Abstract
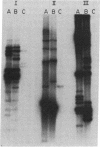
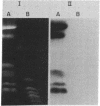
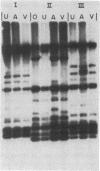
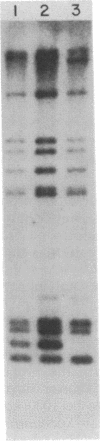
Candida albicans causes a wide variety of infections but can readily be isolated from the skin and mucosa of healthy individuals. To enable high-resolution epidemiologic studies on this common pathogen, a species-specific DNA probe has been isolated from its genome. There are approximately equal to 10 copies of the sequence dispersed among the chromosome-sized DNA molecules resolved by pulsed-field electrophoresis. New DNA polymorphisms in this gene family arise at high rates. As a consequence, this probe will readily distinguish strains from different patients in the same hospital and from various sites in individual patients. The DNA polymorphisms detected by using this probe are largely due to internal changes in members of the family rather than movement to new genomic locations. This suggests recombination or gene conversion rather than transposition as the mechanism producing the observed variation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calos M. P., Miller J. H. Transposable elements. Cell. 1980 Jul;20(3):579–595. doi: 10.1016/0092-8674(80)90305-0. [DOI] [PubMed] [Google Scholar]
- Cameron J. R., Loh E. Y., Davis R. W. Evidence for transposition of dispersed repetitive DNA families in yeast. Cell. 1979 Apr;16(4):739–751. doi: 10.1016/0092-8674(79)90090-4. [DOI] [PubMed] [Google Scholar]
- Carle G. F., Olson M. V. Separation of chromosomal DNA molecules from yeast by orthogonal-field-alternation gel electrophoresis. Nucleic Acids Res. 1984 Jul 25;12(14):5647–5664. doi: 10.1093/nar/12.14.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. E., Jr, Lehrer R. I., Stiehm E. R., Fischer T. J., Young L. S. Severe candidal infections: clinical perspective, immune defense mechanisms, and current concepts of therapy. Ann Intern Med. 1978 Jul;89(1):91–106. doi: 10.7326/0003-4819-89-1-91. [DOI] [PubMed] [Google Scholar]
- Lerner C. W., Tapper M. L. Opportunistic infection complicating acquired immune deficiency syndrome. Clinical features of 25 cases. Medicine (Baltimore) 1984 May;63(3):155–164. doi: 10.1097/00005792-198405000-00002. [DOI] [PubMed] [Google Scholar]
- Magee B. B., D'Souza T. M., Magee P. T. Strain and species identification by restriction fragment length polymorphisms in the ribosomal DNA repeat of Candida species. J Bacteriol. 1987 Apr;169(4):1639–1643. doi: 10.1128/jb.169.4.1639-1643.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odds F. C., Abbott A. B., Stiller R. L., Scholer H. J., Polak A., Stevens D. A. Analysis of Candida albicans phenotypes from different geographical and anatomical sources. J Clin Microbiol. 1983 Oct;18(4):849–857. doi: 10.1128/jcm.18.4.849-857.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder G. S., Fink G. R. Movement of yeast transposable elements by gene conversion. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5621–5625. doi: 10.1073/pnas.79.18.5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaberg D. R., Tompkins L. S., Falkow S. Use of agarose gel electrophoresis of plasmid deoxyribonucleic acid to fingerprint gram-negative bacilli. J Clin Microbiol. 1981 Jun;13(6):1105–1108. doi: 10.1128/jcm.13.6.1105-1108.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer S., Davis R. W. Recombination of dispersed repeated DNA sequences in yeast. Science. 1980 Sep 19;209(4463):1380–1384. doi: 10.1126/science.6251545. [DOI] [PubMed] [Google Scholar]
- Scherer S., Stevens D. A. Application of DNA typing methods to epidemiology and taxonomy of Candida species. J Clin Microbiol. 1987 Apr;25(4):675–679. doi: 10.1128/jcm.25.4.675-679.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. C., Cantor C. R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984 May;37(1):67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- Slutsky B., Buffo J., Soll D. R. High-frequency switching of colony morphology in Candida albicans. Science. 1985 Nov 8;230(4726):666–669. doi: 10.1126/science.3901258. [DOI] [PubMed] [Google Scholar]
- Slutsky B., Staebell M., Anderson J., Risen L., Pfaller M., Soll D. R. "White-opaque transition": a second high-frequency switching system in Candida albicans. J Bacteriol. 1987 Jan;169(1):189–197. doi: 10.1128/jb.169.1.189-197.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell R. G., Wilkins R. J. Separation of chromosomal DNA molecules from C.albicans by pulsed field gel electrophoresis. Nucleic Acids Res. 1986 Jun 11;14(11):4401–4406. doi: 10.1093/nar/14.11.4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiller R. L., Bennett J. E., Scholer H. J., Wall M., Polak A., Stevens D. A. Susceptibility to 5-fluorocytosine and prevalence of serotype in 402 Candida albicans isolates from the United States. Antimicrob Agents Chemother. 1982 Sep;22(3):482–487. doi: 10.1128/aac.22.3.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel E., Dunsmuir P., Rubin G. M. Polymorphisms in the chromosomal locations of elements of the 412, copia and 297 dispersed repeated gene families in Drosophila. Cell. 1979 Jun;17(2):429–439. doi: 10.1016/0092-8674(79)90169-7. [DOI] [PubMed] [Google Scholar]
- Tenover F. C. Plasmid fingerprinting. A tool for bacterial strain identification and surveillance of nosocomial and community-acquired infections. Clin Lab Med. 1985 Sep;5(3):413–436. [PubMed] [Google Scholar]
- Tompkins L. S., Plorde J. J., Falkow S. Molecular analysis of R-factors from multiresistant nosocomial isolates. J Infect Dis. 1980 May;141(5):625–636. doi: 10.1093/infdis/141.5.625. [DOI] [PubMed] [Google Scholar]
- Tompkins L. S. Recombinant DNA and other direct specimen identification techniques. Clin Lab Med. 1985 Mar;5(1):99–107. [PubMed] [Google Scholar]
- Warnock D. W. Typing of Candida albicans. J Hosp Infect. 1984 Sep;5(3):244–252. doi: 10.1016/0195-6701(84)90073-2. [DOI] [PubMed] [Google Scholar]
- Whelan W. L., Kerridge D. Decreased activity of UMP pyrophosphorylase associated with resistance to 5-fluorocytosine in Candida albicans. Antimicrob Agents Chemother. 1984 Oct;26(4):570–574. doi: 10.1128/aac.26.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan W. L., Markie D., Kwon-Chung K. J. Complementation analysis of resistance to 5-fluorocytosine in Candida albicans. Antimicrob Agents Chemother. 1986 May;29(5):726–729. doi: 10.1128/aac.29.5.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichman H. A., Potter S. S., Pine D. S. Mys, a family of mammalian transposable elements isolated by phylogenetic screening. Nature. 1985 Sep 5;317(6032):77–81. doi: 10.1038/317077a0. [DOI] [PubMed] [Google Scholar]
- Wills J. W., Lasker B. A., Sirotkin K., Riggsby W. S. Repetitive DNA of Candida albicans: nuclear and mitochondrial components. J Bacteriol. 1984 Mar;157(3):918–924. doi: 10.1128/jb.157.3.918-924.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]