%, 85%, and 70% decreased, P < 0.05, respectively). IL-6 mRNA expression demonstrated a similar pattern (Figure 6, 75% decreased, P < 0.01). By contrast, PPT-A mRNA expression was virtually the same.
Figure 6.

mRNA expressions of PPT-A, IL-4, IL-6, TNF-α, and IFN-γ in the pinnae from stressed CH mice with injection of anti-L-selectin Ab relative to CH mice without injection. Their expressions of PPT-A, IL-4, IL-6, TNF-α, and IFN-γ were analyzed by real-time PCR. Relative expression of real-time PCR products was determined by the Ct method to compare target gene and GAPDH mRNA expression. All values represent the mean ± SEM, which indicate mRNA expressions in stressed CH mice injected with anti-L-selectin Ab relative to those in stressed CH mice without injection 48 hours after elicitation. These results were obtained from 8 to 10 mice. Stressed CH mice injected with anti-L-selectin Ab showed significant decreases in the expressions of IL-4, IL-6, TNF-α, and IFN-γ mRNAs relative to stressed CH mice without injection (*P < 0.05 and **P < 0.01, respectively). Injection of anti-L-selectin Ab into stressed CH mice failed to decrease the expression of PPT-A mRNA relative to stressed CH mice without injection.
The Effects of NK1R Antagonist on CH Response Augmented by Stress
Next, we performed the inhibition study by using NK1R antagonist to evaluate the role of SP in the stress-induced enhancement of CH responses. We injected NK1R antagonist intrapenitoneally into the wild-type mice before elicitation. Histologically, after being stressed, these mice showed an excellent improvement in edema and the accumulation of infiltrating cells relative to the uninjected CH mice (Figure 7A).
Figure 7.

A: Representative histological sections of CH responses in the ear pinnae from stressed wild-type mice with or without injection of NK1R antagonist 48 hours after oxazolone elicitation. The sections were stained with H&E. Original magnification, ×100. An improvement of edema and leukocyte infiltration was shown in the dermis of ear pinnae from stressed CH mice injected with NK1R antagonist as compared with stressed CH mice without injection. B: Total ear thickness in stressed CH mice with or without injections of NK1R antagonist 48 hours after oxazolon elicitation. The ear thickness was measured 48 hours after oxazolone elicitation. All values represent the mean ± SEM of results obtained from 8 to 10 mice. Stressed CH mice injected with NK1R antagonist showed a significant decrease in ear thickness as compared with stressed CH mice without injection (**P < 0.01).
Ear thickness was measured 48 hours after Ag elicitation, and it was found that these mice also showed a significant decrease comparative to the unstressed mice (Figure 7B, 23% lower, P < 0.01).
In addition, an assessment of the mast cell numbers revealed that these mice showed a significant decrease as compared with stressed CH mice without injection (Figure 8A, 37% lower, P < 0.01).
Figure 8.

A: Mast cell numbers in the dermis of pinnae from stressed CH mice with or without injection of NK1R antagonist 48 hours after oxazolone elicitation in CH responses. Mast cell numbers per dermis section in high-power microscopic field (0.07 mm2) were determined by counting in paraffin sections stained with toluidine blue. Stressed CH mice with NK1R antagonist showed a significant decrease as compared with stressed CH mice without injection in mast cell numbers (P < 0.01). B: mRNA expressions of PPT-A, IL-4, IL-6, TNF-α, and IFN-γ in the pinnae from stressed CH mice with injection of NK1R antagonist relative to CH mice without injection. Their expressions of PPT-A, IL-4, IL-6, TNF-α, and IFN-γ were analyzed by real-time PCR. Relative expression of real-time PCR products was determined by the Ct method to compare target gene and GAPDH mRNA expression. All values represent the mean ± SEM, which indicate mRNA expressions in stressed CH mice injected with NK1R antagonist relative to those in stressed CH mice without injection 48 hours after elicitation. These results were obtained from 8 to 10 mice. Stressed CH mice injected with NK1R antagonist showed significant decreases in the expressions of PPT-A, TNF-α, IFN-γ, IL-4, and IL-6 mRNAs relative to stressed CH mice without injection (*P < 0.05 and **P < 0.01, respectively).
PPT-A, TNF-α, and IFN-γ mRNA expressions were all significantly lower in this group than in the uninjected ones (Figure 8B, 30%, 50%, and 68% lower, P < 0.05, respectively). Lastly, IL-4 and IL-6 mRNA expressions were significantly lower in this group than in the uninjected ones (Figure 8B, 53% and 70% lower, P < 0.01, respectively).
Discussion
We examined the changes in CH brought about by exposure to annoying sound in the present study. We believe this report is the first to reveal the effects of sound stress on CH augmentation in mice, including selectin−/− mice. First, two groups were created to examine the effect of the timing of the stress stimuli. Both groups were housed in special rooms with sound equipment that delivered irritating noise: the first group for 2 days after elicitation and the second group for 9 days both at the sensitization and elicitation phases. Both groups showed significant increases in the ear thickness and mast cell numbers when compared with the unstressed CH. Furthermore, the second group showed a more significant increase in ear thickness and mast cell number than the first group. Thus, we recognized that auditory stress enhanced CH response in the present study as Joachim et al14 mentioned. It also seemed to be likely that stress both at the sensitization and elicitation phases enhanced CH response more than stress at the elicitation phase only. Furthermore, we suspect that this model might be useful in examining the effects of stress on the cutaneous immune response.
Auditory stress experienced both for 2 days and for 9 days induced enhancement of a skin immune function despite the differences in the duration of stress in the present study. We have also performed a preliminary experiment by using a restraint stress, in which ear swelling was also enhanced in stressed CH mice comparatively (data not shown). There have been some stress experiments in which the authors reported the suppression or the exacerbation of immune responses.15–18 Among them, Dhabhar and McEwen15 mentioned that chronic stress has an immunosuppressive and acute stress has an immunoenhance effect. Thus, it was thought that the auditory stress we used could be considered an acute stress according to Dhabhar's and McEwen's15 criteria.
For further analyses, the differences were examined in mRNA levels of PPT-A and cytokines including IL-4, IL-6, TNF-α, and IFN-γ. We selected PPT-A, which was a precursor of SP, IFN-γ as a Th1 cytokine, IL-4 as a Th2 cytokine, and IL-6 and TNF-α as inflammatory cytokines.
Stressed CH mice showed significant increases in the transcript levels of all cytokines as well as PPT-A, compared with the unstressed ones. First, it has been reported that short duration stressors significantly enhanced skin DTH response. Our study's stress-induced increase in IFN-γ mRNA could be considered to support these reports because IFN-γ is a local mediator of an acute stress-induced enhancement of skin DTH,5 and CH was one of the DTH reactions and CH basically showed Th1 type cytokine pattern.
Next, our stressed CH mice showed significant increases in mRNA levels of IL-6 and TNF-α in parallel with the increase of ear thickness and mast cell numbers. IL-6 and TNF-α were selected as proinflammatory cytokines and they were reported to be involved in the pathogenesis of edema formation.19,20 Thus, it was considered that our results supported their results and that the increase of IL-6 and TNF-α mRNAs were related to the edema formation in the dermis of ear pinnae of our stressed CH mice.
PPT-A was a precursor of SP, which is secreted by various cells including mast cells, keratinocytes, and fibroblasts in the skin.21,22 Our stressed CH mice also showed significant increases in mast cell number relative to the unstressed ones. Mast cells release histamine, which can lead to cuatneous edema.23 Therefore, it is possible that mast cells could be involved in the development of ear thickness in stressed CH mice.
IL-4 was selected as a Th2 type cytokine and predominantly produced by mast cells and CD4+ T cells in the chronic CH model.1 Both PPT-A and mast cell numbers were increased in the stressed CH mice as compared with the unstressed ones in the present study. The authors of some reports recognized the low level or reduction of IL-4 in the stressed animals.24–26 There were some studies showing the induction of Th2 dominance in the stress experiments,27,28 but we could not find any studies showing the increase of IL-4 in stress experiments. Although IL-4 mRNA expression was increased by 2.9-fold, IFN-γ mRNA expression was increased four times in the stressed CH mice in the present study. Many studies have showed Th1 dominance in stress experiments.25,29 Our results also indicated Th1 dominance, although the increase in IL-4 mRNA expression was very significant. Further experiments should be conducted to elucidate the extreme variation. SP, IL-4, IL-6, TNF-α, and IFN-γ were increased in the pinnae of stressed CH mice by auditory stress in the present study. There are many authors reporting that such molecules are produced by many cells existing in inflamed skin.21,22,30–33 Thus, our study seems to indicate that many cells, including keratinocytes, fibroblasts, endothelial cells, and circulating cells in inflamed skin, could be affected by stress stimuli.
Next, to reveal the involvement of each selectin molecule in the augmentation of CH response by auditory stress, we conducted the same experiment by using E-, P-, and L-selectin−/− mice, and, in addition, a blocking study with mAb to L-selectin. We recognized a significant decrease in the ear thickness and in the number of mast cells in stressed CH responses in L-selectin−/− mice, but not in E- and P-selectin−/− mice as compared with wild-type mice, although all mice were sensitized, elicitated, and exposed to auditory stress. Interestingly, stressed CH responses in L-selectin−/− mice showed a more dramatic decrease than wild-type mice in the levels of PPT-A, IFN-γ, IL-4, IL-6, and TNF-α mRNA. Furthermore, the injection of mAb to L-selectin induced a significant decrease in the ear thickness as well as a significant decrease in IFN-γ, IL-4, IL-6, and TNF-α transcript levels. By contrast, the injection of mAb to L-selectin did not significantly reduce the number of mast cells (data not shown) or the PPT-A mRNA level, which was the precursor of SP. Mast cells can produce SP and mast cell number might be related to the expression of PPT-A mRNA. NK1R is reported to be a main receptor for SP, although it has been reported that NK2R and NK3R also function as the receptors for SP besides NK1R.34 Thus, we conducted an inhibition study by using NK1R antagonist to evaluate the role of SP in stressed CH response augmented by auditory stress. We selected Spantide I as an NK1R antagonist. The injection of NK1R antagonist into stressed wild-type mice induced a significant decrease in the ear thickness and in the number of mast cells in stressed CH response as well as a significant decrease in PPT-A, IFN-γ, IL-4, IL-6, and TNF-α transcript levels in the inhibition study. Namely, it was recognized that a SP/NK1R pathway was actually involved in the increase of CH response augmented by auditory stress.
Therefore, this failure by the injection of anti-L-selectin Ab might be due to the dose or administration method of anti-L-selectin Ab, or might be explained by the speculation that auditory stress could induce the secretion of SP and that SP could activate an L-selectin involved pathway, which led to the increase of infiltrating inflammatory cells in the lesional skin, although there was no author reporting the induction of L-selectin by SP. Further experiments should be conducted to explain this discrepancy.
Meanwhile, it is also very interesting that auditory stress can affect the hypothalamo- pituitary-adrenocortical axis and lead to the anti-inflammatory action.35,36 It was reported that stress induced SP-dependent neurogenic inflammation and made the brain-skin connection.37 Although we have recognized the involvement of SP in stressed CH mice, unfortunately, we did not examine corticotrophin-releasing hormone, adrenocorticotropic hormone, or corticosterone in the present study. Additional studies should be conducted to clarify such questions in the near future.
Here, we summarize our proposed mechanisms underlying the stress-induced up-regulation of the cutaneous immune system.
First, there have been some authors reporting the induction of L-selectin by IFN-γ in experiments.5,38 Therefore, one possibility is that auditory stress can induce L-selectin expression via IFN-γ activation pathway in the stressed CH mice because the increase of IFN-γ mRNA was actually significant in the stressed CH mice compared with unstressed CH mice in the present study.
Secondly, another possibility is that auditory stress can induce L-selectin expression on mast cells and certain leukocytes, which express SP and inflammatory cytokines, respectively, although we could find no author reporting this. Finally, there are some authors reporting the induction of E- and P-selectin by SP.39,40 Our inhibition study by using NK1R antagonist showed that the injection of it could not reduce the expressions of E- and P-selectin mRNAs (data not shown). Because the injection of anti-L-selectin Ab could not reduce PPT-A mRNA expression in the present study, this may indicate that auditory stress could induce the secretion of SP and that SP could activate an L-selectin involved pathway, which led to the increase of infiltrating inflammatory cells in the lesional skin.
In conclusion, though auditory stress enhances CH response in mice, the results of this study indicate that L-selectin mediates the augmentation of CH response. We suggest that L-selectin be investigated for its potential as an agent to control human skin diseases augmented by stress stimuli such as atopic dermatitis.
Footnotes
Supported by Grant-in-Aid from the Ministry of Education, Science, and Culture of Japan (19591315 to S.J.B.).
S.J.B. and K.S. contributed equally to this study.
References
- 1.Kitagaki H, Ono N, Hayakawa K, Kitazawa T, Watanabe K, Shiohara T. Repeated elicitation of contact hypersensitivity induces a shift in cutaneous cytokine milieu from a T helper cell type 1 to a T helper cell type 2 profile. J Immunol. 1997;159:2484–2491. [PubMed] [Google Scholar]
- 2.Steeber DA, Tang ML, Green NE, Zhang XQ, Sloane JE, Tedder TF. Leukocyte entry into sites of inflammation requires overlapping interactions between the L-selectin and ICAM-1 pathways. J Immunol. 1999;163:2176–2186. [PubMed] [Google Scholar]
- 3.Kitagaki H, Fujisawa S, Watanabe K, Hayakawa K, Shiohara T. Immediate-type hypersensitivity response followed by a late reaction is induced by repeated epicutaneous application of contact sensitizing agents in mice. J Invest Dermatol. 1995;105:749–755. doi: 10.1111/1523-1747.ep12325538. [DOI] [PubMed] [Google Scholar]
- 4.Flint MS, Miller DB, Tinkle SS. Restraint-induced modulation of allergic and irritant contact dermatitis in male and female B6.129 mice. Brain Behav Immun. 2000;14:256–269. doi: 10.1006/brbi.2000.0604. [DOI] [PubMed] [Google Scholar]
- 5.Dhabhar FS, Satoskar AR, Bluethmann H, David JR, McEwen BS. Stress-induced enhancement of skin immune function: a role for gamma interferon. Proc Natl Acad Sci USA. 2000;97:2846–2851. doi: 10.1073/pnas.050569397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakano Y. Stress-induced modulation of skin immune function: two types of antigen-presenting cells in the epidermis are differentially regulated by chronic stress. Br J Dermatol. 2004;151:50–64. doi: 10.1111/j.1365-2133.2004.05980.x. [DOI] [PubMed] [Google Scholar]
- 7.Tedder TF, Steeber DA, Chen A, Engel P. The selectins: vascular adhesion molecules. FASEB J. 1995;9:866–873. [PubMed] [Google Scholar]
- 8.Fiscus LC, Van Herpen J, Steeber DA, Tedder TF, Tang ML. L-selectin is required for the development of airway hyperresponsiveness but not airway inflammation in a murine model of asthma. J Allergy Clin Immunol. 2001;107:1019–1024. doi: 10.1067/mai.2001.114703. [DOI] [PubMed] [Google Scholar]
- 9.Arbones ML, Ord DC, Ley K, Ratech H, Maynard-Curry C, Otten G, Capon DJ, Tedder TF. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity. 1994;1:247–260. doi: 10.1016/1074-7613(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 10.Gallatin WM, Weissman IL, Butcher EC. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983;304:30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- 11.Scholzen TE, Steinhoff M, Sindrilaru A, Schwarz A, Bunnett NW, Luger TA, Armstrong CA, Ansel JC. Cutaneous allergic contact dermatitis responses are diminished in mice deficient in neurokinin 1 receptors and augmented by neurokinin 2 receptor blockage. FASEB J. 2004;18:1007–1009. doi: 10.1096/fj.03-0658fje. [DOI] [PubMed] [Google Scholar]
- 12.Azma T, Matsubara Y, Kinoshita H, Hidaka I, Shiraishi S, Nakao M, Kawamoto M, Yuge O, Hatano Y. Prothrombotic roles of substance-P, neurokinin-1 receptors and leukocytes in the platelet-dependent clot formation in whole blood. J Thromb Thrombolysis. 2009;27:280–286. doi: 10.1007/s11239-008-0215-0. [DOI] [PubMed] [Google Scholar]
- 13.Meijerink J, Mandigers C, van de Locht L, Tonnissen E, Goodsaid F, Raemaekers J. A novel method to compensate for different amplification efficiencies between patient DNA samples in quantitative real-time PCR. J Mol Diagn. 2001;3:55–61. doi: 10.1016/S1525-1578(10)60652-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joachim RA, Quarcoo D, Arck PC, Herz U, Renz H, Klapp BF. Stress enhances airway reactivity and airway inflammation in an animal model of allergic bronchial asthma. Psychosom Med. 2003;65:811–815. doi: 10.1097/01.psy.0000088582.50468.a3. [DOI] [PubMed] [Google Scholar]
- 15.Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav Immun. 1997;11:286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- 16.Dhabhar FS. Stress-induced augmentation of immune function: the role of stress hormones, leukocyte trafficking, and cytokines. Brain Behav Immun. 2002;16:785–798. doi: 10.1016/s0889-1591(02)00036-3. [DOI] [PubMed] [Google Scholar]
- 17.Bowers SL, Bilbo SD, Dhabhar FS, Nelson RJ. Stressor-specific alterations in corticosterone and immune responses in mice. Brain Behav Immun. 2008;22:105–113. doi: 10.1016/j.bbi.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhabhar FS, McEwen BS. Enhancing versus suppressive effects of stress hormones on skin immune function. Proc Natl Acad Sci USA. 1999;96:1059–1064. doi: 10.1073/pnas.96.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol. 1993;54:1–78. doi: 10.1016/s0065-2776(08)60532-5. [DOI] [PubMed] [Google Scholar]
- 20.Falk S, Goggel R, Heydasch U, Brasch F, Muller KM, Wendel A, Uhlig S. Quinolines attenuate PAF-induced pulmonary pressor responses and edema formation. Am J Respir Crit Care Med. 1999;160:1734–1742. doi: 10.1164/ajrccm.160.5.9902033. [DOI] [PubMed] [Google Scholar]
- 21.Bae S, Matsunaga Y, Tanaka Y, Katayama I. Autocrine induction of substance P mRNA and peptide in cultured normal human keratinocytes. Biochem Biophys Res Commun. 1999;263:327–333. doi: 10.1006/bbrc.1999.1285. [DOI] [PubMed] [Google Scholar]
- 22.Bae SJ, Matsunaga Y, Takenaka M, Tanaka Y, Hamazaki Y, Shimizu K, Katayama I. Substance P induced preprotachykinin-a mRNA, neutral endopeptidase mRNA and substance P in cultured normal fibroblasts. Int Arch Allergy Immunol. 2002;127:316–321. doi: 10.1159/000057749. [DOI] [PubMed] [Google Scholar]
- 23.Ohmura T, Tsunenari I, Hayashi T, Satoh Y, Konomi A, Nanri H, Kawachi M, Morikawa M, Kadota T, Satoh H. Role of substance P in an NC/Nga mouse model of atopic dermatitis-like disease. Int Arch Allergy Immunol. 2004;133:389–397. doi: 10.1159/000077359. [DOI] [PubMed] [Google Scholar]
- 24.Nakano Y. Effect of chronic topical exposure to low-dose noxious chemicals and stress on skin sensitivity in mice. J Occup Health. 2007;49:431–442. doi: 10.1539/joh.49.431. [DOI] [PubMed] [Google Scholar]
- 25.Maes M, Song C, Lin A, De Jongh R, Van Gastel A, Kenis G, Bosmans E, De Meester I, Benoy I, Neels H, Demedts P, Janca A, Scharpe S, Smith RS. The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine. 1998;10:313–318. doi: 10.1006/cyto.1997.0290. [DOI] [PubMed] [Google Scholar]
- 26.Buske-Kirschbaum A, Gierens A, Hollig H, Hellhammer DH. Stress-induced immunomodulation is altered in patients with atopic dermatitis. J Neuroimmunol. 2002;129:161–167. doi: 10.1016/s0165-5728(02)00168-6. [DOI] [PubMed] [Google Scholar]
- 27.Kang DH, Fox C. Th1 and Th2 cytokine responses to academic stress. Res Nurs Health. 2001;24:245–257. doi: 10.1002/nur.1027. [DOI] [PubMed] [Google Scholar]
- 28.Iwakabe K, Shimada M, Ohta A, Yahata T, Ohmi Y, Habu S, Nishimura T. The restraint stress drives a shift in Th1/Th2 balance toward Th2-dominant immunity in mice. Immunol Lett. 1998;62:39–43. doi: 10.1016/s0165-2478(98)00021-2. [DOI] [PubMed] [Google Scholar]
- 29.Ackerman KD, Martino M, Heyman R, Moyna NM, Rabin BS. Stressor-induced alteration of cytokine production in multiple sclerosis patients and controls. Psychosom Med. 1998;60:484–491. doi: 10.1097/00006842-199807000-00016. [DOI] [PubMed] [Google Scholar]
- 30.Brown MA, Hural J. Functions of IL-4 and control of its expression. Crit Rev Immunol. 1997;17:1–32. doi: 10.1615/critrevimmunol.v17.i1.10. [DOI] [PubMed] [Google Scholar]
- 31.Kamimura D, Ishihara K, Hirano T. IL-6 signal transduction and its physiological roles: the signal orchestration model. Rev Physiol Biochem Pharmacol. 2003;149:1–38. doi: 10.1007/s10254-003-0012-2. [DOI] [PubMed] [Google Scholar]
- 32.Steinke JW, Borish L. 3. Cytokines and chemokines. J Allergy Clin Immunol. 2006;117:S441–S445. doi: 10.1016/j.jaci.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Korbel DS, Schneider BE, Schaible UE. Innate immunity in tuberculosis: myths and truth. Microbes Infect. 2008;10:995–1004. doi: 10.1016/j.micinf.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 34.Almeida TA, Rojo J, Nieto PM, Pinto FM, Hernandez M, Martin JD, Candenas ML. Tachykinins and tachykinin receptors: structure and activity relationships. Curr Med Chem. 2004;11:2045–2081. doi: 10.2174/0929867043364748. [DOI] [PubMed] [Google Scholar]
- 35.Dhabhar FS, McEwen BS, Spencer RL. Stress response, adrenal steroid receptor levels and corticosteroid-binding globulin levels: a comparison between Sprague-Dawley. Fischer 344 and Lewis rats. Brain Res. 1993;616:89–98. doi: 10.1016/0006-8993(93)90196-t. [DOI] [PubMed] [Google Scholar]
- 36.Burow A, Day HE, Campeau S. A detailed characterization of loud noise stress: intensity analysis of hypothalamo-pituitary-adrenocortical axis and brain activation. Brain Res. 2005;1062:63–73. doi: 10.1016/j.brainres.2005.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joachim RA, Handjiski B, Blois SM, Hagen E, Paus R, Arck PC. Stress-induced neurogenic inflammation in murine skin skews dendritic cells towards maturation and migration: key role of intercellular adhesion molecule-1/leukocyte function-associated antigen interactions. Am J Pathol. 2008;173:1379–1388. doi: 10.2353/ajpath.2008.080105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng G, Wood KJ, Bushell A. Interferon-gamma conditioning ex vivo generates CD25+CD62L+Foxp3+ regulatory T cells that prevent allograft rejection: potential avenues for cellular therapy. Transplantation. 2008;86:578–589. doi: 10.1097/TP.0b013e3181806a60. [DOI] [PubMed] [Google Scholar]
- 39.Smith CH, Barker JN, Morris RW, MacDonald DM, Lee TH. Neuropeptides induce rapid expression of endothelial cell adhesion molecules and elicit granulocytic infiltration in human skin. J Immunol. 1993;151:3274–3282. [PubMed] [Google Scholar]
- 40.Miyazaki Y, Satoh T, Nishioka K, Yokozeki H. STAT-6-mediated control of P-selectin by substance P and interleukin-4 in human dermal endothelial cells. Am J Pathol. 2006;169:697–707. doi: 10.2353/ajpath.2006.051211. [DOI] [PMC free article] [PubMed] [Google Scholar]


