Abstract
Allergic contact dermatitis is a T cell-mediated immune response, which in its relapsing chronic form is of high socioeconomic impact. The phosphoglycoprotein osteopontin (OPN) has chemotactic and Th1 cytokine functions and in various models is essential for robust T cell-mediated immunity. Here we demonstrate that OPN is abundantly expressed by both effector T cells and keratinocytes in allergic contact dermatitis lesions. T cells from nickel-allergic donors secrete high levels of OPN following antigen-specific stimulation. OPN may substitute for missing IFN-γ secretion in T effector cells because low IFN-γ-producing T cell clones secrete high levels of OPN, and OPN down-modulates their interleukin-4 expression. Furthermore, interferon-γ from T effector cells augments OPN in allergic contact dermatitis by inducing OPN in keratinocytes, which in turn polarizes dendritic cells and attracts inflammatory cells. In the murine contact hypersensitivity (CHS) model for allergic contact dermatitis, OPN is strongly induced in antigen-specific proliferating T cells, and OPN null mice display a reduced chronic CHS inflammatory response due to a decreased influx of effector T cells. Importantly, because of its function for chronic allergic contact dermatitis, OPN may well be a therapeutic target, because anti-OPN antibody treatment in part suppresses established chronic CHS.
Allergic contact dermatitis (ACD) is a common disease with high socioeconomic impact, and sufferers carry a heavy burden in their working and social lives.1 Once ACD against a chemical, usually a hapten, occurs, only strict allergen avoidance prevents recurrent disease. To date there is no method to reverse persistent sensitization.2,3 The prognosis of ACD with sensitization to antigens encountered in different professions is poor and often initial occurrence is followed by progressive chronic disease.1,4–6
During the sensitization phase of ACD, myeloid type dendritic cells (mDCs) of the skin, namely Langerhans cells (LCs) and dermal DCs transport haptenated peptides that have penetrated the epidermis to skin draining lymph nodes to present them to naive T cells.7–9 Antigen-specific primed T cells that have acquired the ability to enter the skin through expression of cutaneous lymphocyte antigen recirculate to the skin.3,10,11 When the epidermis is again penetrated by the allergen and immediately recognized by the antigen-specific cells, a rapid expansion and attraction of additional T effector cells occurs, resulting in localized skin inflammation at the contact site.10 With repeated antigen exposure, chronic stimulation of antigen-specific T cells results in severe relapsing or persistent eczema.6
Contact hypersensitivity (CHS), the murine model of ACD, is a well established system to study immune mechanisms of delayed type hypersensitivity reactions, mediated by hapten specific T cells in the skin.3 Furthermore, the pathomechanisms that lead to chronic nonresolving ACD can be studied in this model. In mice sensitized on the abdominal skin against 2,4,6-trinitrochlorobenzene (TNCB), reapplication of the hapten causes skin inflammation (acute CHS) that resolves within a few days. However, when the hapten is repeatedly applied, chronic inflammation is provoked (chronic CHS).3,12
Osteopontin (OPN) is an acidic phosphoglycoprotein with cytokine functions under physiological or pathological conditions that is secreted by different immune cells among them T cells, macrophages, and DCs.13–16 Intracellular isoforms of OPN and secreted isoforms of OPN (sOPN) have been identified in the regulation of cell-mediated immune responses. Macrophages and different DCs subsets produce both sOPN and intracellular isoforms of OPN. Differential functions of both isoforms in myeloid DCs (mDCs) and plasmacytoid DCs (pDCs) have been implicated in regulating the development of Th1 and Th17 effector cells, thereby substantially influencing cell mediated immunity in infectious, autoimmune, and allergic disease.17–23 Shinohara et al24 demonstrated that OPN gene expression in T cells is regulated by the transcription factor T-bet that polarizes T helper cells toward a Th1 phenotype. T-bet-dependent expression of OPN induces the differentiation of CD4+ and CD8+ T helper cells toward the Th1 or Tc1 lineage.
Overexpression of OPN especially by activated T cells is known to be associated with the aggravation of autoimmune diseases, among them systemic lupus erythematosus, inflammatory bowel disease, rheumatoid arthritis, and multiple sclerosis.25–30 OPN null mice have ameliorated disease in Th1- or Th17-dominated immune responses, such as experimental autoimmune encephalomyelitis, autoimmune keratitis, and granuloma formation,19,24,29,31–33 and are less efficient in clearing viral or bacterial disease because of a reduced T cell-mediated immune response. In experimental autoimmune encephalomyelitis models, OPN promotes the survival of T cells by modulating various signaling cascades, for example triggering the NFκb pathway and simultaneously inhibiting the transcription factor Foxo3a.31,34 Furthermore, through its chemotactic properties OPN attracts T cells, monocytes and DCs into inflammatory sites and induces the proangiogenic secretion of interleukin (IL)-1β in monocytes, an effect likely to contribute to increased microvessel formation in chronic inflammation.13,35
We previously investigated OPN function during the sensitization phase of CHS and found that sOPN guides LCs and mDCs into skin-draining lymph nodes, induces mDC activation and polarizes them toward a Th1-inducing phenotype.36,37 Further, we found that OPN null mice are reduced in their capacity to mount full acute CHS response.22 Assuming that OPN secretion is important for the elicitation phase and the chronification of delayed type allergic disease, we studied OPN expression and cell specificity in chronic ACD. We found high levels of OPN secretion on antigen-specific stimulation by memory T cells. In turn Th1 cell-derived interferon-γ (IFN-γ) specifically induced OPN in activated keratinocytes. Finally, antigen-specific OPN expression was important for chronification of ACD in vivo, because OPN null mice have a reduced antigen-specific chronic CHS response and established chronic inflammation is in part suppressed by anti-OPN antibodies.
Materials and Methods
Media and Reagents
RPMI 1640 was supplemented (c-RPMI) with 10% heat-inactivated FCS, 1% nonessential amino acids, 1% penicillin and streptomycin, 1% l-glutamine, 1% HEPES buffer, and 1% sodium pyruvate (all from Life Technologies, Eggenstein, Germany). For Th-polarization assays, c-RPMI was supplemented with 5 × 10−5 M mercaptoethanol (Sigma-Aldrich, Taufkirchen, Germany). For culture of human T cells the above medium was used with 5% heat inactivated human serum. Monoclonal antibodies specific for OPN (MPIIIB10 (1)) were purchased from Developmental Studies Hybridoma Bank at the University of Iowa. The anti-human integrin antibodies: αvβ3 (LM609), αvβ5 (P1FG), β1 (P4G11), α4 (9F10), α9β1 (Y9A2), and CD45RO (UCHL1) were obtained from Chemicon International (Schwalbach, Germany); CD44s (SFF-2) and CD44v6 (VFF-18) from Bender MedSystems (Vienna, Austria); CD4 (RPA-T4), α4 (9F10), IFN-γ-R (GIR-94), and isotype control antibodies (mouse IgM (G155-228)), mouse IgG1 (107.3), and mouse IgG2a (MOPC-21)) were from BD Pharmingen (Heidelberg, Germany). The phosphatidylethanolamine-conjugated anti-murin antibody CD4 (GK1.5) was obtained from Miltenyi Biotec (Bergisch Gladbach, Germany) and the fluorescein isothiocyanate- or antigen-presenting cell-conjugated anti-murine antibody CD8a (53-6.7) from BD Pharmingen.
Monoclonal anti-OPN antibody 35B6 (IgG1) was obtained by immunizing mice with the synthetic peptide VDVPNGRGDSLAYGLR corresponding to the internal sequence of murine OPN. The antibody 5A1 was obtained by immunizing mice with the synthetic peptide IPVKQADSGSSEEKQ corresponding to the internal sequence of human OPN.38
Tissue Donors
The study was approved by the Ethics Committee of the University of Ulm. Formal written consent was obtained from all donors. Skin specimens were obtained by standard biopsy procedures. Biopsies from of acute eczema were taken from patients that underwent routine path testing with 5% NiSO4 in vaselinum album (Almirall Hermal, Reinbek, Germany), 24 hours after application of the allergen that had at least double-positive reactions (++) with erythema, infiltration, papules, and vesicles.6 Chronic eczema specimens were obtained from patients with long-lasting chronic contact dermatitis of the hands with multiple sensitizations against delayed-type allergens before treatment. Plasma samples for OPN enzyme-linked immunosorbent assay (ELISA) were obtained from patients with long-lasting severe chronic ACD of the hands and/or feet or healthy controls without history of eczema. Blood samples for T cell activation assays were obtained from volunteers with sensitization against NiSO4, verified by positive patch test or nickel patch test negative donors as controls.
Immunohistochemistry
Immunohistochemistry was performed as described previously.22 In brief, paraffin embedded sections were pretreated by pressure cooker heating using Dako S1699 target retrieval buffer according to the manufacturers protocol (Dako, Glostrup, Denmark). Following the application of the primary antibody against OPN, αvβ3, β5, CD44s, or CCD44v6, staining was visualized by EnVision detection system (Dako) with 3,3′-diaminobenzidine chromogen as a chromogen for the peroxidase reaction. For double staining, the primary antibody was visualized by EnVision detection system with 3,3′-diaminobenzidine chromogen as a chromogen (brown) and the antibody against the second antigen was visualized with Dako REAL APAAP (Dako) using New Fuchsin substrate (red) System (Dako). Slides were photographed with an Olympus AX70 Microscope, (Olympus, Hamburg, Germany) scanned, and assembled with Corel Draw (Corel, Unterschleissheim, Germany).
Antigen-Specific Stimulation of Human T Cells
Peripheral blood mononuclear cells (PBMCs) were isolated from blood of nickel-allergic patients or healthy donors by Ficoll gradient centrifugation as described previously.39 CD4+ or CD8+ cells were isolated with the CD4- or CD8-negative isolation kit (Miltenyi Biotec) from PBMCs according to the manufacturer's protocol. Autologous CD14+ monocytes were used as antigen-presenting cells, which were isolated from PBMCs by positive selection with anti-CD14 microbeads (Miltenyi Biotec). A total of 8 × 104 CD4+ or CD8+ T cells were cultured with 2 × 104 CD14+ monocytes with or without 10−4 M NiSO4. Nickel-specific proliferation was assessed by [3H]thymidine incorporation. OPN production was analyzed after 48 hours by ELISA (IBL, Takasaki, Japan).
Human T Cell Clones
T cell clones were established from the peripheral blood of patch test-positive donors with a history of ACD against nickel as described previously.40 In brief, blood-derived T cells were stimulated twice with 10−4 M NiSO4 and subsequently cloned by limiting dilution. Cells were plated in 60-well Terasaki microplates (Greiner bio-one, Frickenhausen, Germany) (0.5 cells/well, c-RPMI 1640) with 2 × 104 irradiated allogeneic feeder cells per well, 100 U/ml human IL-2 (PeproTech, London, UK), and 1 μg/ml phytohemagglutinin. T cell clones were cultured with IL-2 and stimulated with phytohemagglutinin and feeder cells twice a month. The antigen-specific proliferation and OPN production of the clones was assessed using irradiated autologous PBMCs in presence or absence of 10−4 M NiSO4. After 48 hours, supernatants were harvested and analyzed for cytokine expression.
Cytokine Activation of Human Keratinocytes
Keratinocytes were generated as described previously.41 In brief, foreskin tissue was cut into small pieces (2 × 2 mm). To separate the epithelium from the underlying fibrous connective tissue, skin was treated with 6 mg/ml dispase (BD Biosciences, Heidelberg, Germany) for 12 hours at 4°C. The epithelium dissociated and was incubated at 37°C in 5 ml of trypsin-EDTA (0.05% trypsin and 0.53 mmol/L EDTA) for 10 minutes. The isolated keratinocytes were grown to 70% confluence in keratinocyte growth medium 2 (Promocell) and were used for experiments from passages 3 to 6. For stimulation experiments keratinocytes were stimulated for 1.5, 3, 6, 12, and 36 hours with IFN-γ (200 U/ml), IL-1α (10 ng/ml), IL-1β (200 U/ml), TNF-α (1000 U/ml), IL-12 (20 ng/ml), IL-4 (12.5 ng/ml), IL-17 (50 ng/ml) (all from PeproTech) or phorbol myristate acetate (10−7 M, Sigma-Aldrich, Munich, Germany) and ionomycine (1 μg/ml; Calbiochem, Darmstadt, Germany). Quantitative real-time PCR of cells harvested on the indicated days was performed with a LightCycler (Roche Diagnostics, Grenzach-Wyhlen, Germany).
Mice
The mice lacking the functional OPN gene (spp1) were described previously.42 For all experiments, C57BL/6 OPN-mutant mice and their wild-type littermates in the ninth generation of backcrossing were used. For T cell transfer experiments C57BL/6 RAG2−/− mice were used. All animal protocols were approved by the Committee of Animal Research (Regierungspräsidium Tuebingen, Germany), which are in accordance with National Institutes of Health guidelines.
Chronic CHS and Antibody Treatment
Induction of chronic CHS was performed as described previously.12 In brief, 6-week-old C57BL/6 OPN-mutant mice or wild-type littermates were painted on their abdominal skin with 100 μl of 7% TNCB in acetone at day 0. On day 7, mice were challenged by painting 10 μl of 1% TNCB on both sides of the ears. Challenge was repeated three times a week for 28 days. Ear thickness was measured with an engineer's micrometer (Mitutoyo, Neuss, Germany) before and at days 7, 8, 9, 16, 17, 18, 28, 29, and 30, and ear-swelling was calculated.43
For antibody treatment of chronic CHS, mice were sensitized on day 0, first challenged on day 5, followed by rechallenge every second day. A total of 400 μg of anti-OPN 35B6 monoclonal antibody that recognizes the GDSLAYGLR epitope or isotype matched control antibody 5K1 was injected i.p. on days 10, 13, and 16.
Adoptive Transfer of T Cells into RAG2−/− Mice
Lymph node T cells from TNCB-sensitized OPN-mutant or wild-type C57BL/6 mice were enriched using Pan T cell isolation kit (Miltenyi Biotec). The purity of T cells was typically ≥95% CD3-positive cells. Purified T cells were washed three times with sterile PBS, and 107 cells were injected i.v. in 0.25 ml of sterile PBS into the tail vein of RAG2−/− mice. Twelve hours after injection an acute CHS response was induced as described above.
Single-Cell Suspension of Ear Skin
Mice were sacrificed and ears were cut off the base and split into dorsal and ventral halves with forceps. Dorsal halves of both ears (epidermis and dermis without cartilage) were cut into small pieces and incubated in HBSS with 1.5 mg/ml collagenase and 0.5 mg/ml hyaluronidase for 1 hour at 37°C on a shaker. The resulting cell suspension was passed through a 40-μm cell strainer and washed three times with PBS, followed by FACS analysis for CD8- or CD4-positive T cell numbers.
Antigen-Specific Stimulation of Murine T Cells
Murine T cells were obtained from 6-week-old C57BL/6 mice 24 hours after the initial elicitation of CHS with TNCB. Splenic cells of the same mice served as antigen-presenting cells and were loaded with 7 mmol/L 2,4,6-trinitrobenzene sulfonic acid (TNBS) for 15 minutes at 37°C. For antigen-specific stimulation, 2 × 105 lymph node cells were incubated with 2 × 105 untreated or TNBS-loaded irradiated splenic cells. For viability control concavalin A (5 μg/ml) was added. CD4+ or CD8+ cells were isolated from lymph nodes with the CD4- or CD8a-negative isolation Kit (Miltenyi Biotec) according to the manufacturer's protocol. A total of 1 × 105 CD4+ or CD8+ T cells was incubated with 1 × 105 untreated or TNBS-loaded irradiated splenic cells. At the indicated time points, supernatants were analyzed by OPN ELISA (IBL), and cells were collected for RNA isolation and quantitative real-time-PCR. Proliferation was assessed by [3H]thymidine incorporation.
Flow Cytometry
Surface receptor expression on T cells was determined by a standard protocol. Cells were stained (4°C, 30 minutes) with fluorescein isothiocyanate- or phosphatidylethanolamine-labeled mAb. A total of 0.1 μg/ml propidium iodide (Sigma-Aldrich) was added to exclude dead cells by appropriate gating. 5 to 10 × 103 or in case of skin cell suspension up to 5 × 105 viable cells/sample were analyzed and mean fluorescence intensities determined by CellQuest software.
Proliferation Assay
For analyzing antigen-specific proliferation T cells were cultured with antigen-presenting cells in presence or absence of antigenic stimulants. After 32 hours, co-cultures were pulsed with 1 μCi/well [3H]thymidine for 18 hours at 37°C. Plates were harvested with a Cell Harvester (Inotech, Nabburg, Germany) compared with filter mates (PerkinElmer, Waltham, MA) and [3H]thymidine incorporation was determined by liquid scintillation spectroscopy using a beta counter 1450 Microbeta Trilux (PerkinElmer).
Cytokine Measurement
Supernatants from stimulated T cells or T cell clones were harvested at the indicated time points and stored at −80°C. Cytokines were quantified by ELISA according to the manufacturer's instructions and measured at an extinction of 450 nm (MR5000 ELISA-reader and Bio-LinxTM Software; Dynatech, Chantilly, VA). The following ELISA kits were used (sensitivity): OPN (5 ng/ml) from IBL, IL-4 (7.8 pg/ml), IFN-γ (4.7 pg/ml), all Opteia from BD Pharmingen.
RNA Isolation and Quantitative Real-Time PCR
RNA isolation of human tissue was performed using TissueLyser (Qiagen, Hilden, Germany) and RNeasy mini kit (Qiagen) according to manufacturer's instructions. Cultured cells were lysed and homogenized using Qiagen QIA-shredder (Qiagen) columns and RNA isolation was performed using the Qiagen RNeasy mini kit. To synthesize cDNA reverse transcription was performed with Omniscript RT kit (Qiagen). Obtained cDNA was used for quantitative real-time PCR with QuantiTect SYBR Green PCR kit and a LightCycler (Roche Diagnostics). Thermocycling conditions: hold at 95°C for 15 minutes, 40 cycles of 95°C for 15 seconds, 60°C for 20 seconds, and 72°C for 10 seconds followed by melting curve. The relative expression of target gene in different samples was normalized to the endogenous glyceraldehyde-3-phosphate dehydrogenase and was calculated with the 2−ΔΔct method.44 Human OPN primers were 5′-AAGAAGTTTCGCAGACCTGACATC-3′ (forward) and 5′-GATGGCCTTGTATGCACCATTC-3′ (reverse). Human glyceraldehyde-3-phosphate dehydrogenase primers were 5′-GCAAATTCCATGGCACCGT-3′ (forward) and 5′-GCCCCACTTGATTTTGGAGG-3′ (reverse). Mouse OPN primers were 5′-GGTGATAGCTTGGCTTATGGACTG-3′ (forward) and 5′-GCTCTTCATGTGAGAGGTGAGGTC-3′ (reverse). Mouse glyceraldehyde-3-phosphate dehydrogenase primers were 5′-TGGCCTTCCGTGTTCCTACC-3′ (forward) and 5′-GGTCCTCAGTGTAGCCCAAGATG-3′ (reverse).
Recombinant OPN Expression in Eukaryotic Cells
Construction of expression plasmid: Human OPN was amplified with upper primer 5′-GGGCTCTGGCTAGCATACCAGTTAAACAGGCTGATTCTGGAAG-3′ and lower primer 5′-CGGGATCCTTAGTGGTGGTGGTGGTGGTGACTCCCATTGACCTCAGAAGATGC-3′.45 The correct sequence was confirmed by DNA sequencing with an ABIprism sequencer (PerkinElmer). Resulting fragments were cloned into the episomal expression vector pCEP-Pu using restriction sites NheI/BamHI. Escherichia coli strain JM109 was used for transformation and propagation of vector constructs. Proteins were expressed in frame with BM-40 signal peptide and 6× Histidin-tag using human embryonic kidney cells.
Cell Culture, Transfection, and Protein Expression
Human embryonic kidney cells expressing the EBV-encoded nuclear Ag-1 protein of Epstein-Barr virus (HEK-293-EBNA) (Invitrogen, Paisley, Scotland) were cultured in Dulbecco's modified Eagle's medium:F12 (Invitrogen) supplemented with 10% fetal bovine serum (PAA Laboratories, Pasching, Österreich), 1% glutamine, and 10 μg/ml penicillin/streptomycin (Biochrom, Berlin, Germany). Cells were transfected with vector constructs and selected with 2 μg/ml puromycin (Sigma-Aldrich) as described previously.46 Confluent bulk cultures of transfected cells were used for protein expression. Serum-free medium was collected from cultures, and new serum-free medium was added every 48 hours. Harvested medium was centrifuged at 2500 × g for 10 minutes, buffered in 20 mmol/L HEPES (pH 7.1) (Biochrom), and stored at −80°C. Harvested medium containing recombinant protein was sterile filtered, dialyzed against 50 mmol/L Tris (pH 7.9), and passed over a column of DEAE-Sepharose (Amersham Biosciences, Uppsala, Sweden). The protein was eluted with 500 mmol/L NaCl. Purification of human OPN-6× His-tag was performed by using His Bind Purification Kit (Novagen, Darmstadt, Germany) according to the manufacturer's instructions. The fractions containing the purified protein were dialyzed against 20 mmol/L Tris (pH 7.4) and 2 mmol/L CaCl2 and analyzed using SDS-PAGE, followed by immunoblot with anti His antibody (13/45/31) and polyclonal horseradish peroxidase-conjugated anti-mouse IgG (both Dianova, Hamburg, Germany) or silver staining.
Results
OPN and OPN Receptors Are Expressed in Acute and Chronic Lesions of ACD
To determine the expression of OPN in acute and chronic ACD, skin biopsies were stained by immunohistochemistry for OPN and OPN receptors. Compared with normal skin (Figure 1, A and B) of the same donor (Figure 1, C–E), OPN was primarily expressed in the inflammatory infiltrate in acute ACD lesions while only minimal staining was detectable within the epidermis. In chronic ACD lesions, in addition to abundant OPN expression throughout the inflammatory infiltrate and on microvascular endothelial cells within the infiltrate, OPN was detected throughout the epidermis (Figure 1, F–H). Analysis of serial sections revealed that OPN-expressing inflammatory cells and keratinocytes also express the OPN receptors CD44s, CD44v6, and β5 integrin (Figure 1, I–N). Membrane associated αvβ3 was expressed in the inflammatory infiltrate. Within the epidermis αvβ3 was detected especially in basal areas. This pattern was confirmed by immunofluorescence staining of cryosectioned specimen (data not shown). The partial nuclear staining of this antibody was only observed in the paraffin embedded sections and was regarded as background staining. The CD44v6 epitope was predominantly expressed by keratinocytes, however, rarely by the inflammatory cells (Figure 1K).
Figure 1.
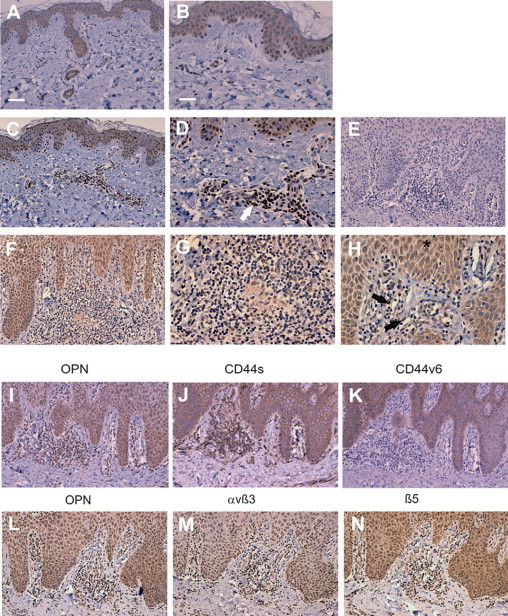
OPN and its receptors are abundantly expressed in chronic allergic contact dermatitis by infiltrating inflammatory cells and keratinocytes. Immunohistochemical staining of skin biopsies was performed with mAb MPIIIB10 against OPN (brown stain) in normal skin (A and B). A similar staining pattern was also observed for the OPN antibody 10A16 (IBL, data not shown). C and D: Acute allergic contact dermatitis (arrow). OPN-expressing infiltrating inflammatory cells. F–H: Chronic allergic contact dermatitis,(arrow) OPN-expressing endothelial cells, and (asterisk) OPN-expressing keratinocytes. E: Chronic allergic contact dermatitis stained with primary isotype control antibody. Serial sections of skin biopsies from donors with chronic allergic contact dermatitis were stained with anti OPN antibody (I and L), anti CD44 antibody (J), anti-CD44v6 antibody (K), anti-αvβ3 antibody (M), or anti-β5 integrin antibody (N). A, C, F, E, I, and J–N: Representative bar in A corresponds to 80 μm. B, D, E, G, and H: Representative bar in B corresponds to 40 μm. Representative data from 5 donors each with chronic and acute ACD and 10 healthy donors.
Especially in patients with autoimmune disease, active disease has been associated with high OPN plasma levels.25,26,47 Compared with age and gender matched healthy donors we detected significantly elevated OPN plasma levels in patients with chronic ACD (Figure 2). These findings suggest that OPN is highly expressed in ACD and contributes to the chronification of the disease.
Figure 2.
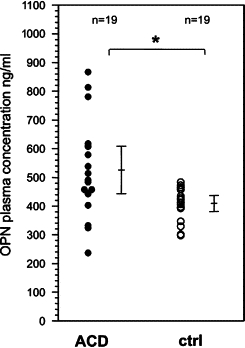
Patients with chronic allergic contact dermatitis have elevated OPN plasma concentrations. OPN concentration was measured in plasma from 19 healthy volunteers (10 male, 9 female, average age 45.8 years) and from 19 donors with allergic contact dermatitis (6 male, 13 female, average age 42.7 years) by OPN ELISA (IBL). Arrow bars indicate the SD The differences between both groups are statistically significant: *P = 0.007, Mann-Whitney rank-sum test.
CD45RO+, CD8+, and CD4+ T Cells Form Nickel-Allergic Donors Secrete OPN on Antigen-Specific Stimulation
To determine which cells within the inflammatory infiltrate are the major OPN-producing cells, immunohistochemical double staining of chronic ACD lesions was performed. CD45RO+ cells, which are predominantly memory T cells, express OPN (Figure 3, A–C). We were now interested to investigate whether OPN expression by memory T cells could be induced by antigen-specific activation. When PBMC from patch test positive nickel-allergic donors were stimulated by NiSO4 both CD8+ and CD4+ T cells sOPN (Figure 3, D and F), whereas T cells from healthy non nickel sensitized donors did not (Figure 3, E and G). Our findings indicate that OPN is induced in T cells on antigen-specific activation. This is in accordance to the original observation that OPN expression is strongly induced in activated T lymphocytes, as reflected in one of its names, Early T lymphocyte activation 1.48
Figure 3.
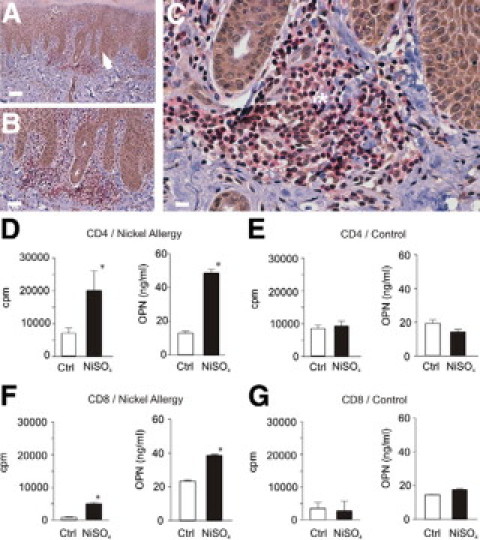
Antigen-specific proliferating CD4+ and CD8+ memory T cells from nickel-allergic donors secrete OPN. Immunohistochemical double staining of paraffin sections from biopsies of chronic allergic contact dermatitis was performed with antibodies specific for OPN (MPIIB10, brown, Envision; Dako) and CD45RO (red, APAAP; Dako) for memory T cells. In chronic allergic contact dermatitis CD45RO+ memory T cells (asterisk) and keratinocytes (arrow) express OPN. Magnifications: ×10 (A), ×20 (B), and ×40 (C). Representative data from five donors. Scale bars correspond to 80 μm in A, 40 μm in B, and 25 μm in C. PBMCs from nickel-allergic donors (D and F) with positive patch test to NiSO4 and healthy donors (E and G) with negative patch test were generated by gradient centrifugation and CD4+ (D and E) or CD8+ T cells (F and G) were purified by MACS negative depletion (purity >95%). Antigen-specific proliferation was determined by [3H]thymidine incorporation (D–G). Forty-eight hours after stimulation with NiSO4 supernatants were obtained and subjected to OPN-specific ELISA. Representative results for eight donors.
OPN Secretion of Nickel-Specific T Cell Clones Correlates with Their Cytokine Phenotype
To investigate how OPN secretion is correlated with the phenotype of antigen-specific T cells, we generated six T cell clones from the peripheral blood of nickel-allergic donors. All generated T cell clones were CD4+. As expected and previously described the clones showed varying phenotypes.49 When investigating the OPN expression of such clones, we found that two clones that produced a Th1 cytokine pattern with higher amounts of IFN-γ-secreted lower amounts of OPN, as shown for representative clone II11B in Figure 4A. Two clones with a Th0 cytokine pattern secreted intermediate amounts of OPN as shown for clone V3G in Figure 4B that produced both IL-4 and IFN-γ on antigen-specific stimulation. However, two other clones for which IV10A in Figure 4C is representative that produced low levels of IFN-γ and some IL-4 (indicating a Th2 phenotype) secreted comparably high amounts of OPN (Figure 4C). In none of the clones did we find modulation of OPN expression by the antigen (Figure 4, A–C). We speculate that this was due to the consolidated phenotype of the clones following repeated IL-2 restimulation during their establishment. These findings indicate that the amount of OPN secretion correlates with a certain phenotype of antigen-specific T cell clones. Investigating the expression of OPN receptors on these clones, we found that they expressed αv integrins (data not shown) v6-containing CD44 isoforms as well as integrin receptors α4β1 and α9β1 for the matricryptic SLAYGLR sequence located in the N-terminal half of thrombin-cleaved OPN (Figure 4D).
Figure 4.
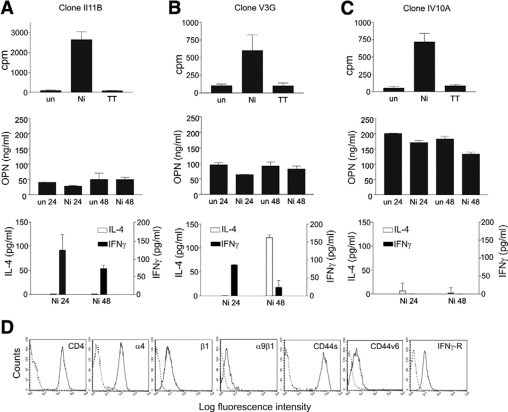
OPN expression by memory T cell clones correlates with their cytokine expression pattern. CD4+CD45RO+ T cell clones were generated from peripheral blood of nickel-allergic donors, identified by NiSO4 patch testing, as described in Materials and Methods. NiSO4 specific proliferation was measured by [3H]thymidine incorporation. Following antigen-specific stimulation, the secretion of OPN, IFN-γ, and IL-4 was measured by ELISA. Abbreviations: un, unstimulated; Ni, stimulated with NiSO4; TT, stimulated with tetanus toxoid. Cytokine expression was analyzed 24 and 48 hours after NiSO4 stimulation. Data for three representative clones (A–C) of a total of six analyzed clones established from two donors are shown. D: FACS analysis of the indicated OPN receptors on a CD4+ T cell clone IV10A. Representative data for three clones.
OPN Stimulation of T Cell Clones Suppresses Their IL-4 Secretion
Because of the described Th1 cytokine functions of OPN, we asked how OPN affects Th1 and Th2 cytokine secretion in nickel-specific T cell clones. To obtain OPN secreted by eukaryotic cells best resembling a form of OPN that is found in inflammatory sites and to avoid effects of contaminating lipopolysaccharide, OPN was transfected into the human EBV-encoded nuclear Ag-carcinoma cell line and purified by His-tag (data not shown). When T cell clones were stimulated with recombinant human OPN, IL-4 secretion decreased, but IFN-γ expression was not significantly affected (Figure 5). Furthermore, following IFN-γ stimulation of the cells, OPN was not significantly modulated. These findings suggest that OPN can modulate antigen-specific T cells to robustly express a Th1 cytokine pattern by suppressing IL-4 secretion.
Figure 5.
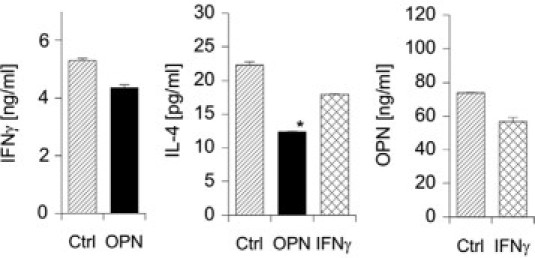
OPN modulates the expression of IL-4 in nickel-specific T cell clones. CD4+CD45RO+ T cell clones were generated from peripheral blood of nickel-allergic donors, identified by NiSO4 patch-testing, as described in Materials and Methods. Cells were washed and then stimulated with OPN (2 μg/ml) or IFN-γ (200 U/ml) for 48 hours. Cytokine secretion was measured by IL-4, IFN-γ, or OPN-specific ELISA. A representative experiment from independent experiments with three clones is shown. (Statistically significant, paired t-test: *P < 0.05.)
Antigen-Specific Stimulation of T Cells from TNCB-Sensitized Mice Induces OPN-Specific Production of OPN in Both CD4+ and CD8+ T Cells
To validate the in vivo significance of our findings in the human system, we investigated the potential impact of OPN in the murine TNCB/TNBS CHS model. T cells from skin-draining lymph nodes of TNCB-sensitized mice were stimulated with TNBS-loaded splenic cells, which induced OPN secretion by bulk-proliferating lymph node cells on the protein (Figure 6, A and B) and mRNA level (Figure 6C). CD8+ T cells are the major effector cells in CHS. We therefore investigated whether CD4+ or CD8+ T cells would differ in their OPN-secreting potential (Figure 6, D and E). When restimulating the CD4+ and CD8+ population with antigen-loaded irradiated splenic cells, we found that both CD4+ and CD8+ T cells proliferating on antigen-specific stimulation produced OPN (Figure 6E). Interestingly, CD8+ T cells produced more than double the amount of CD4+ cells. Our findings indicate that OPN is highly produced by antigen-specific T cells on their activation and that CD8+ cells produce the highest amounts of OPN in this system.
Figure 6.
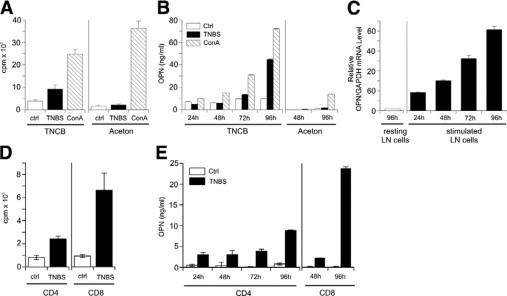
Antigen-specific stimulation of CHS effector T cells from sensitized mice induces their OPN secretion. On day 0, C57BL/6 mice were sensitized on the abdomen with 7% TNCB and challenged with 1% TNCB on both ears on day 5. Twenty-four hours after challenge, lymph node single-cell suspensions were prepared (A–C). Lymph node cells from challenged mice were stimulated with untreated or TNBS-loaded splenic cells or concavalin A (ConA) was added to untreated cells. Proliferation was measured by [3H]thymidine uptake (A), secretion of OPN was detected by ELISA (B), and OPN mRNA expression was analyzed by real-time PCR. Data are given as ratio of OPN mRNA to glyceraldehyde-3-phosphate dehydrogenase housekeeping gene (GAPDH) (C). D and E: CD4+ and CD8+ T cells were isolated by MACS-negative depletion. Cells were cultured with 30-Gy irradiated, TNBS-loaded spleen cells or unloaded spleen cells as controls (Ctrl). Proliferation was measured by [3H]thymidine uptake (D), and secretion of OPN was detected by ELISA (E). The data represent the results of three independent experiments.
IFN-γ Specifically Induces OPN Expression by Keratinocytes
Chemokines and cytokines secreted by keratinocytes during their activation in chronic ACD decisively contribute to the inflammatory response.50 Because we had observed that keratinocytes that normally express only very low levels of OPN, stained intensely with antibodies against OPN, which increased from acute to chronic ACD, we tested the modulation of OPN expression in keratinocyte cultures using a panel of cytokines that are secreted by inflammatory cells in ACD. Using real-time PCR, we found that within 4 hours IFN-γ specifically induced high levels of OPN mRNA in keratinocytes. Additionally, IL-4 induced OPN, however, mRNA levels were only slightly beyond the significance level (Figure 7A). To detect OPN protein produced by keratinocytes, immunostaining of keratinocytes was performed. In contrast to unstimulated cells IFN-γ-activated keratinocytes stained brightly with anti-OPN monoclonal antibody. When OPN-specific ELISA was performed with supernatants, only IFN-γ provoked OPN secretion (Figure 7, B–E). However, levels of OPN were relatively low, which could possibly be explained by OPN binding to CD44 isoforms (Figures 7F and 1, J and K) and αv-integrins (Figure 1, M and N), which are abundantly expressed by keratinocytes. Our findings suggest that OPN expression by keratinocytes is strongly induced by IFN-γ and is involved in perpetuation of the antigen-specific immune response through its chemotactic and Th1 cytokine functions.
Figure 7.
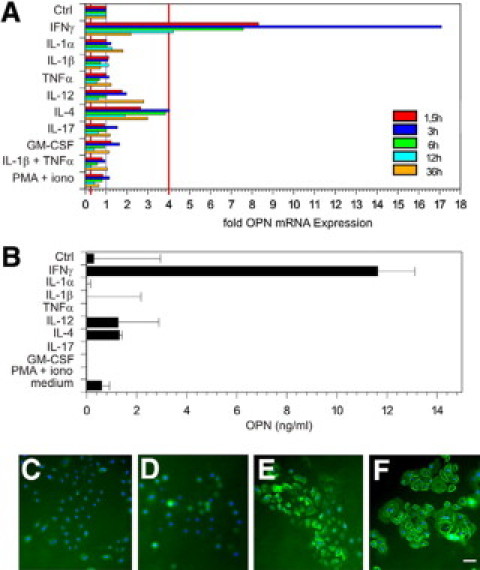
Among proinflammatory cytokines expressed in contact dermatitis, IFN-γ specifically induces OPN expression in keratinocytes. Human-cultured keratinocytes were stimulated with the indicated proinflammatory cytokines and OPN mRNA expression was measured by real-time PCR at time points 1.5, 3, 6, 12, and 36 hours after cytokine stimulation (A) or OPN secretion was measured 48 hours after cytokine stimulation (B). Immunohistochemistry was performed with layers of untreated keratinocytes (D) or with IFN-γ (48 hours) stimulated keratinocytes (C, E, and F). Cells were stained with primary isotype control antibody (C) or antibodies against OPN (D and E) or CD44s (F). Cy2 (green fluorescent) labeled secondary antibody was used to visualize staining (C–F). Cell nuclei are stained in blue (bisBenzidineH 33342 trihydrochloride; Sigma-Aldrich); bar = 15 μm. The data represent the results of three independent experiments with keratinocytes from three different donors.
OPN Null Mice Are Impaired in Their Capacity to Mount Chronic CHS Response
To determine the in vivo importance of OPN expression for the chronification of CHS, we challenged TNCB-sensitized mice every 3 days over a period of 27 days (Figure 8A). The ear-swelling response was determined 24 and 48 hours after challenge. Compared with wild-type mice, OPN null mice showed a significantly reduced CHS response (Figure 8B). Over the time period of 27 days, differences between wild-type and OPN null mice were gradually reduced. An explanation could be that other cytokines gradually substitute for the missing OPN. To investigate T cell-dependent pathomechanism explaining reduced chronic CHS, we investigated the influx of T cells into acute CHS lesions. In OPN null mice, we found that fewer CD4 and CD8 effector cells enter the inflammatory site (Figure 8C). To further confirm that this was at least partially due to a reduced OPN secretion by antigen-specific activated T cells, OPN wild-type, or OPN null T cells were transferred into OPN wild-type RAG2−/− mice (Figure 8D). The mice that had received the OPN null T cells also showed impaired influx of CD4 and CD8 cells into the elicitation site. In conclusion, our data provide evidence that OPN highly secreted by antigen activated T cells in vitro and in vivo is a central factor for a robust chronic allergic immune response at least in part by attracting T effector cells into the skin.
Figure 8.
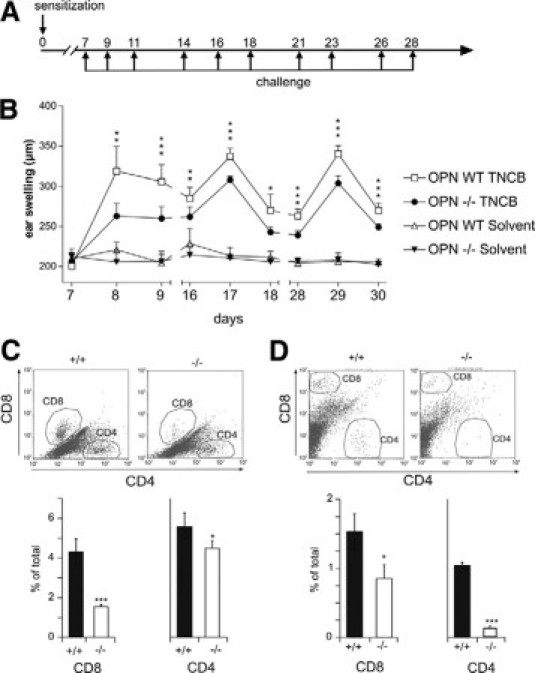
OPN null mice have impaired chronic CHS response. A and B: OPN wild-type (WT) and OPN null (−/−) mice were sensitized on the abdominal skin with 100 μl of TNCB (7% in acetone). On day 7, the mice were challenged with 20 μl of TNCB on both sides of the ear (1% in acetone). To induce chronification of CHS, the challenge was repeated three times per week for 3 weeks. Control animals were treated by solvent alone. Ear swelling was quantified by dial thickness gauge (Mitutoyo) on days 7 (before first challenge), 8, 9, 16, 17, 18, 28, 29, and 30. OPN wild-type TNCB: n = 12, OPN−/− TNCB: n = 5, OPN wild-type solvent: n = 12, OPN−/− solvent: n = 8 mice. C: OPN wild-type (+/+) and OPN null (−/−) mice were sensitized on the abdominal skin with 100 μl of 7% TNCB. After 5 days, the mice were challenged with 20 μl of 1% TNCB on both sides of the ear, and single-cell suspension of ear skin was analyzed for infiltrating CD4+ and CD8+ cells by flow cytometry after 24 hours (n = 4 mice). D: Pan T cells were purified from lymph nodes and spleens of OPN wild-type (+/+) and OPN null (−/−) donor mice and adoptively transferred i.v. into syngeneic Rag2−/− recipient mice. Twelve hours after transfer, recipient mice were sensitized on the abdominal skin with 100 μl of 7% TNCB in acetone or acetone alone. After 5 days, the mice were challenged with 20 μl of TNCB (1% in acetone) on both sides of the ear. Twenty-four hours after challenge, single-cell suspension of ear skin was analyzed by flow cytometry (60,000 to 100,000 events) to assess infiltration of CD4+ and CD8+ (n = 3 recipient mice). (Statistically significant, unpaired t-test: ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001). The data represent the results of two independent experiments.
OPN Antibodies Suppress Established Chronic CHS
To finally investigate whether OPN could be a therapeutic target, chronic CHS was elicited in mice and anti OPN mAbs were injected i.p. (Figure 9A). Monoclonal OPN antibodies were able to significantly suppress the established chronic CHS inflammatory response (Figure 9B). Importantly, antibodies were even effective when applied during repeated antigen challenge that induces a very strong inflammatory response. These findings underline the involvement of OPN in chronic inflammatory CHS responses and hint toward a possible therapeutic application of anti-OPN antibody preparations for chronic unmanageable chronic ACD.
Figure 9.
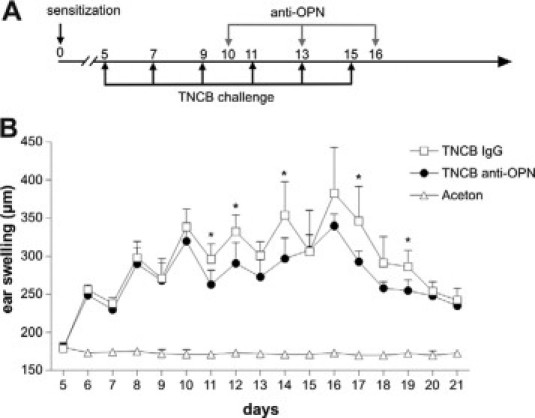
Anti-OPN antibodies suppress established chronic CHS. Mice were sensitized on day 0 with 7% TNCB in acetone or acetone as control. CHS was elicited with 1% TNCB or acetone as indicated in A. TNCB sensitized animals were injected i.p. with 400 μg of anti-OPN monoclonal antibody or isotype matched control antibody on days 10, 13, and 16 (A and B). Ear swelling was measured daily beginning on day 5. TNCB IgG: n = 5, TNCB anti-OPN: n = 5, acetone: n = 8 mice. (Statistically significant, unpaired t-test: ∗P < 0.05.)
Discussion
OPN has been shown to have important functions for the induction of a robust Th1/Th17-mediated immunity in antimicrobial response and in autoimmune diseases.13,14,24,34,51 Here we demonstrated that OPN is highly expressed during the induction of chronic ACD, for example, against NiSO4 and use a murine model of chronic ACD to study the implications of OPN secretion for the induction of chronic antigen-specific inflammation of the skin. Having previously shown that OPN is important during the sensitization phase of CHS by its Th1-polarizing and chemotactic effect on mDCs,36 we here demonstrate that OPN functions as a central cytokine during the elicitation of chronic ACD/CHS when highly induced by antigen-specific T cell activation.
We found that during the elicitation phase of ACD infiltrating CD45RO+ memory T cells secrete OPN. Recently, Shinohara et al24 demonstrated that OPN gene expression in activated T cells is regulated by the transcription factor T-bet that controls CD4+ helper T cell commitment toward a Th1 phenotype. Furthermore, T-bet-dependent expression of OPN is essential for CD4+ and CD8+ effector cells to develop into Th1/Tc1 cells. We therefore investigated whether OPN expression is a signal induced by antigen-specific activation of effector T cells. When specifically stimulating CD4+ or CD8+ T cells from nickel-allergic donors with NiSO4, we found that both T cell subsets highly produced sOPN. OPN secreted at inflammatory sites has been shown to have multiple functions. OPN is a chemo-attractant for various types of immune cells, especially monocytes, mDCs, and T cells.22,52,53 We therefore propose that during the elicitation phase of ACD OPN attracts additional antigen-specific T cells, monocytes, and DCs (Figure 10). Furthermore, through its cytokine functions OPN induces IL-12 secretion in macrophages simultaneously inhibiting their IL-10 production.51 Importantly, we found that OPN is able to induce a Th1-skewing phenotype in myeloid-type DCs by inducing their IL-12 secretion, which may further help to stabilize the Th1 response through antigen presentation by such polarized DCs.36 We therefore propose that OPN highly secreted on antigen-specific stimulation of memory T cells affects monocyte and DC functions to stabilize the Th1-dominated immune response (Figure 10).
Figure 10.
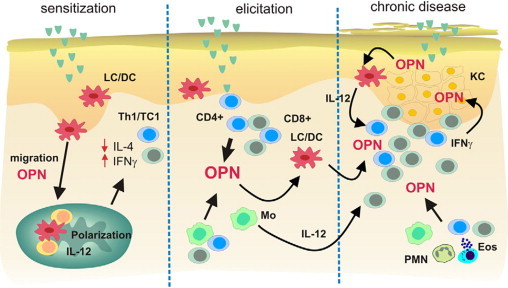
A model for the role of OPN in the chronification of allergic contact dermatitis. During the sensitization-phase OPN interacts with CD44 and αv integrins on LC/DC to guide them from the skin into skin draining lymph nodes.22 Simultaneously OPN induces the polarization of LC/DC toward a Th1-modulating phenotype by inducing their IL-12 secretion.36 During the effector phase, OPN is secreted by activated memory T cells, and as a chemokine, it induces the influx of additional T effector cells, neutrophils, and mast cells. When chronification occurs after repeated application of the allergen, the strong expression of OPN can stabilize the inflammatory response by inducing IL-12 in macrophages and DCs to maintain IFN-γ-dominated Th1 immune response. Simultaneously, IFN-γ secreted by effector cells induces the production of OPN by keratinocytes. Keratinocyte OPN may directly affect the polarization and activation of LCs (epidermal DCs).
To further investigate the mechanisms of OPN for T cell function in CHS nickel-specific T cell clones were generated from the peripheral blood of nickel-allergic donors. To our surprise, in such clones that proliferated specifically, OPN could not be up-regulated by antigen stimulation. We can only speculate that this may occur because such clones were repeatedly restimulated with IL-2 in the process of their generation. These cells may have differentiated into memory T cells that already produce higher amounts of OPN that may not be further up-regulated by TCR activation. This has not been observed previously but will be an interesting topic to investigate in depth in future studies.
However, when comparing different clones, we found that OPN secretion varied among them, correlating with their cytokine-secreting phenotype. We found that clones that secreted very high amounts of OPN secreted low levels of IFN-γ but moderate to high amounts of IL-4. Th1-type clones with high IFN-γ secretion however, produced less than half the amounts of OPN than the Th2-type cells. Interestingly, two clones that produced abundant OPN secreted very low levels of both IL-4 and IFN-γ, indicating that high OPN levels may suppress these cytokines in an autocrine manner. Because these T cell clones express the IFN-γ receptor as well as the OPN receptors CD44 isoforms and OPN-binding integrins, we tested OPN effects on IL-4 and IFN-γ secretion in our clones. When treating clones with recombinant OPN from eukaryotic cells, OPN did not significantly modulate IFN-γ, however, strongly down modulated IL-4 secretion. These findings indicate that in an inflammatory setting OPN highly secreted by antigen-specific T cells may have Th1 cytokine functions that in a loop-like fashion inhibit IL-4 secretion of effector cells, thereby stabilizing the Th1-dominated inflammatory response. Our data are in accordance to recent findings that demonstrated that OPN administration during the challenge phase of allergic airway diseases decreases established Th2 responses, also by down-modulating IL-4 secretion.21 Interestingly, in the human system it was shown that the OPN gene is highly up-regulated during bee venom immunotherapy, which is considered to be effective at least partially by IL-4-inhibiting Th1-skewing effects on the antigen-specific immune response.54
In the TNCB/TNBS model of murine CHS, CD8+ T cells have been shown to be the major effector T cells. In this system, both CD4+ and CD8+ T cells secreted OPN when restimulated by TNBS; however, CD8+ T cells showed stronger antigen-specific proliferation leading to the production of more than twice the amount of OPN that is secreted by the CD4+ cells, which indicates that OPN is an important factor used by CD8+ effector T cells. Hur et al34 demonstrated that in autoimmune disease of the brain, OPN functions as a prosurvival signal for T cells through OPN promoted activation of Nf-κb inhibition of the transcription factor Foxo3a and alteration of the proapoptotic proteins Bim, Bak, and Bax. It seems likely that OPN antiapoptotic functions are implicated in the chronification of ACD/CHS, inhibiting the elimination of T effector cells.
In skin biopsies of ACD we found that OPN was gradually up-regulated by keratinocytes as the disease became more chronic. Activation of keratinocytes is an important pro inflammatory factor during the elicitation of ACD/CHS.50 Various cytokines secreted in the process of the inflammatory response are known to activate keratinocytes.55 Because OPN expression by keratinocytes has not been investigated so far, we stimulated keratinocyte cultures by cytokines typically expressed in ACD lesions and found that IFN-γ quickly and strongly induced keratinocyte OPN mRNA expression. IL-4 in contrast induced only a modest increase in OPN expression in keratinocytes with quantitative mRNA levels only just beyond significance. When determining sOPN in supernatants of cytokine stimulated keratinocytes, we found that cells, when considering the total cell number in cultures, produced relatively moderate amounts of OPN. In addition to αv integrins, keratinocytes highly express CD44 isoforms that have been described to bind OPN. We therefore propose that high amounts of the OPN secreted by keratinocytes are bound on the cell surface by OPN receptors. The staining pattern of cultured keratinocytes as well as the staining of keratinocytes in ACD lesions suggests that keratinocytes may contain an intracellular form of OPN,18,56 its function for keratinocytes, however, remaining subject for future investigations. Functionally OPN secreted by keratinocytes may have implications for the activation of LCs, the DCs of the epidermis, as we have shown that OPN is able to activate LCs on their emigration from the epidermis, and induces their IL-12 secretion.22 This supports our hypothesis that keratinocyte sOPN may be a factor that supports the stabilization of the chronic Th1 response by polarizing Th1-skewing DCs. Because in various cell systems OPN has been shown to have antiapoptotic capacities,34,57–60 one could additionally speculate that keratinocytes use OPN as an antiapoptotic factor, when they are attacked by Fas-expressing CD8+ effector T cells, which however has to be subject of further investigations.
When studying the role of OPN for the induction of chronic CHS in vivo, we found that OPN null mice respond with less severe chronic CHS than wild-type mice, which indicates that OPN is an important factor for the chronification of T cell-mediated inflammatory response in vivo. Indeed, in OPN null mice, less CD4+ and CD8+ effector T cells were found to invade the challenged skin. Furthermore, we found that the OPN secreted by the antigen-activated T cells was a central factor explaining the reduced allergic response, because RAG2−/− mice established with OPN null T cells were also less effective in attracting T cells to the inflamed skin. In conclusion, these findings provide strong evidence that highly secreted sOPN from effector T cells is a major factor for robust establishment of chronic antigen-specific driven allergic inflammation.
The importance of these data are further underlined by our finding that fully established chronic CHS can be suppressed by anti OPN antibodies. The antibody used in this study was designed to bind to the matricryptic SLAYGLR (SVVYGLR in human) sequence of OPN that is only exposed on protease cleavage of OPN. We found that T cells in CHS express receptors for full-length OPN such as αv integrins and CD44 isoforms and further α4β1 and α9β1 integrin that interact with the SLAYGLR sequence of cleaved OPN. Previous work has established that noncleaved and thrombin cleaved forms of OPN are present at inflammatory sites and that especially cleaved OPN that contains the SLAYGLR sequence contributes to cell-mediated inflammation, eg, in T cell-mediated liver disease and rheumatoid arthritis.61,62 We speculate that in CHS OPN protease cleavage at sites of chronic inflammation may enhance the inflammatory response by attracting α4 and α9 integrin-expressing effector cells. Therefore, in addition to general OPN-neutralizing functions, the monoclonal antibody used here may be especially effective by blocking the matricryptic epitope of cleaved OPN, making it an interesting tool for therapeutic approaches in therapy resistant chronic ACD. Interestingly, by OPN antibody suppression of CHS (Figure 9B) was not as effective as total deletion of OPN in OPN null mice (Figure 9B). This may be explained in part by the fact that OPN monoclonal antibodies are able to deplete only sOPN, but the function of intracellular isoforms of OPN is not affected by the antibody.
In conclusion, our data support a model (Figure 10) in which OPN has an important function for Th1 cell-mediated skin inflammation, which may open the perspective to use anti-OPN antibody preparations for the treatment of therapy refractory T cell-mediated skin disease. During the sensitization phase of CHS, OPN is involved in guiding DCs toward skin draining lymph nodes, simultaneously skewing them toward Th1-polarizing abilities. When ACD/CHS is elicited, OPN is highly secreted by antigen-specific effector cells, attracting further inflammatory cells and activating monocytes to secrete IL-12 and simultaneously inhibiting their IL-10 secretion. Attracted T effectors secrete IFN-γ, which then activates keratinocytes to produce OPN that may help T effectors to invade the epidermis and damage keratinocytes. Keratinocyte-derived OPN may influence epidermal LCs to consolidate Th1-driven response, especially as the disease progresses to become chronic.
Acknowledgements
We thank Thomas Ahrens for providing the recombinant OPN and his support in all questions of protein biochemistry. We thank Hermann-Josef Thierse for his kind support in generation of nickel-specific clones and Stefan F. Martin for fruitful discussions and critical review of the manuscript. Josef Schlick and Jan Scheurmann contributed to parts of this work by their expert technical assistance. The MPIIIB10 (1) monoclonal antibody developed by Solursh/Franzen was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by the University of Iowa, Department of Biological Sciences.
Footnotes
Supported by a grant from the Landesstiftung Baden-Württemberg, “Forschungsprogramm Allergologie” grant P-LS-AL/12 (to J.M.W.). A.S. is supported by the Deutsche Forschungsgemeinschaft grant SFB497-C7.
A.M.S. and A.C.R. contributed equally to this work.
References
- 1.Diepgen TL, Coenraads PJ. The epidemiology of occupational contact dermatitis. Int Arch Occup Environ Health. 1999;72:496–506. doi: 10.1007/s004200050407. [DOI] [PubMed] [Google Scholar]
- 2.Kimber I, Dearman RJ. Allergic contact dermatitis: the cellular effectors. Contact Dermatitis. 2002;46:1–5. doi: 10.1034/j.1600-0536.2002.460101.x. [DOI] [PubMed] [Google Scholar]
- 3.Cavani A, Girolomoni G. Immune mechanisms in allergic contact dermatitis. Landes Bioscience; Georgetown, TX: 2005. p. 1. [Google Scholar]
- 4.Hogan DJ, Dannaker CJ, Maibach HI. The prognosis of contact dermatitis. J Am Acad Dermatol. 1990;23:300–307. doi: 10.1016/0190-9622(90)70213-2. [DOI] [PubMed] [Google Scholar]
- 5.Thyssen JP, Johansen JD, Menne T. Contact allergy epidemics and their controls. Contact Dermatitis. 2007;56:185–195. doi: 10.1111/j.1600-0536.2006.01058.x. [DOI] [PubMed] [Google Scholar]
- 6.Frosch PJ, Menne T, Lepoittevin JP. Contact dermatitis. Springer; Berlin: 2007. p. 135. [Google Scholar]
- 7.Romani N, Ebner S, Tripp CH, Flacher V, Koch F, Stoitzner P. Epidermal Langerhans cells—changing views on their function in vivo. Immunol Lett. 2006;106:119–125. doi: 10.1016/j.imlet.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal Langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23:611–620. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Kissenpfennig A, Henri S, Dubois B, Laplace-Builhe C, Perrin P, Romani N, Tripp CH, Douillard P, Leserman L, Kaiserlian D, Saeland S, Davoust J, Malissen B. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Cavani A, Albanesi C, Traidl C, Sebastiani S, Girolomoni G. Effector and regulatory T cells in allergic contact dermatitis. Trends Immunol. 2001;22:118–120. doi: 10.1016/s1471-4906(00)01815-9. [DOI] [PubMed] [Google Scholar]
- 11.Martin SF, Jakob T. From innate to adaptive immune responses in contact hypersensitivity. Curr Opin Allergy Clin Immunol. 2008;8:289–293. doi: 10.1097/ACI.0b013e3283088cf9. [DOI] [PubMed] [Google Scholar]
- 12.Pichler BJ, Kneilling M, Haubner R, Braumuller H, Schwaiger M, Rocken M, Weber WA. Imaging of delayed-type hypersensitivity reaction by PET and 18F-galacto-RGD. J Nucl Med. 2005;46:184–189. [PubMed] [Google Scholar]
- 13.O'Regan AW, Nau GJ, Chupp GL, Berman JS. Osteopontin (Eta-I) in cell mediated immunity: teaching an old dog new tricks. Immunol Today. 2000;21:475–478. doi: 10.1016/s0167-5699(00)01715-1. [DOI] [PubMed] [Google Scholar]
- 14.Denhardt DT, Noda M, O'Regan AW, Pavlin D, Berman JS. Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest. 2001;107:1055–1061. doi: 10.1172/JCI12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang KX, Denhardt DT. Osteopontin: role in immune regulation and stress responses. Cytokine Growth Factor Rev. 2008;19:333–345. doi: 10.1016/j.cytogfr.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Buback F, Renkl AC, Schulz G, Weiss JM. Osteopontin and the skin: -Multiple emerging roles in cutaneous biology and pathology. Exp Dermatol. 2009;18:750–759. doi: 10.1111/j.1600-0625.2009.00926.x. [DOI] [PubMed] [Google Scholar]
- 17.Cantor H, Shinohara ML. Regulation of T helper cell lineage development by osteopontin: the inside story. Nat Rev Immunol. 2009;9:137–141. doi: 10.1038/nri2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shinohara ML, Lu L, Bu J, Werneck MB, Kobayashi KS, Glimcher LH, Cantor H. Osteopontin expression is essential for interferon-α production by plasmacytoid dendritic cells. Nat Immunol. 2006;7:498–506. doi: 10.1038/ni1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shinohara ML, Kim JH, Garcia VA, Cantor H. Engagement of the type I interferon receptor on dendritic cells inhibits T helper 17 cell development: role of intracellular osteopontin. Immunity. 2008;29:68–78. doi: 10.1016/j.immuni.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shinohara ML, Kim HJ, Kim JH, Garcia VA, Cantor H. Alternative translation of osteopontin generates intracellular and secreted isoforms that mediate distinct biological activities in dendritic cells. Proc Natl Acad Sci USA. 2008;105:7235–7239. doi: 10.1073/pnas.0802301105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xanthou G, Alissafi T, Semitekolou M, Simoes DC, Economidou E, Gaga M, Lambrecht BN, Lloyd CM, Panoutsakopoulou V. Osteopontin has a crucial role in allergic airway disease through regulation of dendritic cell subsets. Nat Med. 2007;13:570–578. doi: 10.1038/nm1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiss JM, Renkl AC, Maier CS, Kimmig M, Liaw L, Ahrens T, Kon S, Maeda M, Hotta H, Uede T, Simon JC. Osteopontin is involved in the initiation of cutaneous contact hypersensitivity by inducing Langerhans and dendritic cell migration to lymph nodes. J Exp Med. 2001;194:1219–1229. doi: 10.1084/jem.194.9.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murugaiyan G, Mittal A, Weiner HL. Increased osteopontin expression in dendritic cells amplifies IL-17 production by CD4+ T cells in experimental autoimmune encephalomyelitis and in multiple sclerosis. J Immunol. 2008;181:7480–7488. doi: 10.4049/jimmunol.181.11.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shinohara ML, Jansson M, Hwang ES, Werneck MB, Glimcher LH, Cantor H. T-bet-dependent expression of osteopontin contributes to T cell polarization. Proc Natl Acad Sci USA. 2005;102:17101–17106. doi: 10.1073/pnas.0508666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong CK, Lit LC, Tam LS, Li EK, Lam CW. Elevation of plasma osteopontin concentration is correlated with disease activity in patients with systemic lupus erythematosus. Rheumatology (Oxford) 2005;44:602–606. doi: 10.1093/rheumatology/keh558. [DOI] [PubMed] [Google Scholar]
- 26.Comabella M, Pericot I, Goertsches R, Nos C, Castillo M, Blas NJ, Rio J, Montalban X. Plasma osteopontin levels in multiple sclerosis. J Neuroimmunol. 2005;158:231–239. doi: 10.1016/j.jneuroim.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Xu G, Nie H, Li N, Zheng W, Zhang D, Feng G, Ni L, Xu R, Hong J, Zhang JZ. Role of osteopontin in amplification and perpetuation of rheumatoid synovitis. J Clin Invest. 2005;115:1060–1067. doi: 10.1172/JCI23273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sato T, Nakai T, Tamura N, Okamoto S, Matsuoka K, Sakuraba A, Fukushima T, Uede T, Hibi T. Osteopontin/Eta-1 up-regulated in Crohn's disease regulates the Th1 immune response. Gut. 2005;54:1254–1262. doi: 10.1136/gut.2004.048298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chabas D, Baranzini SE, Mitchell D, Bernard CC, Rittling SR, Denhardt DT, Sobel RA, Lock C, Karpuj M, Pedotti R, Heller R, Oksenberg JR, Steinman L. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science. 2001;294:1731–1735. doi: 10.1126/science.1062960. [DOI] [PubMed] [Google Scholar]
- 30.Kitamura M, Iwabuchi K, Kitaichi N, Kon S, Kitamei H, Namba K, Yoshida K, Denhardt DT, Rittling SR, Ohno S, Uede T, Onoe K. Osteopontin aggravates experimental autoimmune uveoretinitis in mice. J Immunol. 2007;178:6567–6572. doi: 10.4049/jimmunol.178.10.6567. [DOI] [PubMed] [Google Scholar]
- 31.Jansson M, Panoutsakopoulou V, Baker J, Klein L, Cantor H. Cutting edge: attenuated experimental autoimmune encephalomyelitis in eta-1/osteopontin-deficient mice. J Immunol. 2002;168:2096–2099. doi: 10.4049/jimmunol.168.5.2096. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka K, Morimoto J, Kon S, Kimura C, Inobe M, Diao H, Hirschfeld G, Weiss JM, Uede T. Effect of osteopontin alleles on β-glucan-induced granuloma formation in the mouse liver. Am J Pathol. 2004;164:567–575. doi: 10.1016/s0002-9440(10)63146-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Renkl AC, Wussler J, Eggers T, Maier CS, Ahrens T, Martin S, Liaw L, Kon S, Uede T, Hirschfeld G, Simon JC, Weiss JM. Th1 cytokine-like properties of osteopontin on T cells, macrophages, and dendritic cells in type 1 immune responses. Allergy Clin Immunol Int. 2004;Supp 1:97–102. [Google Scholar]
- 34.Hur EM, Youssef S, Haws ME, Zhang SY, Sobel RA, Steinman L. Osteopontin-induced relapse and progression of autoimmune brain disease through enhanced survival of activated T cells. Nat Immunol. 2007;8:74–83. doi: 10.1038/ni1415. [DOI] [PubMed] [Google Scholar]
- 35.Sodek J, Batista Da Silva AP, Zohar R. Osteopontin and mucosal protection. J Dent Res. 2006;85:404–415. doi: 10.1177/154405910608500503. [DOI] [PubMed] [Google Scholar]
- 36.Renkl AC, Wussler J, Ahrens T, Thoma K, Kon S, Uede T, Martin S, Simon JC, Weiss JM. Osteopontin functionally activates dendritic cells and induces their differentiation towards a Th-1 polarizing phenotype. Blood. 2005;106:946–955. doi: 10.1182/blood-2004-08-3228. [DOI] [PubMed] [Google Scholar]
- 37.Schulz G, Renkl AC, Seier A, Liaw L, Weiss JM. Regulated osteopontin expression by dendritic cells decisively affects their migratory capacity. J Invest Dermatol. 2008;128:2541–2544. doi: 10.1038/jid.2008.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kon S, Yokosaki Y, Maeda M, Segawa T, Horikoshi Y, Tsukagoshi H, Rashid MM, Morimoto J, Inobe M, Shijubo N, Chambers AF, Uede T. Mapping of functional epitopes of osteopontin by monoclonal antibodies raised against defined internal sequences. J Cell Biochem. 2002;84:420–432. doi: 10.1002/jcb.10039. [DOI] [PubMed] [Google Scholar]
- 39.Weiss JM, Renkl AC, Denfeld RW, de Roche R, Spitzlei M, Schöpf E, Simon JC. Low-dose UVB radiation perturbs the functional expression of B7.1 and B7.2 co-stimulatory molecules on human Langerhans cells. Eur J Immunol. 1995;25:2858–2862. doi: 10.1002/eji.1830251022. [DOI] [PubMed] [Google Scholar]
- 40.Moulon C, Wild D, Dormoy A, Weltzien HU. MHC-dependent and -independent activation of human nickel-specific CD8+ cytotoxic T cells from allergic donors. J Invest Dermatol. 1998;111:360–366. doi: 10.1046/j.1523-1747.1998.00306.x. [DOI] [PubMed] [Google Scholar]
- 41.Denfeld RW, Hollenbaugh D, Fehrenbach A, Weiss JM, von Leoprechting A, Mai B, Voith U, Schöpf E, Aruffo A, Simon JC. CD40 is functionally expressed on human keratinocytes. Eur J Immunol. 1996;26:2329–2334. doi: 10.1002/eji.1830261009. [DOI] [PubMed] [Google Scholar]
- 42.Liaw L, Birk DE, Ballas CB, Whitsitt JS, Davidson JM, Hogan BL. Altered wound healing in mice lacking a functional osteopontin gene (spp1) J Clin Invest. 1998;101:1468–1478. doi: 10.1172/JCI1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weiss JM, Sleeman J, Renkl AC, Dittmar HC, Termeer CT, Taxis S, Howells N, Hofmann M, Köhler G, Schöpf E, Ponta H, Herrlich P, Simon JC. An essential role for CD44 variant isoforms in epidermal Langerhans cell and blood dendritic cell function. J Cell Biol. 1997;137:1137–1147. doi: 10.1083/jcb.137.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 45.Young MF, Kerr JM, Termine JD, Wewer UM, Wang MG, McBride OW, Fisher LW. cDNA cloning, mRNA distribution and heterogeneity, chromosomal location, and RFLP analysis of human osteopontin (OPN) Genomics. 1990;7:491–502. doi: 10.1016/0888-7543(90)90191-v. [DOI] [PubMed] [Google Scholar]
- 46.Ahrens T, Pertz O, Haussinger D, Fauser C, Schulthess T, Engel J. Analysis of heterophilic and homophilic interactions of cadherins using the c-Jun/c-Fos dimerization domains. J Biol Chem. 2002;277:19455–19460. doi: 10.1074/jbc.M200606200. [DOI] [PubMed] [Google Scholar]
- 47.Chiocchetti A, Indelicato M, Bensi T, Mesturini R, Giordano M, Sametti S, Castelli L, Bottarel F, Mazzarino MC, Garbarini L, Giacopelli F, Valesini G, Santoro C, Dianzani I, Ramenghi U, Dianzani U. High levels of osteopontin associated with polymorphisms in its gene are a risk factor for development of autoimmunity/lymphoproliferation. Blood. 2004;103:1376–1382. doi: 10.1182/blood-2003-05-1748. [DOI] [PubMed] [Google Scholar]
- 48.Patarca R, Freeman GJ, Singh RP, Wei FY, Durfee T, Blattner F, Regnier DC, Kozak CA, Mock BA, Morse HC3. Structural and functional studies of the early T lymphocyte activation 1 (Eta-1) gene: definition of a novel T cell-dependent response associated with genetic resistance to bacterial infection. J Exp Med. 1989;170:145–161. doi: 10.1084/jem.170.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moulon C, Vollmer J, Weltzien HU. Characterization of processing requirements and metal cross-reactivities in T cell clones from patients with allergic contact dermatitis to nickel. Eur J Immunol. 1995;25:3308–3315. doi: 10.1002/eji.1830251216. [DOI] [PubMed] [Google Scholar]
- 50.Albanesi C, Cavani A, Scarponi C, Girolomoni G. In: Multiple roles of keratinocytes in allergic contact dermatitis: immune mechanisms in allergic contact dermatitis. Cavani A, Girolomoni G, editors. Landes Bioscience; Georgetown, TX: 2005. p. 104. [Google Scholar]
- 51.Ashkar S, Weber GF, Panoutsakopoulou V, Sanchirico ME, Jansson M, Zawaideh S, Rittling SR, Denhardt DT, Glimcher MJ, Cantor H. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 2000;287:860–864. doi: 10.1126/science.287.5454.860. [DOI] [PubMed] [Google Scholar]
- 52.O'Regan AW, Chupp GL, Lowry JA, Goetschkes M, Mulligan N, Berman JS. Osteopontin is associated with T cells in sarcoid granulomas and has T cell adhesive and cytokine-like properties in vitro. J Immunol. 1999;162:1024–1031. [PubMed] [Google Scholar]
- 53.Bruemmer D, Collins AR, Noh G, Wang W, Territo M, Arias-Magallona S, Fishbein MC, Blaschke F, Kintscher U, Graf K, Law RE, Hsueh WA. Angiotensin II-accelerated atherosclerosis and aneurysm formation is attenuated in osteopontin-deficient mice. J Clin Invest. 2003;112:1318–1331. doi: 10.1172/JCI18141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Konno S, Golden DB, Schroeder J, Hamilton RG, Lichtenstein LM, Huang SK. Level of osteopontin is increased after bee venom immunotherapy. J Allergy Clin Immunol. 2005;115:1317–1318. doi: 10.1016/j.jaci.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 55.Albanesi C, Scarponi C, Giustizieri ML, Girolomoni G. Keratinocytes in inflammatory skin diseases. Curr Drug Targets Inflamm Allergy. 2005;4:329–334. doi: 10.2174/1568010054022033. [DOI] [PubMed] [Google Scholar]
- 56.Zohar R, Suzuki N, Suzuki K, Arora P, Glogauer M, McCulloch CA, Sodek J. Intracellular osteopontin is an integral component of the CD44-ERM complex involved in cell migration. J Cell Physiol. 2000;184:118–130. doi: 10.1002/(SICI)1097-4652(200007)184:1<118::AID-JCP13>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 57.Burdo TH, Wood MR, Fox HS. Osteopontin prevents monocyte recirculation and apoptosis. J Leukoc Biol. 2007;81:1504–1511. doi: 10.1189/jlb.1106711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee JL, Wang MJ, Sudhir PR, Chen GD, Chi CW, Chen JY. Osteopontin promotes integrin activation through outside-in and inside-out mechanisms: OPN-CD44V interaction enhances survival in gastrointestinal cancer cells. Cancer Res. 2007;67:2089–2097. doi: 10.1158/0008-5472.CAN-06-3625. [DOI] [PubMed] [Google Scholar]
- 59.Rice J, Courter DL, Giachelli CM, Scatena M. Molecular mediators of αvβ3-induced endothelial cell survival. J Vasc Res. 2006;43:422–436. doi: 10.1159/000094884. [DOI] [PubMed] [Google Scholar]
- 60.Cook AC, Tuck AB, McCarthy S, Turner JG, Irby RB, Bloom GC, Yeatman TJ, Chambers AF. Osteopontin induces multiple changes in gene expression that reflect the six “hallmarks of cancer” in a model of breast cancer progression. Mol Carcinog. 2005;43:225–236. doi: 10.1002/mc.20105. [DOI] [PubMed] [Google Scholar]
- 61.Ohshima S, Yamaguchi N, Nishioka K, Mima T, Ishii T, Umeshita-Sasai M, Kobayashi H, Shimizu M, Katada Y, Wakitani S, Murata N, Nomura S, Matsuno H, Katayama R, Kon S, Inobe M, Uede T, Kawase I, Saeki Y. Enhanced local production of osteopontin in rheumatoid joints. J Rheumatol. 2002;29:2061–2067. [PubMed] [Google Scholar]
- 62.Diao H, Kon S, Iwabuchi K, Kimura C, Morimoto J, Ito D, Segawa T, Maeda M, Hamuro J, Nakayama T, Taniguchi M, Yagita H, Van Kaer L, Onoe K, Denhardt D, Rittling S, Uede T. Osteopontin as a mediator of NKT cell function in T cell-mediated liver diseases. Immunity. 2004;21:539–550. doi: 10.1016/j.immuni.2004.08.012. [DOI] [PubMed] [Google Scholar]


